Abstract
Dieulafoy's disease of the bronchus is a relatively rare cause of hemoptysis. It can be completely asymptomatic and diagnosed as an incidental finding on bronchoscopy. At the other end of the spectrum, it can present with potentially fatal hemorrhage. We present a case of a 13-year old boy who suffered from massive hemoptysis. Endobronchial ultrasound (EBUS) and bronchial artery embolization (BAE) proved useful in the initial management. This case may support the role of EBUS in the diagnosis of Dieulafoy's disease as well as other intrapulmonary vascular lesions.
Keywords: Hemoptysis, Endobronchial ultrasound, Bronchial artery embolization, Dieulafoy's disease
1. Introduction
In 1898, Georges Dieulafoy first described the presence of an abnormal, tortuous submucosal artery in the stomach which eroded through the mucosa and caused massive gastrointestinal bleeding. These Dieulafoy lesions were later observed in the small intestine and colon. Similar lesions were first reported in the bronchi of patients presenting with hemoptysis in 1995 [1]. Biopsies of these lesions may prove fatal. Endobronchial ultrasound can be of value in preventing iatrogenic hemoptysis as reported below.
2. Case report
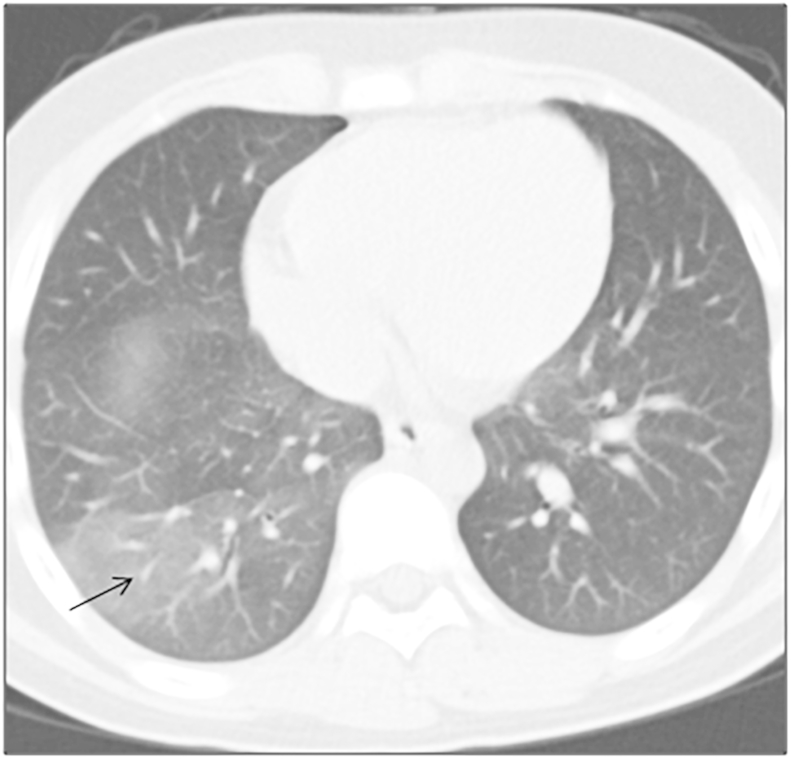
A 13-year old school boy was admitted to a regional hospital with several episodes of hemoptysis over a week. He had no significant past medical illness other than a history of fever which resolved with over-the-counter (OTC) medications one month ago and a two-week long cough productive of yellow sputum. Clinical examination was unremarkable. Computed tomography of the chest showed right posterior basal lobe ground glass opacity (Fig. 1). He developed a further episode of massive hemoptysis, expectorating about 500 ml of frank red blood. The teenager was managed conservatively with prompt resuscitation using intravenous fluids and intravenous hemocoagulase atrox before being transferred to our department.
Fig. 1.
A ground glass opacity in the right posterior basal segmental lobe on CT scan (black arrow).
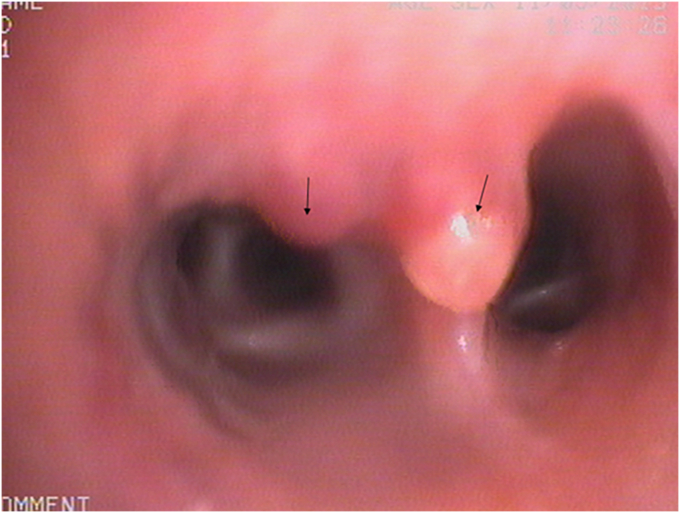
Flexible bronchoscopy revealed two elevated lesions with white caps (Fig. 2). They were located in the right lateral basal bronchus and over the subcarina of the right lateral and posterior basal bronchi; and measured 1.5 and 2.0 mm in diameter respectively. The lesions were not pulsatile and no abnormal vessels were seen through the mucosa. We suspected they could be Dieulafoy's lesions and decided to perform an endobronchial ultrasound scan (EBUS).
Fig. 2.
Two elevated lesions (one at the entrance of the right lateral basal bronchus and one over the subcarina of right lateral and posterior basal bronchus-black arrows) seen at bronchoscopy.
2.1. Technique for endobronchial ultrasound
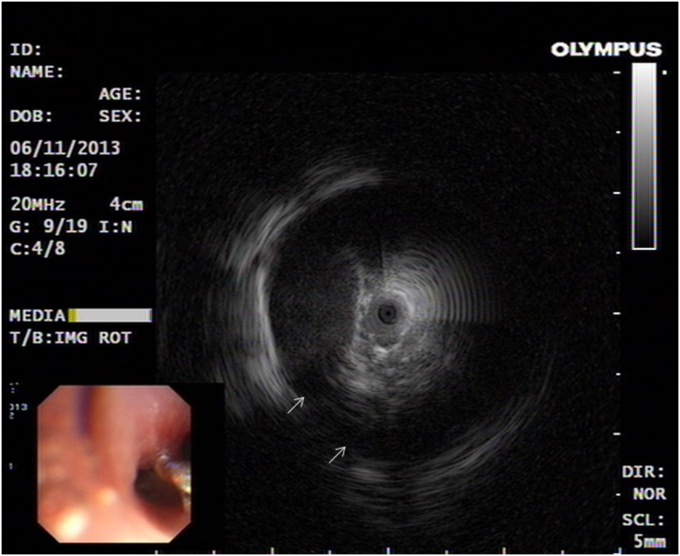
Local anaesthesia of the upper respiratory tract was achieved using 4% lidocaine. EBUS was performed using an endoscopic ultrasound system (EU-ME1; Olympus, Tokyo, Japan), equipped with a 20-MHz mechanical radial-type probe (UM-S20-17S; Olympus), having an external diameter of 1.8 mm. A standard bronchoscope with a working channel of 2.8 mm in diameter was used (BF-1TH190; Olympus). The probe was inserted into a guide sheath (GS). The GS-covered probe was inserted through the working channel of the bronchoscope and advanced towards the elevated lesions. The balloon of the radial probe was inflated with sterile water in order to obtain an EBUS image. The EBUS showed two circular anechoic areas circumscribed with a hyperechoic margin highly suggestive of vascular structures (Fig. 3). No biopsy was taken.
Fig. 3.
Endobronchial ultrasound showed two circular anechoic areas circumscribed with a hyperechoic margin highly suggestive of vascular structures (white arrow).
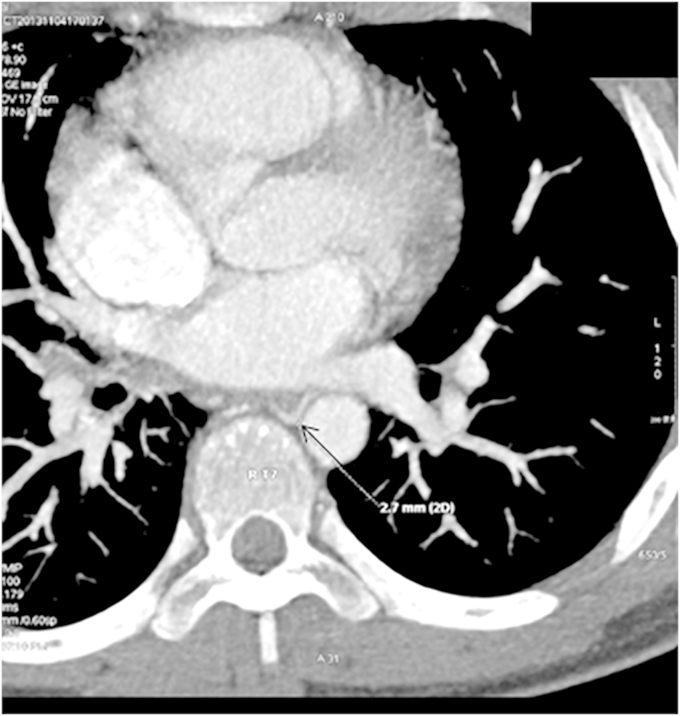
Bronchial artery computed tomo-angiography (BA-CTA) showed an abnormally situated right bronchial artery originating from the anterior wall of the thoracic aorta at the level of the fourth thoracic vertebra. The right bronchial artery was tortuous and dilated, measuring about 2.7 mm in diameter (Fig. 4). Bronchial artery embolization (BAE) was performed guided by digital subtraction angiography (DSA).
Fig. 4.
Computed tomography of bronchial artery showing an enlarged right bronchial artery about 2.7 mm in diameter (black arrow).
2.2. Procedures for BAE
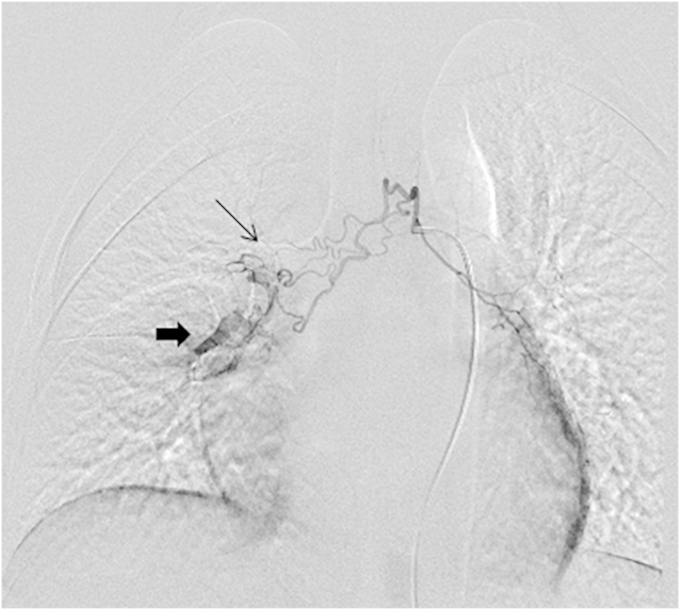
Under local anesthesia, the right common femoral artery was percutaneously punctured, and an introduction sheath was inserted. Using the Seldinger's technique, a 4F cobra Catheter was advanced into the upper part of descending thoracic aorta. Contrast agent was injected. It revealed a right tortuous bronchial artery and a bronchopulmonary shunt (Fig. 5). Transcatheter embolization of the hypertrophic bronchial artery was performed using PolyVinylAlcohol particles (PVA) of 300–500 μm in diameter. After embolization, DSA revealed complete disappearance of the abnormal right bronchial artery (Fig. 6).
Fig. 5.
Right tortuous bronchial artery (thin black arrow) and a bronchopulmonary shunt (thick arrow) on DSA.
Fig. 6.
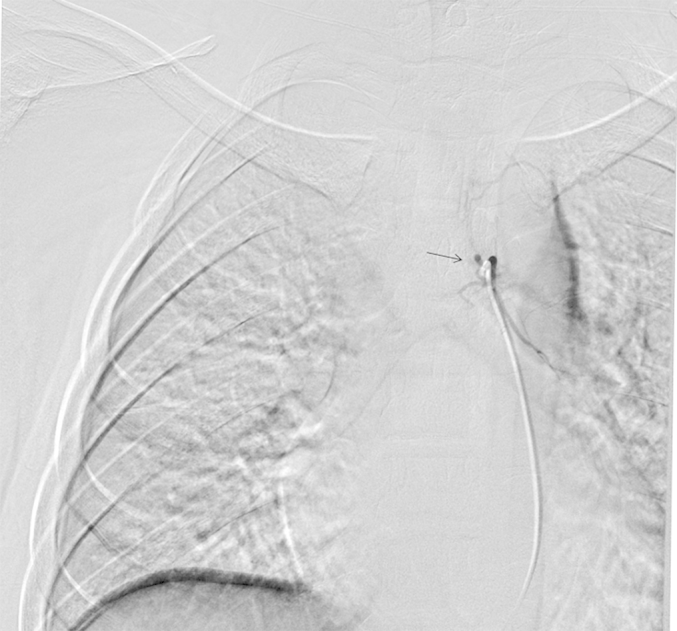
Complete disappearance of the right bronchial artery (black arrow) and bronchopulmonary shunt observed on DSA, after bronchial arterial embolization with PVA.
Massive hemoptysis recurred 3 months later. He underwent urgent thoracotomy with surgical resection of the right middle and lower lobes. Pathology showed a tortuous bronchial artery extending into the bronchial submucosa of the right lateral basal bronchus diagnostic of Dieulafoy's disease. No further episode of hemoptysis was reported.
3. Discussion
Massive hemoptysis is relatively uncommon in teenagers. Common conditions that can present with focal pulmonary hemorrhage in this age group include bronchitis, bronchiectasis, tuberculosis, trauma, pulmonary arteriovenous malformation, foreign bodies, pulmonary embolism, hemangioma and other neoplasms. Volume resuscitation and transfusion are necessary. It is essential to secure the airway and maintain ventilation. Localization of the bleeding is usually accomplished by imaging studies (chest x-ray, BA-CTA, BAE) and bronchoscopy. If the site of hemorrhage can be identified under bronchoscopy, irrigation with cold saline, topical administration of vasoconstrictive agents or endobronchial tamponade can be attempted. However, if the site of bleeding cannot be identified or there is active pulmonary hemorrhage, bronchial angiography followed by embolization should be considered.
Since Dieulafoy's disease of the bronchus was first described by Sweerts et al., in 1995 [1], the number of cases reported has increased gradually. More than 20 cases have been reported in the literature. However, this is the first case occurring in a teenager. The etiopathogenesis of this vascular anomaly remains obscure. It is thought to be congenital in nature. The age of our patient supports this theory. However, most other cases occurred in middle age patients and this may point to the fact that environmental factors may play a role. Respiratory infection and persistent cough may have triggered the bleeding in our patient.
The clinical features are non-specific. The lesion can remain asymptomatic for years. The most frequent presentation is recurrent hemoptysis or massive hemoptysis. It can follow an infectious prodrome or occur spontaneously. It has been seen as a fortuitous finding in patients undergoing bronchoscopy for other lung pathologies. At bronchoscopy, the lesions are elevated, non pulsating nodules with normal overlying mucosa. They may have a white cap [2], [3]. They do not have the look of a typical vascular lesion and can be easily misdiagnosed for a submucous mass or carcinoid tumor. Maxeiner reported an abnormal submucosal bronchial artery presenting as a protrusion in the right middle lobe bronchus leading to atelectasis of the right middle lobe [4]. Biopsy of Dieulafoy's lesions may cause potentially fatal hemorrhage [4], [5]. It is, therefore, important for the endoscopist to recognize these lesions.
Conventional white light bronchoscopy may miss up to 29% of vascular lesions and abnormal vascular patterns associated with dysplasia. The use of autofluorescence technique increases the sensitivity of detecting these lesions as the spectrum of light makes them fluoresce. However, the specificity is low. The combination of narrow band imaging (NBI) with autofluorescence helps to decrease the false positive rates. NBI causes enhancement of blood vessels as it uses wavelengths within the range of hemoglobin [6].
EBUS may help the endoscopist in deciding whether the lesion is vascular [7]. Radial probe EBUS uses a high frequency transducer (20–30 MHz) giving a high resolution of less than 1 mm and a penetration depth of up to 5 cm. It is ideal for diagnosis of submucosal vascular lesions. They can be advanced through the working channel of the bronchoscope. A guide sheath can be used to obtain ultrasound image of peripheral lesions which cannot be visualized by bronchoscopy itself. EBUS may be used along with fluoroscopy in some cases. Vascular lesions are usually hypoechoic or anechoic with hyperechoic or echogenic walls. EBUS bronchoscopes with integrated probes at the distal ends usually have a Doppler mode and can be used to confirm that a lesion is vascular by demonstrating flow. However, these probes have a relatively large diameter and cannot be used for peripheral lung lesions. They also have a lower frequency (5–12 MHz) which give a poorer resolution but a better depth of penetration.
While in all cases undergoing resection and pathological diagnosis, ectatic, tortuous arteries were seen in the bronchial mucosa, the origins of the arteries differ. They may arise from the systemic system (bronchial artery) or the pulmonary system (pulmonary artery) [5], [8]. Bronchial angiography [3] and BA-CTA can show dilated bronchial arteries, abnormal arteries, vascular shunts, fistulae and extravasation of contrast into the lungs. However, lesions of the pulmonary arteries may fail to show up. In these situations, pulmonary angiography may be essential.
The use of bronchial artery embolization has been described for initial management hemoptysis, including massive episodes. Conservative measures followed by BAE can control 90% of hemoptysis [9]. Different methods have been described including the use of spirals, tungsten coils or microparticles. BAE with N-butyl-2-cyanoacrylate may provide higher hemoptysis-free survival rate than PVA [10]. BAE can be performed under local anesthesia and has less complication rates than lobectomies. Morbidity is less and lung function after the procedure is better. Barisione [11] and Kolb [12] did not report recurrence after successful embolization. However, BAE may not always be feasible. In a series of 7 patients, Parrot et al. documented technical failures due to anatomical considerations and unsuccessful cannulations [13]. Reccurrence is common after BAE. Failure of BAE may also be due to an aberrant pulmonary artery instead of a bronchial artery [5]. Bronchopulmonary shunts may also impair success rate of BAE. Revascularization or neo-angiogenesis have been reported. Bhatia et al. successfully managed a case of Dieulafoy's disease of the bronchus with repeated BAE over 10 years [14]. We could have considered BAE for the second episode of massive hemoptysis but the patient was referred to our department after surgery.
Surgery has been the main definitive treatment till now [8]. While most reports claim that there were no post-operative recurrences of hemoptysis, long term follow ups are not documented.
4. Conclusion
Dieulafoy disease can lead to lethal hemoptysis. It is important for the endoscopist and interventional radiologist to recognize suspicious lesions and refrain to biopsy them. EBUS may be helpful in determining if an endobronchial lesion is vascular. BAE should be the first option in treating Dieulafoy's disease. Repeated embolization may be considered for long term management. Lobectomy should be considered in cases where BAE fails or is not feasible.
References
- 1.Sweerts M., Nicholson A.G., Goldstraw P., Corrin B. Dieulafoy's disease of the bronchus. Thorax. 1995;50:697–698. doi: 10.1136/thx.50.6.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gharagozloo F., Rennert D., Margolis M., Tempesta B., Schwartz A., Cole V., Wang K.-P. Dieulafoy lesion of the bronchus: review of the literature and report of the 13th case. J. Bronchology Interventional Pulmonol. 2008;15:38–40. [Google Scholar]
- 3.Hope-Gill B., Prathibha B.V. Bronchoscopic and angiographic findings in Dieulafoy's disease of the bronchus. Hosp. Med. 2002;63:178–179. doi: 10.12968/hosp.2002.63.3.2067. [DOI] [PubMed] [Google Scholar]
- 4.Maxeiner H. Lethal hemoptysis caused by biopsy injury of an abnormal bronchial artery. Chest. 2001;119:1612–1615. doi: 10.1378/chest.119.5.1612. [DOI] [PubMed] [Google Scholar]
- 5.van der Werf T.S., Timmer A., Zijlstra J.G. Fatal haemorrhage from Dieulafoy's disease of the bronchus. Thorax. 1999;54:184–185. doi: 10.1136/thx.54.2.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weisberg M.D., Garza E.G., Tabor M.H., Andrews A., Rumbak M.J. The use of narrow band imaging in patients with benign disease: hereditary hemorrhagic telangiectasia. J. Bronchology Interventional Pulmonol. 2011;18:352–354. doi: 10.1097/LBR.0b013e3182342346. [DOI] [PubMed] [Google Scholar]
- 7.Gurioli C., Casoni G.L., Gurioli C., Tomassetti S., Romagnoli M., Ravaglia C., Poletti V. Endobronchial ultrasound in Dieulafoy's disease of the bronchus: an additional application of EBUS. Monaldi archives for chest disease = Archivio Monaldi per le malattie del torace/Fondazione clinica del lavoro, IRCCS [and] Istituto di clinica tisiologica e malattie apparato respiratorio, Universita di Napoli, Secondo ateneo. 2010;73:166–168. doi: 10.4081/monaldi.2010.287. [DOI] [PubMed] [Google Scholar]
- 8.Stoopen E., Baquera-Heredia J., Cortes D., Green L. Dieulafoy's disease of the bronchus in association with a paravertebral neurilemoma. Chest. 2001;119:292–294. doi: 10.1378/chest.119.1.292. [DOI] [PubMed] [Google Scholar]
- 9.Savale L., Parrot A., Khalil A., Antoine M., Theodore J., Carette M.F., Mayaud C., Fartoukh M. Cryptogenic hemoptysis: from a benign to a life-threatening pathologic vascular condition. Am. J. Respir. Crit. Care Med. 2007;175:1181–1185. doi: 10.1164/rccm.200609-1362OC. [DOI] [PubMed] [Google Scholar]
- 10.Woo S., Yoon C.J., Chung J.W., Kang S.G., Jae H.J., Kim H.C., Seong N.J., Kim Y.J., Woo Y.N. Bronchial artery embolization to control hemoptysis: comparison of N-butyl-2-cyanoacrylate and polyvinyl alcohol particles. Radiology. 2013;269:594–602. doi: 10.1148/radiol.13130046. [DOI] [PubMed] [Google Scholar]
- 11.Barisione E.E., Ferretti G.G., Ravera S.S., Salio M.M. Dieulafoy's disease of the bronchus: a possible mistake. Multidiscip. Respir. Med. 2012;7:40. doi: 10.1186/2049-6958-7-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kolb T., Gilbert C., Fishman E.K., Terry P., Pearse D., Feller-Kopman D., Yarmus L. Dieulafoy's disease of the bronchus. Am. J. Respir. Crit. Care Med. 2012;186:1191. doi: 10.1164/rccm.201206-1016IM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parrot A., Antoine M., Khalil A., Theodore J., Mangiapan G., Bazelly B., Fartoukh M. Approach to diagnosis and pathological examination in bronchial Dieulafoy disease: a case series. Respir. Res. 2008;9:58. doi: 10.1186/1465-9921-9-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bhatia P., Hendy M.S., Li-Kam-Wa E., Bowyer P.K. Recurrent embolotherapy in Dieulafoy's disease of the bronchus. Can. Respir. J. J. Can. Thorac. Soc. 2003;10:331–333. doi: 10.1155/2003/729714. [DOI] [PubMed] [Google Scholar]