Abstract
Pulmonary alveolar microlithiasis is a disorder in which many tiny fragments (microliths) of calcium phosphate gradually accumulate in alveoli. Loss of function mutations in the gene SLC34A2 coding for the sodium phosphate co-transporter (NaPi-IIb) are responsible for genetic forms of alveolar microlithiasis.
We now report a consanguineous Italian family from Calabria with two affected members segregating alveolar microlithiasis in a recessive fashion. We describe, for the first time, a novel loss of function mutation in the gene coding for NaPi-IIb. A careful description of the clinical phenotype is provided together with technical details for direct sequencing of the gene.
Keywords: SLC34A2, NaPi-IIb, Pulmonary alveolar microlithiasis
Abbreviations: OMIM, Online Mendelian inheritance in man; AD, Autosomic dominant; AR, Autosomic recessive; CH, Compound heterozygous; Hsa, Homo sapiens; PAM, Pulmonar alveolar microlithiasis; PAS, Periodic acid-Schiff
1. Introduction
Two protein families, SLC20 and SLC34, act as Na+-dependent, secondary-active co-transporters to transport Pi across cell membranes [1].
The SLC20 family has two members, SLC20A1 (also known as PiT-1, OMIM *137570) and SLC20A2 (also known as PiT-2 OMIM: *158378, #213600 AD). Both proteins are ubiquitous and considered as “housekeeping” transport protein. PiT-1 and PiT-2 are electrogenic [2]. The importance of these channels is highlighted by their tissue-specific activity, regulatory pathways, and by the pathology associated with genetic variants [3]. The SLC34 family has three members: SLC34A1, coding for NaPi-IIa, OMIM: (*182309, #613388 AR #612286 AD), SLC34A2, coding for NaPi-IIb, OMIM: (*624217, #265100 AR), and SLC34A3, coding for NaPi-IIc OMIM: (*609826, #241530 AD/CH). These transporters mediate the translocation of one divalent inorganic phosphate (HPO4 (2-)) together with two (NaPi-IIc) or three sodium ions (NaPi-IIa and NaPi-IIb), respectively. Consequently, phosphate transport by NaPi-IIa and NaPi-IIb is electrogenic whereas NaPi-IIc is electroneutral. NaPi-IIa and NaPi-IIc are expressed mostly on the brush border membrane of the proximal tubule [4]. Homozygous mutations in the gene SLC34A2 coding for NaPi-IIb are responsible for pulmonary alveolar microlithiasis, a rare disease characterized by the deposition of calcium phosphate microliths throughout the lungs.
2. Case report
Here we describe the case of a 58 years old lady reported for genetic counseling by the unit of pneumology of our university hospital.
The lady was totally asymptomatic at the time of evaluation.
Clinical examination revealed a regular facies. During auscultation of the lungs fine expiratory crackles were heard on the whole thorax and especially in the left basal part. Cardiac auscultation was normal, and no cyanosis or peripheral edema was observed. There was no history of smoking or previously known pulmonary diseases. On routine blood examination, blood count and serum chemistry were found normal. Arterial blood gas analysis, ECG, and echocardiography showed no important abnormalities. Pulmonary function tests were also normal.
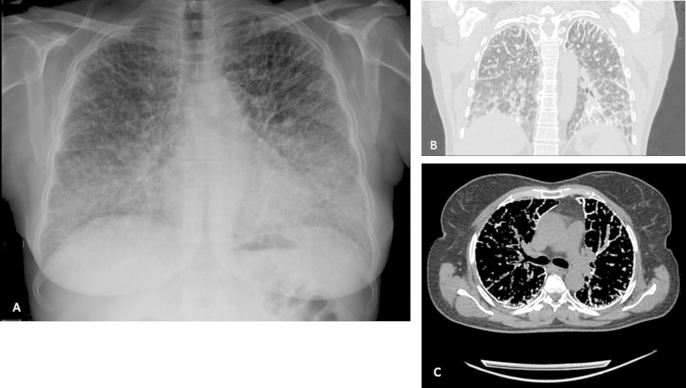
Radiologic findings are shown in Fig. 1.
Fig. 1.
(A) Chest radiograph: symmetric pattern of diffuse fine micronodules in both lungs. (B–C) HRCT images: diffuse symmetric pattern of microlithiasis, consisting of both discrete nodules and areas of ground glass attenuation of the lung parenchyma.
Posteroanterior chest radiographs (Fig. 1A) depicted diffuse, scattered, bilateral areas of micronodular pattern, producing a “sandstorm” appearance, first involving the inferior portions and then middle and upper portions of the lungs [5], [6].
Based on these findings, a high resolution computed tomography scan was performed (Fig. 1B–C) that revealed a diffuse symmetric pattern of microlithiasis, consisting of both discrete nodules and areas of ground glass attenuation of the lung parenchyma. Calcifications were also seen along the vessels, along with crazy paving pattern areas.Multiple sub-pleural cysts were seen along the costal and mediastinal pleura [7], [8].
The patient underwent a bronchoscopy with bronchoalveolar lavage, which produced numerous microliths in the bronchoalveolar lavage liquid, compatible with a diagnosis of pulmonary microlithiasis.
Ultrasound of abdomen showed moderate liver steatosis, a sepimented cyst approximately 2.5 × 2 cm on the lower third of the left kidney and a multinodular uterus.
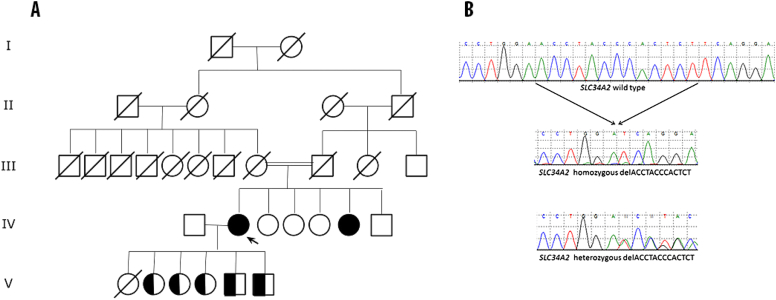
Analysis of the pedigree revealed that our patient was the sister of a woman affected by pulmonary alveolar microlithiasis (Fig. 2A).
Fig. 2.
(A) Family pedigree. The proband (IV-2) is indicated by an arrow. (B) DNA sequences of SLC34A2, exon 3 from the homozygous proband and the heterozygous son V-5, as indicated. The wild type sequence corresponds to NCBI NM_006424.
We designed an optimized high specific sequencing primer set (Table 1) for SLC34A2 gene coding exons using the NCBI platform, AmpliFX bioinformatic software [9] and Repeat Masker web app from the institute for Systems Biology [10].
Table 1.
Primers set for Hsa SLC34A2 coding exons.
| CCCAGTTGATGCTTTGCAACCA | SLC34A2 ex 2FOR |
| CCCAGTTGATGCTTTGCAACCA | SLC34A2 ex 2REV |
| TTGTCTGCAGATCGGCCTTTGT | SLC34A2 ex3 FOR |
| AGGACCCTTGCTTCAAGGGAATCA | SLC34A2 ex3 REV |
| GCCAAACTTCTCAGGGTTTCCA | SLC34A2 ex4 FOR |
| GAGAGGGCTTGCTGAGTTTTAC | SLC34A2 ex4 REV |
| TTTACTCAGTGCCCACCTAATCCC | SLC34A2 ex5 FOR |
| CAAGGCCTGGACATATTCAGAGTG | SLC34A2 ex5 REV |
| GCATAGGTAACTTTAGCCTGCCTC | SLC34A2 ex6 FOR |
| TAGCCATACCTCCCTGTGGTTACT | SLC34A2 ex6 REV |
| TCACATGGTGCCCACTTGCTTA | SLC34A2 ex7 FOR |
| TATATCTGTCAGCTCAGGTAGGGG | SLC34A2 ex7 REV |
| GGATATGCTGATGGTTTCCTGTCT | SLC34A2 ex8 FOR |
| CCTACTGTTTACCTGCATTTGGC | SLC34A2 ex8 REV |
| ATACTGCATGCACCATGGG | SLC34A2 ex9 FOR |
| CTGTCCTTTCCTTCACTTACTGGG | SLC34A2 ex9 REV |
| GGCTGAGAAGGGCTGTCTTGATTT | SLC34A2 ex10 FOR |
| CCACCCAGGATGCCAAGAACATTT | SLC34A2 ex10 REV |
| GATGTACAACCTCACCCCTAAGCC | SLC34A2 ex11 FOR |
| CTCAATGGTTATCACGCCGATTCC | SLC34A2 ex11 REV |
| CACTGCCATTTCCTGTCATCCCAT | SLC34A2 ex12 FOR |
| GTCCTGAAAACTTTACCGTGGCT | SLC34A2 ex12 REV |
| TGATGCCTGCTAGCTTACCT | SLC34A2 ex13.1 FOR |
| GGAAGTTCCAGTTCTGGAGT | SLC34A2 ex13.1 REV |
| TCATCCTGGTACTGTGCCT | SLC34A2 ex13.2 FOR |
| TCGCTAATGGGGAGCAAATC | SLC34A2 ex13.2 REV |
SLC34A2 gene exons were sequenced on an AbiPrism 310 platform. Our patient was homozygous for a novel frameshift deletion in exon 3 (c.212_224 delACCTACCCACTCT pAsn71IlefsX25, Ref seq. ENSG00000157765) that caused a frameshift resulting in a truncated protein at codon 95 (Fig. 2B).
All the sons and daughter of our proband patient were heterozygous for the maternal allele.
3. Results
In the present study we summarize the actual findings of a patient with familiar microlithiasis and describe a mutation in the SLC34A2 gene, coding for NaPi-IIb, (GeneID: 10568). The mutation, a frameshift mutation in the exon 3 (homozygote c.212_224 delACCTACCCACTCT (pAsn71IlefsX25)) that produces a truncated form of the protein, was never reported before in the literature. In this paper a new set of highly specific primers for coding exons of the gene is proposed.
In 2003 and 2004 two monumental reviews were published, detailing 424 cases [11] and 527 cases [5]. After those, mostly case reports were published. Two recent reviews were published by Tachibana et al. [12] and Ferreira et al. [13].
Pulmonary alveolar microlithiasis was found in the Hsa and in other animal species such as dogs, cats, monkeys, birds, fishes and anphibians [5]. PAM is a rare condition, counting about 700 cases in literature. Its geographic distribution is higher in Europe and in Asia, counting together 73% of all cases [11] or 83% [5]. The M:F ratio is 1:1. It can be diagnosed from stillbirth to the VII decade. About 53% of patients present no symptoms on diagnosis. Some cases survived even 44 years after first diagnosis. The familiar form represents around 32% [5] or 36% [11] of all cases.
NaPi-IIb is expressed mostly in type II pneumocytes, the cells that produce the surfactant. Phospholipides are a fundamental component of surfactant and when they degrade they lose Pi. Type II pneumocytes are involved in the reabsorption of exhaust phospholipides. If the ability to reabsorb Pi is altered in these cells, the amount of Pi in the surfactant will raise and then precipitates, thus forming the microlithes.
Microlithes will raise in volume and eventually come into contact with the alveolar walls, starting a fibrotic process.
The intra alveolar lamellar microlithes are PAS positive, formed by concentric lamellae of Ca++ around a granular or amorphous central core. They tend to have spherical or ovoid shape, a diameter between 0.01 and 2.8 mm; Pi:Ca ratio of 1:2, compatible with Calcium phosphate and/or hydroxylapatite [14], [15]. The features above describe stones formed in familial pulmonary alveolar microlithiasis, which are different from the ones caused by sporadic pulmonary alveolar microlithiasis [16] and from the ones caused by metastatic and dystrophic processes [13].
From a macroscopic point of view, the lungs of affected individuals have been found to weigh up to 5 Kgs in the more serious cases, showing reduced elasticity and higher resistance. External surfaces appear wrinkled, ruvid and irregular due to the numerous microlithes. Apical bullae and blebs can be found on the apexes and the forward portions of the lungs. Microlites were also found in the sympathetic ganglia and in the gonads. calcifications of pericardium, epididymis, seminal vesicles and prostate were also reported. In fact the expression of SLC20A2 has been demonstrated in other cells, including enterocytes. In these cells the membrane abundance of the channel is regulated by ubiquitin ligase Nedd4-2 and by serum- and glucocorticoid-dependent kinase 1 [17].
Under a clinical point of view a “clinical-radiological dissociation” has been described, where a very serious radiological situation is accompanied by a modest clinical condition. This condition generally has a slow evolution, and it has been diagnosed in individuals of different age. Serum levels of surfactant proteins A and D correlate with the progression of the disease, and may be a useful monitoring tool [18].
Corticosteroids are generally considered to be ineffective, as well as hydroxychloroquine and budesonide [19]. Long term etidronate treatment has been attempted in two patients and resulted in some radiological benefits [20]. Single or bilateral lung transplantation is an option.
Conflict of interests
All authors declare they have no conflict of interests.
Acknowledgments
We wish to thanks the patients and their families for their precious collaboration, and Dr. Joseph Toaff for writing assistance and manuscript editing.
Contributor Information
Rodolfo Iuliano, Email: iuliano@unicz.it.
Nicola Perrotti, Email: perrotti@unicz.it.
References
- 1.Forster I.C., Hernando N., Biber J. Phosphate transporters of the SLC20 and SLC34 families. Mol. Asp. Med. 2013;34(2–3):386–395. doi: 10.1016/j.mam.2012.07.007. [DOI] [PubMed] [Google Scholar]
- 2.Forster I.C., Hernando N., Biber J. Phosphate transport kinetics and structure-function relationships of SLC34 and SLC20 proteins. Curr. Top. Membr. 2012;70:313–356. doi: 10.1016/B978-0-12-394316-3.00010-7. [DOI] [PubMed] [Google Scholar]
- 3.Traiffort E., O'Regan S., Ruat M. The choline transporter-like family SLC44: properties and roles in human diseases. Mol. Aspects. Med. 2013;34(2–3):646–654. doi: 10.1016/j.mam.2012.10.011. [DOI] [PubMed] [Google Scholar]
- 4.Wagner C.A., Hernando N., Forster I.C. The SLC34 family of sodium-dependent phosphate transporters. Pflugers Arch. 2014;466(1):139–153. doi: 10.1007/s00424-013-1418-6. [DOI] [PubMed] [Google Scholar]
- 5.Mariotta S., Ricci A., Papale M. Pulmonary alveolar microlithiasis: report on 576 cases published in the literature. Sarcoidosis Vasc. Diffuse Lung Dis. 2004;21(3):173–181. [PubMed] [Google Scholar]
- 6.Walters G., Trotter S., McGrath E.E. Pulmonary Alveolar Microlithiasis. Ann. Thorac. Surg. 2013;96(2):702. doi: 10.1016/j.athoracsur.2012.12.052. [DOI] [PubMed] [Google Scholar]
- 7.Siddiqui N.A., Fuhrman C.R. Best cases from the AFIP: pulmonary alveolar microlithiasis. Radiographics. 2011;31(2):585–590. doi: 10.1148/rg.312105157. [DOI] [PubMed] [Google Scholar]
- 8.Abdalla G., Marchiori E., Zanetti G. Pulmonary alveolar microlithiasis: a case report with emphasis on imaging findings. Cas. Rep. Med. 2010;2010:819242. doi: 10.1155/2010/819242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.AmplifX 1.7.0 by Nicolas Jullien, CNRS, Aix-Marseille Université, http://crn2m.univ-mrs.fr/pub/amplifx-dist, Retrieved online, Apr 2015.
- 10.Smit AFA, Hubley R, GreenP. RepeatMasker at http://repeatmasker.org Retrieved online, Apr 2015.
- 11.Castellana G., Lamorgese V. Pulmonary alveolar microlithiasis. World cases and review of the literature. Respiration. 2003;70(5):549–555. doi: 10.1159/000074218. [DOI] [PubMed] [Google Scholar]
- 12.Tachibana T., Hagiwara K., Johkoh T. Pulmonary alveolar microlithiasis: review and management. Curr. Opin. Pulm. Med. 2009;15(5):486–490. doi: 10.1097/MCP.0b013e32832d03bb. [DOI] [PubMed] [Google Scholar]
- 13.Ferreira Francisco F.A., Pereira e Silva J.L., Hochhegger B. Pulmonary alveolar microlithiasis. State-of-the-art review. Respir. Med. 2013;107(1):1–9. doi: 10.1016/j.rmed.2012.10.014. [DOI] [PubMed] [Google Scholar]
- 14.Barnard N.J., Crocker P.R., Blainey A.D. Pulmonary alveolar microlithiasis. A new analytical approach. Histopathology. 1987;11(6):639–645. doi: 10.1111/j.1365-2559.1987.tb02674.x. [DOI] [PubMed] [Google Scholar]
- 15.Pracyk J.B., Simonson S.G., Young S.L. Composition of lung lavage in pulmonary alveolar microlithiasis. Respiration. 1996;63(4):254–260. doi: 10.1159/000196556. [DOI] [PubMed] [Google Scholar]
- 16.Yesner R. Pulmonary alveolar microlithiasis revisited. N. Engl. J. Med. 2003;348(1):84–85. doi: 10.1056/NEJM200301023480121. author reply 84–85. [DOI] [PubMed] [Google Scholar]
- 17.Palmada M., Dieter M., Speil A. Regulation of intestinal phosphate cotransporter NaPi IIb by ubiquitin ligase Nedd4-2 and by serum- and glucocorticoid-dependent kinase 1. Am. J. Physiol. Gastrointest. Liver Physiol. 2004;287(1) doi: 10.1152/ajpgi.00121.2003. G143-50. [DOI] [PubMed] [Google Scholar]
- 18.Takahashi H., Chiba H., Shiratori M. Elevated serum surfactant protein A and D in pulmonary alveolar microlithiasis. Respirology. 2006;11(3):330–333. doi: 10.1111/j.1440-1843.2006.00844.x. [DOI] [PubMed] [Google Scholar]
- 19.Moslehi M.A. Pulmonary alveolar microlithiasis. Lung India. 2013;30(4):375. doi: 10.4103/0970-2113.120616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ozcelik U., Yalcin E., Ariyurek M. Long-term results of disodium etidronate treatment in pulmonary alveolar microlithiasis. Pediatr. Pulmonol. 2010;45(5):514–517. doi: 10.1002/ppul.21209. [DOI] [PubMed] [Google Scholar]