Abstract
[Purpose] The purpose of the study was to design and implement a multichannel dynamic functional electrical stimulation system and investigate acute effects of functional electrical stimulation of the tibialis anterior and rectus femoris on ankle and knee sagittal-plane kinematics and related muscle forces of hemiplegic gait. [Subjects and Methods] A multichannel dynamic electrical stimulation system was developed with 8-channel low frequency current generators. Eight male hemiplegic patients were trained for 4 weeks with electric stimulation of the tibia anterior and rectus femoris muscles during walking, which was coupled with active contraction. Kinematic data were collected, and muscle forces of the tibialis anterior and rectus femoris of the affected limbs were analyzed using a musculoskelatal modeling approach before and after training. A paired sample t-test was used to detect the differences between before and after training. [Results] The step length of the affected limb significantly increased after the stimulation was applied. The maximum dorsiflexion angle and maximum knee flexion angle of the affected limb were both increased significantly during stimulation. The maximum muscle forces of both the tibia anterior and rectus femoris increased significantly during stimulation compared with before functional electrical stimulation was applied. [Conclusion] This study established a functional electrical stimulation strategy based on hemiplegic gait analysis and musculoskeletal modeling. The multichannel functional electrical stimulation system successfully corrected foot drop and altered circumduction hemiplegic gait pattern.
Key words: Hemiplegia, Functional electrical stimulation, Gait
INTRODUCTION
Hemiplegia associated with stroke, cerebral palsy, or polio commonly leads to movement disorders1). Research into rehabilitation for hemiplegic patients has attracted increased attention. The neurological dysfunctions of hemiplegic patients are often related to spasticity and/or contracture of ankle plantarflexors, low muscle activity of dorsiflexors, and loss of selectivity in motor control2). Abnormality is due to interruption of nerve excitability and transmission to muscles through the central nervous system and causes abnormal gait1). Therefore, it has been a challenge to provide effective exercises to paralyzed muscles to improve their functions in walking in clinical rehabilitation practices.
In order to find a solution to this problem, many studies have been performed on gait analysis and functional electrical stimulation (FES). Chen et al.3) showed that poststroke patients had impaired swing initiation of the affected limb and exaggerated trunk elevation during the swing phase. In addition, a shorter duration of single limb support on the affected limb, circumduction gait pattern, asymmetry in step length, and increased step width were also found in the patients. Bensoussan et al.4) investigated the gait initiation patterns of hemiplegic patients and found that the healthy limb supported more body weight than the affected limb and that the affected knee was elevated less than the healthy limb during the swing phase.
Stanic and Trnkoczy5) first reported the results regarding restoration of ankle joint movements of a hemiplegic patient during gait using FES of antagonistic muscle groups and position feedback. Vodovnic et al.6) demonstrated that patients with wrist dorsiflexion weakness were able to achieve a full range of motion when treated with neuromuscular electrical stimulation. Currently, FES is widely used in clinical applications of rehabilitation. Patients with movement disorders receive electrical stimulation induced by electromyography (EMG) activities of their proprioceptors, and repeat movement patterns to help stimulate excitation of the motor cortex, which may cause permanent improvement in movements and posture. It has been proven that real-time control plays a key role in the success of FES. Several previous studies demonstrated that FES significantly increased the step length of affected limbs7,8,9), dorsiflexion at initial contact during walking10), maximal knee flexion9), and EMG activities of muscles related to hemiplegic gait11).
Electrical stimulation devices are commonly used in static and localized applications. However, static electrical stimulation cannot work effectively during dynamic movements. FES can only provide stimulations based on feedback from muscle groups that can still generate contractions. The FES used in hemiplegia treatments is normally capable of reinforcing or rebuilding the proprioceptive biofeedback system to enable relevant signals to be relayed back to the central nervous system and to develop a new proprioception-motion feedback system. The multichannel dynamic electrical stimulation system (M-DESA) developed by our group can provide assistive strength training to specific muscles involved in certain movements, and it was developed based on our first-generation single-channel dynamic electrical stimulation system (S-DESA)12). The system has a total of eight channels and is capable of stimulating eight muscles (or muscle groups) simultaneously. During movement, electrical stimulations are controlled by a computer program using inputs from a foot switch and can be generated according to multi-muscle coordination. Combining voluntary contractions of targeted muscles (as well as those muscles that have lost their contractibility due to hemiplegia) and passive muscle contractions elicited by electrical stimulation, the system is able to provide efficient feedback stimulation to the central nervous system so that more muscle fibers are involved during contractions and more muscle force is generated.
Although FES has been widely used in hemiplegia rehabilitation, the stimulation is often applied to patients statically when they lie on their side or stand with aids. In addition, hemiplegic gait characteristics have been widely investigated. Dynamic modeling and simulation of human movements can be used to estimate muscle forces and activation sequences to provide support for efficacy of FES. Few studies have incorporated musculoskeletal modeling in estimating muscle forces during the gait of hemiplegic patients during or after FES. Therefore, the purpose of this study was to design and implement a multichannel dynamic FES system and investigate acute effects of FES of the tibialis anterior and rectus femoris on ankle and knee sagittal-plane kinematics and related muscle forces of the hemiplegic gait. Our first hypothesis was that the step length of affected limbs would be greater during stimulation compared with that prior to FES. We further hypothesized that the maximum ankle dorsiflexion and knee flexion angles would be greater during FES compared with that before FES and that the muscle forces would be greater during stimulation compared with those before FES.
SUBJECTS AND METHODS
Eight male patients (age, 40.3 ± 9.2 years; height, 175 ± 6.6 cm; and mass, 68.9 ± 7.1 cm) recruited from a local hospital participated in the study. Among the patients, four had cerebral hemorrhage, three cerebral infarction, and one traumatic brain injury. In order to be qualified for the study, patients had to show typical hemiplegic gait patterns, demonstrate involuntary contraction of the tibialis anterior and quadriceps femoris muscles, and be able to walk more than ten meters three times without assistance. The exclusion criteria included cardiopulmonary dysfunctions, renal insufficiency, severe cognitive impairments, speech disorder, inability to give proper informed consent, and neuromuscular diseases. All participating patients signed an informed consent form approved by the local ethics committee.
Development of the dynamic muscle electrical stimulation system: The circular hemiplegic gait is a typical abnormal gait pattern caused by tibialis anterior weakness. We initially designed the S-DESA for the tibialis anterior to target the circular hemiplegic gait, and it is a wearable mini medical device that includes a control unit (ARM7 CPU), stimulation electrode, stimulation electrode cable, foot switch and trigger. Other features include a lithium battery, a clip on case, and a weight of 150 g. The device can be clipped on the belt or strapped to the leg. Its main function is correction of the foot drop gait pattern by stimulating the tibialis anterior of hemiplegic patients. The electrical stimulation can be triggered by either the foot switch or trigger. The stimulation level increases to its peak within 0.2 seconds after heel-off and is maintained until heel-strike. After heel-strike, the stimulation level decrease to zero when foot is flat in about 0.2 seconds. The foot switch has a built-in pressure sensor that can be adjustable to detect the heel-strike and toe-off. The ascent and descent ramp times for stimulation can also be adjusted between 0.1 and 10 seconds.
In order to stimulate multiple muscles/muscle groups simultaneously during a dynamic movement, the M-DESA was developed based on the S-DESA to have 8-channel low frequency current generators. Every channel has two surface electrodes and independent constant output current pulses. All current pulse parameters for each channel, including waveform, amplitude, frequency, pulse width, start and stop time, and stimulation duration, can be controlled and adjusted independently by a computer program. The current pulse stimulation is triggered by a pressure foot switch according to the status of the gait. An emergency shutoff button is used to turn off the current stimulation for all channels when necessary. The M-DESA is based on a single-chip microcomputer (ATTINY2313 and MAX3232E). It receives stimulation parameters and stimulation temporal sequences from a control computer (PC). This system has already been patented in China.
The patients were trained for 4 weeks. During the training period, they were asked to practice walking and contract the tibia anterior and rectus femoris muscles while the election stimulation was applied to them. The motion capture data were collected before and after the training (4 weeks) using a 6-camera motion analysis system (60 Hz, Motion Analysis Corporation, Santa Rosa, CA, USA). A total of 35 reflective markers (Fig. 1) were applied to the patients.
Fig. 1.
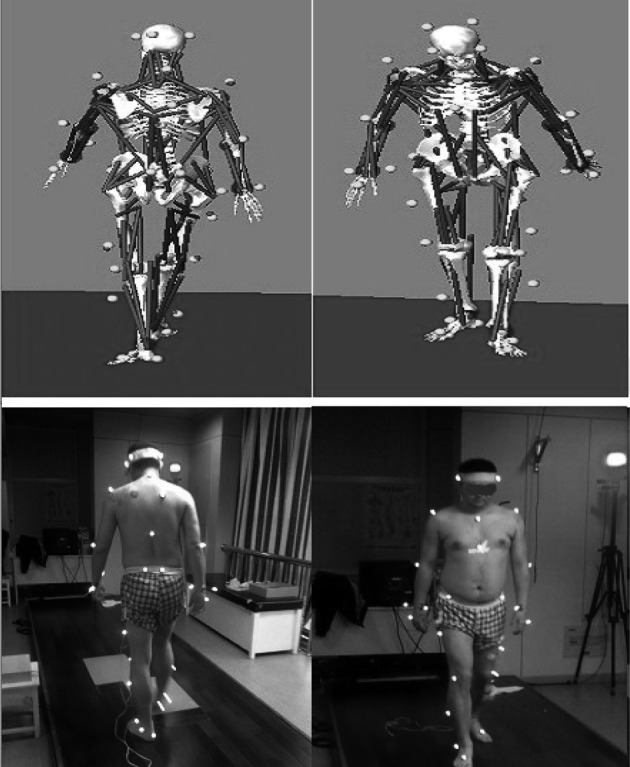
The marker placements and musculoskeletal model
The gait analysis data before and after the training were imported into a musculoskeletal simulation software suite (LIFMOD, LifeModeler, Inc., San Clemente, CA, USA) to establish musculoskeletal and dynamic models to compute the muscle forces of the rectus femoris and tibialis anterior13). The dependent variables included muscle forces of the tibialis anterior and rectus femoris, step length, and maximum knee and ankle angles before and after training. The paired sample t-test was used to detect the differences between before and after training (IBM SPSS Statistics,Version 19) with an alpha level of 0.05.
RESULTS
Before applying stimulation, the step length of the affected limb was 0.1 m less than that of the healthy limb. A significant increase in the step length of the affected limb was found, and the difference between the affected and healthy limbs was no longer significant after the stimulation was applied (p<0.05, Table 1).
Table 1. Comparisons of selected gait kinematic variables and maximum muscle forces before and during stimulation.
| Variables | Before stimulation | During stimulation | ||
|---|---|---|---|---|
| Affected limb | Healthy limb | Affected limb | Healthy limb | |
| Step length (m) | 0.443± 0.147# | 0.543 ± 0.154 | 0.526 ± 0.071* | 0.585 ± 0.078 |
| Lateral displacement (m) | 0.100 ± 0.032 | - | 0.063 ± 0.028* | - |
| Max dorsiflexion angle (°) | 4.95 ± 1.50 | - | 10.03 ± 2.66* | - |
| Max knee flexion angle (°) | 24.50 ± 9.90 | - | 31.42 ± 10.46* | - |
| Max tibia anterior force (kN) | 0.49 ± 0.88# | 0.53 ± 0.96 | 0.62 ± 0.11* | - |
| Max rectus femoris force (kN) | 0.53 ± 0.18# | 0.95 ± 0.53 | 0.81 ± 0.33* | - |
Mean ± SD. #significantly different from healthy limb before stimulation. *significantly different from before stimulation in affected limb. -: data not available
The lateral displacement of the affected limb decreased (by 0.036 m) after stimulation (p<0.05, Table 1). In addition, the maximum dorsiflexion angle and maximum knee flexion angle of the affected limb increased, by 5.08° and 6.92°, respectively, after the stimulation (p<0.05).
Before FES, the maximum muscle forces of the tibia anterior and rectus femoris were significantly greater in the healthy limb compared with the affected limb (p<0.05 for all comparisons, Table 1). In the affected limb, the maximum muscle forces of these muscles increased, by 0.13 and 0.27 kN, respectively, after stimulation (p<0.05).
DISCUSSION
The purpose of this study was to design and implement a multichannel dynamic FES system and investigate acute effects of FES of the tibialis anterior and rectus femoris on ankle and knee sagittal-plane kinematics and related muscle forces of the hemiplegic gait. The first hypothesis was that the step length of the affected limb would be greater during stimulation compared with that prior to stimulation. The results of the study provided support for the hypothesis, showing a longer step length during stimulation compared with that before stimulation.
Before stimulation, the step length of the affected limb was 18% less than that of the healthy limb, and it was 20% less than that of healthy older adults14). When the stimulation was applied, the step length of the affected limbs increased by19% and was similar to that of the healthy limb. The differences in step length between affected and healthy limbs during stimulation was approximate 0.059 m, which was 41% less than that before stimulation. Our results were supported by findings in the literature. A previous study showed that healthy subjects had a 25% longer step length compared with hemiplegic patients15). The effects of FES on the step length of affected limbs have been investigated as well7, 10). Kim et al.7) showed that the step length of affected limbs increased by 7% when FES was applied to both the gluteus medius and tibialis anterior muscles and increased by only 3% when FES was applied to tibialis anterior alone. Mum et al.8) also showed that stimulating the tibialis anterior and gluteus medius together was much more effective than stimulating the tibialis anterior alone during walking.
The hemiplegic gait is characterized by in a semicircular pattern during the swing phase of the affected limb. The limb circumduction is reflected in the increased lateral displacement of the affected limb to maintain foot clearance3). A previous study showed that the lateral displacement of the foot of the affected limb was 0.046 m, which was approximately 3 times that of healthy limbs3). In the current study, the lateral displacement of the affect limb during stimulation decreased by 37% from the level before stimulation (0.1 m).
The second hypothesis of this study was that the maximum dorsiflexion angle and knee flexion angle would be greater during FES compared with that before stimulation. The hypothesis was supported by the results showing a greater peak dorsiflexion angle and knee flexion angle during stimulation compared with before stimulation. In the current study, the maximum dorsiflexion angle during FES was 10.0°, which was 102% greater than that before FES of the tibialis anterior. The maximum knee flexion angle during FES was 31.4°, which was 28% greater than that before stimulation of the rectus femoris. With simultaneous stimulation of both the ankle dorsiflexor and hip flexor, the affected ankle and knee were able to achieve greater degrees of flexion. These results are supported by findings in the literature. It was shown that the maximum dorsiflexion angle at initial contact increased significanly by 13.0° when FES was applied to the peroneal and hamstrings compared with no stimulation10). When FES was only applied to the dorsiflexor muscle during the swing phase of gait, the peak maximum dorsiflexion angle increased by approximately 6.0°, and the peak knee flexion angle barely changed16). The maximum knee flexion angle was also shown to be significantly increased by 18° when FES was applied to the affected dorsiflexors of patients walking with robot-assisted gait training9).
Our third hypothesis was that the muscle forces would be greater during stimulation compared with those before stimulation. This hypothesis was supported by the fact that the maximum muscle forces of both the tibia anterior and rectus femoris increased significantly during stimulation compared with those before FES was applied. During stimulation, the maximum muscle forces of the tibialis anterior and rectus femoris of the affected limb were 0.62 and 0.81 kN, which were increased by 26.5% and 50.0%, respectively, after applying stimulation. Although no studies seem to have examined muscle forces as a results of FES, surface EMG activities of muscles related to hemiplegic gait before and after FES have been investigated11). Sabut et al.11) showed that the maximum root mean square EMG signal of the tibia anterior using a single-channel FES showed an approximately 66% increase compared with prior to stimulation. Furthermore, dorsiflexors were significantly stronger at the end of a three-month FES intervention program for hemiplegic patients compared with before the intervention (p<0.04)2).
The present study has a few limitations. The study enrolled a small number of patients, so the results may not be readily applicable to a general hemiplegic population. In addition, the hemiplegic patients demonstrated exaggerated frontal plane movements, such as foot circumduction which may be related to exaggerated hip movements. Future studies need to evaluate the effects of FES on frontal plane kinematic and kinetic variables. Studies of the long-term benefits of FES are also warranted.
This study successfully established a functional electrical stimulation strategy based on musculoskeletal modeling and 3D hemiplegic gait analysis for each individual. As a result of FES, muscle forces of the tibialis anterior and rectus femoris increased, and therefore the dorsiflexion during the stance phase and knee flexion during the swing phase of the gait cycle were both improved. Consequently, the step length was increased to correct foot drop and alter the circumduction gait pattern. In addition, the M-DESA system developed by our group is capable of stimulating multiple muscles/muscle groups simultaneously during level walking.
Acknowledgments
The work described in this paper was substantially supported by a grant from the National Natural Science Foundation of China (No. 31270998).
REFERENCES
- 1.Pia L, Neppi-Modona M, Ricci R, et al. : The anatomy of anosognosia for hemiplegia: a meta-analysis. Cortex, 2004, 40: 367–377. [DOI] [PubMed] [Google Scholar]
- 2.Embrey DG, Holtz SL, Alon G, et al. : Functional electrical stimulation to dorsiflexors and plantar flexors during gait to improve walking in adults with chronic hemiplegia. Arch Phys Med Rehabil, 2010, 91: 687–696. [DOI] [PubMed] [Google Scholar]
- 3.Chen G, Patten C, Kothari DH, et al. : Gait differences between individuals with post-stroke hemiparesis and non-disabled controls at matched speeds. Gait Posture, 2005, 22: 51–56. [DOI] [PubMed] [Google Scholar]
- 4.Bensoussan L, Mesure S, Viton JM, et al. : Kinematic and kinetic asymmetries in hemiplegic patients’ gait initiation patterns. J Rehabil Med, 2006, 38: 287–294. [DOI] [PubMed] [Google Scholar]
- 5.Stanic U, Trnkoczy A: Closed-loop positioning of hemiplegic patient’s joint by means of functional electrical stimulation. IEEE Trans Biomed Eng, 1974, 21: 365–370. [DOI] [PubMed] [Google Scholar]
- 6.Vodovnik L, Crochetiere WJ, Reswick JB: Control of a skeletal joint by electrical stimulation of antagonists. Med Biol Eng, 1967, 5: 97–109. [DOI] [PubMed] [Google Scholar]
- 7.Kim JH, Chung Y, Kim Y, et al. : Functional electrical stimulation applied to gluteus medius and tibialis anterior corresponding gait cycle for stroke. Gait Posture, 2012, 36: 65–67. [DOI] [PubMed] [Google Scholar]
- 8.Mun BM, Kim TH, Lee JH, et al. : Comparison of gait aspects according to FES stimulation position applied to stroke patients. J Phys Ther Sci, 2014, 26: 563–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bae YH, Ko YJ, Chang WH, et al. : Effects of robot-assisted gait training combined with functional electrical stimulation on recovery of locomotor mobility in chronic stroke patients: a randomized controlled trial. J Phys Ther Sci, 2014, 26: 1949–1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Springer S, Vatine JJ, Wolf A, et al. : The effects of dual-channel functional electrical stimulation on stance phase sagittal kinematics in patients with hemiparesis. J Electromyogr Kinesiol, 2013, 23: 476–482. [DOI] [PubMed] [Google Scholar]
- 11.Sabut SK, Lenka PK, Kumar R, et al. : Effect of functional electrical stimulation on the effort and walking speed, surface electromyography activity, and metabolic responses in stroke subjects. J Electromyogr Kinesiol, 2010, 20: 1170–1177. [DOI] [PubMed] [Google Scholar]
- 12.Qian JG, Yu X: The function of functional electrical stimulation to lower extremity of hemiplegia. J Nanjing Inst Phy Ed, 2009, 8: 116–117(Natural Science). [Google Scholar]
- 13.Qian JG, Li Z, Zhang H, et al. : Effectiveness of selected fitness exercises on stress of femoral neck using musculoskeletal dynamics simulations and finite element model. J Hum Kinet, 2014, 41: 59–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Judge JO, Davis RB, 3rd, Ounpuu S: Step length reductions in advanced age: the role of ankle and hip kinetics. J Gerontol A Biol Sci Med Sci, 1996, 51: M303–M312. [DOI] [PubMed] [Google Scholar]
- 15.Boudarham J, Roche N, Pradon D, et al. : Variations in kinematics during clinical gait analysis in stroke patients. PLoS ONE, 2013, 8: e66421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Awad LN, Kesar TM, Reisman D, et al. : Effects of repeated treadmill testing and electrical stimulation on post-stroke gait kinematics. Gait Posture, 2013, 37: 67–71. [DOI] [PMC free article] [PubMed] [Google Scholar]


