Abstract
[Purpose] The purpose of this study was to elucidate the cathepsin-D involvement in signaling pathways for the survival and apoptosis of myofibers in rats with hindlimb-unloading in a low-temperature environment. [Subjects and Methods] Wistar rats were divided into two groups: a control group and a group that underwent hindlimb unloading in a low-temperature environment to induce muscle apoptosis. Cathepsin-D localization in the soleus and extensor digitorum longus muscles, along with the expression of cathepsin-D in apoptotic myofibers, was examined. Expression of the active and inactive forms of cathepsin-D was also analyzed. [Results] Cathepsin-D was mainly expressed in type I myofibers and was observed to have punctate patterns in the control group. In the hindlimb unloading in a low-temperature environment group, the type I myofiber composition ratio decreased, and caspase-3 activation and TUNEL-positive apoptotic myofibers were observed. In caspase-3-activated myofibers, cathepsin-D overexpression and leakage of it into the cytoplasm were observed. In the hindlimb unloading in a low-temperature environment group, the amount of inactive cathepsin-D decreased, whereas that of the active form increased. [Conclusion] Cathepsin-D was deduced to be indicative of a myofiber-type classification and a factor related to myofiber type maintenance. In addition, cathepsin-D leakage into the cytoplasm was appeared to be involved in caspase-3 activation in the hindlimb unloading in a low-temperature environment group.
Key words: Cathepsin-D, Apoptosis, Muscle fiber type
INTRODUCTION
Cathepsin-D is an aspartic endopeptidase, a type of lysosomal proteolytic enzyme found in almost all animal cells. In cells, it is localized in the endoplasmic reticulum or endosome as a soluble fraction or in a membrane-bound form1). Cathepsin-D is synthesized in the rough endoplasmic reticulum as an inactive precursor, preprocathepsin-D, and after removal of the signal peptide, mannose 6-phosphate is tagged in the face cisternae of the Golgi to form procathepsin-D. This precursor is then transferred to the late endosome, forming the single-chain intermediate active enzyme form, after cleavage of the N-terminal propeptide under acidic conditions. Procathepsin-D is further degraded by the lysosome into a light chain and a heavy chain, which are the mature forms that demonstrate proteolytic activity2,3,4).
Although an in vitro experiment revealed that cathepsin-D degrades connectin, which is localized in the sarcomere I-band5), Z-band6), myosin, α-actinin, tropomyosin, troponin T, and troponin I7, 8), the role and localization of cathepsin-D in skeletal muscle remain largely unknown. Therefore, this study investigated the localized characteristics of cathepsin-D.
Cathepsin-D has previously been considered to be responsible for the nonspecific proteolysis in lysosomes. However, studies involving cathepsin-D knockout mice showed normal growth without any abnormalities from after birth to 2 weeks, although growth cessation was observed at 3 weeks. These animals died approximately 26 days after birth9), suggesting that cathepsin-D specifically catalyzes the limited degradation of proteins that are essential for growth or homeostasis and is also involved in signal transduction pathways associated with survival.
In staurosporine-induced apoptosis of fibroblasts, cathepsin-D specifically cleaves Phe24, Trp48, and Phe183 of an apoptosis induction factor, Bid, resulting in its activation, and this, leads to the release of cytochrome c from the mitochondria as well as the induction of apoptosis10). Furthermore, in naphthazarin-induced apoptosis of human fibroblasts, leakage of cathepsin-D was observed in the cytoplasm11), and overexpression of inactive cathepsin-D suppresses H2O2-induced apoptosis12). Based on these research results, cathepsin-D was revealed to be involved in not only the signal transduction pathway for cell survival but also the signal transduction pathway for apoptosis.
As for apoptosis induced by disuse muscle atrophy, apoptosis caused by immobilization-induced limb muscle atrophy decreased in caspase-3 knockout mice13). In the case of denervation-induced atrophied muscle, the amount of apoptosis-inducing factor released from the mitochondria decreased, and apoptosis was suppressed14). However, the involvement of cathepsin-D in the apoptosis mechanism induced in disuse muscle atrophy remains uncertain.
In our previous study describing the preventive method for disuse muscle atrophy using low-temperature environment (LTE) therapy, it was revealed that rats with hindlimb unloading (HU) treated with LTE therapy acclimated to low temperatures without shivering, and the progress of muscle atrophy in the soleus was reduced in comparison with that in HU rats kept in a normal-temperature environment15). However, LTE therapy for HU rats caused the occurrence of apoptosis in the soleus muscle as a side effect; histological analysis revealed cathepsin-D leakage in the caspase-3-positive myofibers in HU in an LTE (HULT). These were assumed to be associated with apoptosis, which is caused by LTE16), although the role of cathepsin-D in myofiber survival and death is unclear. Hence, the present study revealed histological and biochemical characterizations of the expression of cathepsin-D in the normal soleus muscle and in the soleus muscle of HU rats treated using LTE therapy.
SUBJECTS AND METHODS
This investigation was conducted in accordance with the ethical guidelines for the experimental treatment of animals of Hiroshima University.
Nine-week-old male Wistar rats weighing 278 ± 8 g were housed in individual cages and randomly assigned to four groups (5 rats/group). Rats underwent hindlimb unloading in a low-temperature environment (10 °C, HULT) for 3 weeks, whereas the control groups was not subjected to hindlimb unloading and was maintained in a normal-temperature environment (25 °C, CON). Hindlimb unloading was achieved by elevating the hindlimbs and preventing them from touching a supporting surface. The forelimbs maintained contact with a grid floor, which allowed the animals a full range of motion. Food and water were provided ad libitum.
At the end of the experimental period, the rats were anesthetized with an intraperitoneal injection of 50 mg/kg pentobarbital sodium17) (Abbott Laboratories, North Chicago, IL, USA), weighed, and sacrificed by laparotomy followed by transection of the abdominal aorta. The soleus muscles were immediately excised and cut transversely into two segments. Each segment was individually mounted on cork using optimum cutting temperature compound (Sakura Finetek USA, Torrance, CA, USA), and then the blocks were rapidly snap frozen in isopentane cooled in liquid nitrogen and stored at −80 °C.
To compare the ratio of fiber type compositions in the soleus muscle of CON and HULT rats, 10-μm-thick serial transverse cryosections were cut from tissue blocks using a cryostat microtome at −25 °C and were fixed in cold acetone at 4 °C. The sections were stained using the VECTASTAIN Elite ABC system (Vector Laboratories, Burlingame, CA, USA) according to the manufacturer’s protocol. Briefly, the endogenous peroxidase activity in tissue was blocked by incubation in 0.3% (v/v) hydrogen peroxide (H2O2) in absolute methanol, and then nonspecific immunoglobulin (Ig) binding sites were blocked by 1% normal bovine serum albumin (BSA, Sigma-Aldrich, St. Louis, MO, USA) in phosphate-buffered saline (PBS). The sections were immersed in a mouse monoclonal anti-slow myosin antibody (Sigma) or a mouse monoclonal anti-fast myosin antibody (Sigma) in PBS containing 1% BSA at RT, incubated in a secondary biotinylated anti-mouse IgG, and treated with an ABC reagent. Immunoreactivity was visualized using Tris-HCl buffer containing 0.1% 3,3′-diaminobenzidine tetrahydrochloride (DAB) (Sigma) and 0.05% H2O2. Myofiber types were manually classified for almost all myofibers using a light microscope, and the total number of slow- and fast-type myofibers were counted using the NIH Image Ver. 1.62 (National Institutes of Health) software.
To observe the cathepsin-D and fast myosin expression in the apoptotic myofibers of atrophied soleus muscle, immunofluorescence staining was performed by the same procedure as described above. Some sections were routinely stained with hematoxylin and eosin (HE) prior to fixation18). The primary antibodies used were a rabbit polyclonal anti-caspase-3 antibody (Promega, Madison, WI, USA), a mouse monoclonal anti-dystrophin antibody (Novocastra Laboratories, Newcastle, UK), a mouse monoclonal anti-cathepsin-D antibody (Transduction Laboratories, Lexington, KY, USA), a mouse monoclonal anti-slow myosin antibody (Sigma), and a mouse monoclonal anti-fast myosin antibody (Sigma). The secondary antibodies used were Alexa 488-conjugated anti-mouse IgG or Alexa 546-conjugated anti-rabbit IgG (Molecular Probes, Eugene, OR, USA). For double-labeling immunofluorescence, incubations with primary and secondary antibodies were performed as described above. The sections were examined using a confocal laser scanning microscope (LSM-510, Carl Zeiss, Jena, Germany). The numbers of caspase-3-positive myofibers were counted on digitized images from fluorescence microscopy.
Internucleosomally degraded DNA was detected by the terminal deoxynucleotidyl transferase (TdT)-mediated dUTP nick end labeling (TUNEL) technique (DeadEndTM Fluorometric TUNEL System, Promega) according to the manufacturer’s instructions.
For western blot analyses, whole muscle lysates were isolated by homogenizing freshly excised muscle in lysis buffer on ice. Homogenates were centrifuged at 10,000 ×g for 10 min at 4 °C to remove nuclear fragments and tissue debris without precipitating the plasma membrane, and the supernatants were used. The protein concentrations were determined by the Bradford method19, 20). All proteins were denatured by boiling at 90 °C in sample buffer. Denatured proteins were electrophoresed in 12.5% SDS-polyacrylamide gels and transferred to nitrocellulose membranes (Bio-Rad, Hercules, CA, USA). The membranes were blocked overnight with blocking buffer, incubated in an anti-cathepsin-D antibody or an anti-actin antibody (sigma), probed with a secondary biotinylated anti-mouse antibody, and incubated with ABC reagent. Labeling was detected with 0.01% DAB and 0.05% H2O2 in 50 mM Tris–HCl buffer. Quantification of the signal was performed by densitometric analysis using the NIH Image (Ver. 1.59) software.
All values are expressed as the mean ± SE. The results obtained from all experiments were analyzed by Welch’s t-test21, 22). Data were considered significantly differrent when p < 0.05.
RESULTS
In the CON group, the fiber-type composition ratio in the soleus muscles was 90.8% for type I myofibers and 9.2% for type II myofibers. In the HULT group, the fiber-type composition ratio in the soleus muscles was 72.9% for type I myofibers and 27.1% for type II myofibers (Table 1). Compared with the CON group, the composition ratio of type I myofibers decreased in the HULT group, whereas that of type II increased.
Table 1. Ratio of fiber-type composition and number of apoptotic myofibers.
| CON | HULT | ||
|---|---|---|---|
| Ratio of fiber-type composition | Type I | 90.8 ± 4.2 | 72.9 ± 5.4† |
| Type II | 9.2 ± 4.2 | 27.1 ± 5.4† | |
| Number of active-caspase-3 positive myofibers | 0 | 3.0 ± 2.0* | |
The values are means ± standard deviation. P-values were assessed between the CON and HULT groups by unpaired student’s t-test. *Significant difference between the CON and HULT groups (p < 0.05). †Significant difference between the CON and HULT groups (p < 0.001).
The rate of occurrence of caspase-3-expressed myofibers in the total number of myofibers in the HULT group was 0.27 ± 0.17%. Apoptotic myofibers were absent in the CON group, and this was accompanied by the presence of TUNEL-positive myonuclei and caspase-3 activation; on the other hand, apoptotic myofibers were present in the HULT group, which showed a statistically significant difference (Table 1).
In the soleus muscles of the CON group, images of tissue staining for cathepsin-D showed two types of myofibers that could be clearly differentiated in terms of fluorescent intensity; some myofibers with low fluorescence intensity were observed, whereas myofibers with high fluorescence intensity predominated (Fig. 1a). Fluorescence signals of cathepsin-D were observed to have punctate patterns within myofibers within the vicinity of the sarcolemma, as well as within the sarcoplasm (Fig. 1a, c).
Fig. 1.
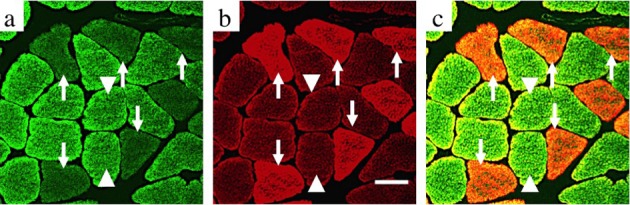
Conformity with fiber type and cathepsin-D expression by immunohistochemical analysis in the normal soleus muscle (a–c). Transverse serial sections of normal soleus muscles stained with anti-cathepsin-D and anti-fast myosin antibodies. The immunofluorescence of cathepsin-D is shown in green (a), and that of fast myosin is shown in red (b). The left panels show merged images for cathepsin-D and fast myosin (c). Arrows indicate the same myofibers as fiber type II myofibers expressing both weak immunofluorescence reactivity for cathepsin-D and a positive reaction for fast myosin in the serial sections. Arrowheads indicate the same myofibers as type I myofibers expressing strong immunofluorescence reactivity for cathepsin-D and a negative reaction for fast myosin in the serial sections. Scale bar: 20 μm.
The muscle tissues showed positive staining for fast myosin, which was in accordance with the myofibers with low fluorescence intensity of cathepsin-D, and in contrast, they demonstrated a negative reaction for fast myosin, which was consistent with the myofibers with a high fluorescence intensity for cathepsin-D (Fig. 1a–c). Based on these results, myofibers with low fluorescence intensity for cathepsin-D were determined to be fast myosin-positive type II myofibers, and on the other hand, myofibers with high fluorescence intensity for cathepsin-D were determined to be fast myosin-negative type I myofibers.
In the serial cross sections of rat soleus muscles that had been subjected to hindlimb unloading in a low temperature environment for 3 weeks to induce apoptosis, decreased staining of the myonucleus, collapse of the fine network pattern of the sarcoplasm, and an obscure myocyte periphery, aggregated sarcoplasm, and fragments of denatured myofibers were observed by HE staining (Fig. 2d, e).
Fig. 2.
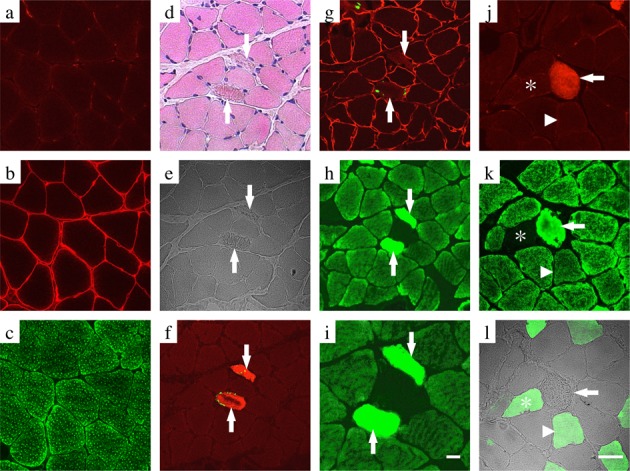
Conformity of cathepsin-D expression and apoptotic myofibers by immunohistochemical analysis in the soleus muscle of the CON (a–c) and HULT (d–l) groups. Transverse serial sections of the muscle were labeled with both anti-active caspase-3 antibody and TUNEL (a, f), both anti-dystrophin antibody and TUNEL (b, g), anti-cathepsin-D antibody (c, h, i, k), anti-active caspase-3 antibody (j), and fast myosin antibody (l). A higher magnification of panel e is depicted in panel i. Some of the serial sections were routinely stained with eosin–hematoxylin solution (d). Arrows indicate apoptotic myofibers expressing a positive reaction for both the active caspase-3 (red) and TUNEL-labeling (green) in serial sections (d–i) and a positive reaction for the active caspase-3 (red) in serial sections (j–l). Asterisks indicate type II myofibers exhibiting a negative reaction for both cathepsin-D (green) and positive reaction for fast myosin (green) in the serial sections (j–l). Arrowheads indicate a hybrid-type myofiber exhibiting a positive reaction for both cathepsin-D and fast myosin in the serial sections (j–l). Scale bar: 20 μm.
A collapsed dystrophin, a protein that constitutes the sarcolemma, and disappearance of the line that forms the sarcolemma were observed in the myofibers that were positive for TUNEL labeling and active caspase-3 in immunofluorescence staining (Fig. 2f, g). Therefore, these myofibers indicated the immunohistological features of apoptotic myofibers.
In these apoptotic myofibers, cathepsin-D was overexpressed, and images were observed in which cathepsin-D leaked from the lysosome or endoplasmic reticulum into the cytoplasm and was widely diffused (Fig. 2h, i). The overexpression of cathepsin-D and its leakage into the sarcoplasm, as well as punctuate expression of cathepsin-D, were observed in caspase-3-active myofibers but were fuzzy in non-apoptotic, caspase-3-negative myofibers (Fig. 2k). The caspase-3-active myofibers were type I myofibers that stained negative for fast myosin (Fig. 2j, l).
In western blot analysis, generally, cathepsin-D in rodents is detected as an approximately 43 kDa preprocathepsin-D of inactive form that is cleaved and glycosylated to form an approximately 46 kDa procathepsin-D, a 28 kDa heavy chain, and a 15 kDa light chain3, 23, 24). Bands of preprocathepsin-D and procathepsin-D, which are inactive forms of cathepsin-D, as well as the heavy chain, which is the active form of cathepsin-D, were observed in the soleus muscles of the CON and HULT groups (Fig. 3a). The light chain was not detected in these groups.
Fig. 3.
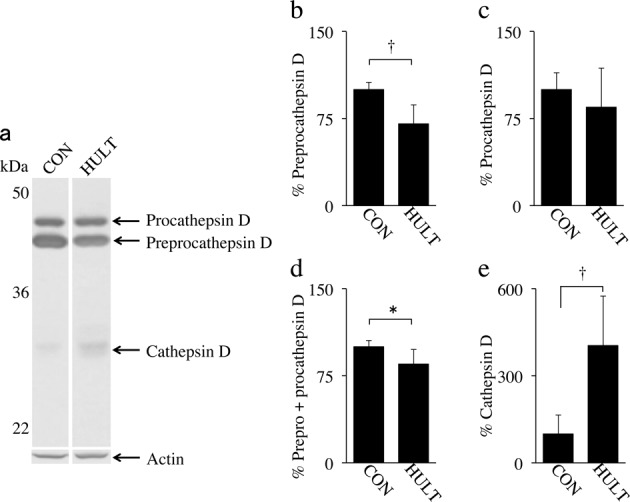
Western blot analysis of cathepsin-D in tissue supernatant fluids from normal and degenerative soleus muscles. The three forms of cathepsin-D, which are preprocathepsin-D, procathepsin-D, and cathepsin-D mature form (heavy chain), were detected in the CON and HULT rats (a). Preprocathepsin-D level decreased in the HULT group (b). Procathepsin-D level showed no significant difference between the CON and HULT groups (c). The total inactive form of cathepsin-D, which is the amount of preprocathepsin-D and procathepsin-D, decreased in the HULT group (d), and the active form of cathepsin-D increased drastically in the HULT group (e). The results represent the mean ± SD. *Significant difference between the CON and HULT groups (p < 0.05). †Significant difference between the CON and HULT groups (p < 0.001).
Compared with the CON group, the expression of preprocathepsin-D significantly decreased in the HULT group (Fig. 3b). No significant difference in the expression of procathepsin-D was observed between the two groups (Fig. 3c). The total expression of the inactive forms, namely, preprocathepsin-D and procathepsin-D, significantly decreased in the HULT group compared with that in the CON group (Fig. 3d). On the other hand, the expression of the heavy chain in the HULT group significantly increased compared with that in the CON group (Fig. 3e). Therefore, it was concluded that the amount of inactive cathepsin-D decreased in the atrophied muscles in the HULT group, whereas that of active cathepsin-D increased.
DISCUSSION
In the present study, two distinct types of myofibers were observed in rat soleus muscles, which showed a clear immunohistological difference in cathepsin-D fluorescence intensity. This difference in fluorescence intensity represented the abundance of antigen–antibody complex reactions of the anti-cathepsin-D antibody. Therefore, the observed strong fluorescence intensity of type I myofibers and the weak fluorescence intensity of type II myofibers are likely due to differences in cathepsin-D content, which depends on myofiber type, and this suggests that the cathepsin-D content in type I myofibers was higher than that in type II myofibers. Uchiyama et al.25) reported that the content of the cathepsin group varies in each organ, and our results suggest that cathepsin-D concentrations were determined by the type of myofibers.
In the microscopic analysis, the expression of cathepsin-D in fiber type I myofibers was detected throughout transversely sectioned myofibers (Fig. 1a). Early and late endosomes, phagosomes, and lysosomes in muscle are present in the sarcolemma and sarcoplasm26). Preprocathepsin-D is synthesized in the rough endoplasmic reticulum, procathepsin-D is present in both the Golgi apparatus and prelysosomal compartments, single chain and mature cathepsin-D is present in lysosomes, and procathepsin D is distributed into them through the sarcoplasm27). Hence, the detection of cathepsin-D in the present study is suggested to be a result of detecting all the cathepsin-D forms existing in the sarcolemma and sarcoplasm.
In contrast, myofibers in skeletal muscles can be classified as MHC-1, MHC-2A, MHC-2X/2D, and MHC-2B based on differences in myosin heavy chain (MHC) isoforms using methods such as electrophoresis and immunohistological staining28, 29) and as types I, IIA, IIB, and IID (X), depending on the difference in myosin ATPase activity, using the myosin ATPase staining method30,31,32,33). Moreover, succinate dehydrogenase staining and nicotinamide tetrazolium reductase staining are known to classify myofibers as types I and II34, 35). These identification methods are based on differences in MHC isoforms or mitochondrial enzyme activity, and no other method that identifies the type of myofibers has previously been established. Our findings demonstrated that the immunohistological staining of cathepsin-D was a useful method to clearly identify and classify myofibers into types I and II.
Tissues with high turnover rates of protein metabolism contain high levels of cathepsin-D36), and the soleus muscle is known to contain high levels of myonucleus, ribosome, and total RNA compared with the extensor digitorum longus muscles37). Based on these facts, one of the reasons why cathepsin-D was noticeably seen in type I myofibers compared with type II myofibers is presumed to be that type I myofibers have high protein metabolic turnover. Therefore, the strong fluorescence intensity of cathepsin-D in non-apoptotic myofibers of the HULT and CON groups seem to represent comprehensive results of slow MHC and protein expressions involved in the synthesis of slow MHC. The cathepsin-D is presumed to play a significant role in slow MHC synthesis and degradation.
In addition to MHC isoforms and protein metabolic turnover, type I and II myofibers are known to have distinct features such as their mitochondrial number, cytochrome c content, and nicotinamide adenine dinucleotide activity38). Furthermore, myofibers with high oxidation capacity show higher cathepsin activity compared with myofibers with low oxidation capacity39). Hence, one of the reasons why cathepsin-D fluorescence intensity was higher in type I myofibers than in type II myofibers is that type I myofibers have high rates of energy metabolism.
In the present study, the composition ratio of type I myofibers decreased in the HULT group, and western blot analysis revealed a decrease in the inactive-form cathepsin-D in the HULT group. In addition, the percentage of type I myofibers showing an extremely strong immunoreaction for cathepsin-D were decreased in the HULT rats, whereas type II myofibers showing a weak immunoreaction for cathepsin-D were increased in these rats. In disuse muscle atrophy, the turnover of muscle protein metabolism40) and muscle energy metabolism41) is lower, and the decrease in the level of inactive cathepsin-D observed in the present study may be caused by a decrease in the number of type I myofibers, increase in the number of type II myofibers, and decrease in the turnover rate of muscle protein metabolism and muscle energy metabolism. In addition, the fiber-type composition during development is known to transform from a fast type to a slow type, whereas that of muscle atrophy shifts from a slow type to a fast type42,43,44). The reasons for this are assumed to be that the switch of MHC isoforms occurs within individual fibers and not by replacement with fibers of another fiber type45, 46) and that the individual myofibers of muscle atrophy degenerate the individual myofibers. Hence, the alteration of fiber-type composition in the present study is considered to have resulted from transformation from slow MHC to fast MHC.
Although the actual amount of apoptosis occurring has not been previously reported, some apoptotic myofibers have been observed in the muscle of untreated aged rats47). In contrast, apoptotic myofibers in normal muscles of the CON rats could not be found in our microscope observation. This result was mostly in agreement with other studies48); the appearances of apoptotic myonuclei have been observed extremely rarely in the normal muscle of young adult rats. Immunofluorescence staining showed overexpression of cathepsin-D in caspase-3-active myofibers and leakage of it into the cytoplasm in the HULT group. Western blot analysis demonstrated an increase in the active-form cathepsin-D. In staurosporine-induced apoptosis in fibroblasts, cathepsin-D mediates cytochrome c release from the mitochondria and caspase-3 activation, and cytochrome c release and activation of caspase-9 and caspase-3 are inhibited by pepstatin A, which is a cathepsin-D inhibitor49). In thallium-induced apoptosis of rat pheochromocytoma (PC12 cells), lysosome damage leads to the leakage of cathepsin-D into the cytoplasm, which triggers apoptosis via the degradation of Bid, a proapoptotic protein of the Bcl-2 family. This apoptotic event is known to be completely inhibited by pepstatin A, whereas E-64d, an inhibitor of cathepsin B, only partially inhibits apoptosis50). Moreover, in naphthazarin-induced apoptosis in cultured myocardial cells, cathepsin-D leakage from the lysosome activates caspase-351), suggesting that cathepsin-D leakage into the cytoplasm causes cytochrome c release from mitochondria, activates caspase-9 and caspase-3, and further triggers apoptosis. The expression of apoptosis in the present study is therefore considered to be caused by caspase-3 activation due to cathepsin-D leakage.
In the present study, muscle lysate analysis detected a decrease in the inactive forms of cathepsin-D in the HULT group together with an increase in the active form. However, immunofluorescence staining showed overexpression of either the inactive or active form or both forms of cathepsin-D in apoptotic myofibers, which further leaked into the cytoplasm. In another study, when Y1-Ad12 cancer cells overexpressing the inactive form of cathepsin-D were treated with etoposide, a topoisomerase II inhibitor, cathepsin-D leaked into the cytoplasm, which was followed by the leakage of cytochrome c from the mitochondria and the activation of caspase-9 and caspase-3, and this resulted in an increase in the number of apoptotic cells52). When the mature form or inactive form of cathepsin-D was microinjected into the cytoplasm of fibroblasts or HeLa cells, both the mature and inactive forms induced apoptosis53). These results thus suggest that the expression of apoptosis is independent of the activation status of cathepsin-D and that the leakage of cathepsin-D into the cytoplasm is a key event. The expression mechanism of apoptosis in the present study is also considered to involve cathepsin-D leakage as a major event.
Lastly, the presence of cathepsin-D in the endoplasmic reticulum, endosome, or lysosome can be indicative of myofiber type and further suggests that cathepsin-D is involved in the mechanism governing the differentiation of myofiber types. In addition, apoptosis occurred in the soleus muscles that underwent hindlimb unloading in a low temperature environment in this study and cathepsin-D leakage appeared to play a key role in the mechanism of this apoptotic event.
REFERENCES
- 1.Diment S, Leech MS, Stahl PD: Cathepsin D is membrane-associated in macrophage endosomes. J Biol Chem, 1988, 263: 6901–6907. [PubMed] [Google Scholar]
- 2.Erickson AH, Conner GE, Blobel G: Biosynthesis of a lysosomal enzyme. Partial structure of two transient and functionally distinct NH2-terminal sequences in cathepsin D. J Biol Chem, 1981, 256: 11224–11231. [PubMed] [Google Scholar]
- 3.Hasilik A, Neufeld EF: Biosynthesis of lysosomal enzymes in fibroblasts. Phosphorylation of mannose residues. J Biol Chem, 1980, 255: 4946–4950. [PubMed] [Google Scholar]
- 4.Metcalf P, Fusek M: Two crystal structures for cathepsin D: the lysosomal targeting signal and active site. EMBO J, 1993, 12: 1293–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim K, Homma Y, Ikeuchi Y, et al. : Cleavage of connectin by calpain and cathepsin D. Biosci Biotechnol Biochem, 1995, 59: 896–899. [DOI] [PubMed] [Google Scholar]
- 6.Matsukura U, Matsumoto T, Tashiro Y, et al. : Morphological changes in myofibrils and glycerinated muscle fibers on treatment with cathepsins D and L. Int J Biochem, 1984, 16: 957–962. [DOI] [PubMed] [Google Scholar]
- 7.Jones TL, Ogunro EA, Samarel AM, et al. : Susceptibilities of cardiac myofibrillar proteins to cathepsin D-catalyzed degradation. Am J Physiol, 1983, 245: H294–H299. [DOI] [PubMed] [Google Scholar]
- 8.Matsumoto T, Okitani A, Kitamura Y, et al. : Mode of degradation of myofibrillar proteins by rabbit muscle cathepsin D. Biochim Biophys Acta, 1983, 755: 76–80. [DOI] [PubMed] [Google Scholar]
- 9.Saftig P, Hetman M, Schmahl W, et al. : Mice deficient for the lysosomal proteinase cathepsin D exhibit progressive atrophy of the intestinal mucosa and profound destruction of lymphoid cells. EMBO J, 1995, 14: 3599–3608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Appelqvist H, Johansson AC, Linderoth E, et al. : Lysosome-mediated apoptosis is associated with cathepsin D-specific processing of bid at Phe24, Trp48, and Phe183. Ann Clin Lab Sci, 2012, 42: 231–242. [PubMed] [Google Scholar]
- 11.Roberg K, Johansson U, Ollinger K: Lysosomal release of cathepsin D precedes relocation of cytochrome c and loss of mitochondrial transmembrane potential during apoptosis induced by oxidative stress. Free Radic Biol Med, 1999, 27: 1228–1237. [DOI] [PubMed] [Google Scholar]
- 12.Hah YS, Noh HS, Ha JH, et al. : Cathepsin D inhibits oxidative stress-induced cell death via activation of autophagy in cancer cells. Cancer Lett, 2012, 323: 208–214. [DOI] [PubMed] [Google Scholar]
- 13.Zhu S, Nagashima M, Khan MA, et al. : Lack of caspase-3 attenuates immobilization-induced muscle atrophy and loss of tension generation along with mitigation of apoptosis and inflammation. Muscle Nerve, 2013, 47: 711–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O’Leary MF, Vainshtein A, Carter HN, et al. : Denervation-induced mitochondrial dysfunction and autophagy in skeletal muscle of apoptosis-deficient animals. Am J Physiol Cell Physiol, 2012, 303: C447–C454. [DOI] [PubMed] [Google Scholar]
- 15.Nagano K, Suzaki E, Nagano Y, et al. : A low temperature environment delays the changes in muscle fiber type composition induced by unloading. Acta Histochem Cytochem, 2005, 38: 305–312. [Google Scholar]
- 16.Nagano K, Suzaki E, Nagano Y, et al. : The activation of apoptosis factor in hindlimb unloading-induced muscle atrophy under normal and low-temperature environmental conditions. Acta Histochem, 2008, 110: 505–518. [DOI] [PubMed] [Google Scholar]
- 17.Miyachi R, Yamazaki T: Effects of static interventions on disuse atrophy of the rat soleus muscle at different sites along its longitudinal axis. J Phys Ther Sci, 2015, 27: 2317–2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sakaguchi A, Ookawara T, Shimada T: Inhibitory effect of a combination of thermotherapy with exercise therapy on progression of muscle atrophy. J Phys Ther Sci, 2010, 22: 17–22. [Google Scholar]
- 19.Bradford MM: A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem, 1976, 72: 248–254. [DOI] [PubMed] [Google Scholar]
- 20.Nonaka K, Akiyama J, Tatsuta N, et al. : Carbon dioxide-rich water bathing increases myonuclear number and muscle fiber size in regenerating skeletal muscles. J Phys Ther Sci, 2012, 24: 1295–1298. [Google Scholar]
- 21.Ruxton GD: The unequal variance t-test is an underused alternative to Student’s t-test and the Mann-Whitney U test. Behav Ecol, 2006, 17: 688–690. [Google Scholar]
- 22.Welch BL: The significance of the difference between two means when the population variances are unequal. Biometrika, 1938, 29: 350–362. [Google Scholar]
- 23.Beaujouin M, Baghdiguian S, Glondu-Lassis M, et al. : Overexpression of both catalytically active and -inactive cathepsin D by cancer cells enhances apoptosis-dependent chemo-sensitivity. Oncogene, 2006, 25: 1967–1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Conner GE: Isolation of procathepsin D from mature cathepsin D by pepstatin affinity chromatography. Autocatalytic proteolysis of the zymogen form of the enzyme. Biochem J, 1989, 263: 601–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Uchiyama Y, Waguri S, Sato N, et al. : Cell and tissue distribution of lysosomal cysteine proteinases, cathepsins B, H, and L, and their biological roles. Acta Histochem Cytochem, 1994, 27: 287–308. [Google Scholar]
- 26.Jacobs BL, Goodman CA, Hornberger TA: The mechanical activation of mTOR signaling: an emerging role for late endosome/lysosomal targeting. J Muscle Res Cell Motil, 2014, 35: 11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vetvicka V, Vashishta A, Saraswat-Ohri S, et al. : Procathepsin D and cancer: from molecular biology to clinical applications. World J Clin Oncol, 2010, 1: 35–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hämäläinen N, Pette D: Patterns of myosin isoforms in mammalian skeletal muscle fibres. Microsc Res Tech, 1995, 30: 381–389. [DOI] [PubMed] [Google Scholar]
- 29.Kammoun M, Cassar-Malek I, Meunier B, et al. : A simplified immunohistochemical classification of skeletal muscle fibres in mouse. Eur J Histochem, 2014, 58: 2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lind A, Kernell D: Myofibrillar ATPase histochemistry of rat skeletal muscles: a “two-dimensional” quantitative approach. J Histochem Cytochem, 1991, 39: 589–597. [DOI] [PubMed] [Google Scholar]
- 31.Okumura Y, Kanazashi M, Kanazawa Y, et al. : Differential effects of astaxanthin on oxidative key enzyme and capillarization in the deep and superficial layers of unloading-induced atrophied muscle. J Phys Ther Sci, 2013, 25: 349–353. [Google Scholar]
- 32.Tanaka S, Tachino K, Kawahara E, et al. : Hepatocyte growth factor in mouse soleus muscle increases with reloading after unloading. J Phys Ther Sci, 2006, 18: 33–41. [Google Scholar]
- 33.Yokogawa M, Yamazaki T, Inoue K, et al. : Age-associated changes in atrophy of the extensor digitorum longus muscle in hindlimb-suspended rats. J Phys Ther Sci, 2008, 20: 129–133. [Google Scholar]
- 34.Schiaffino S, Reggiani C: Fiber types in mammalian skeletal muscles. Physiol Rev, 2011, 91: 1447–1531. [DOI] [PubMed] [Google Scholar]
- 35.Wank V, Bauer R, Punkt K, et al. : Enzyme activity patterns of myosin ATPase, alpha-glycerophosphate dehydrogenase and succinate dehydrogenase within different muscle fibre types. Acta Histochem, 1994, 96: 213–218. [DOI] [PubMed] [Google Scholar]
- 36.Bechet D, Tassa A, Taillandier D, et al. : Lysosomal proteolysis in skeletal muscle. Int J Biochem Cell Biol, 2005, 37: 2098–2114. [DOI] [PubMed] [Google Scholar]
- 37.Habets PE, Franco D, Ruijter JM, et al. : RNA content differs in slow and fast muscle fibers: implications for interpretation of changes in muscle gene expression. J Histochem Cytochem, 1999, 47: 995–1004. [DOI] [PubMed] [Google Scholar]
- 38.Picard M, Hepple RT, Burelle Y: Mitochondrial functional specialization in glycolytic and oxidative muscle fibers: tailoring the organelle for optimal function. Am J Physiol Cell Physiol, 2012, 302: C629–C641. [DOI] [PubMed] [Google Scholar]
- 39.Kominami E, Tsukahara T, Bando Y, et al. : Distribution of cathepsins B and H in rat tissues and peripheral blood cells. J Biochem, 1985, 98: 87–93. [DOI] [PubMed] [Google Scholar]
- 40.Goldspink DF: The influence of immobilization and stretch on protein turnover of rat skeletal muscle. J Physiol, 1977, 264: 267–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yoshida N, Ikata T, Sairyo K, et al. : Evaluation of disuse atrophy of rat skeletal muscle based on muscle energy metabolism assessed by 31P-MRS. J Physiol Anthropol Appl Human Sci, 2001, 20: 247–252. [DOI] [PubMed] [Google Scholar]
- 42.Chopard A, Leclerc L, Pons F, et al. : Effects of 14-day spaceflight on myosin heavy chain expression in biceps and triceps muscles of the rhesus monkey. J Gravit Physiol, 2000, 7: S47–S49. [PubMed] [Google Scholar]
- 43.Delp MD, Pette D: Morphological changes during fiber type transitions in low-frequency-stimulated rat fast-twitch muscle. Cell Tissue Res, 1994, 277: 363–371. [DOI] [PubMed] [Google Scholar]
- 44.Falempin M, Mounier Y: Muscle atrophy associated with microgravity in rat: basic data for countermeasures. Acta Astronaut, 1998, 42: 489–502. [DOI] [PubMed] [Google Scholar]
- 45.Ashley Z, Sutherland H, Lanmüller H, et al. : Atrophy, but not necrosis, in rabbit skeletal muscle denervated for periods up to one year. Am J Physiol Cell Physiol, 2007, 292: C440–C451. [DOI] [PubMed] [Google Scholar]
- 46.Michel RN, Parry DJ, Dunn SE: Regulation of myosin heavy chain expression in adult rat hindlimb muscles during short-term paralysis: comparison of denervation and tetrodotoxin-induced neural inactivation. FEBS Lett, 1996, 391: 39–44. [DOI] [PubMed] [Google Scholar]
- 47.Alway SE, Bennett BT, Wilson JC, et al. : Epigallocatechin-3-gallate improves plantaris muscle recovery after disuse in aged rats. Exp Gerontol, 2014, 50: 82–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bruusgaard JC, Egner IM, Larsen TK, et al. : No change in myonuclear number during muscle unloading and reloading. J Appl Physiol 1985, 2012, 113: 290–296. [DOI] [PubMed] [Google Scholar]
- 49.Johansson AC, Steen H, Ollinger K, et al. : Cathepsin D mediates cytochrome c release and caspase activation in human fibroblast apoptosis induced by staurosporine. Cell Death Differ, 2003, 10: 1253–1259. [DOI] [PubMed] [Google Scholar]
- 50.Hanzel CE, Almeira Gubiani MF, Verstraeten SV: Endosomes and lysosomes are involved in early steps of Tl(III)-mediated apoptosis in rat pheochromocytoma (PC12) cells. Arch Toxicol, 2012, 86: 1667–1680. [DOI] [PubMed] [Google Scholar]
- 51.Ollinger K: Inhibition of cathepsin D prevents free-radical-induced apoptosis in rat cardiomyocytes. Arch Biochem Biophys, 2000, 373: 346–351. [DOI] [PubMed] [Google Scholar]
- 52.Beaujouin M, Liaudet-Coopman E: Cathepsin D overexpressed by cancer cells can enhance apoptosis-dependent chemo-sensitivity independently of its catalytic activity. Adv Exp Med Biol, 2008, 617: 453–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schestkowa O, Geisel D, Jacob R, et al. : The catalytically inactive precursor of cathepsin D induces apoptosis in human fibroblasts and HeLa cells. J Cell Biochem, 2007, 101: 1558–1566. [DOI] [PubMed] [Google Scholar]


