Abstract
[Purpose] It has been well-established that exercise-induced muscle damage occurs following intense exercise. Massage is commonly used to manage muscle damage resulting from exercise. However the effect of massage after exercise is still not clear. The purpose of this study was to examine the effect of manual lymph drainage on muscle damage and on the removal of blood lactate following submaximal exercise (SE), as part of a solution to the challenging problem in sports medicine of muscular recovery after exercise. [Subjects and Methods] Eighteen healthy male students, with moderate exercise training, were randomly assigned to either receive manual lymph drainage (MLD) or serve as controls. Both groups were subjected to a graded exercise test, performed on a treadmill ergometer, to determine each subject’s individual anaerobic threshold (IAT). Seven days later, all subjects were made to run for 30 minutes on the same treadmill ergometer, at a running speed equivalent to the IAT. One group received MLD treatment, while the control subjects received no treatment. [Results] Following an increase immediately after exercise, lactic acid (LA) and lactate dehydrogenase (LDH) serum levels dropped rapidly and significantly at the end of MLD application and two hours after SE in the subjects receiving MLD. The course of creatine kinase (CK) and myoglobin levels was comparable, and with myoglobin showing a significant difference at 2 h after SE, and CK at 24 h after SE. [Conclusion] Manual lymph drainage after SE correlated with a more rapid fall in LA and of the muscular enzymes of LDH, CK and myoglobin, and may have resulted in an improvement in the regenerative processes elicted by structural damage to the muscle cells.
Key words: Manual lymph drainage, Recovery, Submaximal exercise
INTRODUCTION
Exhaustive and/or unaccustomed exercises (particularly those involving high intensity muscle contractions) are known to induce temporary muscle damage. This damage includes a series of events occurring. It causes damage to the sarcolemma and the muscle cell membrane. This damage results in the release of biochemical markers of muscle damage including (e.g. lactate dehydrogenase (LDH), creatine kinase (CK), and myoglobin (Mb)1, 2). Coaches, athletic trainers, and athletes should seek scientific support for therapeutic interventions which claim to help reduce the effects of muscle damage and speed of recovery from exercise and athletic endeavors2). Therefore, improving muscle recovery after physical exercise is an important topic in sports medicine.
Many researchers investigated various intervention to treat muscle damage such as active recovery, massage, cryotherapy, electrotherapy, hydrotherapy, supplementation and compression garments3,4,5,6,7,8,9,10). Massage is also commonly assumed to enhance muscle recovery from intense exercise11). However the effect of massage after delayed onset muscle soreness (DOMS) is still not clear12). A recently review and meta-analysis demonstrated that therapeutic massage is the only intervention that has a positive effect on the recovery of “muscle soreness” and function; however, its mean effect is too small to be considered clinically relevant1). The potential benefits of massage on recovery include increased blood circulation and venous return, greater lactate clearance, decreased pain sensation, and general well-being13).
Manual Lymph Drainage (MLD) is a massage technique that involves the skin surface only and follows the anatomic lymphatic pathways of the body. Unlike other types of massage, it produces neither blush nor pain and promotes lymph flow14, 15). Some biochemical markers, such as CK, LDH, aspartate aminotransferase (AST) and Mb are particularly significant in the evaluation of tissue damage16). Creatine kinase, a dimeric enzyme with a particularly large molecule (80,000 Da) cannot penetrate enter into the bloodstream following muscular injury. And under injury conditions, it passes into the lymphatic system from the intercellular fluid and is transferred to the general circulation from the thoracic lymph nodes. This has led to the hypothesis of an inverse relationship between lymphatic circulation and CK levels17). Havas et al. studied the effect of peripheral lymphatic flow reduction, achieved by bed rest, on serum CK after exercise. They concluded that physical inactivity of short duration reduces the lymphatic transport of CK and the secretion of muscle enzymes18). Currently, there is a lack of controlled research to support the efficacy of manual lymph massage in accelerating the rate of post-exercise blood clearance of lactate and biochemical markers such as CK, LDH, and AST19). Although acute submaximal exercise (SE) may induce tissue damage in trained individuals, MLD applied immediately after exercise can reduce tissue or muscle damage and also help the removal of blood lactate. In this study, we have investigated the effect of MLD on reducing tissue or muscle damage and removing blood lactate and muscle enzymes after SE in moderately trained individuals was investigated.
SUBJECTS AND METHODS
Eighteen healthy and moderately trained male students took part in this study. The subjects were randomly divided into two groups, an MLD group and a control group. They are illustrated in (Fig. 1). None of the subjects had any history of cardiovascular, metabolic or musculoskeletal injury, or disease. This study was approved by the Human Research Ethics Committee of the Faculty of Medicine, Abant Izzet Baysal University, Bolu, Turkey. All the subjects gave their informed consent before the study started. Subjects were instructed to avoid heavy exercise in the 72 hours before coming on all occasions. The physical characteristics of the subjects are shown in Table 1.
Fig. 1.
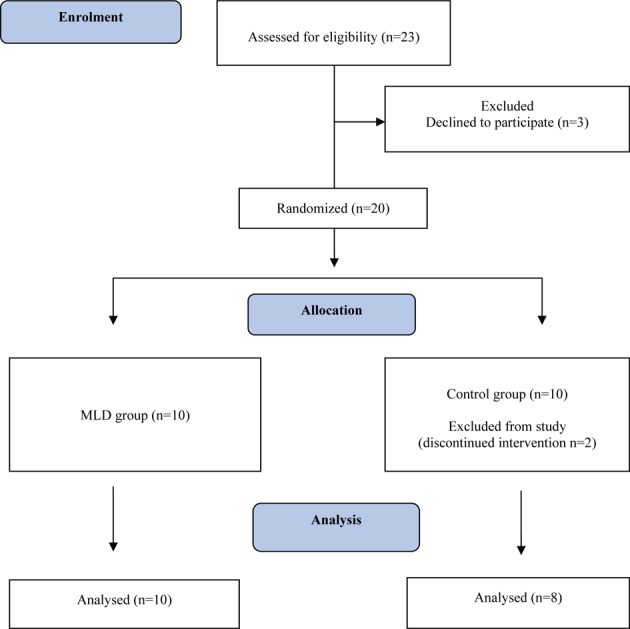
Study flow diagram
Table 1. Physical characteristics of the subjects.
| MLD (n=10) | CG (n=8) | |
|---|---|---|
| X±SD | X±SD | |
| Age (years) | 21.1 ± 1.1 | 21.9 ± 1.2 |
| Height (m) | 1.77 ± 0 | 1.78 ± 0.1 |
| Body weight (kg) | 69.2 ± 7 | 69.4 ± 6.6 |
| Anaerobic threshold (km-hr) | 13.1 ± 0.9 | 12.4 ± 1.3 |
MLD: Manual lymph drainage group, CG: Control group
The subjects performed a graded exercise test on a treadmill ergometer (Hp Mercury, Germany) under standardized conditions to determine the anaerobic threshold. The exercise test began at 8 km·hr−1 and increased by 1 km·hr−1 every three minutes. Heart rate was recorded by a Polar monitor (Electro, Finland). Blood lactate levels were measured in capillary blood drawn from a hyperemic ear lobe, at rest and at the end of each exercise level. The subjects ran until their blood lactate levels exceeded 5 mmol/L. Seven days after the graded exercise test, all subjects ran 30 minutes on the same treadmill ergometer, at a running velocity equivalent to the IAT. Capillary blood samples to determine the lactate concentrations were taken from a hyperemic earlobe before the beginning of each test procedure.
MLD was applied by a trained physiotherapist, who used specialized hand movements in a range of different sequences. The treatment was carried out as very light, completely pain-free, rhythmical translational movements of the skin in the flow direction of the lymph vessels. The subjects lay in the supine position with their knees bent. The MLD started with abdominal lymph drainage after which central lymph stimulation was performed. MLD was consecutively applied to the right and left lower extremities (leg) consecutively. Each treatment session lasted approximately 30 minutes. The control group did not receive any therapy.
Blood samples were drawn from the antecubital vein shortly before MLD. Serum and plasma were separated by centrifugation at 1,500 × g for 15 minutes. All serum and the plasma samples were stored at −80 °C until analysis. Serum levels of AST, LDH, CK and plasma levels of lactate were determined by an autoanalyzer (Architect c8000, Abbott Inc., Abbott Park IL, USA), using test kits by the same manufacturer. Serum myoglobin level was determined by a chemiluminescentce immunoassay analyzer (Architect i2000, Abbott Laboratories, Abbott Park, Illinois, USA) using the commercial kits recommended by the manufacturer. The blood samples were taken: 1) directly before the submaximal exercise (B-SE); 2) directly after the submaximal exercise (A-SE); 3) after 30 min rest / after the MLD application (A-MLD-R); 4) 2 hours after the submaximal exercise (2hA-SE) 5); 24 hours after the submaximal exercise (24hA-SE) and 6) 48 hours after the submaximal exercise (48hA-SE)
The SPSS 16 statistical package was used for statistical analyses. The Mann and Whitney U-test for two independent samples was performed for intergroup comparisons. Differences between the groups and over time were examined by repeated measurements analysis of variance and stepwise multivariate tests. The probability level (p-value) for significance was chosen as 0.05.
RESULTS
There were no significant differences in the physical characteristics of two groups (p>0.05) (Table 1). Changes in LA and the muscle enzymes, (LDH, myoglobin and CK) are presented in Table 2. Statistically significant differences from baseline data were found at the repeated measurements over time of LA, LDH, myoglobin and CK in both groups (p<0.05), except for CK in the control group, which showed no significant differences.
Table 2. Changes in lactic acid (LA) and muscle enzymes (LDH, Mb, CK).
| Measure | Group | LA (IU·ml1) | LDH (IU·ml1) | Mb (IU·ml1) | CK (IU·ml1) |
|---|---|---|---|---|---|
| B-SE | MLD | 13.1±6 | 151.2±14.1 | 40±21 | 190.1±87.3 |
| CG | 11.4±1.7 | 156.5±18.4 | 34.1±7.2 | 275.7±312.2 | |
| A-SE | MLD | 71.2±28.6 | 182.1±23.1 | 80.7±46.9 | 235.8±111.2 |
| CG | 54.3±10.1 | 185.5±21.4 | 60.3±14.5 | 325±320.6 | |
| A-MLD | MLD | 21.3±7.7 | 155±33.2 | 120.9±63.7 | 196.6±95.7 |
| CG | 29.6±7.5 | 185.1±15.6 | 88.4±34.3 | 310.7±306.2 | |
| * | * | ||||
| 2hA-SE | MLD | 12.5±4.3 | 143.6±21.7 | 193.3±190.4 | 192.2±41.6 |
| CG | 19.9±7.5 | 174.4±24.8 | 94.3±38 | 347.5±279.5 | |
| * | * | * | |||
| 24hA-SE | MLD | 11.4±1.9 | 153.4±16.8 | 69.5±51.2 | 145.1±38.8 |
| CG | 19.1±10.6 | 156±12.1 | 52.6±28.6 | 235.7±89.9 | |
| * | |||||
| 48hA-SE | MLD | 13.9±7.3 | 142±18.6 | 64.4±86.6 | 182.4±94 |
| CG | 15±8.2 | 152.6±8.6 | 44.1±14.9 | 208.2±139.4 | |
* Significant difference between the MLD group and CG (p <0.05). Before submaximal exercise (B-SE), after submaximal exercise (A-SE), after manual lymph drainage (A-MLD), two hours after submaximal exercise (2hA-SE), 24 hours after submaximal exercise (24hA-SE), 48 hours after submaximal exercise (48hA-SE), lactic acid (LA), lactate dehydrogenase (LDH), myoglobin (Mb), creatine kinase (CK)
Significant differences were found between the two groups in baseline LA (p=0.027) and LDH (p=0.016); two hours after SE in LA (p=0.034), LDH (p=0.012) and myoglobin (p=0.034); and 24 h after SE in CK (p=0.043). No other significant differences were found between the groups. LA increased directly after SE in both groups. The increase was followed by a significant decrease in the MLD group after MLD, a difference that remained significant two hours after SE. A similar pattern was observed for LDH. Change in the myoglobin level was only significant at 2 h after SE, and in CK only 24 h at SE.
DISCUSSION
This study was performed to assess the effect of MLD on the reduction of tissue or muscle damage and the removal of blood lactate and muscle enzymes after SE, in moderately trained individuals. The importance of this study is in its being the first to show the influence of MLD on LA. The results demonstrate that MLD might be effective at removing blood lactate and some muscle enzymes after SE.
MLD was used to accelerate lymphatic circulation in this study. MLD is a type of massage used to increase lymph flow and reabsorption without increasing ultrafiltration; and it requires special training. It is hypothesized that the specific handling at the skin level causes contraction of the superficial lymph vessels, increasing lymphatic drainage20). It was reported that MLD application not only speeds up the blood circulation but may also lead to faster elimination of metabolic waste from the body and increase oxygen transport to the muscle21).
Another study of the effect of MLD, not too familiar a concept in sport medicine, on muscle injury arising during SE and the disposal of metabolic waste, has been published. Two important aspects differentiate this study from the former: first our evaluation of LA levels in addition to the muscular enzymes (LDH, CK, myoglobine), and second, the drawing of blood samples immediately after the MLD application. Thus, evaluated both LA levels and the immediate effect of MLD were evaluated. Blood samples were drawn 2 h after the MLD application in the study of Schillinger et al.19); however, it was reported that MLD effect peaks within 30 minutes following MLD22). We observed a significant fall in both LA and LDH immediately after MLD, and their application, with levels remained low at the end of the second hour. This is an aspect in which this study offers a superior feature. LDH levels fell after 48 hours in Schillinger’s study.
The main cause of fatigue developing during exercise is LA builds up. Accumulation of LA increases the H− concentration, thus reducing action potentials. This in turn reduces calcium secretion from the sarcoplasma and muscle enzyme activity. The first sign of fatigue that follows is a loss of strength in voluntary muscle contraction23). According to Dierking and Bemben, LA is also responsible for the muscle pain accompanying delayed fatigue, which continues for 24–48 hours after exercise24). Exercise also depletes cellular ATP. This results in increased cellular permeability and in increases in the serum concentrations of skeletal muscle enzymes such as CK, LDH and AST17), an increase which are also affected also by the type and intensity of exercise and the duration of muscular activity. The overall serum CK evolution shows an increase especially at 24 h after exercise, and the effect that may persist for more than 4 days25). While Schillinger’s study did not establish a significant difference in CK levels, the present study showed that MLD was followed by a decrease in CK within 24 hours, and a reduction in delayed muscular fatigue. The results of both studies suggested that MLD contributed to mitigation of muscular damage and enhanced the regenerative process of muscle cells. In our opinion, such a contribution is significant, especially for athletes who have to strive daily to present their best performance in competition. However, Nogueira et al. analyzed CK before and (except CK), 24 h, 48 h, 72 h and 96 h after an exercise protocol. They classified individuals as “low” and “high” responders at the peak of maximum voluntary concentric muscle contraction. The classification of the subjects into low and high responders groups was important for demonstrating that the most responsive group to strength decrease after eccentric exercise also showed higher DOMS levels. This notwithstanding, a relationship with CK could not be established26).
Different methods including massage, cold application, tensing and light exercise are used to help recovery after exercise. Studies of these methods are as yet insufficient for the purpose of drawing definitive conclusions. Published studies considering massage performed post-exercise have investigated its effect on variables such as delayed muscle pain, sensitivity, and muscular function and performance. Abad et al. reported there was no effect of post-exercise massage on delayed muscle pain reduction27); Farr et al. indicated that massage has no effect on muscle performance and function, it does diminish pain intensity28). Mancinelli et al. investigated the effect of massage on post-exercise symptoms of 22 trained women using the pressure pain threshold, shuttle race times and vertical leaps as outcome measures, and reported it resulted in muscular pain and an improvement in vertical leaps29). Emiliano Ce et al. performed a study comparing the effects of deep/superficial massage with those of passive tension and active to passive recovery on the lactate level in the blood. They concluded that neither of the two massage methods (deep vs superficial) or the passive static tension application was correlated to a significant change in lactate levels; in other words, that they could be used as an alternative to active recovery (at 50% VO2max)30). These contradictory results can be explained by the absence of a standard procedure for the different techniques used under the description of “massage” and the small numbers of subjects in the different studies31).
Another application thought to be potentially useful for recovery is intermittent pneumatic compression (IPC). A study performed by Draper investigated the contribution of intermittent pneumatic compression on the muscle inflammation marker C-reactive protein (CRP) and DOMS in trained athletes after long distance running. The results indicated statistically there was no significant difference in CRP or pain levels between the control run and treatment run2). These results aren’t compatible with our results. However, the data suggests the test subjects recovered one day after receiving IPC than to the control, and a full day recovery for a professional athlete is very important. Studies with greater numbers of subjects comparing MLD to different recovery methods, following up values, especially CK serum levels, up to 120 hours are still needed in order to proceed to a further advance our understanding of muscular recovery.
REFERENCES
- 1.Torres R, Ribeiro F, Alberto Duarte J, et al. : Evidence of the physiotherapeutic interventions used currently after exercise-induced muscle damage: systematic review and meta-analysis. Phys Ther Sport, 2012, 13: 101–114. [DOI] [PubMed] [Google Scholar]
- 2.Draper SN: Effects of intermittent pneumatic compression on delayed onset muscle soreness (DOMS) in long distance runners. Cleveland State University. PhD Thesis. 2014. [PMC free article] [PubMed]
- 3.Pastre CM, Bastos FD, Netto JJ, et al. : Post-exercise recovery methods: a systematic review. Rev Bras Med Esporte. 2009, 15: 138–144. [Google Scholar]
- 4.Nelson N: Delayed onset muscle soreness: is massage effective? J Bodyw Mov Ther, 2013, 17: 475–482. [DOI] [PubMed] [Google Scholar]
- 5.Glasgow PD, Ferris R, Bleakley CM: Cold water immersion in the management of delayed-onset muscle soreness: is dose important? A randomised controlled trial. Phys Ther Sport, 2014, 15: 228–233. [DOI] [PubMed] [Google Scholar]
- 6.Rashid SA, Quddus N: Effect of brief intense TENS and cryotherapy on the symptoms associated with delayed onset of muscle soreness in healthy male subjects. Indian J Physiotherapy Occup Therapy-An Int J, 2013, 7: 1–5. [Google Scholar]
- 7.Felismino AS, Costa EC, Aoki MS, et al. : Effect of low-level laser therapy (808 nm) on markers of muscle damage: a randomized double-blind placebo-controlled trial. Lasers Med Sci, 2014, 29: 933–938. [DOI] [PubMed] [Google Scholar]
- 8.Cuesta-Vargas AI, Travé-Mesa A, Vera-Cabrera A, et al. : Hydrotherapy as a recovery strategy after exercise: a pragmatic controlled trial. BMC Complement Altern Med, 2013, 13: 180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pasiakos SM, Lieberman HR, McLellan TM: Effects of protein supplements on muscle damage, soreness and recovery of muscle function and physical performance: a systematic review. Sports Med, 2014, 44: 655–670. [DOI] [PubMed] [Google Scholar]
- 10.Hill J, Howatson G, van Someren K, et al. : Compression garments and recovery from exercise-induced muscle damage: a meta-analysis. Br J Sports Med, 2014, 48: 1340–1346. [DOI] [PubMed] [Google Scholar]
- 11.Hemmings B, Smith M, Graydon J, et al. : Effects of massage on physiological restoration, perceived recovery, and repeated sports performance. Br J Sports Med, 2000, 34: 109–114, discussion 115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Han JH, Kim MJ, Yang HJ, et al. : Effects of therapeutic massage on gait and pain after delayed onset muscle soreness. J Exerc Rehabil, 2014, 10: 136–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Delextrat A, Hippocrate A, Leddington-Wright S, et al. : Including stretches to a massage routine improves recovery from official matches in basketball players. J Strength Cond Res, 2014, 28: 716–727. [DOI] [PubMed] [Google Scholar]
- 14.Martín ML, Hernández MA, Avendaño C, et al. : Manual lymphatic drainage therapy in patients with breast cancer related lymphoedema. BMC Cancer, 2011, 11: 94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shim JM, Kim SJ: Effects of manual lymph drainage of the neck on EEG in subjects with psychological stress. J Phys Ther Sci, 2014, 26: 127–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martin NA, Zoeller RF, Robertson RJ, et al. : The comparative effects of sports massage, active recovery, and rest in promoting blood lactate clearance after supramaximal leg exercise. J Athl Train, 1998, 33: 30–35. [PMC free article] [PubMed] [Google Scholar]
- 17.Alibeyoğlu A: Düzenli spor yapmayan genç erkeklerde akut dayanıklılık egzersizi sonrası hematolojik ve serum enzim değerlerindeki değişikliklerin incelenmesi. Kafkas University. Graduate School of Health Sciences. Master of Science Thesis. Kars; 2008.
- 18.Havas E, Komulainen J, Vihko V: Exercise-induced increase in serum creatine kinase is modified by subsequent bed rest. Int J Sports Med, 1997, 18: 578–582. [DOI] [PubMed] [Google Scholar]
- 19.Schillinger A, Koenig D, Haefele C, et al. : Effect of manual lymph drainage on the course of serum levels of muscle enzymes after treadmill exercise. Am J Phys Med Rehabil, 2006, 85: 516–520. [DOI] [PubMed] [Google Scholar]
- 20.Rose KE, Taylor HM, Twycross RG: Long-term compliance with treatment in obstructive arm lymphoedema in cancer. Palliat Med, 1991, 5: 52–55. [Google Scholar]
- 21.Földi M, Strössenreuther R: Foundations of manual lymph drainage, 3rd ed. New York: Elsevier; 2005, pp 4–49. [Google Scholar]
- 22.Földi M, Kubik S: Lehrbuch der Lymphologie, 4th ed. Jena, Urban Fischer Publisher; 2001, pp 530–557. [Google Scholar]
- 23.Gültekin Z, İşler AK, Sürenkök Ö, et al. : Effect of electrical stimulation with high voltage pulsed galvanic current and Russian currents on lactic acid accumulation: a preliminary study. Fizyoter Rehabil. 2006, 17: 89–94. [Google Scholar]
- 24.Dierking JK, Bemben MG, Bemben DA, et al. : Validity of diagnostic ultrasound as a measure of delayed onset muscle soreness. J Orthop Sports Phys Ther, 2000, 30: 116–122, discussion 123–125. [DOI] [PubMed] [Google Scholar]
- 25.Hazar S: Egzersize bağlı iskelet ve kalp kası hasarı. Spormetre, 2004, II: 119–126. [Google Scholar]
- 26.Nogueira FR, Chacon-Mikahil MP, Vechin FC, et al. : Muscle soreness and creatine kinase activity after eccentric actions: a cluster analysis. Rev Bras Med Esporte. 2014, 20: 257–261. [Google Scholar]
- 27.Abad CC, Ito LT, Barroso R, et al. : Effect of classical massage on subjective perceived soreness, edema, range of motion and maximal strength after delayed onset muscle soreness. Rev Bras Med Esporte. 2010, 16: 36–40. [Google Scholar]
- 28.Farr T, Nottle C, Nosaka K, et al. : The effects of therapeutic massage on delayed onset muscle soreness and muscle function following downhill walking. J Sci Med Sport, 2002, 5: 297–306. [DOI] [PubMed] [Google Scholar]
- 29.Mancinelli CA, Davis DS, Aboulhosn L, et al. : The effects of massage on delayed onset muscle soreness and physical performance in female collegiate athletes. Phys Ther Sport, 2006, 7: 5–13. [Google Scholar]
- 30.Cè E, Limonta E, Maggioni MA, et al. : Stretching and deep and superficial massage do not influence blood lactate levels after heavy-intensity cycle exercise. J Sports Sci, 2013, 31: 856–866. [DOI] [PubMed] [Google Scholar]
- 31.Best TM, Hunter R, Wilcox A, et al. : Effectiveness of sports massage for recovery of skeletal muscle from strenuous exercise. Clin J Sport Med, 2008, 18: 446–460. [DOI] [PubMed] [Google Scholar]


