Abstract
Interstitial lung disease (ILD) is rare in infancy or early childhood. Differentiating between the different types of ILD is important for reasons of treatment, monitoring of clinical course and prognosis. We present a case of a 5-month old female with tachypnea and hypoxemia. The clinical suspicion of neuroendocrine cell hyperplasia of infancy (NEHI) was confirmed by high-resolution chest CT and subsequent lung biopsy. We conclude that high-resolution chest CT has characteristics findings that can be used as a non-invasive test to support the clinical diagnosis of neuroendocrine cell hyperplasia of infancy.
Keywords: Interstitial lung disease, Neuroendocrine cell hyperplasia of infancy, High-resolution chest CT
1. Case report
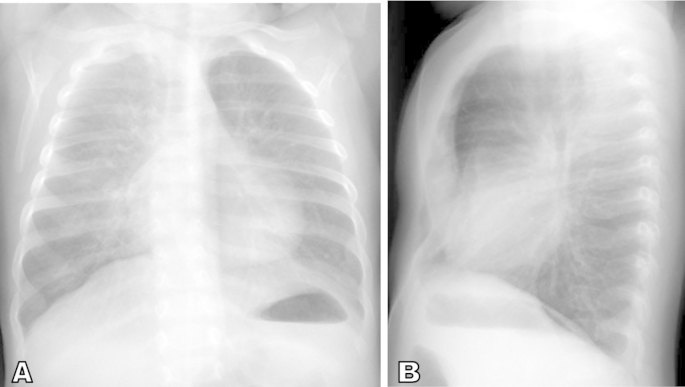
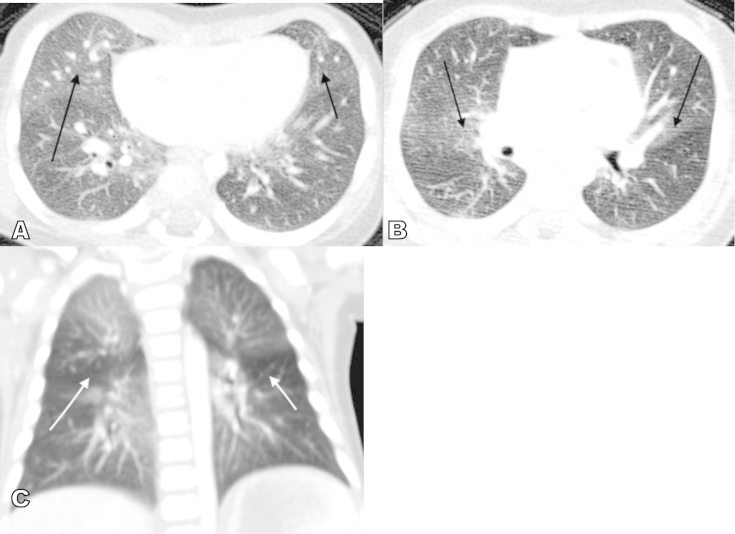
Our patient is a 5-month old female with a history of hypoxemia and poor weight gain. At three months of age, she presented with respiratory tract infection and was noted to have oxygen saturations in the high 80s to low 90 s at room air. Chest x-ray done at that time suggested pneumonia and so the pediatrician prescribed a course of Amoxicillin but the patients respiratory symptoms persisted. The patient was then referred to pediatric pulmonary clinic at five months of age and was noted to have de-saturation on room air that resolved with supplemental oxygen therapy. Chest radiograph done at the initial pulmonology clinic visit showed hyper-inflated lungs with diffuse interstitial thickening initially attributed to small airways disease, possibly secondary to viral bronchiolitis or asthma (Fig. 1). However, lab work-up for allergy and infectious etiology were negative. She was subsequently admitted and high-resolution CT of the chest was done to evaluate the possibility of an interstitial lung disease. The chest CT showed scattered areas of ground glass opacification and mosaic attenuation likely from air trapping (Fig. 2). These findings raised the possibility of Neuroendocrine cell hyperplasia of infancy. A lung biopsy was performed to confirm the diagnosis.
Fig. 1.
AP and lateral chest radiographs. Non-specific perihilar interstitial thickening and pulmonary hyperaeration suggestive of infectious or reactive small airways disease. Incidental finding of pectus excavatum.
Fig. 2.
High-resolution chest CT. A. Axial image shows patchy ground glass opacification involving the right middle lobe (long black arrow) and lingula (short black arrow). B. Ground glass opacities are likewise predominantly central in location (arrows). C. Coronal image shows areas of air-trapping (white arrows).
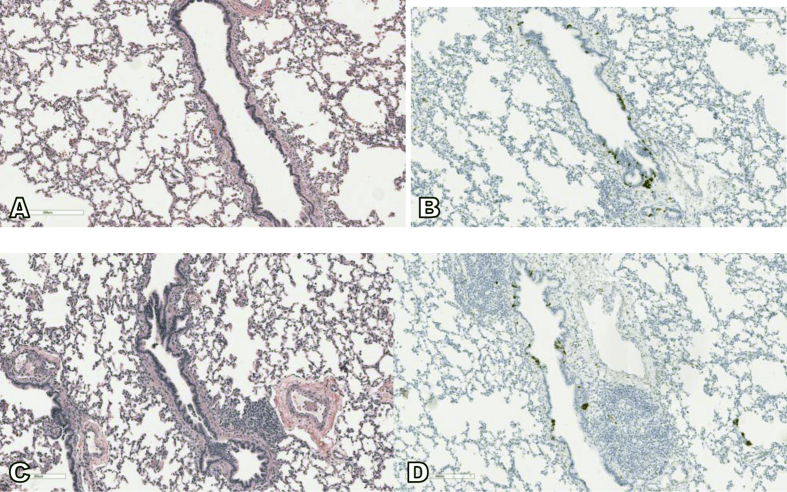
Sections of surgical biopsies from right middle and lower lobes demonstrate generally similar histologic features. Both are composed of large wedge biopsies of lung parenchyma with normal alveolar architecture. The prominent histological findings are reactive lymphoid hyperplasia occasionally with germinal centers associated with small and large bronchioles. The pulmonary arteries and veins show no significant pathologic abnormalities. The immunohistochemical staining for synaptophysin demonstrates a mild and diffuse increase in neuroendocrine cells (NECs), including NECs in >70% of bronchioles and up to 16% NECs within one individual airway (40 NECs/250 total bronchial epithelial cells). The clusters of NECs and occasional large neuroendocrine bodies are noted in both normal bronchioles and bronchioles with reactive lymphoid hyperplasia (Fig. 3).
Fig. 3.
The wedge biopsy of right middle lobe of lung shows generally normal alveolar architecture and bronchioles (A) with reactive lymphoid hyperplasia (C). The immunohistochemistry for synaptophysin highlights multiple small clusters of neuroepithelial cells within both normal bronchioles and bronchioles with reactive lymphoid hyperplasia (B and D).
2. Discussion
Interstitial lung disease (ILD) is rare with a reported prevalence rate of 0.4 cases/100,000 children [1]. It tends to present with rapid shallow breathing with retractions, persistent oxygen requirement and failure to thrive without any other obvious medical illness. Differentiating between the different types of ILD is important for reasons of treatment, monitoring of clinical course and prognosis. The new classification system of interstitial lung disease in infants include 1) diffuse developmental disorders (acinar and alveolar dysplasia), 2) alveolar growth abnormalities, 3) surfactant dysfunction disorders and 4) specific conditions of unknown or poorly understood pathology. The fourth subtype includes neuroendocrine cell hyperplasia and pulmonary interstitial glycogenosis [2].
Neuroendocrine cell hyperplasia of infancy (NEHI) is a form of ILD involving the small airways. Infants affected tend to present with persistent cough, tachypnea, dyspnea and hypoxemia commonly after a respiratory infection [3]. Poor growth requiring nutritional supplements and gastro-esophageal reflux are also common [4]. The mean age when symptoms occur is about 4 months [5].
Clinical features associated with neuroendocrine cell hyperplasia include tachypnea and hypoxemia. Our patient presented at 3 months of age with tachypnea requiring prolonged oxygen supplementation but has since improved symptomatically. This is consistent with several case series where patients have persistent symptoms needing oxygen therapy with most patients slowly improving over time [2], [5]. The etiology of neuroendocrine cell hyperplasia of infancy is unknown and corticosteroids are ineffective in treating the disease. However, the prognosis is excellent and there has been no reported mortality for this condition.
Diagnosis is made based on clinical, imaging and histologic examinations. Pulmonary function test typically shows air trapping and airflow obstruction [4]. Radiologic findings consist of hyperinflation with variable increased peri-hilar opacity on chest radiograph. Although the definitive diagnosis of NEHI relies on the histologic finding of increased number of bombesin immune-positive neuroendocrine cells in bronchioles [6], high-resolution chest CT appears to be the most reliable non-invasive imaging that can suggest the diagnosis of neuroendocrine cell of hyperplasia. The findings of multi-lobar ground-glass opacity predominantly involving the right middle lobe and lingula as well as mosaic pattern of air-trapping are characteristic findings on CT with a reported sensitivity of 83% and specificity of 100% [7]. More importantly, chest CT can differentiate NEHI from other types of interstitial lung disease.
3. Conclusion
High-resolution chest CT has characteristics findings that can be used as a non-invasive test to support the clinical diagnosis of neuroendocrine cell hyperplasia of infancy. This could potentially obviate the need for invasive procedures such as bronchoscopy and lung biopsy.
References
- 1.Griese M., Haug M., Brasch F. Incidence and classification of pediatric diffuse parenchymal lung disease in Germany. Orphanet J. Rare Dis. 2009;4:26. doi: 10.1186/1750-1172-4-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yee E.Y. Interstitial lung disease in infants: new classification system, imaging techniques, clinical presentation and imaging findings. Pediatr. Radiol. 2013;43:3–13. doi: 10.1007/s00247-012-2524-x. [DOI] [PubMed] [Google Scholar]
- 3.Gomes V.C., Silva M.C., Maia Filho J.H. Diagnostic criteria and follow-up in neuroendocrine cell hyperplasia of infancy: a case series. J. Bras. Pneumol. 2013;39:569–578. doi: 10.1590/S1806-37132013000500007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Deterding R.R. Infants and young children with children's interstitial lung. Pediatr. Allergy Immunol. Pulmonol. 2010;23:25–31. doi: 10.1089/ped.2010.0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Glasser S.W., Hardie W.D., Hagood J.S. Pathogenesis of interstitial lung disease in children and adults. Pediatr. Allergy Immunol. Pulmonol. 2010;23:9–14. doi: 10.1089/ped.2010.0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deterding R.R.1, Pye C., Fan L.L., Langston C. Persistent tachypnea of infancy is associated with neuroendocrine cell hyperplasia. Pediatr. Pulmonol. 2005;40:157–165. doi: 10.1002/ppul.20243. [DOI] [PubMed] [Google Scholar]
- 7.Brody A.S.1, Guillerman R.P., Hay T.C. Neuroendocrine cell hyperplasia of infancy: diagnosis with high-resolution CT. AJR Am. J. Roentgenol. 2010;194:238–244. doi: 10.2214/AJR.09.3385. [DOI] [PMC free article] [PubMed] [Google Scholar]