Abstract
Cystic Fibrosis (CF) is a multisystem disease predominantly affecting the airways and predisposing patients to recurrent infections with various multidrug resistant organisms. Mycobacterium tuberculosis (MTB) infection is rarely seen, but considered a potential pathogen in CF patients. We report a 26 year old pregnant CF patient on Ivacaftor who was admitted with symptoms suggestive of tuberculosis. Three years prior to the current admission, she had completed four drug anti- MTB therapy for pulmonary tuberculosis and was considered cured as her sputum cultures after six months of treatment were negative. Genotype analysis revealed the current MTB strain to be different from the strain causing the previous infection. After receiving first line anti-tuberculous regimen for nine months, the patient's condition markedly improved culminating in an uneventful pregnancy and delivery. To our knowledge, this is the only reported case of reinfection tuberculosis in a CF patient.
Keywords: Cystic fibrosis, Mycobacterium tuberculosis, AFB, GeneXpert MTB/RIF assay, Genotyping
1. Introduction
Cystic Fibrosis (CF) is an autosomal recessive disorder caused by a mutation in Cystic Fibrosis Transmembrane Conductance Regulator [CFTR] gene, a complex chloride channel and regulatory protein. Typically, CF leads to multisystem involvement, predominantly affecting the airways with inability to clear viscous secretions, mucus plugging, and intense inflammation due to lack of the regulatory protein. This predisposes them to recurrent infections with various multidrug resistant organisms, leading to bronchiectasis and fibrotic lung disease. Several studies have shown that Non Tuberculous Mycobacteria (NTM) occur in CF patients, but Mycobacterium Tuberculosis (MTB) infection is rarely seen in these patients and only a few cases have been reported in the literature [1], [2], [3], [4]. We report a CF patient with Δ G551D mutation, on Ivacaftor, previously treated with a full course of Anti Tuberculous Therapy (ATT), who was reinfected with a new strain of MTB three years later.
2. Case report
A 26-year-old Caucasian female at 18 weeks of gestation and a history of CF with Δ G551D mutation on Ivacaftor, a CF Transmembrane Conductance Regulator (CFTR) potentiator, was found to have recurrent admissions to the hospital with exacerbations of her pulmonary symptoms of cough and purulent sputum production, dyspnea on exertion, pleuritic chest pain and small amount of hemoptysis. The vital signs were stable except for a low grade fever of 99.5 °F. Blood cultures were negative, but she was known to harbor multi-drug resistant Pseudomonas aeruginosa, Methicillin Resistant Staphylococcus aureus (MRSA), Achromobacter species and Aspergillus fumigatus in her bronchial airways, and had been treated multiple times in the past for these infections. She was not on any immunosuppressive drugs and did not have any other medical problems. Her chest radiograph revealed bilateral diffuse reticulonodular opacities which had remained stable over the past two years (Fig. 1). She had been treated for pulmonary tuberculosis three years ago, with a regimen that included Isoniazid (INH), Rifampin (RIF), Ethambutol (EMB) and Pyrazinamide (PZA) in the intensive phase for two months, followed by four months of INH and RIF in the continuation phase. Her sputum cultures after completion of therapy were negative for MTB. Two sputum cultures during the current admission revealed MTB, but on genotype analysis, it was found to be a different MTB strain from the one she had before, pointing towards reinfection after successful completion of antitubercular treatment. Drug susceptibility testing showed sensitivity to all first-line drugs (INH, RIF, EMB, PZA). Due to her pregnancy, she was given a three drug regimen of INH, RIF and EMB for nine months. Her repeat sputum cultures were negative for MTB six weeks after therapy was initiated. She tolerated the regimen well with an improvement in her symptoms and delivered a full term male fetus without any complications. The patient was lost to follow-up after completion of therapy and all efforts to contact her thereafter were unsuccessful.
Fig. 1.
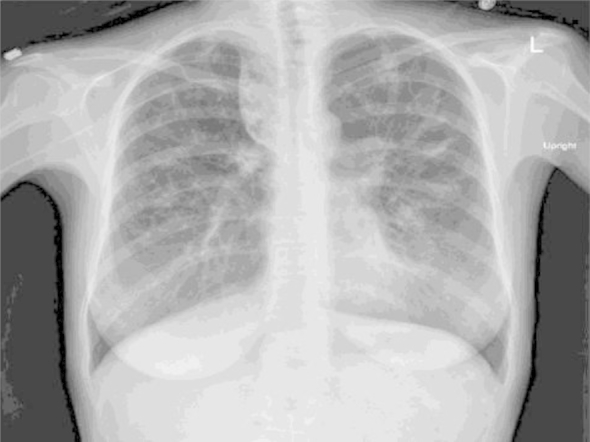
Chest Radiograph with bilateral reticulonodular changes and chronic right upper lobe paratracheal consolidation during this admission.
3. Discussion
NTM infections are seen in approximately 7–13% of CF patients [4]. In vitro studies have shown that NTM invades respiratory epithelium with a protein that attaches to fibronectin within the exposed extracellular matrix on the damaged mucosal surfaces, unlike MTB that adheres to intact mucosa [5]. However, despite having multiple risk factors such as malnutrition, diabetes mellitus, steroid use and chronic lung disease, MTB is rarely seen among CF patients [4], [6]. The etiology for this rarity is not well defined, but several pathways have been suggested that point to a genetic component of the disease or a defect in enzyme activity [7], [8]. It was reported in one study that carriers of this recessive gene are resistant to Mycobacterium tuberculosis infection, which is attributed to the production of excessive amounts of hyaluronic acid in these individuals [8]. Another possible explanation is the diminished activity of arylsulfatase, a lysosomal enzyme, in CF patients compared to normal population [9]. Reduction in the activity of this enzyme by decreasing the availability of sulfate may have evolved as a mechanism to confer protection against M. tuberculosis proliferation in CF patients [9], [10]. Mutation in the G551D gene in CF patients is caused by the substitution of the amino acid glycine by aspartate at position 551 in the nucleotide binding domain-1 of the CFTR gene. Ivacaftor, a CFTR potentiator, specifically targets the G551D gating mutation by improving the chloride transport and increasing the channel opening probability through a non-conventional ATP independent mechanism [11]. Targeted therapy with Ivacaftor normalizes the respiratory mucosa making them more susceptible to MTB infections than NTM. Our patient was not on this therapy when she had her initial MTB infection, but was on it during the current admission. Literature review reveals no cases of MTB infections that have been reported with the use of this therapy.
TB genotyping is a laboratory-based approach used to examine the genetic material of M. tuberculosis. Population-based genotyping using two Polymerase Chain Reaction based methods, spoligotyping and Mycobacterial Interspersed Repetitive Units, has been available in USA since 2004. Specific sections of the genetic content help in distinguishing between dissimilar strains of M. tuberculosis based on the distinct genetic patterns formed [12]. The isolate from the second episode was genotype G07350, which was in a cluster with a pleural TB case from another county in Arkansas reported in May of 2010. Given that she completed treatment in August 2011 and we diagnosed her with a second episode of TB in March 2013, her most likely source of re-exposure was a TB case outside Arkansas. In the time frame above, four TB cases of the same genotype were reported from Tennessee, three from Mississippi and one from Oklahoma. However, there were no other TB cases nationwide. Thus, this cluster is limited to contiguous states, but we could not find any epidemiological links between these cases. With the help of genotype analysis of the specimen, we were able to identify that our patient was infected with different strains of MTB on the two occasions, first with G08667 and then with G07350. This confirmed that the current MTB was a reinfection and not a relapse of the previous infection. Laboratory cross-contamination is considered unlikely since two separate sputum cultures grew MTB at different points of time.
The diagnosis of M. tuberculosis is established by a combination of Interferon Gamma Release Assays (IGRA), Tuberculin Skin Test (TST), Acid Fast Bacillus (AFB) sputum smear and culture examination, and more recently the GeneXpert MTB/RIF assay (Cepheid Inc.) [13]. The standard regimen for MTB is an initial two month intensive phase followed by a four month continuation phase [14]. The duration of therapy is prolonged by factors like cavitation on initial chest X-ray and positive cultures on completion of two months of the initial phase of therapy. It is recommended that molecular drug susceptibility testing of the initial MTB isolate be conducted for all patients and should be repeated at three months if no significant clinical improvement is observed [15]. Failure to complete MTB therapy is associated with high recurrence rate and drug resistance, thus ensuring medication compliance of all patients with MTB infection is essential [7].
The treatment of MTB in CF patients follows general guidelines and the standard therapeutic regimen of six months duration. Recurrent MTB requires another cycle of standard regimen, which in some cases like our patient, needs an extended length of therapy.
Conflicts of interest
The authors disclose no financial or personal relationships with other people or organizations that could inappropriately influence or bias this work.
Acknowledgments
None.
References
- 1.Girón R.M., Máiz L., Barrio I., Martínez M.T., Salcedo A., Prados C. Nontuberculous mycobacterial infection in patients with cystic fibrosis: a multicenter prevalence study. Arch. Bronconeumol. 2008;44:679–684. [PubMed] [Google Scholar]
- 2.Pierre-Audigier C., Ferroni A., Sermet-Gaudelus I., Le Bourgeois M., Offredo C., Vu-Thien H. Age-related prevalence and distribution of nontuberculous mycobacterial species among patients with cystic fibrosis. J. Clin. Microbiol. 2005;43:3467–3470. doi: 10.1128/JCM.43.7.3467-3470.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Asherova I.K., Feigelson J., Vasilyeva L.A., Gabitov V.J. Cystic fibrosis complicated by multiresistant tuberculosis. Acta Paediatr. 2006;95:1513–1514. doi: 10.1080/08035250600724515. [DOI] [PubMed] [Google Scholar]
- 4.Manika K., Giouleka P., Zarogoulidis K., Kioumis I. Multidrug-resistant tuberculosis in an adult with cystic fibrosis. Respiration. 2013;85:350–353. doi: 10.1159/000338846. [DOI] [PubMed] [Google Scholar]
- 5.Middleton A.M., Chadwick M.V., Nicholson A.G., Dewar A., Groger R.K., Brown E.J. Inhibition of adherence of Mycobacterium avium complex and Mycobacterium tuberculosis to fibronectin on the respiratory mucosa. Respir. Med. 2004;98:1203–1206. doi: 10.1016/j.rmed.2004.04.013. [DOI] [PubMed] [Google Scholar]
- 6.Smith M.J., Efthimiou J., Hodson M.E., Batten J.C. Mycobacterial isolations in young adults with cystic fibrosis. Thorax. 1984;39:369–375. doi: 10.1136/thx.39.5.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Patil N., Marco A., Montales M.T., Bhaskar N., Mittadodla P., Mukasa L.N. Pulmonary tuberculosis in a patient with cystic fibrosis. North Am. J. Med. Sci. 2015;7:233–235. doi: 10.4103/1947-2714.157494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tobacman J.K. Does deficiency of arylsulfatase B have a role in cystic fibrosis? Chest. 2003;123:2130–2139. doi: 10.1378/chest.123.6.2130. [DOI] [PubMed] [Google Scholar]
- 9.Rogiers V., Vercruysse A., Dab I., Baran D. Abnormal fatty acid pattern of the plasma cholesterol ester fraction in cystic fibrosis patients with and without pancreatic insufficiency. Eur. J. Pediatr. 1983;141:39–42. doi: 10.1007/BF00445666. [DOI] [PubMed] [Google Scholar]
- 10.Meindl R.S. Hypothesis: a selective advantage for cystic fibrosis heterozygotes. Am. J. Phys. Anthropol. 1987;74:39–45. doi: 10.1002/ajpa.1330740104. [DOI] [PubMed] [Google Scholar]
- 11.Eckford P.D.W., Li C., Ramjeesingh M., Bear C.E. Cystic fibrosis transmembrane conductance regulator (CFTR) potentiator VX-770 (ivacaftor) opens the defective channel gate of mutant CFTR in a phosphorylationdependent but ATP-independent manner. J. Biol. Chem. 2012;287:36639–36649. doi: 10.1074/jbc.M112.393637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Berzkalns A., Bates J., Ye W., Mukasa L., France A.M., Patil N. The road to tuberculosis (Mycobacterium tuberculosis) elimination in Arkansas; a re-examination of risk groups. PLoS One. 2014;9 doi: 10.1371/journal.pone.0090664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Patil N., Saba H., Marco A., Samant R., Mukasa L. Initial experience with GeneXpert MTB/RIF assay in the Arkansas tuberculosis control program. Australas. Med. J. 2014;7:203–207. doi: 10.4066/AMJ.2014.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patil N., Marco A., Saba H., Samant R., Mukasa L. TB or not TB; don't miss the obvious. J. Ark. Med. Soc. 2014;111:112–114. [PubMed] [Google Scholar]
- 15.American thoracic society/centers for disease control and prevention/infectious diseases society of America. Am. J. Respir. Crit. Care Med. 2003;167(4):603–662. doi: 10.1164/rccm.167.4.603. [DOI] [PubMed] [Google Scholar]


