Abstract
[Purpose] The aim of this study was to investigate the effect of exercise on glycemic control using data from fifth Korea National Health and Nutrition Examination Survey and to provide appropriate exercise guidelines for patients with type 2 diabetes mellitus in Korea. [Subjects and Methods] We selected 1,328 patients from the fifth Korea National Health and Nutrition Examination Survey database who had type 2 diabetes and ranged in age from 30 to 90 years. Statistical analyses included χ2 tests, multiple linear regression, and logistic regression. [Results] Factors found to be significantly related to glycemic control included income level, physical activity based on intensity of aerobic exercise, use of diabetes medicine, presence of hypertension, duration of diabetes, and waist circumference. In addition, engaging in combined low- and moderate-intensity aerobic exercise when adjusted for resistance exercise was found to lower the risk of glycemic control failure. [Conclusion] Patients with type 2 diabetes mellitus in Korea should engage in combined low- and moderate-intensity aerobic exercise such as walking for 30 minutes or more five times a week. Physical activity is likely to improve glycemic control and thus prevent the acute and chronic complications of diabetes mellitus.
Key words: Exercise, Glycemic control, Type 2 diabetes mellitus
INTRODUCTION
The number of persons with diabetes continues to increase worldwide1, 2). Diabetes, a metabolic disorder characterized by hyperglycemia, is divided into two types. Type 1 diabetes occurs when the pancreas fails to secrete insulin, and type 2 diabetes occurs when the pancreas retains some capacity to secrete insulin but insulin resistance is increased for a variety of reasons3, 4). Insulin controls blood glucose by facilitating glucose intake or inhibiting glucogenesis. Insulin resistance is a condition in which the action of insulin is decreased, although the level of insulin is not insufficient5). Chronic hyperglycemia is definitely related to complications of diabetes that damage the kidneys, eyes, nerves, heart, and blood vessels6, 7). Therefore, glycemic control is the most fundamental way to manage this disease.
Exercise has been considered one of the three cornerstones in the management of diabetes, the other two being dietary measures and drug therapies8, 9). The American Diabetes Association (ADA)10) now recommends that patients with type 2 diabetes engage in moderate to rigorous aerobic exercise of moderate intensity (50 to 70% of maximal heart rate) for at least 150 minutes spread out over at least three days a week, with no more than two consecutive days between exercise sessions. Resistance exercises at least twice a week on nonconsecutive days are also recommended. Exercise can rapidly increase the expression of GLUT4 (glucose transporter type 4), the sugar transporter in skeletal muscles, thus increasing the activity of AMPK (adenosine monophosphate-activated protein kinase) in these muscles11, 12). Although patients with type 2 diabetes have a defect in insulin signal transmission in the skeletal muscles, the exercise-induced activation of AMPK in these muscles can achieve normal action13), and the oxidation of fatty acids and glucose absorption will be accelerated14). The increase in insulin sensitivity after exercise is known to be maintained for about 60 hours and returns to pre-exercise levels after 3 to 5 days. However, when exercise of moderate intensity is repeatedly performed, the ability to maintain greater insulin sensitivity can be extended. Thus, repeating exercise within a time interval of 48 to 60 hours may help control blood glucose levels over the long term15). In a meta-analysis of the results of 16 randomized controlled trials (RCTs), Qiu et al.16) found that walking lowered the concentration of glycated hemoglobin (HbA1c) in patients with type 2 diabetes. Measurement of a person’s HbA1c concentration reflects the average glucose concentration for the past 2 to 3 months. An HbA1c level lower than 6.5% is considered good control. A decrease in HbA1c of 1% can decrease the occurrence of the major cardiovascular diseases by 15 to 20%17) and the occurrence of microvascular complications by 37%18). A meta-analysis of studies involving 12 aerobic exercises and 2 resistance exercises conducted by Boulé et al.19) revealed that the HbA1c concentration in their exercise group was 0.66% lower than that in their control group after an intervention in patients with type 2 diabetes (p < 0.001). In a review of the literature by Eves and Plotnikoff20), a prescription of resistance exercise was found to improve both glycemic control and insulin sensitivity in patients type 2 diabetes. However, it was difficult to generalize their results because most of the studies they reviewed were individually conducted under the supervision of experts and the sample sizes were relatively small. Thus, based on the studies cited above, large-scale, population-based studies are still needed to determine the best approach to glycemic control by means of exercise. Thomas et al.21) conducted a meta-analysis of 14 RCTs and found that exercise could decrease the concentration of HbA1c in patients with type 2 diabetes by 0.6% as compared with controls (p < 0.05). Although a number of studies on exercise and glycemic control have been conducted, such research using national data are lacking. Therefore, our objectives were to investigate the effect of exercise (physical activity) on glycemic control using data from the fifth Korea National Health and Nutrition Examination Survey (KNHANES V) and to provide appropriate exercise guidelines for patients with type 2 diabetes in Korea.
SUBJECTS AND METHODS
Subjects
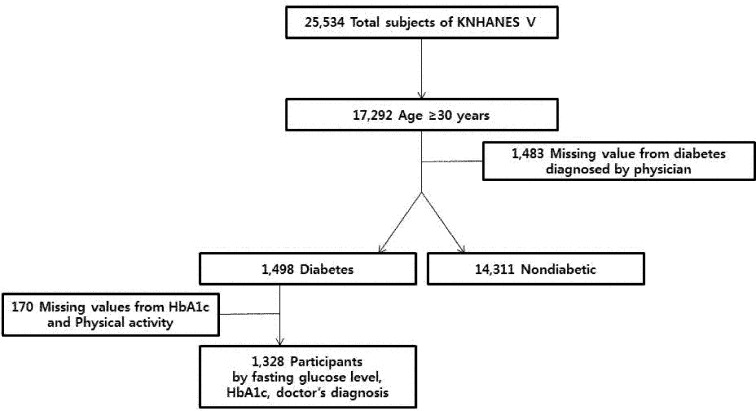
This study used raw data from the KNHANES V, a survey conducted in 2010 to 201222). Use of this survey was approved by the Korea Center for Disease Control and Prevention (KCDC) Research Ethics Review Committee (2010-02CON-21-C, 2011-02CON-06-C, 2012-01EXP-01-2C). Written informed consent was obtained from all participants. KNHANES V surveyed three areas: health, nutrition, and health examination. Because there is no factor in the KNHANES for identifying the type of diabetes a person might have, subjects under 29 years of age who had been diagnosed with diabetes were assumed to have type 1 diabetes based on an existing national study of epidemiological characteristics of diabetes in Korea23). Out of a total 25,534 subjects, we therefore excluded those who had been diagnosed before the age of 29. A total of 1,328 patients given a diagnosis of type 2 diabetes at age 30 or older based on fasting glucose level, HbA1c concentration, and a physician’s diagnosis were selected for study (Fig. 1).
Fig. 1.
Study population framework
Methods
The criteria for glycemic control were an HbA1c concentration below 6.5%, the goal set by the International Diabetes Federation24) and the Korean Diabetes Association3). Physical activity was classified into six groups: no physical activity, walking, moderate activity, vigorous activity, walking plus moderate activity, and walking plus vigorous activity. Walking consisted of sessions of 30 minutes or more 5 days a week during a recent week and included all forms of walking (e.g., when commuting, in daily activities, and as exercise). Moderate physical activity consisted of activity that was more strenuous or made one breathe harder than usual and involved 30-minute sessions five times or more a week during a recent week (e.g., slow swimming, playing tennis doubles, volleyball, badminton, table tennis, transporting light objects, etc.). Vigorous physical activity referred to engaging in intense physical activity that made one very tired or breathe much harder than usual and involved 20-minute sessions (or longer each time) 3 days a week during a recent week (e.g., running, jogging, mountain climbing, fast cycling, fast swimming, playing soccer, playing basketball, skipping rope, playing squash or singles tennis, transporting heavy objects, etc.). Resistance exercise meant doing push-ups or sit-ups or lifting dumbbells or barbells twice or more per week during a recent week.
Smokers were defined as subjects who were currently smoking or those who had smoked 100 cigarettes (5 packs) at some time. Drinkers were defined as those who consumed one glass of an alcoholic beverage or more during their lifetime. A family history of diabetes was defined as having parents, brothers, or sisters who have or had diabetes.
Data were analyzed using PASW Statistics version 18.0 (SPSS Inc., Chicago, IL, USA). The level of significance was set at p < 0.05. To identify the relationship between glycemic control and other factors, we performed chi-square tests (χ2-tests) and multiple linear regressions analyses. To investigate the effect of each factor on glycemic control after adjustment, logistic regression analyses were carried out.
RESULTS
The 1,328 subjects included 677 (53.6%) males and 651 (46.4%) females with a mean age of 61.8 years. Of these subjects, 23.8% had a history of smoking, and 79.5% had a history of alcohol consumption. With regard to exercise, 28.6% of the subjects performed low-intensity aerobic exercises such as walking, 2.8% performed moderate-intensity aerobic exercise, and 5.0% performed high-intensity (vigorous) aerobic exercise; 2.8% of subjects combined walking with moderate aerobic exercise, and 4.6% combined walking with vigorous aerobic exercise. A total of 34.8% had a family history of diabetes, and 96.4% took oral hypoglycemic agents. The percentages of subjects with high blood pressure, stroke, coronary artery disease, and chronic kidney disease were 56.7%, 5.5%, 8.8%, and 0.9%, respectively. The mean duration of diabetes was 7.9 years, and the mean concentration of HbA1c was 7.4%. The mean waist circumference was 87.0 cm, and the mean BMI was 24.9. Daily fat intake averaged 30.2 g, and daily carbohydrate intake averaged 317.1 g (Table 1).
Table 1. Characteristics of the patients with diabetes (N=1,328).
| Characteristics | N | % | |
|---|---|---|---|
| Gender | Male | 677 | 53.6 |
| Female | 651 | 46.4 | |
| Age, yr | Mean±SD | 61.76±0.41 | |
| Quartile of household income | Low | 462 | 31.8 |
| Middle-low | 332 | 26.1 | |
| Middle-high | 267 | 21.8 | |
| High | 257 | 20.3 | |
| Education level | Elementary | 630 | 44.9 |
| Middle school | 216 | 16.5 | |
| High school | 311 | 25.6 | |
| College | 170 | 13.0 | |
| Smoking history | No | 1,056 | 76.2 |
| Yes | 268 | 23.8 | |
| Alcohol history | No | 313 | 20.5 |
| Yes | 1,012 | 79.5 | |
| Physical activity | No activity | 702 | 56.2 |
| Walking | 391 | 28.6 | |
| Moderate | 41 | 2.8 | |
| Vigorous | 55 | 5.0 | |
| Walking+moderate | 38 | 2.8 | |
| Walking+vigorous | 60 | 4.6 | |
| Walking+vigorous | 60 | 4.6 | |
| Resistance training | No | 993 | 75.1 |
| Yes | 330 | 24.9 | |
| Family history of DM | No | 728 | 65.2 |
| Yes | 325 | 34.8 | |
| Oral hypoglycemic agent | No | 51 | 3.6 |
| Yes | 1,144 | 96.4 | |
| Hypertension | No | 516 | 43.3 |
| Yes | 811 | 56.7 | |
| Stroke | No | 1,243 | 94.5 |
| Yes | 84 | 5.5 | |
| Coronary artery disease | No | 1,192 | 91.2 |
| Yes | 135 | 8.8 | |
| Chronic kidney disease | No | 1,317 | 99.1 |
| Yes | 11 | 0.9 | |
| DM duration, yr | Mean±SD | 7.93±0.24 | |
| HbA1c, % | Mean±SD | 7.38±0.05 | |
| Waist circumference, cm | Mean±SD | 86.98±0.36 | |
| Body mass index, kg/m2 | Mean±SD | 24.86±0.15 | |
| Daily fat intake, g | Mean±SD | 30.22±0.93 | |
| Daily carbohydrate intake, g | Mean±SD | 317.08±4.55 | |
Unweighted number, weighted %. DM: diabetes mellitus
The mean age of subjects whose HbA1c concentration was 6.4% or lower was 63.6 years, and that of subjects whose HbA1c concentration was 6.5% or higher was 61.1 years (p = 0.007). The mean duration of diabetes in the subjects whose HbA1c concentration was 6.4% or below was 6.2 years compared with the mean duration of 8.6 years for those whose HbA1c concentration was 6.5% or above, a significant difference (p < 0.001). The mean waist circumference was 85.1 cm in subjects with HbA1c concentrations of 6.4% or lower compared with the mean circumference of 87.7 cm in those with HbA1c concentrations of 6.5% or higher, indicating a significant difference between two groups (p < 0.001). Also statistically significant was the difference between these two groups in their mean BMI values: 24.2 in the subjects with HbA1c concentrations of 6.4% or lower versus 25.1 in those with an HbA1c value of 6.5% or higher (p = 0.003). The daily carbohydrate intakes in these two groups of subjects were 298.0 g and 324.1 g, respectively (p = 0.006). Education level, family history of diabetes, and presence of hypertension were also found to be related to glycemic control (p < 0.05) (Table 2).
Table 2. Relationship between glycemic control according to HbA1c concentration and other factors (N=1,328).
| Characteristic | Glycemic control | ||
|---|---|---|---|
| HbA1c≥6.5 | HbA1c<6.5 | ||
| Gender | Male | 502 (74.5) | 175 (25.5) |
| Female | 477 (71.3) | 174 (28.7) | |
| Age, yrs** | Mean±SD | 61.08±0.44 | 63.58±0.85 |
| Quartile of household income | Low | 317 (68.0) | 145 (32.0) |
| Middle-low | 215 (73.5) | 81 (26.5) | |
| Middle-high | 206 (76.8) | 61 (23.2) | |
| High | 197 (76.0) | 60 (24.0) | |
| Education level* | Elementary | 443 (67.8) | 187 (32.2) |
| Middle school | 168 (75.9) | 48 (24.1) | |
| High school | 238 (77.7) | 73 (22.3) | |
| College | 130 (79.1) | 40 (20.9) | |
| Smoking history | No | 771 (72.6) | 285 (27.4) |
| Yes | 207 (75.1) | 61 (24.9) | |
| Alcohol history | No | 239 (75.8) | 74 (24.2) |
| Yes | 739 (72.5) | 273 (27.5) | |
| Physical activity | No activity | 517 (73.7) | 185 (26.3) |
| Walking | 298 (75.0) | 93 (25.0) | |
| Moderate | 29 (62.7) | 12 (37.3) | |
| Vigorous | 38 (68.5) | 17 (31.5) | |
| Walking+moderate | 25 (52.7) | 13 (47.3) | |
| Walking+vigorous | 47 (79.0) | 13 (21.0) | |
| Walking+vigorous | 47 (79.0) | 13 (21.0) | |
| Resistance training | No | 734 (73.4) | 259 (26.6) |
| Yes | 244 (73.0) | 86 (27.0) | |
| Family history of DM* | No | 539 (72.2) | 189 (27.8) |
| Yes | 250 (79.3) | 75 (20.7) | |
| Oral hypoglycemic agent | No | 42 (85.4) | 9 (14.6) |
| Yes | 864 (74.7) | 280 (25.3) | |
| Hypertension** | No | 401 (77.9) | 115 (22.1) |
| Yes | 577 (69.3) | 234 (30.7) | |
| Stroke | No | 919 (73.2) | 324 (26.8) |
| Yes | 59 (71.0) | 25 (29.0) | |
| Coronary artery disease | No | 886 (73.4) | 306 (26.6) |
| Yes | 92 (68.9) | 43 (31.1) | |
| Chronic kidney disease | No | 970 (72.9) | 347 (27.1) |
| Yes | 9 (85.6) | 2 (14.4) | |
| DM duration, yrs*** | Mean±SD | 8.57±0.27 | 6.20±0.41 |
| Waist circumference, cm*** | Mean±SD | 87.67±0.46 | 85.10±0.54 |
| Body mass index, kg/m2** | Mean±SD | 25.10±0.18 | 24.22±0.22 |
| Daily fat intake, g | Mean±SD | 31.16±1.12 | 27.64±1.87 |
| Daily carbohydrate intake, g** | Mean±SD | 324.05±5.56 | 298.02±7.63 |
Unweighted number, weighted %. DM: diabetes mellitus. *p < 0.05 by χ2 test; **p < 0.01 by χ2 test; ***p < 0.001 by χ2 test
On logistic regression analysis with glycemic control as a dependent variable, we found that income level, physical activity according to intensity of aerobic exercise, use of diabetes medicine, presence of hypertension, duration of diabetes, and waist circumference were related to glycemic control after adjusting for all factors, and these relationships were statistically significant (p < 0.05) (Table 3).
Table 3. Relationship between glycemic control and other factors based on logistic regression analysis (N=1,328).
| Characteristic | Odds ratio | 95% CI | |
|---|---|---|---|
| Gender | Male | 0.927 | 0.561–1.531 |
| Female | 1.000 | ||
| Age, yr | Mean±SD | 1.001 | 0.975–1.028 |
| Quartile of household Income | Low | 0.987 | 0.488–1.995 |
| Middle-low | 1.411 | 0.762–2.610 | |
| Middle-high* | 2.115 | 1.081–4.139 | |
| High | 1.000 | ||
| Education level | Elementary | 0.629 | 0.298–1.329 |
| Middle school | 1.142 | 0.515–2.533 | |
| High school | 0.757 | 0.381–1.565 | |
| College | 1.000 | ||
| Smoking history | No | 0.686 | 0.397–1.183 |
| Yes | 1.000 | ||
| Alcohol history | No | 1.640 | 0.985–2.733 |
| Yes | 1.000 | ||
| Physical activity | No activity | 1.000 | |
| Walking | 1.404 | 0.874–2.256 | |
| Moderate* | 0.317 | 0.103–0.982 | |
| Vigorous | 0.790 | 0.332–1.879 | |
| Walking+moderate* | 0.248 | 0.084–0.734 | |
| Walking+vigorous | 1.415 | 0.437–4.583 | |
| Resistance training | No | 1.273 | 0.788–2.055 |
| Yes | 1.000 | ||
| Family history of DM | No | 0.947 | 0.603–1.489 |
| Yes | 1.000 | ||
| DM medication | No medication | 1.000 | |
| Only insulin*** | 20.406 | 4.533–91.855 | |
| Only OHA** | 2.636 | 1.497–4.640 | |
| Insulin+OHA** | 19.301 | 3.352–111.131 | |
| Hypertension*** | No | 2.440 | 1.574–3.785 |
| Yes | 1.000 | ||
| DM duration, yrs** | Mean±SD | 1.047 | 1.014–1.081 |
| Waist circumference, cm* | Mean±SD | 1.027 | 1.006–1.049 |
| Daily carbohydrate intake, g* | Mean±SD | 1.002 | 1.000–1.003 |
*p < 0.05 by multiple linear regression analysis; **p < 0.01 by multiple linear regression analysis; ***p < 0.001 by multiple linear regression analysis. CI: confidence interval; OHA: oral hypoglycemic agent; DM: diabetes mellitus
DISCUSSION
Because reinforcing insulin action, absorbing glucose into muscle by aerobic exercise, and increasing the amount of muscle by resistance exercise are all effective in decreasing glucose, combining aerobic exercise and resistance exercise is thought to facilitate glycemic control more effectively than one type of exercise alone25). Snowling and Hopkins26) conducted a meta-analysis of the results from 27 RCTs and concluded that aerobic, resistance, and combined exercises all had beneficial effects on glycemic control in patients with type 2 diabetes. However, combination exercises provided additional benefits.
In our study, the number of subjects whose HbA1c concentration was 6.4% or below was 349 (27.0%), as compared with the 979 (73.0%) subjects whose HbA1c concentration was 6.5% or above, indicating that about 3/4 of the entire cohort did not have proper glycemic control. In addition, 702 (56.2%) subjects performed no exercise at all.
After adjusting for all variables in the logistic regression analysis, with glycemic control as a dependent variable, the factors found to be related to glycemic control with statistical significance included income level, physical activity according to intensity of aerobic exercise, use of diabetes medicine, presence of hypertension, duration of diabetes, and waist circumference (p < 0.05). Obesity, lack of exercise, and unhealthy dietary habits associated with low socioeconomic status (SES)27) can be responsible for type 2 diabetes. In this study, we found that the risk of failure of glycemic control in subjects with a middle-to-high income was 2.115 times higher than that in subjects with a high income. If a patient with type 2 diabetes gets moderate exercise, the amount of glucose in peripheral use will become greater than the level of glucogenesis in the liver, thus decreasing blood glucose. Although the secretion of blood insulin is also decreased, hypoglycemia will rarely occur15). However, high-intensity aerobic exercise can promote glucogenesis and induce hyperglycemia by increasing the concentration of blood catecholamine. Exercise-induced hyperglycemia can continue for about 1 to 2 hours28,29,30). In this study, performance of low- and moderate-intensity aerobic exercises at the same time was found to lower the risk of glycemic control failure by 0.248 times (95% CI: 0.084–0.734) (p < 0.05). On the other hand, performance of low- and high-intensity aerobic exercise at the same time did not have a significant effect. Subjects who combined moderate- and high-intensity aerobic exercises and those who combined low-, moderate-, and high-intensity aerobic exercises were excluded from the analysis because their sample sizes were too small.
Moderate aerobic exercise is recommended by the ADA. In this study, we also found that doing one type of moderate aerobic exercise lowered the risk of glycemic control failure by 0.317 times (95% CI: 0.103–0.982). Sigal et al.31) reported that a single performance of aerobic exercise and resistance exercise would improve glycemic control in patients with type 2 diabetes, with combined exercise providing the greatest improvement. The discrepancy between our results and those reported by Sigal et al. might be due to the fact that our total exercise period of combined exercises was longer than that of the combined aerobic and resistance exercises. In addition, our study was not a blinded experiment. The results reported by Snowling and Hopkins26) would be difficult to generalize because the studies included in their meta-analysis involved those with a small number of subjects32) or those in which subjects were limited to only females33), only males34), or only the elderly35).
In our study, if a subject was taking an oral hypoglycemic agent, the risk of glycemic control failure was increased by 2.636 times because hypoglycemic medicine cannot be prescribed for all patients just who are poorly controlled type 2 diabetes. The risk of glycemic control failure in subjects with hypertension was 2.440 times higher.
Hypertension is a common chronic disease that is present in at least 60% of patients with type 2 diabetes. When a person has both hypertension and type 2 diabetes, the risk of vascular complications is increased by 66 to 100% as compared with those with only one of these conditions36). Patients should have paid more attention to their health when they have more than one chronic disease.
In the present study, a 1 year increase in the duration of diabetes resulted in the risk of glycemic control failure increasing by 1.047 times (95% CI: 1.014–1.081), and a 1-cm increase in the waist circumference resulted in the risk of glycemic control failure increasing by 1.027 times (95% CI: 1.006–1.049). It is well known that obesity increases the occurrence of type 2 diabetes as well as the risk of complications3).
This study had several limitations. First, it was a cross-sectional study, so it may be difficult to explain causal relationships. In particular, patients with diabetes are likely to change their exercise behavior based on their doctors’ recommendations. Thus, the finding of a relationship between physical activity and diabetic control status was more evident than that exercise reduces the risk of glycemic control failure. However, in our view, this study was significant in that it examined the effect on glycemic control of combined aerobic exercises at different intensities. Second, we did not investigate the effects of exercise supervised by individual specialists, even though empirical evidence was found in the statistical data based on the extensive national sample representing Koreans that physical activity could alter diabetic control status. Therefore, further studies will be needed to overcome the limitations of this study and to determine the apparent causal relationship between physical activity and glycemic control.
REFERENCES
- 1.International Diabetes Federation: IDF Diabetes Atlas. Brussels: International Diabetes Federation, 2013. [Google Scholar]
- 2.Lee SS, Kang S: Effects of regular exercise on obesity and type 2 diabete mellitus in Korean children: improvements glycemic control and serum adipokines level. J Phys Ther Sci, 2015, 27: 1903–1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Korean Diabetes Association: www.diabetes.or.kr.
- 4.Karoline de Morais P, Sales MM, Alves de Almeida J, et al. : Effects of aerobic exercise intensity on 24-h ambulatory blood pressure in individuals with type 2 diabetes and prehypertension. J Phys Ther Sci, 2015, 27: 51–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.DeFronzo RA, Bonadonna RC, Ferrannini E: Pathogenesis of NIDDM. A balanced overview. Diabetes Care, 1992, 15: 318–368. [DOI] [PubMed] [Google Scholar]
- 6.Yki-Järvinen H: Toxicity of hyperglycaemia in type 2 diabetes. Diabetes Metab Rev, 1998, 14: S45–S50. [PubMed] [Google Scholar]
- 7.Alsenany S, Al Saif A: Incidence of diabetes mellitus type 2 complications among Saudi adult patients at primary health care center. J Phys Ther Sci, 2015, 27: 1727–1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Joslin EP, Root EF, White P: The Treatment of Diabetes Mellitus. Philadelphia: Lea & Febiger, 1959. [Google Scholar]
- 9.Murano I, Asakawa Y, Mizukami M, et al. : Factors increasing physical activity levels in diabetes mellitus: a survey of patients after an inpatient diabetes education program. J Phys Ther Sci, 2014, 26: 695–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.American Diabetes Association: Standards of medical care in diabetes-2014. Diabetes Care, 2014, 37: S31. [DOI] [PubMed] [Google Scholar]
- 11.Kennedy JW, Hirshman MF, Gervino EV, et al. : Acute exercise induces GLUT4 translocation in skeletal muscle of normal human subjects and subjects with type 2 diabetes. Diabetes, 1999, 48: 1192–1197. [DOI] [PubMed] [Google Scholar]
- 12.Musi N, Fujii N, Hirshman MF, et al. : AMP-activated protein kinase (AMPK) is activated in muscle of subjects with type 2 diabetes during exercise. Diabetes, 2001, 50: 921–927. [DOI] [PubMed] [Google Scholar]
- 13.Gang HJ: Clinical exercise physiology mechanism of diabetes. Korean J Health Promot Dis Prev, 2006, 6: S374–S378. [Google Scholar]
- 14.Kahn BB, Alquier T, Carling D, et al. : AMP-activated protein kinase: ancient energy gauge provides clues to modern understanding of metabolism. Cell Metab, 2005, 1: 15–25. [DOI] [PubMed] [Google Scholar]
- 15.Min KW, Park SW: Physical activity and type 2 diabetes mellitus. Diabetes Metab J, 2006, 30: 1–9. [Google Scholar]
- 16.Qiu S, Cai X, Schumann U, et al. : Impact of walking on glycemic control and other cardiovascular risk factors in type 2 diabetes: a meta-analysis. PLoS ONE, 2014, 9: e109767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Selvin E, Marinopoulos S, Berkenblit G, et al. : Meta-analysis: glycosylated hemoglobin and cardiovascular disease in diabetes mellitus. Ann Intern Med, 2004, 141: 421–431. [DOI] [PubMed] [Google Scholar]
- 18.Stratton IM, Adler AI, Neil HA, et al. : Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ, 2000, 321: 405–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boulé NG, Haddad E, Kenny GP, et al. : Effects of exercise on glycemic control and body mass in type 2 diabetes mellitus: a meta-analysis of controlled clinical trials. JAMA, 2001, 286: 1218–1227. [DOI] [PubMed] [Google Scholar]
- 20.Eves ND, Plotnikoff RC: Resistance training and type 2 diabetes: considerations for implementation at the population level. Diabetes Care, 2006, 29: 1933–1941. [DOI] [PubMed] [Google Scholar]
- 21.Thomas DE, Elliott EJ, Naughton GA: Exercise for type 2 diabetes mellitus. Cochrane Database Syst Rev, 2006, 3: CD002968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Korea National Health and Nutrition Examination Survey. https://knhanes.cdc.go.kr/knhanes.
- 23.Rhee B: Epidemiological characteristics of diabetes mellitus among Korean population. J Korean Diabetes Assoc, 2003, 27: 173–179. [Google Scholar]
- 24.International Diabetes Federation: https://www.idf.org.
- 25.Kim KS, Park SW: Exercise and type 2 diabetes: ACSM and ADA joint position statement. J Korean Diabetes, 2012, 13: 61–68. [Google Scholar]
- 26.Snowling NJ, Hopkins WG: Effects of different modes of exercise training on glucose control and risk factors for complications in type 2 diabetic patients: a meta-analysis. Diabetes Care, 2006, 29: 2518–2527. [DOI] [PubMed] [Google Scholar]
- 27.Demakakos P, Marmot M, Steptoe A: Socioeconomic position and the incidence of type 2 diabetes: the ELSA study. Eur J Epidemiol, 2012, 27: 367–378. [DOI] [PubMed] [Google Scholar]
- 28.Baynard T, Franklin RM, Goulopoulou S, et al. : Effect of a single vs multiple bouts of exercise on glucose control in women with type 2 diabetes. Metabolism, 2005, 54: 989–994. [DOI] [PubMed] [Google Scholar]
- 29.Marliss EB, Vranic M: Intense exercise has unique effects on both insulin release and its roles in glucoregulation: implications for diabetes. Diabetes, 2002, 51: S271–S283. [DOI] [PubMed] [Google Scholar]
- 30.Black LE, Swan PD, Alvar BA: Effects of intensity and volume on insulin sensitivity during acute bouts of resistance training. J Strength Cond Res, 2010, 24: 1109–1116. [DOI] [PubMed] [Google Scholar]
- 31.Sigal RJ, Kenny GP, Boulé NG, et al. : Effects of aerobic training, resistance training, or both on glycemic control in type 2 diabetes: a randomized trial. Ann Intern Med, 2007, 147: 357–369. [DOI] [PubMed] [Google Scholar]
- 32.Maiorana A, O’Driscoll G, Goodman C, et al. : Combined aerobic and resistance exercise improves glycemic control and fitness in type 2 diabetes. Diabetes Res Clin Pract, 2002, 56: 115–123. [DOI] [PubMed] [Google Scholar]
- 33.Cuff DJ, Meneilly GS, Martin A, et al. : Effective exercise modality to reduce insulin resistance in women with type 2 diabetes. Diabetes Care, 2003, 26: 2977–2982. [DOI] [PubMed] [Google Scholar]
- 34.Loimaala A, Huikuri HV, Kööbi T, et al. : Exercise training improves baroreflex sensitivity in type 2 diabetes. Diabetes, 2003, 52: 1837–1842. [DOI] [PubMed] [Google Scholar]
- 35.Ligtenberg PC, Hoekstra JB, Bol E, et al. : Effects of physical training on metabolic control in elderly type 2 diabetes mellitus patients. Clin Sci (Lond), 1997, 93: 127–135. [DOI] [PubMed] [Google Scholar]
- 36.Mourad JJ, Le Jeune S: Blood pressure control, risk factors and cardiovascular prognosis in patients with diabetes: 30 years of progress. J Hypertens Suppl, 2008, 26: S7–S13. [PubMed] [Google Scholar]