Abstract
Tumor-infiltrating lymphocytes (TILs) are vital in limiting cancer progression and may supplement the TNM classification. CD45RO+ memory TILs show major prognostic impact in various malignancies but have not been extensively explored in non–small cell lung cancer (NSCLC). In this study, we aimed to evaluate their potential in a NSCLC TNM-Immunoscore. Tissue microarrays were constructed from tumor tissue samples from two cohorts including in total 536 patients (University Hospital of North Norway, n = 285; Nordland Hospital, n = 251) with primary resected stage I to IIIA NSCLC. The density of CD45RO+ and CD8+ TILs in tumor epithelial and stromal compartments of the tumors was evaluated by immunohistochemistry. In univariate analyses, intraepithelial CD45RO+ TIL density (T-CD45RO) was a significant prognostic factor for disease-specific survival (P = .007), limited to the squamous cell carcinoma (SCC) histology subgroup (P < .001), where it was significant in both cohorts (University Hospital of North Norway, P = .003; Nordland Hospital, P = .022). Combining T-CD45RO and stromal CD8+ TIL density (S-CD8) increased the prognostic impact in SCC (P < .001) and showed a significant impact within all pathological stages (I, P = .025; II, P < .001; III, P = .001). In the multivariate analysis, T-CD45RO was an independent positive prognostic factor for SCC (hazard ratio 2.65, 95% confidence interval 1.64-4.28, P < .001), and in combination with S-CD8, the prognostic impact increased vastly (high + high versus low + low: hazard ratio 6.50, 95% confidence interval 3.54-11.91, P < .001). In conclusion, T-CD45RO was an independent prognostic factor for SCC NSCLC. When combined with S-CD8, the prognostic impact increased and was significant within each pathological stage. We propose CD45RO as a candidate marker for TNM-Immunoscore in SCC NSCLC.
Introduction
Lung cancer is the most lethal malignancy worldwide, causing approximately one in five cancer deaths [1]. To improve outcome for patients with non–small cell lung cancer (NSCLC), there is an urgent need for novel prognostic factors, which may assist medical decision making by allowing individualized risk stratification and contribute to the discovery of novel treatment options.
Data on tumor burden (T), presence of cancer cells in regional lymph nodes (N), and evidence of metastases (M) are combined in the American Joint Committee on Cancer/Union Internationale Contre le Cancer TNM classification. Together with histological subtyping, the TNM classification is currently the best determinant of the NSCLC patient prognosis available, supplying the most reliable guidelines for treatment decision making. Nevertheless, a supplement to the TNM status is warranted because clinical outcome may vary significantly among patients within the same pathological stage [2].
Immune cell infiltration can critically influence cancer patient outcome [3], and studies of the “immune contexture”, defined as the type, density, and location of immune cells, have allowed the identification of immune cell subsets influencing prognosis in favorable as well as deleterious ways [3], [4]. Tumor-infiltrating lymphocytes (TILs), especially, have in many human malignancies been associated with improved survival [3]. In colorectal cancer, a combination of the density and location of CD3+, CD45RO+, and CD8+ TILs was found to predict outcome. This combination was independent of, and more powerful than, the standard pathological TNM classification [5], [6]. Presently, an international consortium is prospectively validating and promoting the Immunoscore (TNM-I) in the routine clinical colorectal cancer setting [7]. Furthermore, in breast cancer, stromal TILs were found to be predictive of treatment effect, and consensus recommendations for the evaluation of TILs in breast cancer research and clinical trials have been issued [8].
In NSCLC, several studies have investigated the importance of the immune contexture. Positive associations with survival have been found for tumors with infiltration of immune cells, most consistently CD3+, CD4+, and CD8+ T-lymphocytes, but also CD20+ and tertiary lymphoid structures (TLS), all potential candidates of an Immunoscore [3], [9], [10]. Recently, we as well as others have demonstrated stromal CD8+ TIL density (S-CD8) to be a strong independent prognostic factor for survival supplementing the TNM classification [10], [11]. To our knowledge, CD8 is so far the only prognostic marker validated in a NSCLC TNM-I setting. However, further validation in prospective studies is needed before potential clinical use.
CD45 functions as a protein tyrosine phosphatase in leukocyte signaling, and isoforms are expressed in a cell-type specific manner, depending on their stage of differentiation and activation [12]. T memory cells are CD45RO+ T lymphocytes which have encountered antigen and respond faster and with increased intensity on antigenic stimulation compared with (CD45RA+) naïve T cells [13]. Based on the colorectal cancer TNM-I research, CD45RO+ TIL also appears to be a candidate immune marker for NSCLC, for which its prognostic role has been explored to a limited extent [14]. Thus, we wanted to investigate the prognostic impact of T memory lymphocytes in a large patient material of NSCLC. The aim of this study was to evaluate the prognostic impact of CD45RO+ TIL density, alone and in combination with CD8+ TIL density, in the tumor epithelial and stromal compartments in primary resected stage I to IIIA NSCLC patients, assessing its potential in an NSCLC TNM-I.
Materials and Methods
Patients and Clinical Samples
In this retrospective study, primary tumor tissues from patients who underwent radical resection for NSCLC pathologic stage I to IIIA at the University Hospital of North Norway (UNN) and Nordland Hospital (NH) from 1990 through 2010, including a recent expansion of patients treated during 2005 to 2010, were collected and anonymized. The tumors were staged according to the seventh edition International Union Against Cancer TNM classification [15] and histologically classified according to the World Health Organization guidelines on classification of lung cancer [16]. In this period, 633 patients were diagnosed with NSCLC, according to the hospital pathological databases. Ninety-seven patients were excluded from the study because of 1) radiotherapy or chemotherapy before surgery (n = 15), 2) other malignancy within 5 years before NSCLC diagnoses (n = 39), 3) inadequate paraffin-embedded fixed tissue blocks (n = 25), or 4) adenocarcinoma in situ, before 2011 classified as bronchioloalveolar carcinoma (formerly known as BAC) ≤ 3 cm (n = 18) [17]. Thus, 536 patients with complete medical records and adequate paraffin-embedded tissue blocks were eligible. This report includes follow-up data as of October 1, 2013. Median follow-up time of survivors was 86 months (range, 34-267 months). The Norwegian Data Protection Authority and the Regional Committee for Medical and Health Research Ethics approved the study (Protocol ID: 2011/2503). The reporting of clinicopathologic varables, survival data, and biomarker expressions was conducted in accordance with the REMARK guidelines [18].
Microarray Construction
All cases were histologically reviewed by two pathologists (S.A.S. and K.A.S.), and the most representative areas of viable neoplastic epithelial cells and of tumor stroma were carefully selected. The tissue microarrays (TMAs) were assembled using a tissue-arraying instrument (Beecher Instruments, Silver Springs, MD). The detailed methodology has been previously reported [19]. Briefly, we used a 0.6 mm–diameter stylet, and the study specimens were routinely sampled with two replicate core samples from different areas of neoplastic tissue and two of tumor stroma. Multiple 4-μm sections were cut with a Micron microtome (HM355S) and stained by specific antibodies for immunohistochemistry (IHC) analysis.
Immunohistochemistry
IHC assays for CD45RO were performed on Ventana Discovery Ultra automated immunostainer (Ventana Medical Systems, Tucson, AZ). Slides were deparaffinized in three 8-minute cycles. On-board antigen retrieval was not required and excluded from protocol. Endogenous peroxidase was blocked by Discovery inhibitor (Cat#760-4840, Ventana) for 8 minutes. The primary antibody, prediluted CD45RO mouse monoclonal antibody (Cat#790-2930, clone UCHL1, Ventana), was applied, and slides were incubated for 20 minutes at 37°C. Slides were developed using Ultramap anti-rabbit HRP (Cat#760-4315, Ventana) for 20 minutes and were detected using ChromoMap DAB (Cat#760-159, Ventana). Finally, to visualize the nuclei, slides were counterstained with Ventana Hematoxylin II reagent for 8 minutes followed by a Bluing reagent for 4 minutes and dehydrated, cleared, and mounted as in routine processing.
The prognostic impact of CD8+ TILs has been previously published in 335 of the patients in this cohort [11], [20]. The expansion of the patient material required renewed CD8 staining of the entire cohort (n = 536), this time performed by a new clone of anti-CD8 antibody. The CD8 antibody (Cat# 790-4460, clone SP57, Roche) is well validated and in routine clinical use at included hospitals. The staining method applied has been previously described [11].
Two different controls for our staining method were used. Firstly, control staining of the sections with an isotype-matched control antibody without the primary antibody was performed. Secondly, multiple organ tissue microarray as positive and negative tissue controls was used to verify the specificity of the staining in every staining procedure. The positive tissue controls comprised tonsil and lymph nodes, and negative tissue controls were samples of normal brain tissue. For each antibody, staining was done in one single experiment. Immunostaining was performed on adjacent sections to allow comparison of regional distributions of TIL subset infiltrates.
Scoring of IHC
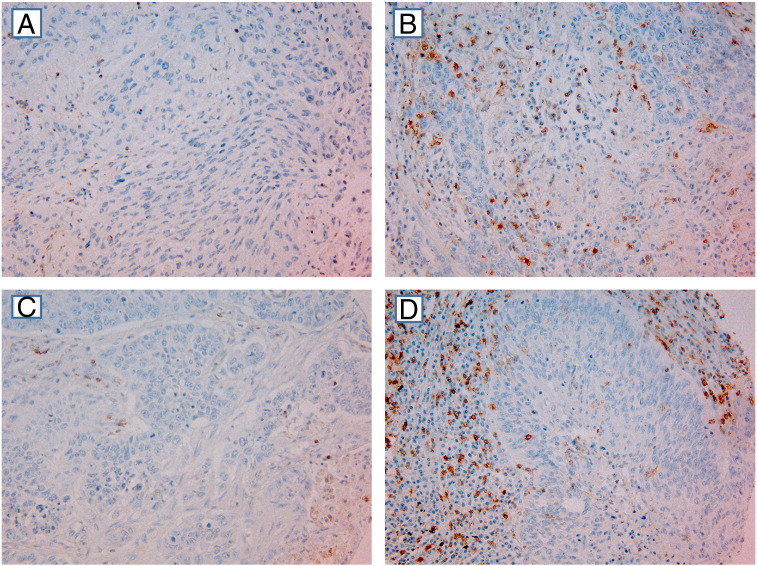
Samples from both cohorts were anonymized and independently scored, under supervision of an experienced pathologist (S.A.S.) by, for CD45RO, one pathologist (R.J.) and one biologist (M.R.) and, for CD8, one biologist (M.R.) and one oncologist (E.P.). When assessing one marker in a given core, both observers were blinded to the scores of the other markers as well as to patient outcome. By light microscopy, representative and viable tissue cores were scored semiquantitatively for degree of infiltration of CD45RO+ or CD8+ lymphocytes. Manually, the percentages of CD45RO+ and CD8+ lymphocytes compared with the total amount of nucleated cells (density) in the stromal and tumor epithelial compartments of each core were assessed separately. Based on our previous experience [11], scoring cutoffs for the density of CD8+ TILs in the stromal compartment was set as follows; 0% to 5% positive cells = 0, 6% to 25% = 1, 26% to 50% = 2, and > 50% = 3. In the epithelial compartment, where immune cells were more sparse, scoring cutoffs were set as follows: absent = 0, 1% to 5% = 1, 6% to 25% = 2, 26% to 50% = 3. The same scoring method was used for CD45RO (Figure 1). No cores had a T-CD45RO score of > 2.
Figure 1.
IHC analysis of NSCLC representing different scores for tumor cell and stromal expression. (A) Low intraepithelial CD45RO score (SCC). (B) High intraepithelial CD45RO score (ADC). (C) Low stromal CD45RO score (ADC). (D) High stromal CD45RO score (SCC) (magnification × 200). In most tumor cores, as well as in some stromal cores, there was a mixture of stromal cells and tumor cells. However, by morphological criteria, we have scored only density of positive immune cells within the tumor epithelial compartment in cores with tumor tissue present and density of positive immune stromal cells within cores with stromal tissue present.
The maximum score approach, defined as the single highest score of the scores available, has been previously validated for S-CD8 [11]. Our aim was to compare and combine CD45RO scores with CD8 scores; thus, we present maximum scores for both. Based on a minimal P value approach, high T-CD45RO was defined as a maximum score of ≥ 1, and a maximum score of ≥ 2 was defined as high for S-CD8, CD8+ TILs in epithelium (T-CD8), and CD45RO+ TILs in stroma (S-CD45RO).
Statistical Methods/Considerations
All statistical analyses were performed using the SPSS statistical package (version 22; SPSS, Chicago, IL). The IHC scores from each observer were compared for interobserver reliability by use of a two-way random effects model with absolute agreement definition. The intraclass correlation coefficient (reliability coefficient) was obtained from these results. Disease-specific survival (DSS), disease-free survival (DFS), and overall survival (OS) were defined as the time from surgery to lung cancer death, to first lung cancer relapse, and to death of any cause, respectively.
The χ2 test or the Fisher exact test was used to examine the association between molecular marker expression and various clinicopathological parameters, applying Monte Carlo simulation when expected values were < 5 in > 20% of cells (confidence level 95%, 10,000 samples, two-sided value). Spearman rank correlation was used to examine the associations between marker expressions. Univariate analysis of survival according to CD45RO+ or CD8+ TIL density was done using the Kaplan-Meier method, and statistically significant differences between survival curves were assessed by the log-rank test. Multivariate analysis was performed using the Cox proportional hazards model, testing the simultaneous influence on survival of all covariates found to be significant in the univariate analyses. Model fitting was done, applying the backward conditional method. Probability for stepwise entry and removal was set at .05 and .10, respectively. P values < .05 were considered statistically significant.
Results
Patient Characteristics
Demographic, clinical, and histopathological variables are presented in Table 1. Median age was 67 (range 28-85) years. The majority of patients were men (68%), but the rate of women increased from 24% to 44% from the period 1990 to 2004 to the period 2005 to 2010. The NSCLC tumors comprised 289 squamous cell carcinomas (SCCs), 201 adenocarcinomas (ADCs), and 46 large-cell carcinomas (LCCs). Seventy-six patients (14%) received postoperative radiotherapy, and 43 patients (20%) were given adjuvant chemotherapy during 2005 to 2010 (1990-2004: data not registered).
Table 1.
Clinicopathologic Variables as Predictors of DSS in 536 NSCLC Patients (Univariate Analyses; Log-Rank Test, Unadjusted Cox Proportional Hazard Ratios)
| All Patients |
SCC |
ADC |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N (%) | 5 Years | Median | HR(95% CI) | P | N (%) | 5 Years | Median | HR(95% CI) | P | N (%) | 5 Years | Median | HR(95% CI) | P | |
| Age | .711 | .654 | .505 | ||||||||||||
| ≤ 65 | 227 (42) | 57 | 127 | 1 | 106 (37) | 64 | 235 | 1 | 102 (51) | 48 | 54 | 1 | |||
| > 65 | 309 (58) | 58 | NA | 0.95 (0.73-1.24) | 183 (63) | 66 | NA | 0.91 (0.61-1.36) | 99 (49) | 49 | 57 | 0.87 (0.59-1.3) | |||
| Sex | .026 | .108 | .050 | ||||||||||||
| Female | 170 (32) | 63 | 190 | 1 | 73 (25) | 73 | NA | 1 | 83 (41) | 56 | 190 | 1 | |||
| Male | 366 (68) | 55 | 88 | 1.4 (1.06-1.84) | 216 (75) | 63 | 235 | 1.49 (0.96-2.31) | 118 (59) | 43 | 51 | 1.5 (1.01-2.23) | |||
| ECOG perf. status | .015 | .158 | .003 | ||||||||||||
| 0 | 310 (58) | 62 | 235 | 1 | 158 (55) | 69 | 235 | 1 | 122 (61) | 56 | NA | 1 | |||
| 1 | 190 (35) | 52 | 71 | 1.45 (1.09-1.93) | 110 (38) | 61 | 114 | 1.47 (0.97-2.23) | 67 (33) | 40 | 50 | 1.57 (1.02-2.4) | |||
| 2 | 36 (7) | 48 | 36 | 1.61 (0.83-3.09) | 21 (7) | 67 | NA | 1.08 (0.45-2.6) | 12 (6) | 17 | 25 | 3.25 (0.96-11.03) | |||
| Smoking | .039 | .19 | .68 | ||||||||||||
| Never | 17 (3) | 44 | 20 | 1 | 7 (2) | 50 | 19 | 1 | 9 (5) | 44 | 21 | 1 | |||
| Previous | 342 (64) | 62 | 235 | 0.56 (0.25-1.24) | 182 (63) | 69 | 235 | 0.58 (0.14-2.37) | 125 (62) | 50 | 68 | 0.69 (0.26-1.84) | |||
| Present | 177 (33) | 51 | 71 | 0.75 (0.33-1.7) | 100 (35) | 60 | 114 | 0.82 (0.2-3.41) | 67 (33) | 45 | 57 | 0.73 (0.27-1.99) | |||
| Weight loss | .961 | .689 | .536 | ||||||||||||
| < 10% | 480 (90) | 58 | 127 | 1 | 257 (89) | 66 | 235 | 1 | 184 (92) | 49 | 57 | 1 | |||
| ≥ 10% | 55 (10) | 59 | NA | 0.99 (0.63-1.56) | 32 (11) | 62 | NA | 1.14 (0.57-2.28) | 17 (8) | 40 | 47 | 1.24 (0.59-2.63) | |||
| Surgical procedure | < .001 | < .001 | < .001 | ||||||||||||
| Wedge/lobectomy | 394 (74) | 63 | 190 | 1 | 197 (68) | 72 | 235 | 1 | 161 (80) | 54 | 104 | 1 | |||
| Pulmonectomy | 142 (26) | 42 | 30 | 1.98 (1.43-2.74) | 92 (32) | 50 | 35 | 1.99 (1.28-3.09) | 40 (20) | 25 | 24 | 2.66 (1.46-4.84) | |||
| Margins | .129 | .252 | .018 | ||||||||||||
| Free | 489 (91) | 59 | 190 | 1 | 257 (89) | 67 | 235 | 1 | 189 (94) | 50 | 68 | 1 | |||
| Not free | 47 (9) | 47 | 57 | 1.39 (0.85-2.29) | 32 (11) | 57 | 114 | 1.39 (0.73-2.63) | 12 (6) | 0 | 35 | 2.33 (0.81-6.69) | |||
| Tstage | < .001 | < .001 | < .001 | ||||||||||||
| 1 | 168 (31) | 72 | 235 | 1 | 83 (29) | 78 | 235 | 1 | 74 (37) | 67 | 190 | 1 | |||
| 2 | 265 (49) | 57 | 91 | 1.74 (1.3-2.32) | 147 (51) | 66 | NA | 1.88 (1.22-2.89) | 94 (47) | 43 | 47 | 1.94 (1.27-2.95) | |||
| 3 | 97 (18) | 36 | 30 | 2.84 (1.87-4.31) | 56 (19) | 46 | 33 | 2.93 (1.62-5.31) | 31 (15) | 16 | 25 | 3.48 (1.76-6.9) | |||
| 4 | 6 (0) | 20 | 15 | 4.89 (0.89-26.9) | 3 (1) | 0 | 10 | 17.41 (0.22-1371.77) | 2 (1) | 50 | 13 | 1.76 (0.23-13.27) | |||
| Nstage | < .001 | < .001 | < .001 | ||||||||||||
| 0 | 364 (68) | 69 | 235 | 1 | 198 (69) | 77 | 235 | 1 | 133 (66) | 60 | 190 | 1 | |||
| 1 | 118 (22) | 36 | 35 | 2.76 (1.93-3.94) | 73 (25) | 45 | 35 | 3.26 (1.99-5.35) | 39 (19) | 25 | 30 | 2.41 (1.38-4.2) | |||
| 2 | 54 (10) | 21 | 19 | 4.23 (2.43-7.37) | 18 (6) | 18 | 13 | 7.12 (2.44-20.77) | 29 (15) | 23 | 24 | 2.88 (1.42-5.82) | |||
| Pathological stage | < .001 | < .001 | < .001 | ||||||||||||
| I | 256 (48) | 72 | 235 | 1 | 127 (44) | 82 | 235 | 1 | 105 (52) | 65 | 190 | 1 | |||
| II | 194 (36) | 53 | 84 | 1.89 (1.42-2.51) | 126 (44) | 60 | 114 | 2.5 (1.66-3.77) | 56 (28) | 34 | 43 | 2.07 (1.3-3.28) | |||
| IIIA | 86 (16) | 20 | 17 | 4.58 (2.87-7.32) | 36 (12) | 23 | 15 | 7.15 (3.23-15.84) | 40 (20) | 16 | 24 | 3.37 (1.8-6.33) | |||
| Histology | .040 | ||||||||||||||
| SCC | 289 (54) | 65 | 235 | 1 | |||||||||||
| ADC | 201 (37) | 48 | 57 | 1.43 (1.08-1.89) | |||||||||||
| LCC | 46 (9) | 50 | 83 | 1.29 (0.8-2.08) | |||||||||||
| Differentiation | < .001 | .033 | .006 | ||||||||||||
| Poor | 231 (43) | 49 | 51 | 1 | 104 (36) | 57 | 84 | 1 | 81 (40) | 38 | 43 | 1 | |||
| Moderate | 240 (45) | 63 | 190 | 0.67 (0.5-0.89) | 155 (54) | 70 | 235 | 0.63 (0.41-0.97) | 85 (42) | 50 | 68 | 0.69 (0.44-1.07) | |||
| Well | 65 (12) | 70 | NA | 0.44 (0.29-0.66) | 30 (10) | 72 | NA | 0.47 (0.24-0.94) | 35 (18) | 69 | NA | 0.36 (0.21-0.63) | |||
| Vascular infiltration | < .001 | .029 | .012 | ||||||||||||
| No | 437 (82) | 62 | 235 | 1 | 231 (80) | 69 | 235 | 1 | 172 (86) | 52 | 71 | 1 | |||
| Yes | 97 (18) | 38 | 35 | 1.89 (1.29-2.78) | 58 (20) | 53 | 71 | 1.65 (0.97-2.82) | 27 (13) | 26 | 27 | 1.9 (1-3.62) | |||
| Missing | 2 (0) | 2 (1) | |||||||||||||
Note: Bold numbers are significant results. Five-year survival (%). Median survival (months). Abbreviations: ECOG perf. status, Eastern Cooperative Oncology Group performance status; N, number; Nstage, nodal stage; Tstage, tumor stage.
Density of CD45RO+ and CD8+ TILs and Their Correlations
The density of CD45RO+ and CD8+ TILs is presented in Table 2. There was a significant correlation between the maximum score of T-CD45RO and S-CD45RO (r = 0.494, P < .001). T-CD45RO was significantly correlated with T-CD8 and S-CD8 stromal (r = 0.265, and 0.223, both P < .001). S-CD45RO was significantly correlated with T-CD8 and S-CD8 (r = 0.252 and 0.559, both P < .001). There were no major differences in clinicopathological characteristics between patients with high versus low CD45RO score in the total material as well as in the SCC and ADC subgroups (Supplementary Table 1).
Table 2.
The Prognostic Impact of Tumor Epithelial and Stromal CD45RO+ and CD8+ TILs on DSS in All Patients and Stratified by Histology (Univariate Analyses; Log-Rank Test, Unadjusted Cox Proportional Hazard Ratios)
| All Patients |
SCC |
ADC |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N (%) | 5 Years | Median | HR (95% CI) | P | N (%) | 5 Years | Median | HR (95% CI) | P | N (%) | 5 Years | Median | HR (95% CI) | P | |
| T-CD45RO | .007 | .0003 | .843 | ||||||||||||
| High | 423 (79) | 59 | 190 | 1 | 226 (78) | 69 | 235 | 1 | 162 (81) | 48 | 57 | 1 | |||
| Low | 81 (15) | 45 | 36 | 1.62 (1.14-2.29) | 46 (16) | 42 | 30 | 2.34 (1.46-3.77) | 29 (14) | 48 | 51 | 1.06 (0.59-1,91) | |||
| Missing | 32 (6) | 17 (6) | 10 (5) | ||||||||||||
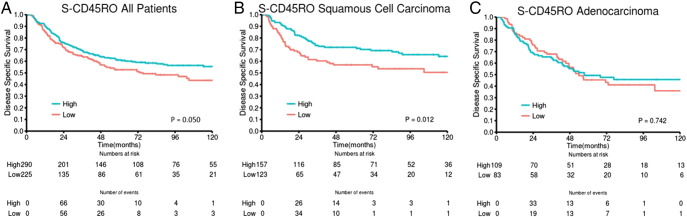
| S-CD45RO | .050 | .012 | .742 | ||||||||||||
| High | 290 (54) | 61 | 235 | 1 | 157 (54) | 72 | 235 | 1 | 109 (54) | 49 | 57 | 1 | |||
| Low | 225 (42) | 53 | 75 | 1.31 (1.00-1.72) | 123 (43) | 57 | NR | 1.67 (1.12-2.50) | 83 (41) | 45 | 54 | 1.17 (0.71-1.60) | |||
| Missing | 21 (4) | 9 (3) | 9 (5) | ||||||||||||
| T-CD8 | .036 | .015 | 1 | .943 | |||||||||||
| High | 146 (27) | 66 | 235 | 1 | 78 (27) | 78 | 235 | 1 | 53 (26) | 49 | 57 | ||||
| Low | 359 (67) | 53 | 91 | 1.39 (1.02-1.90) | 196 (68) | 59 | NR | 1.81 (1.16-2.95) | 136 (68) | 47 | 57 | 1.02 (0.65-1.59) | |||
| Missing | 31 (6) | 15 (5) | 12 (6) | ||||||||||||
| S-CD8 | .0002 | .0002 | .320 | ||||||||||||
| High | 370 (69) | 63 | 235 | 1 | 204 (70) | 73 | 235 | 1 | 137 (68) | 50 | 73 | 1 | |||
| Low | 148 (28) | 44 | 47 | 1.70 (1.28-2.26) | 77 (27) | 46 | 41 | 2.17 (1.43-3.29) | 57 (28) | 42 | 50 | 1.24 (0.81-1.90) | |||
| Missing | 18 (3) | 8 (3) | 7 (4) | ||||||||||||
| T-CD45R0 + S-CD8 | .0001 | < .0001 | .466 | ||||||||||||
| High + high | 315 (62) | 64 | 235 | 1 | 175 (65) | 74 | 235 | 1 | 117 (62) | 76 | 73 | 1 | |||
| Intermediate | 153 (31) | 47 | 51 | 1.60 (1.19-2.14) | 74 (27) | 56 | 105 | 1.66 (1.04-2.64) | 63 (33) | 51 | 50 | 1.10 (0.40-3.05) | |||
| Low + low | 34 (7) | 37 | 28 | 2.36 (1.45-3.84) | 22 (8) | 19 | 18 | 4.77 (2.66-8.56) | 10 (5) | 41 | 71 | 1.42 (0.50-4.00) | |||
Note: Bold numbers are significant results. Five-year survival (%). Median survival (months). Abbreviations: NR, not reached; S, stroma; T, tumor.
Interobserver Reliability
The interobserver reliability coefficients indicated moderate to excellent agreement between scorers: intraclass correlation coefficient for S-CD45RO, 0.889 (P < .001); for T-CD45RO, 0.642 (P < .001); for S-CD8, 0.878 (P < .001); and for T-CD8, 0.760 (P < .001).
Univariate Analyses: Prognostic Impact of Memory TILs in Tumor and Stroma
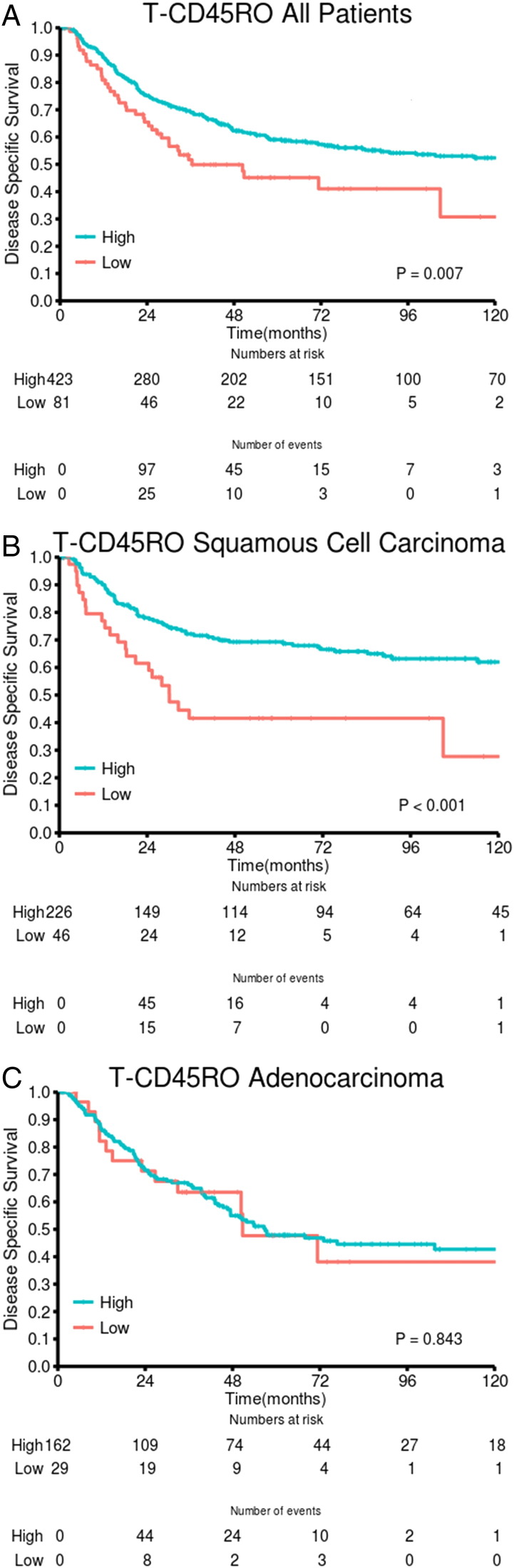
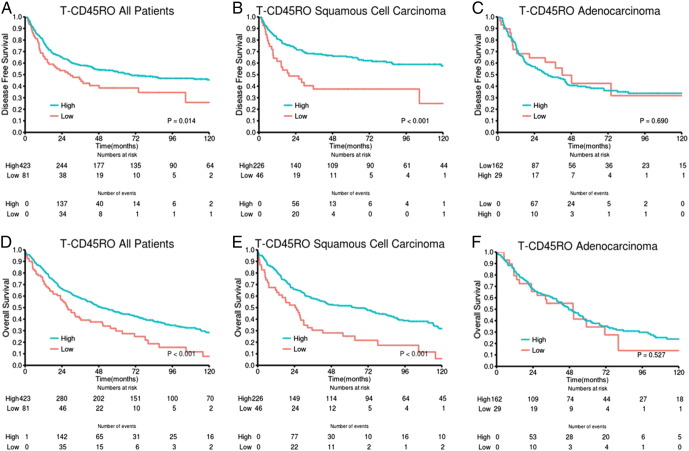
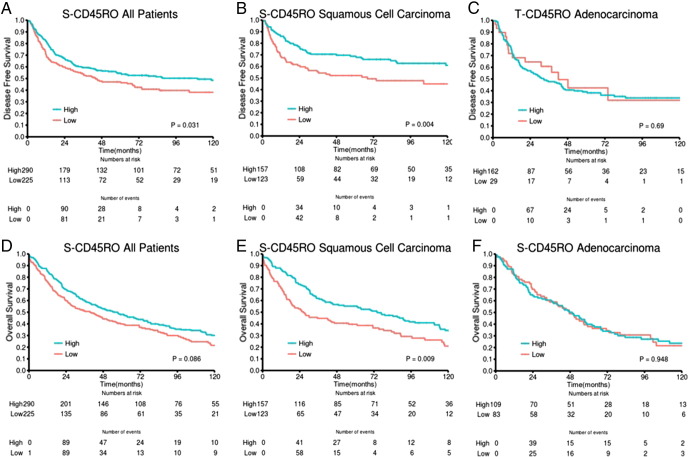
Results of the univariate analyses are presented in Table 2. In the whole cohort, statistically significant associations between high T-CD45RO and improved DSS (P = .007) as well as DFS (P = .014) and OS (P = .001) were observed. In the stromal compartment, association with survival was limited to a trend (Table 2). Combining the scores in tumor epithelium and stroma yielded a slightly less significant association with survival (three-tiered: DSS P = .014, DFS P = .009, OS P = .001) compared with the epithelial scores.
In both epithelial and stromal compartments, we found major prognostic differences between histological subgroups. For patients with SCC, there was a highly significant association between T-CD45RO and DSS (P = .0003), DFS (P < .001), and OS (P = .0003). In the SCC subgroup, the positive impact of high T-CD45RO on DSS was present across pathological stages [I: P = .088 (trend), II: P = .005, IIIA: P = .011] and in both cohorts (UNN: P = .003, NH: P = .022). The S-CD45RO score had a significant prognostic value in SCC but less so than T-CD45RO (Table 2). Also, when combining the epithelial and stromal scores, the association with survival became stronger (DSS, DFS, OS: all P ≤ .0003) than in the whole cohort. In contrast, we found no significant association with survival in ADC and LCC (not presented).
With regard to CD8, we found high density of CD8+ TILs to be associated with improved survival, most significant in the stromal compartment and limited to the SCC subgroup (Table 2).
Univariate Analyses: Prognostic Impact of Epithelial CD45RO+ Combined with CD8+ TILs in Tumor and Stroma
When combining the two most significant settings for each of the two markers, T-CD45RO and S-CD8, a highly significant association with patient outcome emerged, achieving a stratification of patient survival surpassing that of each marker (Table 2). A stratification into three categories: high + high (high T-CD45RO score and high S-CD8 score), intermediate (high + low or low + high), or low + low, revealed substantial differences in DSS, DFS, and OS (all: P < .001). Again, when stratifying into histological subgroups, low P values for all outcomes were seen in the SCC subgroup (DSS, DFS, OS: all P < .001) and across all pathological stages (DSS: I: P = .025, II: P < .001, III: P = .001). For ADC, no association with survival was found (Figure 2, Table 2).
Figure 2.
DSS curves are shown according to density of T-CD45RO in (A) all patients, (B) SCC, and (C) ADC.
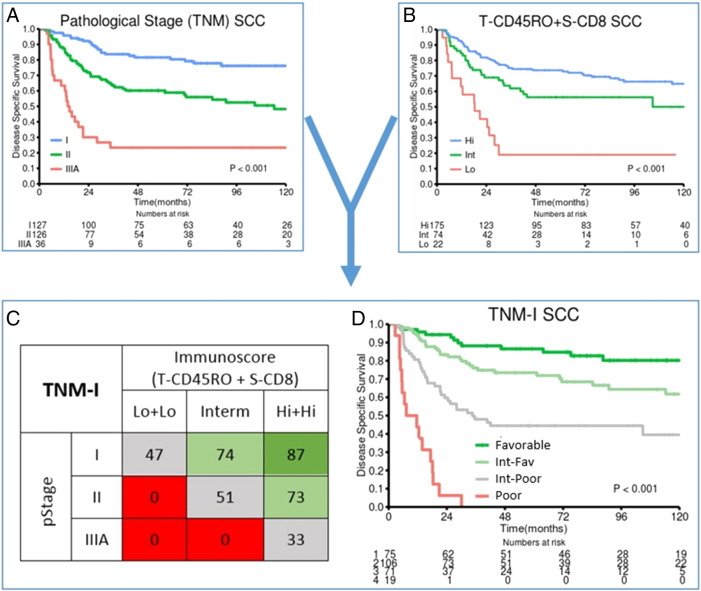
Figure 3 illustrates how combining the immune score (coexpression T-CD45RO and S-CD8) with pathological (TNM) stage for SCC NSCLC increases stratification and adds prognostic impact. The median 5-year DSS of the four TNM-I subgroups was 87% (n = 75), 75% (n = 106), 44% (n = 71), and a dismal 0% (n = 19) versus 82% (pStage I), 60% (pStageII), and 23% (pStage IIIA). According to the immune score (T-CD45RO and S-CD8), there is a 40% difference in 5-year DSS within the pStage I patient group (low + low: 47%, intermediate: 74%, high + high: 87%). In pStage II, the differences in 5-year DSS are even greater (0%, 51%, 73%) when stratified for immune score. In fact, the TNM-I approach facilitates the identification of the most disadvantageous prognostic patient subgroup, which did not reach 5-year DSS (Figure 3, C and D; 16 of 19 patients died of lung cancer within 30 months).
Figure 3.
From TNM to TNM-I in SCC NSCLC.
DSS curves of the SCC patient subgroup, according to (A) pathological (TNM) stage and (B) immunoscore (combination of T-CD45RO and S-CD8 score). The combination of A and B results in a “TNM-I” survival table (C) identifying subgroups of patients with greatly differing 5-year DSS within the same pathological stages. The colors of the boxes in the table (C) represent patient subgroups with similar survival [favorable prognosis (green), intermediate-favorable prognosis (light green), intermediate-poor prognosis (gray), poor prognosis (red)]. Constructing survival curves (DSS) for these patient subgroups (D) illustrates how the immunoscore adds significant prognostic impact across each pathological stage.
Multivariate Analysis: Independent Prognostic Impact of T-CD45RO and S-CD8
All significant demographic, clinicopathologic, and lymphocyte infiltrate variables from the univariate analyses were entered into the multivariate Cox regression analysis. Data are presented in Table 3. In the whole cohort, high T-CD45RO was an independent prognostic factor for DSS [hazard ratio (HR) 1.80, 95% confidence interval (CI) 1.26-2.57, P = .001], DFS (HR 1.79, 95% CI 1.28-2.50, P = .001), and OS (HR 1.61, 95% CI 1.22-2.13, P = .001). In the SCC histological subgroup, the prognostic impact for DSS (HR 2.65, 95% CI 1.64-4.28, P < .001) as well as for DFS (HR 2.36, 95% CI 1.50-3.73) and OS (HR 2.06, 95% CI 1.43-2.96, P < .001) was further increased.
Table 3.
Results of Cox Regression Analysis Summarizing Significant Independent Prognostic Factors for DSS
| All Patients |
SCC |
ADC |
||||
|---|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | |
| Pathological stage | < .001⁎ | < .001⁎ | < .001⁎ | |||
| I | 1.00 | 1.00 | 1.00 | |||
| II | 1.79 (1.31-2.46) | < .001 | 2.49 (1.53-4.06) | < .001 | 2.20 (1.37-3.53) | .001 |
| IIIA | 4.08 (2.83-5.89) | < .001 | 7.38 (4.17-13.07) | < .001 | 3.33 (1.94-5.72) | < .001 |
| Histology | .003⁎ | |||||
| SCC | 1.00 | |||||
| ADC | 1.62 (1.21-2.19) | .001 | ||||
| LCC | 1.00 (0.60-1.67) | .996 | ||||
| Vascular infiltration | .001⁎ | .081⁎ | .048⁎ | |||
| No | 1 | 1.00 | 1.00 | |||
| Yes | 1.79 (1.29-2.50) | 1.51 (0.95-2.41) | 1.72 (1.01-2.94) | |||
| Differentiation | .006⁎ | .303⁎ | .098⁎ | |||
| Well | 1.00 | 1.00 | 1.00 | |||
| Moderate | 1.75 (1.02-3.01) | .042 | 1.31 (0.59-2.89) | .201 | 1.99 (0.98-4.03) | |
| Poor | 2.32 (1.35-3.99) | .001 | 1.69 (0.76-3.78) | .507 | 2.13 (1.06-4.26) | |
| Sex | .001⁎ | NE | .023⁎ | |||
| Female | 1.00 | 1.64 (1.07-2.52) | ||||
| Male | 1.68 (1.24-2.29) | |||||
| ECOG performance status | .005⁎ | NE | .003⁎ | |||
| 0 | 1.00 | 1.00 | ||||
| 1 | 1.54 (1.16-2.03) | .003 | 1.64 (1.08-2.50) | .021 | ||
| 2 | 1.78 (0.99-3.18) | .052 | 3.59 (1.58-8.16) | .002 | ||
| Smoking | .013⁎ | NE | NE | |||
| Never | 1.00 | |||||
| Present | 0.36 (0.18-0.71) | .003 | ||||
| Former | 0.41 (0.20-0.82) | .012 | ||||
| Margins | NE | NE | .362⁎ | |||
| Free | 1.00 | |||||
| Not free | 1.42 (0.67-2.93) | |||||
| T-CD45RO⁎⁎ | .001⁎ | < .001⁎ | NE | |||
| High | 1.00 | 1.00 | ||||
| Low | 1.80 (1.26-2.57) | 2.65 (1.64-4.28) | ||||
| S-CD45RO⁎⁎ | NE | .003⁎ | NE | |||
| High | 1.00 | |||||
| Low | 1.85 (1.23-2.78) | |||||
| T-CD45RO + S-CD8⁎⁎ | < .001⁎ | < .001⁎ | NE | |||
| High + high | 1.00 | 1.00 | ||||
| Intermediate | 1.62 (1.29-2.18) | .002 | 2.08 (1.29-3.37) | .003 | ||
| Low + low | 2.43 (1.46-4.02) | .001 | 6.50 (3.54-11.91) | < .001 | ||
Abbreviation: ECOG, eastern cooperative oncology group; NE, not entered.
Overall significance as a prognostic factor.
In separate models.
The coexpression of T-CD45RO and S-CD8 had a highly significant independent prognostic value in the whole cohort (DSS, DFS, OS: all P < .001). In the SCC histological subgroup, the HR for high + high versus intermediate score was 2.08 (95% CI 1.29-3.37, P = .003), and for high + high versus low + low, it was 6.50 (95% CI 3.54-11.91, P < .001). When stratifying SCC patients into pathological stages, the combination was significant for DSS in multivariate analysis for all stages (high + high versus low + low for pStage I: HR 4.35, P = .012; pStage II: HR 8.24, P < .001; pStage IIIA: HR 9.52, P = .002) and for both centers (high + high versus low + low, UNN: HR: 4.82, P = .001; NH: HR 5.94, P < .001).
Discussion
Our study demonstrates a positive association between the presence of CD45RO+ TILs and survival in a large, unselected, and representative stage I to IIIA NSCLC patient cohort. For tumors in the SCC histological subgroup, high in situ density of epithelial CD45RO+ TILs was a strong prognostic factor for all evaluated end points (DSS, DFS, and OS) in two different cohorts and in both univariate and multivariate analyses. When combined with stromal CD8+ TIL density in SCC, the prognostic impact increased further and remained an independent prognostic factor across all pathological stages (I-IIIA).
Well-validated IHC antibodies used for the staining of immune markers in the routine clinical and research setting were used in this study. The staining method is simple, and the manual, semiquantitative manner of IHC scoring was found to be reproducible, with good interobserver agreement. This demonstrates that simple IHC assessment of immune cell infiltrates in routinely processed and stained tissue from primary tumors can provide valuable prognostic information.
The presence of TILs related to various compartments within the tumor is frequently a matter of debate. In the present study, the prognostic impact of tumor epithelial CD45RO+ TILs clearly surpasses the stromal prognostic impact, although their densities were strongly correlated (r = 0.494). Patients with high stromal score (n = 287) rarely presented a low tumor epithelial score (n = 12), fitting the biological model of TILs infiltrating the epithelial compartment via the stromal compartment. Interestingly, 148 of the 217 tumors with low stromal score had a high tumor score, demonstrating that a strong stromal infiltrate is not a prerequisite for epithelial infiltration, and separate analysis of the compartments seems required. Although our study does not offer an explanation for the difference in prognostic impact depending on location, hopefully it can be explored further in functional studies.
Evaluating the prognostic impact of TILs in different tumor localizations is another important issue. Our TMAs represent a mixture of cores from tissue in the central tumor and at the invasive margins. Assessing localizations separately, as recommended by the colorectal Immunoscore worldwide task force [21], could improve the prognostic impact of immune markers and should be explored for CD45RO in future NSCLC studies. Evaluating whole slides might allow inclusion and analysis of areas from both the central tumor and the invasive margin and is easily available in the clinical routine. Nevertheless, for research purposes, TMA techniques appear to be representative and adequate, and provide a high throughput method [8]. Finally, digital automated evaluation of IHC staining may provide important advantages such as standardization and time efficiency [21].
CD45RO is broadly accepted as the most suitable single marker for the whole memory T cell population [12], [13], [22]. Evaluated by immunochemistry, high expression of CD45RO+ T memory cells has been associated with improved disease-related outcomes in various human cancers, including esophageal (ADC and SCC) [23], [24], colorectal [5], [6], [25], endometrial [26], gastric [27], [28], ovarian [29], and hepatocellular cancer [30]. In renal cell carcinoma, it was associated with worse prognosis [31].
The prognostic effect of the T memory lymphocyte subset in NSCLC has been reported in few studies. In a study on tumor-induced bronchus-associated lymphoid tissues in stage I and IIA NSCLC patient tumors, Dieu-Nosjean et al. [32] reported that the density of mature dendritic cell, a specific marker of TLS, was predictive of survival. In 24 evaluated patient samples, they found CD45RO to be expressed by most CD3+ T cells, whereas the density of CD45RA+ (naïve T cells) was low, in contrast to the equal proportions of conventional lymphoid organs. Although no association between CD45RO and survival could be found, their results pointed to TLS-derived memory cells being involved in developing a central memory and systemic response postsurgery. Goc et al. [33] also found, in a study on 458 human primary lung tumors (stage I-IV), that high density of TLS mature dendritic cells correlated with long-term survival as well as with a strong infiltration of T cells, predominantly of the effector-memory subtype (CD45RA−CCR7-CD27+/−CD28+/−, n = 54). Djenidi et al. [34] found CD8+CD103+ TIL to be characteristic of tissue-resident memory T cells. Their study suggested CD103 to be involved in recruitment and activation of tumor-specific T cells in lung tumor islets and that a high expression of CD103+ TILs correlates with improved stage I NSCLC patient survival (regardless of localization: epithelial or stromal).
To our knowledge, only one study has reported the prognostic impact of in situ CD45RO+ TILs in NSCLC patients [22]. In 956 stage I ADC NSCLC patients, Suzuki et al. [14] found no association between CD45RO+ memory T cells and recurrence-free survival. Reassuringly, in our study, the result was confirmed for this subgroup of patients (stage I ADC: n = 101). In general, we observed no significant prognostic value of CD45RO+ TILs in ADC patients, regardless of stage or end point.
In contrast, for tumors with SCC histology, we found T-CD45RO to be a strong positive prognostic factor in uni- and multivariate analyses across all pathological stages and in both cohorts. The differences in 5-year DSS, DFS, and OS between high and low T-CD45RO scores were 27%, 27%, and 23%, respectively.
Interestingly, similar studies in colorectal cancer show increased prognostic effect of the immune score when combining CD45RO+ and CD8+ TILs [6], [35]. Based on recently published results by our group on S-CD8 as a promising candidate marker for NSCLC TNM-I [11], patient stratification could be further refined by combining T-CD45RO score with S-CD8 score (Figure 3B). In the SCC subgroup, the combined immune score revealed considerable and significant differences in outcome between the three score groups (high + high, intermediate, low + low) and was in multivariate analysis a robust indicator for improved prognosis (Table 3) in all pathological stages.
The marked difference in 5-year DSS of patients across stage I, II, and IIIA, respectively, clearly illustrates the association between density of CD45RO+ and CD8+ TILs and outcome (Figure 3D). This may indicate a potential adjuvant treatment benefit in patients with low immune score, which calls for further studies. For the most dismal TNM-I prognosis group (Figure 3, C and D), the demand for new treatment strategies seems crucial. However, the numbers of these subgroups are too small to draw firm conclusions.
Preventing postoperative recurrence in patients with NSCLC is essential to improve prognosis, and recent research fuels great expectations of immunotherapy as an effective, specific cancer treatment with less toxicity [36]. Research on the dynamics of TILs, including memory T cells, before and after anticancer therapy will be pivotal for assessing their value as predictive markers regarding adjuvant treatment, including both chemotherapy and immunotherapy. Induction or maintenance of memory T cells might be a novel treatment strategy, and ensuring that anticancer treatment does not inhibit tumor-specific T memory cell subsets should at least be considered.
Because of its design, this study cannot solve the question of causality regarding the differences in prognostic impact in histological subgroups. Associations to factors known to differ between SCC versus non-SCC NSCLC, such as likelihood of smoking, growth patterns, pemetrexed sensitivity, antiangiogenic-associated toxicity, and frequency of response to epidermal growth factor receptors, could be explored in future studies. So should the functional importance of these markers in NSCLC subgroups.
In summary, our study is the first to reveal T-CD45RO as a strong prognosticator in NSCLC patients, though limited to the SCC histological subgroup. Interestingly, when combined with S-CD8, the independent positive prognostic impact improved convincingly in all pathological stages (I-IIIA), thus representing a robust immunological indicator of a significantly improved survival time. Furthermore, within each pathological stage, there were significant survival differences according to the immune score. CD45RO+ T memory lymphocytes are valuable prognosticators in NSCLC and are attractive for supplementing the TNM classification in the routine clinical setting as well as for research purposes. We propose CD45RO as a candidate marker for a novel TNM-I in squamous NSCLC.
The following are the supplementary data related to this article.
Association between Tumor Epithelial and Stromal CD45RO Expression and Clinicopathological Characteristics in NSCLC Patients.
Supplementary Figure 1.
DSS curves according to density of stromal CD45RO+ TILs (S-CD45RO) in (A) all patients, (B) SCC, and (C) ADC.
Supplementary Figure 2.
DFS and OS according to density of intraepithelial CD45RO+ TILs density (T-CD45RO) in (A, D) all patients, (B, E) SCC, and (C, F) ADC.
Supplementary Figure 3.
OS and DFS according to density of stromal CD45RO+ TILs (S-CD45RO) and in (A, D) all patients, (B, E) SCC and (C) ADC.
Conflicts of interest
The authors have no conflict of interest or financial disclosure to declare.
Footnotes
Conflict of interest and source of funding: The authors declare no conflict of interest.
Contributor Information
Erna-Elise Paulsen, Email: epa014@post.uit.no.
Thomas Kilvaer, Email: kilvaer@gmail.com.
Mehrdad Rakaee Khanehkenari, Email: mkh011@post.uit.no.
Ramona Johansen Maurseth, Email: ramonajohansen1@hotmail.com.
Samer Al-Saad, Email: samer.al-saad@unn.no.
Sigurd M. Hald, Email: sigurd.hald@uit.no.
Khalid Al-Shibli, Email: khalidshibli@gmail.com.
Sigve Andersen, Email: sigve.andersen@uit.no.
Elin Richardsen, Email: elin.richardsen@unn.no.
Lill-Tove Busund, Email: lill-tove.busund@unn.no.
Roy Bremnes, Email: roy.bremnes@uit.no.
Tom Donnem, Email: tom.donnem@uit.no.
References
- 1.Stewart B, Wild CP. Vol. International Agency for Research on Cancer; Lyon, FRA: 2014. World Cancer Report 2014. [Google Scholar]
- 2.Goldstraw P. The 7th edition of the TNM classification for lung cancer: proposals from the IASLC staging project. EJC Suppl. 2007;5:15–22. [Google Scholar]
- 3.Fridman WH, Pages F, Sautes-Fridman C, Galon J. The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer. 2012;12:298–306. doi: 10.1038/nrc3245. [DOI] [PubMed] [Google Scholar]
- 4.Galon J, Angell HK, Bedognetti D, Marincola FM. The continuum of cancer immunosurveillance: prognostic, predictive, and mechanistic signatures. Immunity. 2013;39:11–26. doi: 10.1016/j.immuni.2013.07.008. [DOI] [PubMed] [Google Scholar]
- 5.Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pages C, Tosolini M, Camus M, Berger A, Wind P. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960–1964. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 6.Pages F, Kirilovsky A, Mlecnik B, Asslaber M, Tosolini M, Bindea G, Lagorce C, Wind P, Marliot F, Bruneval P. In situ cytotoxic and memory T cells predict outcome in patients with early-stage colorectal cancer. J Clin Oncol. 2009;27:5944–5951. doi: 10.1200/JCO.2008.19.6147. [DOI] [PubMed] [Google Scholar]
- 7.Galon J, Pages F, Marincola FM, Angell HK, Thurin M, Lugli A, Zlobec I, Berger A, Bifulco C, Botti G. Cancer classification using the Immunoscore: a worldwide task force. J Transl Med. 2012;10:205. doi: 10.1186/1479-5876-10-205. (3 October 2012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Salgado R, Denkert C, Demaria S, Sirtaine N, Klauschen F, Pruneri G, Wienert S, Van den Eynden G, Baehner FL, Penault-Llorca F. The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: recommendations by an International TILs Working Group 2014. Ann Oncol. 2015;26:259–271. doi: 10.1093/annonc/mdu450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Remark R, Becker C, Gomez JE, Damotte D, Dieu-Nosjean MC, Sautes-Fridman C, Fridman WH, Powell CA, Altorki NK, Merad M. The non–small cell lung cancer immune contexture. A major determinant of tumor characteristics and patient outcome. Am J Respir Crit Care Med. 2015;191:377–390. doi: 10.1164/rccm.201409-1671PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schalper KA, Brown J, Carvajal-Hausdorf D, McLaughlin J, Velcheti V, Syrigos KN, Herbst RS, Rimm DL. Objective measurement and clinical significance of TILs in non–small cell lung cancer. J Natl Cancer Inst. 2015;107 doi: 10.1093/jnci/dju435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Donnem TP, Hald SM, Paulsen EE, Richardsen E, Al-Saad S, Kilvaer TK, Brustugun OT, Helland AP, Lund-Iversen M, Poehl M. Stromal CD8 + t cell density — a promising supplement to TNM STAGING in non–small cell lung cancer. Clin Cancer Res. 2015;21:2635–2643. doi: 10.1158/1078-0432.CCR-14-1905. [DOI] [PubMed] [Google Scholar]
- 12.Michie CA, McLean A, Alcock C, Beverley PC. Lifespan of human lymphocyte subsets defined by CD45 isoforms. Nature. 1992;360:264–265. doi: 10.1038/360264a0. [DOI] [PubMed] [Google Scholar]
- 13.Sallusto F, Geginat J, Lanzavecchia A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu Rev Immunol. 2004;22:745–763. doi: 10.1146/annurev.immunol.22.012703.104702. [DOI] [PubMed] [Google Scholar]
- 14.Suzuki K, Kadota K, Sima CS, Nitadori J, Rusch VW, Travis WD, Sadelain M, Adusumilli PS. Clinical impact of immune microenvironment in stage I lung adenocarcinoma: tumor interleukin-12 receptor beta2 (IL-12Rbeta2), IL-7R, and stromal FoxP3/CD3 ratio are independent predictors of recurrence. J Clin Oncol. 2013;31:490–498. doi: 10.1200/JCO.2012.45.2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rami-Porta R, Chansky K, Goldstraw P. Updated lung cancer staging system. Future Oncol. 2009;5:1545–1553. doi: 10.2217/fon.09.131. [DOI] [PubMed] [Google Scholar]
- 16.Travis WD, Brambilla E, Muller-Hermelink HK, Harris CC. IARC Press: City; 2004. World Health Organization Classification of Tumours. Pathology and Genetics of Tumours of the Lung, Pleura and Heart. [Google Scholar]
- 17.Travis WD, Brambilla E, Van Schil P, Scagliotti GV, Huber RM, Sculier JP, Vansteenkiste J, Nicholson AG. Paradigm shifts in lung cancer as defined in the new IASLC/ATS/ERS lung adenocarcinoma classification. Eur Respir J. 2011;38:239–243. doi: 10.1183/09031936.00026711. [DOI] [PubMed] [Google Scholar]
- 18.McShane LM, Altman DG, Sauerbrei W, Taube SE, Gion M, Clark GM, Statistics Subcommittee of the NCIEWGoCD Reporting recommendations for tumor marker prognostic studies (REMARK) J Natl Cancer Inst. 2005;97:1180–1184. doi: 10.1093/jnci/dji237. [DOI] [PubMed] [Google Scholar]
- 19.Donnem T, Al-Saad S, Al-Shibli K, Delghandi MP, Persson M, Nilsen MN, Busund LT, Bremnes RM. Inverse prognostic impact of angiogenic marker expression in tumor cells versus stromal cells in non small cell lung cancer. Clin Cancer Res. 2007;13:6649–6657. doi: 10.1158/1078-0432.CCR-07-0414. [DOI] [PubMed] [Google Scholar]
- 20.Al-Shibli KI, Donnem T, Al-Saad S, Persson M, Bremnes RM, Busund LT. Prognostic effect of epithelial and stromal lymphocyte infiltration in non–small cell lung cancer. Clin Cancer Res. 2008;14:5220–5227. doi: 10.1158/1078-0432.CCR-08-0133. [DOI] [PubMed] [Google Scholar]
- 21.Galon J, Mlecnik B, Bindea G, Angell HK, Berger A, Lagorce C, Lugli A, Zlobec I, Hartmann A, Bifulco C. Towards the introduction of the 'Immunoscore' in the classification of malignant tumours. J Pathol. 2014;232:199–209. doi: 10.1002/path.4287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jia Q, Yang Y, Wan Y. Tumor-infiltrating memory T-lymphocytes for prognostic prediction in cancer patients: a meta-analysis. Int J Clin Exp Med. 2015;8:1803–1813. [PMC free article] [PubMed] [Google Scholar]
- 23.Rauser S, Langer R, Tschernitz S, Gais P, Jutting U, Feith M, Hofler H, Walch A. High number of CD45RO + tumor infiltrating lymphocytes is an independent prognostic factor in non-metastasized (stage I-IIA) esophageal adenocarcinoma. BMC Cancer. 2010;10:608. doi: 10.1186/1471-2407-10-608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Enomoto K, Sho M, Wakatsuki K, Takayama T, Matsumoto S, Nakamura S, Akahori T, Tanaka T, Migita K, Ito M. Prognostic importance of tumour-infiltrating memory T cells in oesophageal squamous cell carcinoma. Clin Exp Immunol. 2012;168:186–191. doi: 10.1111/j.1365-2249.2012.04565.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pages F, Berger A, Camus M, Sanchez-Cabo F, Costes A, Molidor R, Mlecnik B, Kirilovsky A, Nilsson M, Damotte D. Effector memory T cells, early metastasis, and survival in colorectal cancer. N Engl J Med. 2005;353:2654–2666. doi: 10.1056/NEJMoa051424. [DOI] [PubMed] [Google Scholar]
- 26.de Jong RA, Leffers N, Boezen HM, ten Hoor KA, van der Zee AG, Hollema H, Nijman HW. Presence of tumor-infiltrating lymphocytes is an independent prognostic factor in type I and II endometrial cancer. Gynecol Oncol. 2009;114:105–110. doi: 10.1016/j.ygyno.2009.03.022. [DOI] [PubMed] [Google Scholar]
- 27.Wakatsuki K, Sho M, Yamato I, Takayama T, Matsumoto S, Tanaka T, Migita K, Ito M, Hotta K, Nakajima Y. Clinical impact of tumor-infiltrating CD45RO(+) memory T cells on human gastric cancer. Oncol Rep. 2013;29:1756–1762. doi: 10.3892/or.2013.2302. [DOI] [PubMed] [Google Scholar]
- 28.Lee HE, Chae SW, Lee YJ, Kim MA, Lee HS, Lee BL, Kim WH. Prognostic implications of type and density of tumour-infiltrating lymphocytes in gastric cancer. Br J Cancer. 2008;99:1704–1711. doi: 10.1038/sj.bjc.6604738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leffers N, Gooden MJ, de Jong RA, Hoogeboom BN, ten Hoor KA, Hollema H, Boezen HM, van der Zee AG, Daemen T, Nijman HW. Prognostic significance of tumor-infiltrating T-lymphocytes in primary and metastatic lesions of advanced stage ovarian cancer. Cancer Immunol Immunother. 2009;58:449–459. doi: 10.1007/s00262-008-0583-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gao Q, Zhou J, Wang XY, Qiu SJ, Song K, Huang XW, Sun J, Shi YH, Li BZ, Xiao YS. Infiltrating memory/senescent T cell ratio predicts extrahepatic metastasis of hepatocellular carcinoma. Ann Surg Oncol. 2012;19:455–466. doi: 10.1245/s10434-011-1864-3. [DOI] [PubMed] [Google Scholar]
- 31.Hotta K, Sho M, Fujimoto K, Shimada K, Yamato I, Anai S, Konishi N, Hirao Y, Nonomura K, Nakajima Y. Prognostic significance of CD45RO + memory T cells in renal cell carcinoma. Br J Cancer. 2011;105:1191–1196. doi: 10.1038/bjc.2011.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dieu-Nosjean MC, Antoine M, Danel C, Heudes D, Wislez M, Poulot V, Rabbe N, Laurans L, Tartour E, de Chaisemartin L. Long-term survival for patients with non–small-cell lung cancer with intratumoral lymphoid structures. J Clin Oncol. 2008;26:4410–4417. doi: 10.1200/JCO.2007.15.0284. [DOI] [PubMed] [Google Scholar]
- 33.Goc J, Germain C, Vo-Bourgais TK, Lupo A, Klein C, Knockaert S, de Chaisemartin L, Ouakrim H, Becht E, Alifano M. Dendritic cells in tumor-associated tertiary lymphoid structures signal a Th1 cytotoxic immune contexture and license the positive prognostic value of infiltrating CD8 + T cells. Cancer Res. 2014;74:705–715. doi: 10.1158/0008-5472.CAN-13-1342. [DOI] [PubMed] [Google Scholar]
- 34.Djenidi F, Adam J, Goubar A, Durgeau A, Meurice G, de Montpreville V, Validire P, Besse B, Mami-Chouaib F. CD8+CD103 + tumor-infiltrating lymphocytes are tumor-specific tissue-resident memory T cells and a prognostic factor for survival in lung cancer patients. J Immunol. 2015;194:3475–3486. doi: 10.4049/jimmunol.1402711. [DOI] [PubMed] [Google Scholar]
- 35.Mlecnik B, Tosolini M, Kirilovsky A, Berger A, Bindea G, Meatchi T, Bruneval P, Trajanoski Z, Fridman WH, Pages F. Histopathologic-based prognostic factors of colorectal cancers are associated with the state of the local immune reaction. J Clin Oncol. 2011;29:610–618. doi: 10.1200/JCO.2010.30.5425. [DOI] [PubMed] [Google Scholar]
- 36.Anagnostou VK, Brahmer JR. Cancer immunotherapy: a future paradigm shift in the treatment of non–small cell lung cancer. Clin Cancer Res. 2015;21:976–984. doi: 10.1158/1078-0432.CCR-14-1187. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Association between Tumor Epithelial and Stromal CD45RO Expression and Clinicopathological Characteristics in NSCLC Patients.