Abstract
The rhizosphere is the infection court where soil-borne pathogens establish a parasitic relationship with the plant. To infect root tissue, pathogens have to compete with members of the rhizosphere microbiome for available nutrients and microsites. In disease-suppressive soils, pathogens are strongly restricted in growth by the activities of specific rhizosphere microorganisms. Here, we sequenced metagenomic DNA and RNA of the rhizosphere microbiome of sugar beet seedlings grown in a soil suppressive to the fungal pathogen Rhizoctonia solani. rRNA-based analyses showed that Oxalobacteraceae, Burkholderiaceae, Sphingobacteriaceae and Sphingomonadaceae were significantly more abundant in the rhizosphere upon fungal invasion. Metatranscriptomics revealed that stress-related genes (ppGpp metabolism and oxidative stress) were upregulated in these bacterial families. We postulate that the invading pathogenic fungus induces, directly or via the plant, stress responses in the rhizobacterial community that lead to shifts in microbiome composition and to activation of antagonistic traits that restrict pathogen infection.
Suppressive soils have been described for various soil-borne pathogens and occur worldwide (Mazzola, 2007; Mendes et al., 2011; Berendsen et al., 2012; Mendes et al., 2013). Disease suppressiveness to soil-borne fungal pathogens is in many cases microbial in origin and develops in the field after several disease outbreaks (Mazzola, 2007). Hence, interactions between the fungal pathogen, the plant and the rhizosphere microbiome are key elements in shaping a plant-protective microbiome. To date, however, the responsible microbes and the underlying mechanisms are largely unknown. To understand how pathogen invasion affects the composition and activities of rhizobacterial communities, we sequenced metagenomic DNA and RNA of the rhizosphere microbiome of sugar beet seedlings grown in a soil suppressive to the fungal pathogen Rhizoctonia solani (Supplementary Figure 1 and Supplementary Table 1). The soil used in this study was described in Mendes et al. (2011) and shown to be suppressive to R. solani. Sugar beet seedlings were grown under controlled conditions in suppressive soil without (S) or with inoculation of R. solani (Sr). Whereas in conducive soils disease incidence typically reaches levels above 60% (Mendes et al., 2011), disease incidence in the pathogen-inoculated suppressive soils was 4.2% (±7.2% s.d., N=3) in the bioassay used for the metagenome analysis and 26.4% (±20% s.d., N=3) in the bioassay used for the metatranscriptome analyses. No R. solani-infected plants were observed in the non-inoculated treatment (S) in both independent bioassays. Analysis of the fungal rRNA reads confirmed that the more abundant fungus in the Sr treatment was R. solani (Thanatephorus cucumeris) (Supplementary Figure 2). R. solani was thus able to invade the rhizosphere of sugar beet seedlings growing in the suppressive soil but caused little disease.
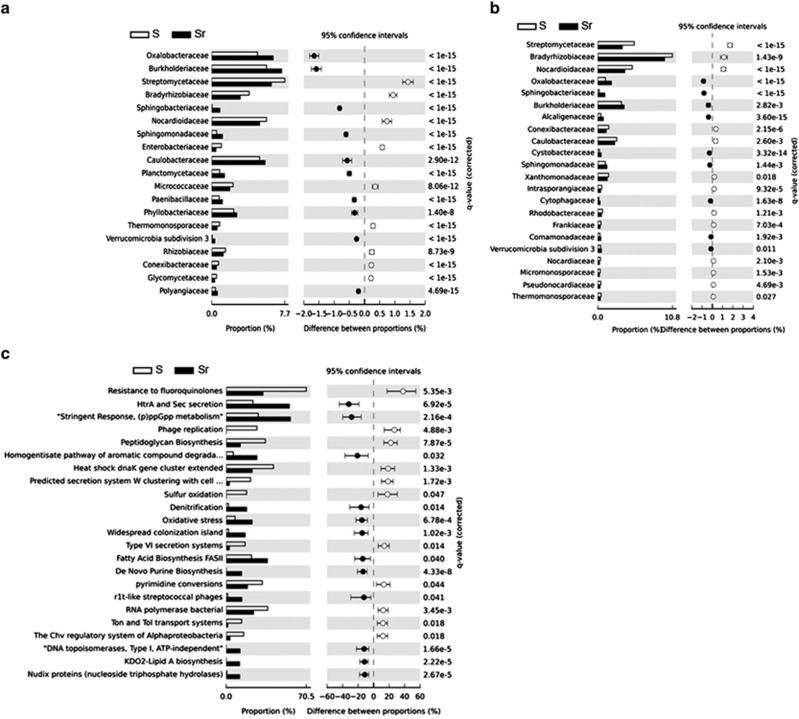
Comparative metagenomic DNA analysis of the rhizobacterial family composition revealed no significant differences between S and Sr. Taxonomic assignment of the annotated rRNA and mRNA reads, however, revealed significant differences between S and Sr, with Oxalobacteraceae, Sphingobacteriaceae, Burkholderiaceae and Sphingomonadaceae as the bacterial families that were consistently more abundant in Sr than in S (Figures 1a and b). Among the Oxalobacteraceae, members of the genera Collimonas and Janthinobacterium are known for their ability to inhibit fungal growth and protect plant roots from fungal infection (Leveau et al., 2010; Johnsen et al., 2010). Similarly, members of the Burkholderiaceae are well studied for the production of diverse metabolites with activity against fungi, including R. solani (El-Banna and Winkelmann, 1998). Also, Sphingobacteria such as Chitinophaga and Pedobacter are known to exhibit fungicidal activities or induce antagonistic traits in other bacterial taxa (Garbeva et al., 2011).
Figure 1.
Rhizosphere bacterial families and functions responding to the inoculation with the soil-borne fungal pathogen R. solani. Bacterial family classifications based on (a) rRNA and (b) mRNA analyses. (c) Functional classification based on mRNA-annotated sequences.
To get a more in-depth understanding of the functions activated in these abundant bacterial families, we analyzed the annotated functions of the mRNA sequence reads. The overall SEED subsystem-based classification showed that protein, RNA and DNA metabolism were the most represented categories, reflecting the active basal cellular machinery of the rhizosphere microbiome (Supplementary Figure 3). To uncover specific functions induced by R. solani, the mRNA reads that binned to the abundant bacterial families described above were extracted from the Sr data set and compared with the overall bacterial mRNA dataset obtained for the S treatment (as an unchallenged rhizosphere community) (Figure 1c). The analysis was also performed in the reverse order by using the functional classification as the starting point before identifying the corresponding contributing bacterial families (Supplementary Figures 4A–C). Both approaches showed that at least three functions were significantly more expressed by the more abundant bacterial families in Sr than in S: these were HtrA/Sec secretion systems, Guanosine-3,5-bis-pyrophosphate ((p)ppGpp) metabolism and oxidative stress response (Figure 1c). The functional analysis of the metagenomes confirmed that genes associated with the stringent response (ppGpp metabolism) and Htr/Sec secretion were more abundant in Sr than in S (Supplementary Figure 5).
The Sec translocase proteic complex is essential for secretion of virulence factors or extracellular lytic enzymes in various bacteria. To our knowledge, no information is available yet on the roles of the Sec system in the more abundant bacterial families detected here. (p)ppGpp metabolism, also referred to as the ‘bacterial alarmone', is a highly conserved mechanism associated with stress perception and stress response regulation. Initial work on ppGpp metabolism in Escherichia coli focused on its role in nutrient stress, but over the past decade it has become evident that ppGpp metabolism has other regulatory functions in different bacterial genera. These include regulation of type III and type IV secretion (Pizarro-Cerda and Tedin, 2004; Dozot et al., 2006) as well as regulation of antagonistic compound release (Manuel et al., 2012). Overrepresentation of ppGpp metabolism in Sr suggests that invasion of the rhizosphere by R. solani directly or indirectly triggered a stress response in several of the abundant rhizobacterial families. Potential triggers of the ppGpp signaling pathway in these bacterial families need to be elucidated, but we propose that oxidative and/or acidic stress via oxalic and phenylacetic acid produced by R. solani itself or by compounds released from plant roots under attack may be the key triggers. The abundant taxa and in particular the Oxalobacteraceae and Burkholderiaceae are known to metabolize oxalate as a carbon source. Evidence for oxalate catabolism (for example, oxalyl-coA decarboxylase and glyoxylate carboligase; Stewart et al., 2004) was found in the metatranscriptome data in particular for members of the α- and β-proteobacteria, with the Burkholderiaceae and Oxalobacteraceae as differential contributors to the function in Sr (Supplementary Figure 4D). In the metatranscriptome, plant functional reads represented 20% and 33% of the annotated mRNA reads in the S and Sr data sets, respectively. Several markers related to the plants' response to oxalic acid, including oxalate oxydase-like germins (known as oxalate-detoxifying and peroxide-producing enzymes; Livingstone et al., 2005) as well as carbonic anhydrase and dihydrolipoamide dehydrogenase (Liang et al., 2009), were only detected in the rhizosphere of plants inoculated with R. solani (Supplementary Table 2). Also, glycolate oxidase, an enzyme implicated in reactive oxygen species-mediated defenses (Rojas et al., 2012), was exclusively found in Sr (Supplementary Table 2).
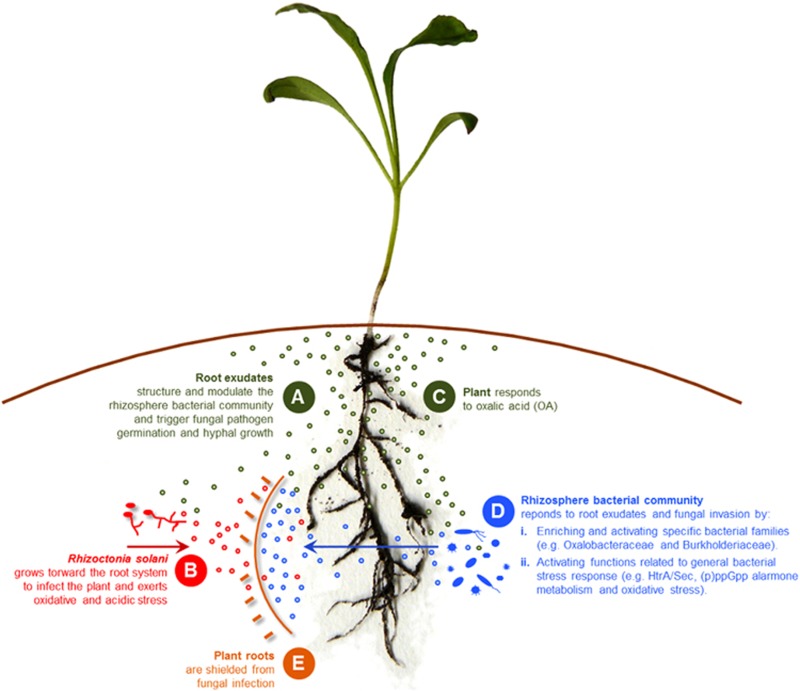
In light of our results, we propose a model (Figure 2) in which R. solani, during hyphal growth toward the plant root, produces oxalic and phenylacetic acid that feed and activate specific rhizobacterial families present in the suppressive rhizosphere microbiome, and, directly or indirectly, exert oxidative stress in specific rhizobacterial families and in the plant (Figure 2). This stress in turn triggers a response in these bacterial families via the ppGpp signaling pathway, leading to the activation of survival strategies such as motility, biofilm formation and the production of secondary metabolites (Figure 2). These compositional and functional changes: (i) adversely affect fungal growth; (ii) induce a plant resistance response; and/or (iii) co-activate other microorganisms in the rhizosphere microbiome to ward off the fungal invader (Figure 2). Future studies will focus on investigating these hypotheses by, among others, re-constructing dynamic rhizobacterial communities with the ultimate goal to elucidate if and how these rhizobacteria restrict fungal infection of the host plant.
Figure 2.
Model illustrating the proposed sequence of events (A thru E) taking place in the rhizosphere of plants grown in a disease suppressive soil during fungal pathogen invasion. Depicted are the changes in microbial community composition and activities that restrict fungal growth and plant infection.
Acknowledgments
This project was financially supported by the EU-funded program ‘EcoFINDERS: Ecological Function and Biodiversity Indicators in European Soils' and in part by the national Dutch BioBased-Economy research program BE-Basic.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on The ISME Journal website (http://www.nature.com/ismej)
Supplementary Material
References
- Berendsen RL, Pieterse CM, Bakker PA. (2012). The rhizosphere microbiome and plant health. Trends Plant Sci 17: 478–486. [DOI] [PubMed] [Google Scholar]
- Dozot M, Boigegrain RA, Delrue RM, Hallez R, Ouahrani-Bettache S, Danese I et al. (2006). The stringent response mediator Rsh is required for Brucella melitensis and Brucella suis virulence, and for expression of the type IV secretion system virB. Cell Microbiol 8: 1791–1802. [DOI] [PubMed] [Google Scholar]
- El-Banna N, Winkelmann G. (1998). Pyrrolnitrin from Burkholderia cepacia: antibiotic activity against fungi and novel activities against streptomycetes. J Appl Microbiol 85: 69–78. [DOI] [PubMed] [Google Scholar]
- Garbeva P, Silby MW, Raaijmakers JM, Levy SB, Boer W. (2011). Transcriptional and antagonistic responses of Pseudomonas fluorescens Pf0-1 to phylogenetically different bacterial competitors. ISME J 5: 973–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnsen MG, Hansen OC, Stougaard P. (2010). Isolation, characterization and heterologous expression of a novel chitosanase from Janthinobacterium sp. strain 4239. Microb Cell Fact 9: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leveau JH, Uroz S, de Boer W. (2010). The bacterial genus Collimonas: mycophagy, weathering and other adaptive solutions to life in oligotrophic soil environments. Environ Microbiol 12: 281–292. [DOI] [PubMed] [Google Scholar]
- Liang Y, Strelkov SE, Kav NN. (2009). Oxalic acid-mediated stress responses in Brassica napus L. Proteomics 9: 3156–3173. [DOI] [PubMed] [Google Scholar]
- Livingstone DM, Hampton JL, Phipps PM, Grabau EA. (2005). Enhancing resistance to Sclerotinia minor in peanut by expressing a barley oxalate oxidase gene. Plant Physiol 137: 1354–1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manuel J, Selin C, Fernando WG, de Kievit T. (2012). Stringent response mutants of Pseudomonas chlororaphis PA23 exhibit enhanced antifungal activity against Sclerotinia sclerotiorum in vitro. Microbiology 158: 207–216. [DOI] [PubMed] [Google Scholar]
- Mazzola M. (2007). Manipulation of rhizosphere bacterial communities to induce suppressive soils. J Nematol 39: 213–220. [PMC free article] [PubMed] [Google Scholar]
- Mendes R, Kruijt M, de Bruijn I, Dekkers E, van der Voort M, Schneider JH et al. (2011). Deciphering the rhizosphere microbiome for disease-suppressive bacteria. Science 332: 1097–1100. [DOI] [PubMed] [Google Scholar]
- Mendes R, Garbeva P, Raaijmakers JM. (2013). The rhizosphere microbiome: significance of plant beneficial, plant pathogenic, and human pathogenic microorganisms. FEMS Microbiol Rev 37: 634–663. [DOI] [PubMed] [Google Scholar]
- Pizarro-Cerda J, Tedin K. (2004). The bacterial signal molecule, ppGpp, regulates Salmonella virulence gene expression. Mol Microbiol 52: 1827–1844. [DOI] [PubMed] [Google Scholar]
- Rojas CM, Senthil-Kumar M, Wang K, Ryu CM, Kaundal A, Mysore KS. (2012). Glycolate oxidase modulates reactive oxygen species-mediated signal transduction during nonhost resistance in Nicotiana benthamiana and Arabidopsis. Plant Cell 24: 336–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart CS, Duncan SH, Cave DR. (2004). Oxalobacter formigenes and its role in oxalate metabolism in the human gut. FEMS Microbiol Lett 230: 1–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.