Abstract
[Purpose] A butterfly vertebra is a rare congenital anomaly resulting from a symmetric fusion defect. Only a few cases of butterfly vertebra have been described. This anomaly may be isolated or associated with Pfeiffer, Jarcho-Levins, Crouzon, or Alagille syndrome. [Subject and Methods] We herein describe a 38-year-old man who presented with neck and low back pain and was found to have butterfly vertebrae at the T9 and L3 levels. He also had Behçet’s disease and psoriasis. [Results] The patient’s symptoms improved with analgesics and physiotherapy. [Conclusion] To our knowledge, butterfly vertebrae at two levels have never been reported. Butterfly vertebrae may be confused with vertebral fractures in lateral radiographs, and awareness of this anomaly is important for a correct diagnosis.
Key words: Butterfly vertebra, Spine, Anomaly
INTRODUCTION
A butterfly vertebra (BV) is a rare congenital anomaly resulting from a symmetric fusion defect. Also known a sagittal cleft vertebra, this anomaly is usually asymptomatic and detected incidentally. A BV may be isolated or associated with other spinal anomalies such as kyphoscoliosis, hemivertebrae, or spina bifida1); other congenital syndromes2,3,4,5); or chromosomal defects6). This anomaly was first described in 1844 by Rokitansky, who examined the 12th thoracic vertebra of a 55-year-old man7). Since then, only a few cases have been reported in the English-language literature. We herein present a case of BVs at two levels in a 38-year-old man who was also diagnosed with Behçet’s disease and psoriasis.
SUBJECT AND METHODS
The Ethics Committee of Bezmialem Vakif University approved this case study, and informed consent was obtained from the patient.
A 38-year-old man presented with a 4-month history of neck and low back pain. The pain did not radiate to the arms or legs. His symptoms were aggravated by standing and walking for long periods. He did not have morning stiffness. There was no history of trauma. He had had psoriasis since early childhood and Behçet’s disease for 15 years; his symptoms were controlled with colchicine at 1.5 mg/day.
On physical examination, he was 158 cm tall with a short neck and mild kyphosis of the thoracic spine. The range of motion of the cervical and lumbar spine was normal, but painful. The straight-leg-raise test was negative. The motor and sensory examination findings and deep tendon reflexes were normal. An anteroposterior X-ray revealed split vertebrae at T8 and L3 with two lateral halves, suggesting BVs. Lateral X-rays showed anterior wedging of the T8 and L3 vertebrae, kyphosis at the T8 level, and loss of lumbar lordosis (Fig. 1). Computed tomography (CT) of the thoracolumbar region revealed two hemivertebrae, each with a complete central cleft, at T8 and L3 and complementary vertebral shapes at the adjacent vertebrae (Fig. 2). Cranial and spinal magnetic resonance imaging (MRI) was also performed to identify any associated anomalies. Cranial MRI was normal, but C4–5 central protrusion was observed on cervical MRI. Thoracolumbosacral MRI confirmed incarcerated T8 and L3 BVs and showed bulging at the L4–5 and L5–S1 levels (Fig. 3). Examination of other systems and hematological evaluation findings were normal.
Fig. 1.
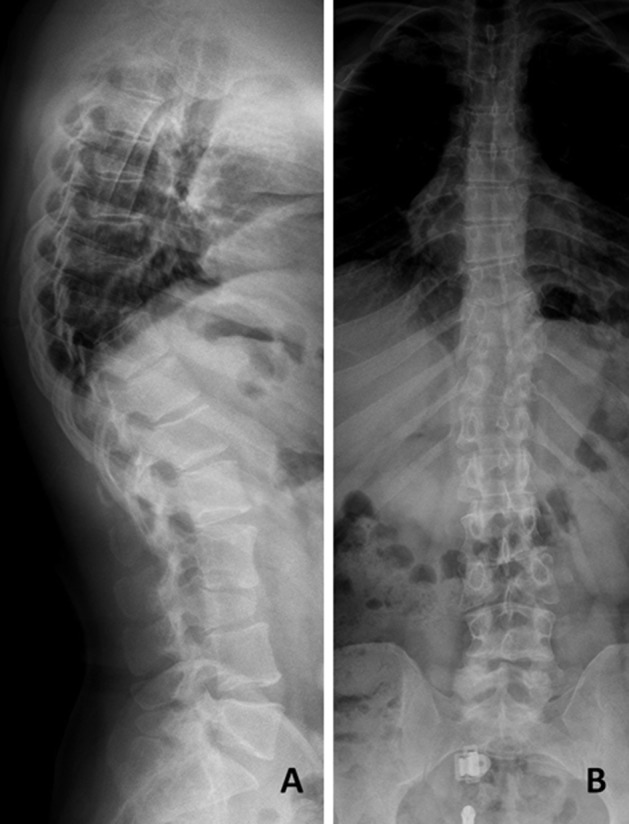
A: Lateral radiograph of the spine showing anterior wedging at the L3 and T9 vertebrae. B: Anteroposterior radiograph of the lumbosacral spine showing the BV at L3
Fig. 2.
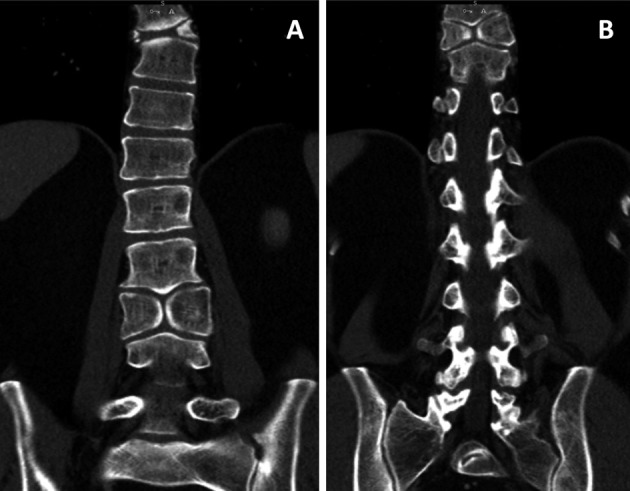
Coronal CT showing the BVs at L3 (A) and T9 (B) and the complementary shapes of the adjacent vertebrae
Fig. 3.
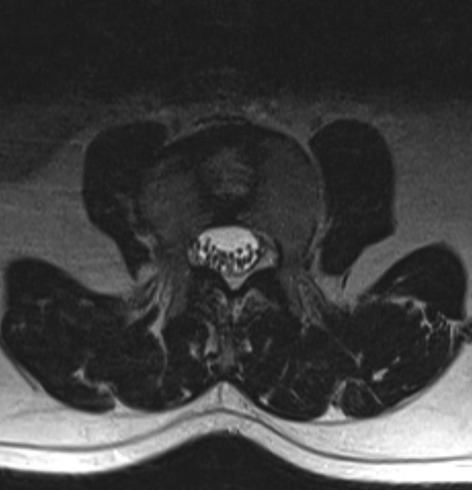
Axial T1-weighted MRI showing the sagittal cleft at the L3 vertebra
RESULTS
The patient’s symptoms improved with analgesics and physiotherapy. He was informed that the abnormal changes in the thoracic and lumbar spine were benign conditions and that there was no need for further treatment.
DISCUSSION
Although congenital vertebral anomalies are common (global prevalence of 0.5–1/1,000 live births), a BV anomaly presenting as a sagittal cleft vertebra is rare8). This defect is thought to occur at 3 to 6 weeks of gestation. A sagittal cleft is caused by persistence of the notochord7). Developing vertebral bodies have two chondrification centers that would normally fuse. If one fails to develop, a hemivertebra occurs. If both centers fail to fuse at the midline, a BV results9). Other organ systems could be affected during this period. A BV may be associated with complex congenital syndromes, such as Pfeiffer, Jarcho-Levins, Crouzon, or Alagille syndrome, and may also be associated with chromosomal defects such as a 22q11 deletion2,3,4,5,6). In our case, clinical examination of other systems revealed no abnormalities or signs of the abovementioned syndromes; therefore, a chromosomal analysis was not performed.
Diastematomyelia, kyphoscoliosis, bars, supernumerary lumbar vertebrae, and spina bifida can each be associated with BV1). In our case, spinal MRI was performed to identify other possible vertebral anomalies. BVs were found at two levels, resulting in kyphosis at the T8 level and a loss of lumbar lordosis at the L3 level. To our knowledge, BVs at two levels have never been reported.
Compensation for the anomalous vertebra by the adjacent cranial and caudal vertebrae is called incarceration. While a non-incarcerated hemivertebra or BV may impair the spinal curve and cause a progressive spinal deformity, an incarcerated anomalous vertebra usually does not cause progressive spinal deformity10).
In lateral radiographs, a BV may be confused with a compression fracture due to trapezoidal or cuneiform anterior wedging. However, a fractured bone with osteoporotic compression results in irregular collapse of the vertebral body, and the upper and lower margins are not involved to similar extents. Pathological vertebral fractures secondary to a neoplasm or infection can lead to extensive bony destruction and disc space narrowing11). MRI reveals altered signal intensity of bone and soft tissue components, which can result from trauma or pathological conditions such as infections and neoplasms. A BV is easily detected in anteroposterior radiographs because the split vertebra has two halves that are not displaced laterally, and they are usually of the same size, resembling the wings of a butterfly.
Delgado et al.12) and Sonel et al.9) described an L3 BV with lumbar disc protrusion at the L4–5 level. Cho et al.13) described symptomatic disc herniation arising from the sagittal cleft of the L4 vertebra and confirmed the existence of nucleus pulposus in the sagittal cleft. Similarly, our patient had an L3 BV and bulging at the L4–5 and L5–S1 levels. A BV is considered asymptomatic, but the spinal biomechanics may be altered, increasing the incidence of disc herniation and chronic low back pain. In patients with chronic low back pain, trunk and lumbar stabilization exercises improve the activities of daily living and quality of life14, 15). Our patient also presented with low back pain, and his symptoms resolved with appropriate medical and physical therapy. Given the rarity of this anomaly, biomechanical and exercise studies on a large series of patients would be extremely difficult.
The literature includes two cases of BV associated with ankylosing spondylitis16). To our knowledge, no association of this anomaly with rheumatic diseases other than ankylosing spondylitis has been described. Our patient also had Behçet’s disease and psoriasis. We believe that these diseases occurred coincidentally.
Despite the rarity of BV, awareness of this congenital anomaly is important because it can be confused with vertebral fractures. Informing the patient about this anomaly may avoid further invasive and noninvasive procedures.
REFERENCES
- 1.Sepulveda W, Kyle PM, Hassan J, et al. : Prenatal diagnosis of diastematomyelia: case reports and review of the literature. Prenat Diagn, 1997, 17: 161–165. [PubMed] [Google Scholar]
- 2.Anderson PJ, Hall CM, Evans RD, et al. : Cervical spine in Pfeiffer’s syndrome. J Craniofac Surg, 1996, 7: 275–279. [DOI] [PubMed] [Google Scholar]
- 3.Lawson ME, Share J, Benacerraf B, et al. : Jarcho-Levin syndrome: prenatal diagnosis, perinatal care, and follow-up of siblings. J Perinatol, 1997, 17: 407–409. [PubMed] [Google Scholar]
- 4.Anderson PJ, Hall C, Evans RD, et al. : The cervical spine in Crouzon syndrome. Spine, 1997, 22: 402–405. [DOI] [PubMed] [Google Scholar]
- 5.Alagille D, Estrada A, Hadchouel M, et al. : Syndromic paucity of interlobular bile ducts (Alagille syndrome or arteriohepatic dysplasia): review of 80 cases. J Pediatr, 1987, 110: 195–200. [DOI] [PubMed] [Google Scholar]
- 6.Ming JE, McDonald-McGinn DM, Megerian TE, et al. : Skeletal anomalies and deformities in patients with deletions of 22q11. Am J Med Genet, 1997, 72: 210–215. [DOI] [PubMed] [Google Scholar]
- 7.Müller F, O’Rahilly R, Benson DR: The early origin of vertebral anomalies, as illustrated by a ‘butterfly vertebra’. J Anat, 1986, 149: 157–169. [PMC free article] [PubMed] [Google Scholar]
- 8.Zuo K, Asenjo JF, Colmegna I: Butterfly vertebra. Arthritis Rheum, 2013, 65: 196. [DOI] [PubMed] [Google Scholar]
- 9.Sonel B, Yalçin P, Oztürk EA, et al. : Butterfly vertebra: a case report. Clin Imaging, 2001, 25: 206–208. [DOI] [PubMed] [Google Scholar]
- 10.Kaplan KM, Spivak JM, Bendo JA: Embryology of the spine and associated congenital abnormalities. Spine J, 2005, 5: 564–576. [DOI] [PubMed] [Google Scholar]
- 11.Resnick D, Niwayama G: Osteoporosis. Bone and Joint Imaging. Philadelphia: Saunders, 1992, pp 569–588. [Google Scholar]
- 12.Delgado A, Mokri B, Miller GM: Butterfly vertebra. 1996, 6: 56–58. [DOI] [PubMed] [Google Scholar]
- 13.Cho HL, Kim JS, Paeng SS, et al. : Butterfly vertebra with lumbar intervertebral disc herniation. J Neurosurg Spine, 2011, 15: 567–570. [DOI] [PubMed] [Google Scholar]
- 14.Hwangbo G, Lee CW, Kim SG, et al. : The effects of trunk stability exercise and a combined exercise program on pain, flexibility, and static balance in chronic low back pain patients. J Phys Ther Sci, 2015, 27: 1153–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ota M, Kaneoka K, Hangai M, et al. : Effectiveness of lumbar stabilization exercises for reducing chronic low back pain and improving quality-of-life. J Phys Ther Sci, 2011, 23: 679–681. [Google Scholar]
- 16.Qian BP, Qiu Y, Wang B, et al. : Unusual association of ankylosing spondylitis with congenital spinal deformity. Spine, 2010, 35: E1512–E1515. [DOI] [PubMed] [Google Scholar]


