Abstract
[Purpose] The main objective of this study was to determine the contributions and extent to which certain physical measurements explain performance in the 6-minute walk test in healthy older adults living in a geriatric nursing home and for older adults dwelling in the community. [Subjects] The subjects were 122 adults aged 65 and older with no cognitive impairment who were independent in their daily activities. [Methods] The 6-minute walk test, age, body mass index, walking speed, chair stand test, Berg Balance Scale, Timed Up-and-Go test, rectus femoris cross-sectional area, Short Physical Performance Battery, and hand-grip strength were examined. [Results] Strong significant associations were found between mobility, lower-limb function, balance, and the 6-minute walk test. A stepwise multiple regression on the entire sample showed that lower-limb function was a significant and independent predictor for the 6-minute walk test. Additionally, lower-limb function was a strong predictor for the 6-minute walk test in our nursing home group, whereas mobility was found to be the best predictor in our community-dwelling group. [Conclusion] Better lower-limb function, balance, and mobility result in a higher distance covered by healthy older adults. Lower-limb function and mobility appeared to best determine walking performance in the nursing home and community-dwelling groups, respectively.
Key words: 6-minute walk test, Physical performance, Older adults
INTRODUCTION
The life expectancy of the world population is rising1). In developed countries, such as European countries or the U.S., the proportion of older adults has rapidly increased in recent years2). Current estimates project that the number of older adults will double in the next 30 years3), changing the structure of the elderly population. The aging process is accompanied by a gradual decrease in exercise capacity and changes in function4), which may affect the ability to perform daily tasks and maintenance of personal independence5, 6). The ability to walk has demonstrated important implications in the preservation of function and independence7), and it is a key component of health-related quality of life8).
The 6-minute walk test (6MWT) is a quick and inexpensive performance-based measure widely used in exercise rehabilitation9) and clinical research10), both in healthy11,12,13,14) and impaired15,16,17,18) older adults. Originally developed by Butland et al. in 198219), the 6MWT measures the total distance walked during a 6-minute period20). The test has established a good reliability21), and it is a valid measure for overall physical functional performance20) and exercise capacity at levels corresponding to efforts commonly performed during daily tasks22). It has been reported that performance in the 6MWT is determined by a range of factors, including age, gender, height, and weight11, 23). Additionally, correlations between the 6MWT and mobility-related and physical measures have been found in community-dwelling older adults and nursing-home residents10, 24, 25). These observations suggest that performance in the 6MWT may be influenced by different demographic and physical measures. However, to the best of our knowledge, it has not been established which factors better explain 6MWT performance in older adults’ populations. It seems important to identify physical-function measures that better explain the capacity to walk so that therapists can include them in the assessment protocol for the aged population and therefore design the most appropriate interventions aimed at mitigating the walking decline associated with age.
The main purpose of the present study was to investigate a range of demographic factors and physical measures for their relative contributions and extent to which they may explain the results of the 6MWT in older adults. A secondary purpose of this study was to compare the factors explaining the 6MWT between older adults dwelling in the community and those living in a geriatric nursing home.
SUBJECTS AND METHODS
The design of this cross-sectional analysis (ClinicalTrials.gov ID: NCT02218411) and the informed consent procedure were approved by the Bioethics and Clinical Research Committee of UCH-CEU University. All participants provided a signed written informed-consent statement regarding their participation in the study.
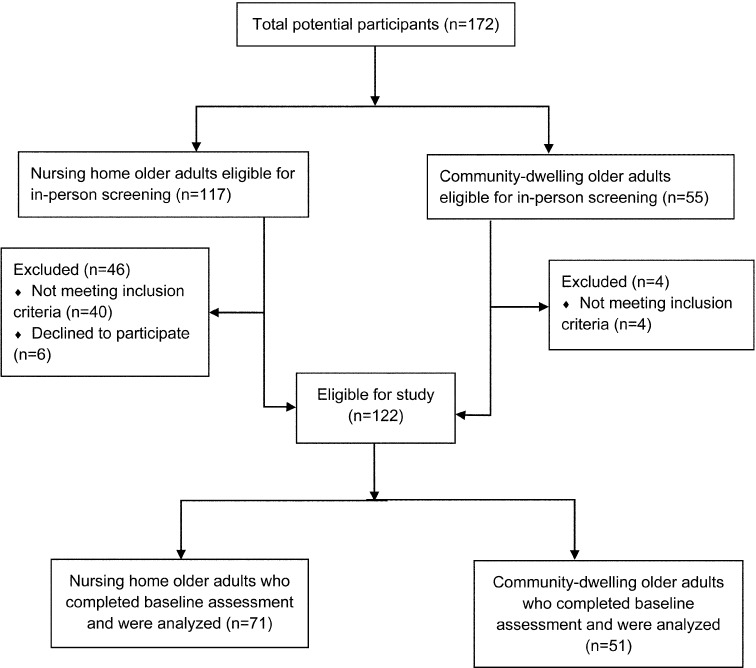
The sample for this analysis consisted of healthy nursing home (NH) residents and community-dwelling (CD) older adults aged 65 years or older from Valencia, Spain. The participants were volunteers who were recruited through advertisements on the bulletin boards in a local community center and a geriatric nursing home, as well as through presentations by researchers in both centers. The sample recruitment started in September 2012 and was completed in January 2013. A physical therapist (PT) with 27 years of clinical experience conducted individual interviews to screen all potential participants for inclusion. All participants received detailed information about the purpose of the study and its objectives. Participants who 1) were ˃65 years of age; 2) were able to ambulate independently without walking aids, 3) had no severe medical contraindications to performance of physical activities, 4) were able to communicate, and 5) provided a signed informed-consent statement were included in the study. Participants who 1) were unable to ambulate independently, 2) had a Mini-Mental State Examination (MMSE) score of <2426), 3) had a Barthel Index (BI) score <8027), 4) had an unstable cardiovascular disease or a neurological disorder that could compromise them during the performance of physical activities, or 5) had an upper- or lower-limb fracture in the past year, were excluded. One hundred twenty-two of the 172 eligible participants met the inclusion criteria and were enrolled in the study. Figure 1 shows the flow of the participants through the trial.
Fig. 1.
Flow chart of participants and screening
Five independent assessors recorded all measurements in a single assessment session, except for the rectus femoris cross-sectional area (CSA), which was assessed on a consecutive day. On the first day, participants were assessed for baseline demographics, health status, and physical measures. To enable assessment of the participants, a total of four functional test stations were set up in a large indoor room, except for the 6MWT, which was performed in a long indoor corridor next to the assessment room. Measurements were chronologically organized in order to minimize fatigue, and they were recorded in the following order.
Balance was assessed using the Berg Balance Scale (BBS). This test consists of 14 tasks common in everyday life with varied difficulty of balance (5 static and 9 dynamic). An experienced PT administered the test following the published guidelines28). Each task was graded on a 5-point scale of 0 (“unable to perform” or “need assistance”) to 4 (“able to perform independently”) according to the participant’s performance or the time taken to complete the task. At the end of the test, individual task scores were summed for a potential maximal score of 56 points (higher scores representing better performance).
Mobility was assessed using the Timed Up-and-Go test (TUG)29), which measures the time needed to rise from a chair, walk 3 meters as quickly as possible to reach a plastic cone, turn around the cone, return to the chair, and sit down again. Participants were instructed to start the test seated in a chair with their arms resting on the armrests and feet flat on the floor. One practice trial was conducted before the participants performed three test trials. The assessor recorded the time from the command “go” until the participant’s back touched the backrest of the chair. Although permitted to use walking aids during the test, no participant required them. The quickest time in seconds was recorded.
Hand-grip strength was assessed using a JAMAR hydraulic hand dynamometer (JAMAR, Sammons Preston Rolyan, Chicago, IL, USA). The dominant hand was defined as the hand preferred in performing daily tasks and was chosen for assessment. The second handle position of the dynamometer (at a fixed value of 5.5 cm) was set for all participants measurements30). The testing procedure was conducted in accordance with the procedure in a previous report31), and the mean score of three trials was recorded in kilograms. Finally, body mass index (BMI) was calculated as weight (kg)/height in meters squared (m2).
Lower-limb function was assessed using the Short Physical Performance Battery (SPPB)32). This measure consists of three tasks representing standing balance, walking speed, and repeated chair stands. Each component was scored according to the participant’s performance or the time needed to complete the task on a scale of 0 to 4. Additionally, a total summary score, which ranged from 0 to 12, was determined for the 3 components, with higher scores representing better functioning. To measure standing balance, participants were instructed to stand and maintain their feet in side-by-side (feet together), semi-tandem (heel of one foot against and touching the side of the big toe of the other foot), and tandem stand (heel of one foot in front of and touching the other foot) positions for ≥10 seconds each (maximum score awarded for ≥10 seconds). Walking speed was measured over a 4-m walking course delimited by two tape lines. Participants were instructed to stand with their feet next to the starting point, designated by a plastic cone placed 2 meters behind the first tape line. After the command “go”, participants walked past the end of the course to reach a second cone placed two meters behind the second tape line. Timing started after the first foot of the participant crossed the first tape line and stopped when the same foot completely crossed the second tape line. The shortest time (in seconds) of two trials was recorded (shorter time to complete the task representing better performance) and converted to meters per second. Finally, the repeated chair-stand test (STS-5) measured the time needed to rise from a chair and sit down again five consecutive times without using the arms. Participants were instructed to perform this test as fast as possible while keeping their arms folded across their chest and their feet flat on the floor. Timing started after the command “go” with the participant seated, and the test finished when the participant stood up for the fifth time. The time (in seconds) was recorded, and higher scores were given for shorter performance times.
The 6MWT evaluates the maximum distance that can be covered along a 30-m long corridor during a 6-minute period33). Two plastic cones delimited the corridor, and two-meter distance intervals were indicated by pieces of tape. A PT with specific experience administered and supervised the test. Participants were instructed to walk along the walkway as fast as possible, and to stop when needed. The assessor walked alongside the participants to ensure their safety and provided them with standardized verbal encouragement at 1, 3, and 5 min (“you are doing well” and “keep up the good work”). The test ended at the end of the 6-min period, and it was stopped immediately if chest pain, dizziness, or dyspnea was reported by the participant. The total distance covered (in meters) was recorded.
A portable ultrasound unit (Sonosite Inc., Bothell, WA, USA) was used to measure the rectus femoris CSA of each participant’s right leg. The rectus femoris was chosen due to its superficiality, accessibility, and facility with respect to visualization and measurement by ultrasonography34). An assessor with 17 years of clinical experience conducted the procedures and instructed the participants to remain in a supine position with their legs extended and relaxed and their toes pointing toward the ceiling. Three consecutive measurements were performed with the transducer placed perpendicularly to the skin surface and positioned midway between the epicondylus lateralis and the greater trochanter of the femur35). The mean area of the three measurements was recorded in centimeters.
Data analysis was conducted using SPSS 17.0 for Windows (SPSS Inc., Chicago, IL, USA). Descriptive statistics (mean ± SD) were generated to summarize demographic, heath-related, and physical measures data for all participants. The Student’s t test or Mann-Whitney U test and χ2 test were used to determine baseline significant differences between the NH and CD groups. The normality of the distribution of the data was determined with the Kolmogorov-Smirnov test before performing parametric or nonparametric analysis.
In order to determine the independent relationship between the 6MWT and the demographic and physical measures, bivariate correlations were calculated for all participants and for the NH and CD groups (the Pearson product moment correlation coefficient (r) was used for normally distributed data, and Spearman’s rho was used for non-normally distributed data).
Stepwise linear regression analyses were conducted to construct a model for identifying independent contributors to performance in the 6MWT. Analyses were conducted for the whole sample as well as for the NH and CD groups separately. Demographic and physical measures that could be associated with the 6MWT were used as independent variables (age, BMI, walking speed, BBS, TUG, rectus femoris CSA, STS-5, SPPB, and hand-grip strength), and the 6MWT was used as a dependent variable. Furthermore, variable selection was preceded by checking for correlation coefficients between the independent variables and the 6MWT. If a significant correlation was found for a variable, it was chosen for further analysis. The alpha level for significance was set at p<0.05.
RESULTS
The complete baseline data are summarized in Table 1. Participants had a mean age of 78 years (SD=8.8) and an age range of 65 to 95 years, and 70% of them were female. The initial sample consisted of 172 participants; however, 50 participants were excluded (n=44, due to not meeting the inclusion criteria) or declined to participate (n=6). Of the final 122 participants, 41.8% were living in their own houses, while 58.2% were living in a geriatric nursing home at the time of inclusion. Based on the mean scores of the BI and MMSE, the sample had high levels of performance in basic daily activities (BI 93.4, SD=10.3) and presented with no cognitive impairment (MMSE ≥24 points). Significant differences were found between the NH and CD groups with regard to demographic and physical measures except for gender, marital status, and heart rate. Data were non-normally distributed except for the 6MWT. During the 6MWT, 8 participants reported fatigue, and two participants reported ankle pain and dizziness.
Table 1. Descriptive statistics at baseline.
| All participants (N=122) X±SD | Nursing home group (N=71) X±SD | Community-dwelling group (N=51) X±SD | |
|---|---|---|---|
| Age (years)α ** | 78 ± 8.8 | 84.4 ± 4.9 | 69.1 ± 3.7 |
| Weight (kg)α ** | 68.8 ± 13.1 | 64.6 ± 10.5 | 74.7 ± 14.2 |
| Height (cm)α * | 155.9 ± 8.6 | 154.2 ± 8.4 | 158.1 ± 8.4 |
| BMI (kg/m2)α * | 28.3 ± 4.5 | 27.1 ± 3.8 | 29.8 ± 4.9 |
| Female † | 69.7% | 64.8% | 76.5% |
| Marital status † | |||
| Married | 21.3% | 16.9% | 27.5% |
| Widowed | 61.5% | 67.6% | 54.9% |
| Single | 17.2% | 15.5% | 17.6% |
| Barthel Index score (0–100)α ** | 93.4 ± 10.3 | 89 ± 11.5 | 99.7 ± 0.9 |
| 6MWT(m)α ** | 324.7 ± 103.9 | 290.6 ± 110.7 | 371.4 ± 71.7 |
| Gait speed (m/s)α ** | 0.8 ± 0.3 | 0.6 ± 0.2 | 1.1 ± 0.2 |
| BBS (0–56)α ** | 49.1 ± 5.7 | 46.9 ± 5.8 | 52 ± 3.9 |
| TUG (s)α ** | 12.6 ± 6.0 | 15.2 ± 6.7 | 9.2 ± 1.7 |
| Rectus femoris CSA (cm2)α ** | 4.6 ± 1.9 | 3.4 ± 1.3 | 6.2 ± 1.6 |
| STS-5 (s)α ** | 14.1 ± 6.6 | 15.9 ± 7.9 | 11.7 ± 2.9 |
| SPPB (0–12)α ** | 8.59 ± 2.9 | 6.8 ± 2.5 | 10.9 ± 1.5 |
| Hand-grip strength (kg)α ** | 23.2 ± 8.2 | 20 ± 7.4 | 27.7 ± 7.2 |
BMI: body mass index; 6MWT: 6-min walk test; BBS: Berg Balance Scale; TUG: Timed Up and Go test; CSA: cross-sectional area; STS-5: sit-to-stand test with five repetitions; SPPB: Short Physical Performance Battery total score. αStudent’s t test or Mann-Whitney U test between groups. †χ2 test. **p≤0.01; *p≤0.05
Associations between the 6MWT and demographics and physical measures for all participants are summarized in Table 2. Spearman’s rho correlation showed a significant strong36) association between the TUG, SPPB, BBS, and 6MWT in the entire sample (r=0.723 to −0.850, p≤0.01). Additionally, moderate-to-high associations were found between the 6MWT and walking speed, age, hand-grip strength, rectus femoris CSA, and STS-5 (r=−0.435 to 0.657, p≤0.01). Subgroup analyses for the NH and CD groups are summarized in Table 3. Analysis for the participants in the NH group showed significant strong association of the TUG, SPPB, and BBS with the 6MWT (r=0.761 to −0.880, p≤0.01), whereas in the analysis for the participants in the CD group, a higher association with the 6MWT was found for the TUG (r=−0.668, p≤0.01). The 6MWT was not significantly associated with the BMI either in the whole sample or the NH or CD groups. Additionally, in the CD group, an insignificant association was found between the 6MWT and the rectus femoris CSA and hand-grip strength.
Table 2. Correlation coefficients for the 6MWT and independent variables in all participants.
| Variables | Age | BMI | Gait speed | BBS | TUG | Rectus femoris CSA | STS-5 | SPPB | Hand-grip strength |
|---|---|---|---|---|---|---|---|---|---|
| 6MWT | −0.508** | 0.003 | 0.657a** | 0.723** | −0.850** | 0.449** | −0.435** | 0.752** | 0.456** |
| Age | −0.296** | −0.752** | −0.566** | 0.675** | −0.674** | 0.402** | −0.705** | −0.507** | |
| BMI | 0.199* | 0.032 | −0.132 | 0.316** | −0.037 | 0.235* | 0.150 | ||
| Gait speed | 0.684** | −0.860** | 0.613** | −0.504** | 0.904** | 0.550** | |||
| BBS | −0.730** | 0.461** | −0.419** | 0.712** | 0.416** | ||||
| TUG | −0.574** | 0.529** | −0.874** | −0.530** | |||||
| Rectus femoris CSA | −0.315** | 0.621** | 0.556** | ||||||
| STS-5 | −0.615** | −0.313** | |||||||
| SPPB | 0.558** |
**p≤0.01; *p≤0.05. 6MWT: 6-min walk test; BMI: body mass index; BBS: Berg Balance Scale; TUG: Timed Up and Go test; CSA: cross-sectional area; STS-5: sit-to-stand test with five repetitions; SPPB: Short Physical Performance Battery total score. aPearson correlation coefficients
Table 3. Correlation coefficients for the 6MWT and independent variables in the nursing home and community-dwelling groups.
| Variables | Group | Age | BMI | Gait speed | BBS | TUG | Rectus femoris CSA | STS-5 | SPPB | Hand-grip strength |
|---|---|---|---|---|---|---|---|---|---|---|
| 6MWT | Nursing home | −0.338a** | −0.158a | 0.697a** | 0.761** | −0.880** | 0.262* | −0.255* | 0.807** | 0.442** |
| Community dwelling | −0.315* | −0.118 | 0.500a** | 0.521** | −0.668** | 0.159a | −0.483a** | 0.372** | −0.010a | |
| Age | Nursing home | −0.112a | −0.357a* | −0.402** | 0.321** | −0.144 | 0.032 | −0.362** | −0.181 | |
| Community dwelling | −0.231 | −0.108 | −0.185 | 0.076 | −0.335* | 0.193 | −0.179 | −0.158 | ||
| BMI | Nursing home | −0.021a | −0.038 | 0.103 | 0.116 | 0.199 | −0.059 | −0.060 | ||
| Community dwelling | −0.056 | −0.210 | 0.075 | 0.314* | −0.150 | 0.187 | 0.130 | |||
| Gait speed | Nursing home | 0.645** | −0.761** | 0.430** | −0.148 | 0.813** | 0.508** | |||
| Community dwelling | 0.425** | −0.660** | −0.283 | −0.737** | 0.761** | −0.096 | ||||
| BBS | Nursing home | −0.749** | 0.225 | −0.274* | 0.768** | 0.380** | ||||
| Community dwelling | −0.470** | −0.017 | −0.400** | 0.348* | −0.108 | |||||
| TUG | Nursing home | −0.305* | 0.287* | −0.820** | −0.461** | |||||
| Community dwelling | 0.114 | 0.550** | −0.522** | 0.077 | ||||||
| Rectus femoris CSA | Nursing home | −0.134 | 0.324* | 0.485** | ||||||
| Community dwelling | 0.039 | −0.087 | 0.090 | |||||||
| STS-5 | Nursing home | −0.343** | −0.216 | |||||||
| Community dwelling | −0.917** | −0.057 | ||||||||
| SPPB | Nursing home | 0.425** | ||||||||
| Community dwelling | 0.003 |
**p≤0.01; *p≤0.05. 6MWT: 6-min walk test; BMI: body mass index; BBS: Berg Balance Scale; TUG: Timed Up and Go test; CSA: cross-sectional area; STS-5: sit-to-stand test with five repetitions; SPPB: Short Physical Performance Battery total score. aPearson correlation coefficients
The stepwise linear regression analyses are summarized in Table 4. When the whole sample was analyzed, the stepwise multiple regression revealed that the SPPB was a significant and independent predictor for the 6MWT (AdjR2=0.595 β=0.774, p<0.001). Model 1 explains over half (59.5%) of the variation in the 6MWT compared with other variables. The same statistical analysis was conducted for the NH and CD groups. The SPPB (AdjR2=0.684, β=0.831, p<0.001) was revealed to be a strong predictor for the 6MWT when data from the NH group were analyzed. However, in the CD group analysis, the TUG (AdjR2=0.484, β=−0.703 p<0.001) was shown to be the best predictor for the 6MWT. These results indicate that higher lower-limb function, measured with the SPPB (which explains 68.4% of the 6MWT variation in the NH group), and shorter time to complete the TUG (which explains 48.4% of the 6MWT variation in the CD group), are associated with the capacity to cover longer distances in the 6MWT in older adults dwelling in the community or in a geriatric nursing home.
Table 4. Multiple stepwise linear regression analyses with the 6MWT as a dependent variable.
| Independent variables | 6-min walk test | ||||
|---|---|---|---|---|---|
| R2 | Adjusted R2 | R2 change | Unstandardized β coefficient (standard error) | Standardized β coefficient | |
| All participants | |||||
| Model 1 | 0.599 | 0.595 | 145.069 | ||
| SPPB | 26.597 (2.208) | 0.774** | |||
| Model 2 | 0.639 | 0.631 | 10.521 | ||
| SPPB | 18.448 (3.279) | 0.537** | |||
| TUG | −5.080 (1.566) | −0.310* | |||
| Nursing home group | |||||
| Model 1 | 0.690 | 0.684 | 122.484 | ||
| SPPB | 35.738 (3.229) | 0.831** | |||
| Model 2 | 0.717 | 0.707 | 5.235 | ||
| SPPB | 32.258 (3.463) | 0.750** | |||
| Hand-grip strength | 2.784 (1.217) | 0.184* | |||
| Community-dwelling group | |||||
| Model 1 | 0.495 | 0.484 | 0.495 | ||
| TUG | −29.192 (4.216) | −0.703** | |||
**β significance p≤0.01; *β significance p≤0.05. TUG: Timed Up and Go test; SPPB: Short Physical Performance Battery total score
DISCUSSION
We found that all physical measurement results were significantly lower in older adults living in a geriatric nursing home compared with those of older adults dwelling in the community. In the bivariate analyses, more distance covered in the 6MWT was associated with better results in the BBS, TUG, and SPPB (higher in the NH group compared with the CD group), better walking speed and hand-grip strength, and a higher rectus femoris CSA. After adjusting for age, rectus femoris CSA, hand-grip strength, BBS, walking speed, and STS-5, the SPPB and TUG were independently related to the 6MWT. The SPPB explained 59.5% of the change in the distance covered during the test. When the NH group was analyzed separately, both the SPPB and hand-grip strength were independently related to the 6MWT, while in the CD group, only the TUG was independently associated with the 6MWT. Therefore, in the light of these results, a lower SPPB score explains less distance covered in the 6MWT in the elderly population. The TUG appears to be an important measure for those individuals dwelling in the community.
The data analysis showed a significant difference in the 6MWT between the study groups. We found that the distance covered by the CD group was very similar to that reported in a prior study on well-functioning community-dwelling older adults (65±2 yrs)37). On the other hand, participants in the NH group reported a lower distance covered (290.6 ± 110.7 m) compared with the CD group (371.4 ± 71.7 m). Additionally, a considerable range in distance covered (ranging 49 to 645 m in the NH group and 172 to 508 m in the CD group) was found in both study groups. This variability between groups was probably due to differences in age38,39,40) or baseline values for the physical measures20, 25). Heterogeneity in health status is a characteristic of the older adults’ population. “Apparently healthy” elderly persons could present with a large diversity in health status, and their exercise capacities could be substantially influenced22).
Although previous studies have assessed the implications of physical measures on 6MWT performance in older adults, their target populations (suffering from multiple sclerosis or strokes)17, 41,42,43,44) or methods (non-standardized procedures for balance or lower-limb function)10, 12, 22, 25) were somewhat different, making direct comparison difficult, and factors explaining the differences in 6MWT performance between two different geriatric groups (older adults living in a geriatric home and those dwelling in the community) have not been previously reported.
Moderate to high (r between 0.5 and ˃0.7)36) correlations between the 6MWT and age, walking speed, BBS, TUG, and SPPB were found, but the correlations were lower with the rectus femoris CSA, STS-5 and hand-grip strength (r <0.5). In agreement with previous research, there was a negative correlation between age and 6MWT11, 45) and a positive correlation between physical performance and 6MWT distance10, 25, 41, 43, 44). Hence, Harada et al.25) observed significant correlations between related individual tasks from the SPPB (standing balance r=0.52, gait speed r=0.73, and chair stands r=0.67) and the 6MWT in a sample of healthy older adults living in retirement homes or dwelling in the community. Additionally, Lord et al.10) reported that overall mobility (which included balance, sensorimotor, and lower-limb strength measures) was significantly associated with the 6MWT in a sample of older adults living in retirement villages. Furthermore, other studies have reported similar observations in impaired older adults. Wetzel et al.44) reported significant correlations between the 6MWT and static balance (r=0.39) and the multiple sit-to-stand test (r=0.57) in community dwelling individuals who had moderate disabilities and had been diagnosed with multiple sclerosis. Langhammer et al.41) observed a strong correlation between the 6MWT and TUG in a sample of older adults with acute stroke. Finally, Kluding et al.43) observed a significant correlation with the BBS (r=0.67) in a sample of patients who had a mean age 57.6 years and were suffering from chronic stroke. Despite differences in the characteristics of the samples in the various studies, these results support the importance of physical performance on the walking ability when covering long distances, especially in low-functioning older adults. Therefore, the present study confirms and extends the findings reported by previous research concerning demographic and physical measures explaining 6MWT performance in a samples of healthy older adults.
Although our study was cross-sectional, the inclusion of participants across the spectrum of aging yielded some interesting observations that might shed some light on the main functional aspects that influence walking distance among older adults. Multivariate analyses showed different results depending on the subgroup analysis. While the 6MWT was independently associated with the SPPB in the NH group, it was associated with the TUG in the CD group. The walking ability of older adults living in a geriatric nursing home is better explained by the SPPB than by age, rectus femoris CSA, or other physical performance variables. To the best of our knowledge, no study has investigated the variance of the 6MWT with the SPPB as a single measure. However, a previous study25) showed that the three individual tasks of the SPPB (balance, gait speed, and chair stands) explained 69% of the variance in the 6MWT in healthy older adults dwelling in the community and living in retirement homes. While Harada et al. analyzed the results for a mixed-population sample from community centers and retirement homes the two types of populations were also analyzed separately in our study. Differences in the variance may be accounted for by other disparities in the analysis of measures and samples of the studies. This finding reveals that the capacity to perform daily activities such as walking, standing up from a chair, or maintaining standing balance, may be reflected by the ability to walk a certain distance. Therefore, the SPPB could be used to evaluate lower-limb functioning as well as walking ability in older adults, with special attention to populations living in geriatric nursing homes. Regarding CD older adults, the TUG was the variable that better explained the variance in the 6MWT. The absence of studies examining the extent to which the TUG influences the 6MWT in healthy older adults prevents us from comparing our results with previous research. However, one study10) reported that overall mobility appeared to provide a measure of 6MWT in a sample of community-dwelling older adults. The regression model of this study (which included age, lower-limb strength, simple reaction time, and postural sway and balance range) explained 52.5% of the variance in the 6MWT. These results are in agreement with our study, which explained 48.4% of the variance in the 6MWT with the TUG. However, care should be taken in comparing these results because of the differences between study populations and assessment measures. The positive correlation between the TUG and 6MWT is not surprising because during the 6MWT, participants walked as far as possible and the test included “turns around a cone” at the end of the corridor. According to our results, it seems that, in lower-functioning older adults, balance and SPPB components are of high importance to improvement of walking distance, while in higher-functioning samples, walking distance relies on mobility and walking speed. Hence, these results suggest that, to improve walking performance in healthy older adults, an intervention could focus on enhancement of balance, mobility, and lower-limb function. This will need to be confirmed in prospective and longitudinal studies.
This study has several limitations. Firstly, the study participants were volunteers and relatively healthy older adults, a sample that may not represent the characteristics of people living in a geriatric nursing home or dwelling in the community. A possible selection bias may explain differences in the participants’ levels of physical performance. The wide inclusion criteria lend support to the external validity of these results when making comparisons to typical older adults living in nursing homes or in the community. Secondly, although we assessed some components of lower-limb physical performance, the measured variables may not cover all physical information that explains the variability in the 6MWT. To confirm the results of this study, future analyses should consider other physical measures, such as the ability to modify balance while walking in the presence of external demands (Dynamic Gait Index)46), fast walking speed (10-Meter Walk Test)47), or muscle strength and CSA of other muscles involved in walking performance, like the vastus medialis. These factors may play an important role in the 6MWT and walking performance. Thirdly, the sample size was relatively small (NH; n=71, CD; n=51). Consequently, caution is warranted when interpreting or generalizing the results of this study, especially to frail or impaired older adults.
In conclusion, the findings of the present study revealed that higher lower-limb function, balance, and mobility are associated with better walking ability and distance covered in healthy older adults. The SPPB is the test that best determines the walking performance in low-functioning older adult populations, while the TUG best determines the walking performance in community-dwelling older adults. Future studies should clarify if improving the results of the SPPB and TUG results in an increased distance in the 6MWT in older adults.
Acknowledgments
The authors would like to acknowledge and thank all individuals who contributed to this study, especially the participants, the directors, and the staff of the “Centro Municipal de Actividades para Personas Mayores Sant Pau” and the “Hermanitas de los Ancianos Desamparados,” for their cooperation and participation. We would also like to thank Carmen Puerto Rentero, who assisted during the data collection, and Katia Esteve Mallent and the Department of Physiotherapy of UCH-CEU University, who provided the much needed support that enabled a considerable contribution to this manuscript.
REFERENCES
- 1.Lubitz J, Cai L, Kramarow E, et al. : Health, life expectancy, and health care spending among the elderly. N Engl J Med, 2003, 349: 1048–1055. [DOI] [PubMed] [Google Scholar]
- 2.Christensen K, Doblhammer G, Rau R, et al. : Ageing populations: the challenges ahead. Lancet, 2009, 374: 1196–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Caffrey C, Sengupta M, Moss A, et al. : Home health care and discharged hospice care patients: United States, 2000 and 2007. Natl Health Stat Rep, 2011, 27: 1–27. [PubMed] [Google Scholar]
- 4.Marcell TJ: Sarcopenia: causes, consequences, and preventions. J Gerontol A Biol Sci Med Sci, 2003, 58: M911–M916. [DOI] [PubMed] [Google Scholar]
- 5.Guralnik JM, Ferrucci L, Simonsick EM, et al. : Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med, 1995, 332: 556–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Janssen I, Baumgartner RN, Ross R, et al. : Skeletal muscle cutpoints associated with elevated physical disability risk in older men and women. Am J Epidemiol, 2004, 159: 413–421. [DOI] [PubMed] [Google Scholar]
- 7.Tinetti ME, Inouye SK, Gill TM, et al. : Shared risk factors for falls, incontinence, and functional dependence. Unifying the approach to geriatric syndromes. JAMA, 1995, 273: 1348–1353. [PubMed] [Google Scholar]
- 8.Guralnik JM, Branch LG, Cummings SR, et al. : Physical performance measures in aging research. J Gerontol, 1989, 44: M141–M146. [DOI] [PubMed] [Google Scholar]
- 9.Alison JA, Kenny P, King MT, et al. : Repeatability of the six-minute walk test and relation to physical function in survivors of a critical illness. Phys Ther, 2012, 92: 1556–1563. [DOI] [PubMed] [Google Scholar]
- 10.Lord SR, Menz HB: Physiologic, psychologic, and health predictors of 6-minute walk performance in older people. Arch Phys Med Rehabil, 2002, 83: 907–911. [DOI] [PubMed] [Google Scholar]
- 11.Troosters T, Gosselink R, Decramer M: Six minute walking distance in healthy elderly subjects. Eur Respir J, 1999, 14: 270–274. [DOI] [PubMed] [Google Scholar]
- 12.Camarri B, Eastwood PR, Cecins NM, et al. : Six minute walk distance in healthy subjects aged 55–75 years. Respir Med, 2006, 100: 658–665. [DOI] [PubMed] [Google Scholar]
- 13.Benavent-Caballer V, Rosado-Calatayud P, Segura-Ortí E, et al. : Effects of three different low-intensity exercise interventions on physical performance, muscle CSA and activities of daily living: a randomized controlled trial. Exp Gerontol, 2014, 58: 159–165. [DOI] [PubMed] [Google Scholar]
- 14.Ihira H, Shimada H, Suzukawa M, et al. : Differences between proximal and distal muscle activity of the lower limbs of community-dwelling women during the 6-minute walk test. J Phys Ther Sci, 2012, 24: 205–209. [Google Scholar]
- 15.Jung Y, Lee K, Shin S, et al. : Effects of a multifactorial fall prevention program on balance, gait, and fear of falling in post-stroke inpatients. J Phys Ther Sci, 2015, 27: 1865–1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mossberg KA: Reliability of a timed walk test in persons with acquired brain injury. Am J Phys Med Rehabil, 2003, 82: 385–390, quiz 391–392. [DOI] [PubMed] [Google Scholar]
- 17.Savci S, Inal-Ince D, Arikan H, et al. : Six-minute walk distance as a measure of functional exercise capacity in multiple sclerosis. Disabil Rehabil, 2005, 27: 1365–1371. [DOI] [PubMed] [Google Scholar]
- 18.Cahalin LP, Mathier MA, Semigran MJ, et al. : The six-minute walk test predicts peak oxygen uptake and survival in patients with advanced heart failure. Chest, 1996, 110: 325–332. [DOI] [PubMed] [Google Scholar]
- 19.Butland RJ, Pang J, Gross ER, et al. : Two-, six-, and 12-minute walking tests in respiratory disease. Br Med J (Clin Res Ed), 1982, 284: 1607–1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rikli RE, Jones CJ: The reliability and validity of a 6-minute walk test as a measure of physical endurance in older adults. J Aging Phys Act, 1998, 6: 363–375. [Google Scholar]
- 21.Kervio G, Carre F, Ville NS: Reliability and intensity of the six-minute walk test in healthy elderly subjects. Med Sci Sports Exerc, 2003, 35: 169–174. [DOI] [PubMed] [Google Scholar]
- 22.Bautmans I, Lambert M, Mets T: The six-minute walk test in community dwelling elderly: influence of health status. BMC Geriatr, 2004, 4: 6–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Enright PL, McBurnie MA, Bittner V, et al. Cardiovascular Health Study: The 6-min walk test: a quick measure of functional status in elderly adults. Chest, 2003, 123: 387–398. [DOI] [PubMed] [Google Scholar]
- 24.Duncan PW, Chandler J, Studenski S, et al. : How do physiological components of balance affect mobility in elderly men? Arch Phys Med Rehabil, 1993, 74: 1343–1349. [DOI] [PubMed] [Google Scholar]
- 25.Harada ND, Chiu V, Stewart AL: Mobility-related function in older adults: assessment with a 6-minute walk test. Arch Phys Med Rehabil, 1999, 80: 837–841. [DOI] [PubMed] [Google Scholar]
- 26.Folstein MF, Folstein SE, McHugh PR: “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res, 1975, 12: 189–198. [DOI] [PubMed] [Google Scholar]
- 27.Wade DT, Collin C: The Barthel ADL Index: a standard measure of physical disability? Int Disabil Stud, 1988, 10: 64–67. [DOI] [PubMed] [Google Scholar]
- 28.Berg KO, Wood-Dauphinee SL, Williams JI, et al. : Measuring balance in the elderly: validation of an instrument. Can J Public Health, 1992, 83: S7–S11. [PubMed] [Google Scholar]
- 29.Podsiadlo D, Richardson S: The timed “Up & Go”: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc, 1991, 39: 142–148. [DOI] [PubMed] [Google Scholar]
- 30.Ruiz-Ruiz J, Mesa JL, Gutiérrez A, et al. : Hand size influences optimal grip span in women but not in men. J Hand Surg Am, 2002, 27: 897–901. [DOI] [PubMed] [Google Scholar]
- 31.Mathiowetz V, Weber K, Volland G, et al. : Reliability and validity of grip and pinch strength evaluations. J Hand Surg Am, 1984, 9: 222–226. [DOI] [PubMed] [Google Scholar]
- 32.Guralnik JM, Simonsick EM, Ferrucci L, et al. : A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol, 1994, 49: M85–M94. [DOI] [PubMed] [Google Scholar]
- 33.Rikli RE, Jones CJ: Development and validation of a functional fitness test for community-residing older adults. J Aging Phys Act, 1999, 7: 129. [Google Scholar]
- 34.Bemben MG: Use of diagnostic ultrasound for assessing muscle size. J Strength Cond Res, 2002, 16: 103–108. [PubMed] [Google Scholar]
- 35.e Lima KM, da Matta TT, de Oliveira LF: Reliability of the rectus femoris muscle cross-sectional area measurements by ultrasonography. Clin Physiol Funct Imaging, 2012, 32: 221–226. [DOI] [PubMed] [Google Scholar]
- 36.Fleiss JL: The Design and Analysis of Clinical Experiments. New York: John Wiley & Sons, 1986, pp 1–33. [Google Scholar]
- 37.Thaweewannakij T, Wilaichit S, Chuchot R, et al. : Reference values of physical performance in Thai elderly people who are functioning well and dwelling in the community. Phys Ther, 2013, 93: 1312–1320. [DOI] [PubMed] [Google Scholar]
- 38.Rikli RE, Jones CJ: Functional fitness normative scores for community-residing older adults, ages 60–94. J Aging Phys Act, 1999, 7: 162. [Google Scholar]
- 39.Steffen TM, Hacker TA, Mollinger L: Age- and gender-related test performance in community-dwelling elderly people: Six-Minute Walk Test, Berg Balance Scale, Timed Up & Go Test, and gait speeds. Phys Ther, 2002, 82: 128–137. [DOI] [PubMed] [Google Scholar]
- 40.Tveter AT, Dagfinrud H, Moseng T, et al. : Health-related physical fitness measures: reference values and reference equations for use in clinical practice. Arch Phys Med Rehabil, 2014, 95: 1366–1373. [DOI] [PubMed] [Google Scholar]
- 41.Langhammer B, Lindmark B, Stanghelle JK: The relation between gait velocity and static and dynamic balance in the early rehabilitation of patients with acute stroke. Adv Physiother, 2006, 8: 60–65. [Google Scholar]
- 42.Danielsson A, Willén C, Sunnerhagen KS: Is walking endurance associated with activity and participation late after stroke? Disabil Rehabil, 2011, 33: 2053–2057. [DOI] [PubMed] [Google Scholar]
- 43.Kluding P, Gajewski B: Lower-extremity strength differences predict activity limitations in people with chronic stroke. Phys Ther, 2009, 89: 73–81. [DOI] [PubMed] [Google Scholar]
- 44.Wetzel JL, Fry DK, Pfalzer LA: Six-minute walk test for persons with mild or moderate disability from multiple sclerosis: performance and explanatory factors. Physiother Can, 2011, 63: 166–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Enright PL, Sherrill DL: Reference equations for the six-minute walk in healthy adults. Am J Respir Crit Care Med, 1998, 158: 1384–1387. [DOI] [PubMed] [Google Scholar]
- 46.Shumway-Cook A, Baldwin M, Polissar NL, et al. : Predicting the probability for falls in community-dwelling older adults. Phys Ther, 1997, 77: 812–819. [DOI] [PubMed] [Google Scholar]
- 47.Peters DM, Fritz SL, Krotish DE: Assessing the reliability and validity of a shorter walk test compared with the 10-Meter Walk Test for measurements of gait speed in healthy, older adults. J Geriatr Phys Ther, 2013, 36: 24–30. [DOI] [PubMed] [Google Scholar]