Abstract
Benign metastasizing leiomyomatosis (BML) is a rare cause of pulmonary lesions found in reproductive age women who have undergone a hysterectomy for uterine leiomyoma. Given the relative rarity of the disease, the management of these lesions varies from surgical (oopherectomy) or medical antiestrogen hormonal therapy to clinical observation and survelliance. The disease generally presents asymptomatically with multiple, well-defined pulmonary nodules discovered incidentally on imaging. We report an atypical presentation of a 46-year-old woman with incidentally found bilateral pulmonary cavitating nodules and cysts, concerning for lymphangioleiomyomatosis (LAM), who was ultimately diagnosed with BML.
Keywords: Benign metastasizing leiomyomatosis (BML), Lymphangioleiomyomatosis (LAM), Benign or congenital lesions, Lung pathology, Lung histology
Abbreviations: BML, Benign metastasizing leiomyomatosis; LAM, Lymphangioleiomyomatosis; CTPA, Computed tomography pulmonary angiogram; BAL, Bronchoalveolar lavage; VATS, Video-assisted thoracoscopic surgery; SMA, Smooth muscle actin; ER, Estrogen receptor; PR, Progesterone receptor; HMB-45, Human melanoma black-45; POD, Post-operative day; GnRH, Gonadotropin-releasing hormone
1. Introduction
Benign metastasizing leiomyomatosis (BML) is a rare condition, first described in 1939, and characterized by disseminated uterine smooth muscle tumors in the lungs, vasculature, abdomen, and pelvis in women with a history of uterine leiomyoma, often after hysterectomy [1], [2]. The disease generally presents asymptomatically with multiple, well-defined pulmonary nodules discovered incidentally on imaging. Lymphangioleiomyomatosis (LAM) is a similar condition characterized by smooth muscle proliferation within pulmonary parenchyma, lymphatics, and vasculature, also affecting women of childbearing age. However, unlike BML, LAM presents with progressively coalescing and enlarging thin-walled pulmonary cysts [3]. We present the case of a 46-year old woman with multiple cavitary and cystic pulmonary nodules who was ultimately diagnosed with benign metastasizing leiomyomatosis (BML).
2. Clinical summary
A 46-year-old woman with a history of asthma, atrial fibrillation and hypertension presented to an outside hospital with several months of dyspnea and cough without constitutional symptoms. The patient's surgical history was only notable for hysterectomy one-year prior for an intramural leiomyoma. She was treated with bronchodilator therapy and a corticosteroid taper without improvement in her symptoms. A follow up computed tomography pulmonary angiogram (CTPA) was performed which showed bilateral scattered solid pulmonary nodules, some of which demonstrated eccentric cavitation. Due to concern for infectious etiologies, a bronchoscopy with bronchoalveolar lavage (BAL) was performed. At that time, BAL cultures, Quantiferon Gold test, and blood cultures were negative.
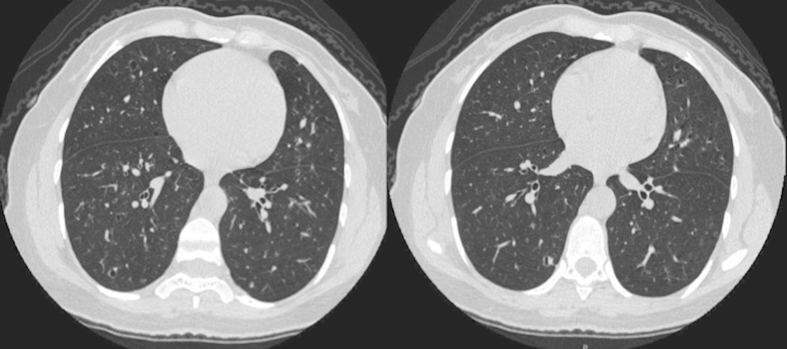
Five months after her initial presentation, a high-resolution chest CT was performed which revealed progression of the previously seen cavitary nodules into irregularly-shaped pulmonary cysts (Fig. 1). She subsequently underwent a radio-tracer labeled video-assisted thoracoscopic surgery (VATS) wedge-resection of the right lower lobe. The specimens were submitted to surgical pathology for intraoperative consultation.
Fig. 1.
Two cross-sectional images from chest computerized tomography (CT) scan demonstrating multiple, bilateral, irregularly-shaped pulmonary cysts and scattered pulmonary nodules.
Gross examination revealed well-circumscribed firm white-tan nodules measuring up to 0.5 cm in maximum dimension, without evidence of hemorrhage or necrosis. Frozen section revealed a low-grade spindle cell proliferation in a peribronchial distribution and histologic examination demonstrated a proliferation of bland eosinophilic spindle cells with elongate nuclei and tapered ends consistent with smooth muscle (Fig. 2). Cytologic atypia, necrosis and mitotic figures were not identified.
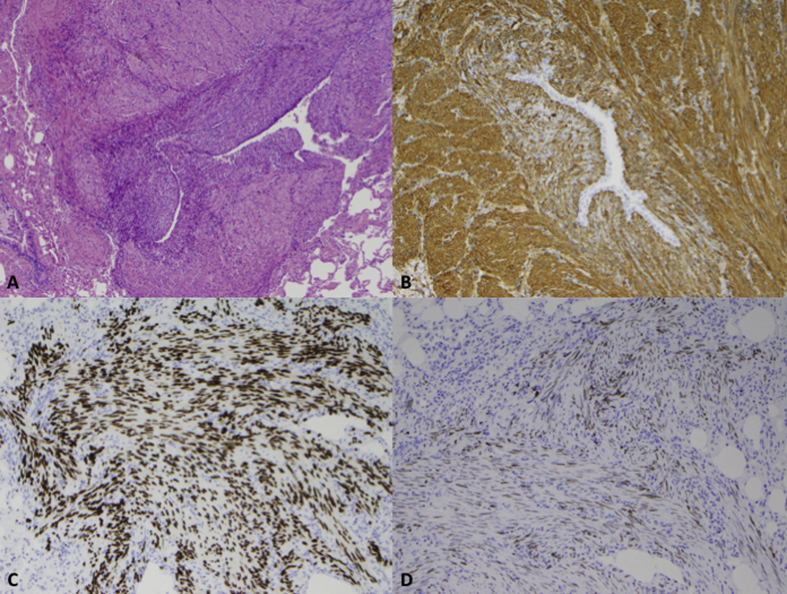
Fig. 2.
(A) Histologic examination revealed peribronchial proliferations comprised of bland eosinophilic spindle cells resembling smooth muscle [hematoxylin and eosin (H&E) stain, 40X]. (B) Smooth muscle actin demonstrated strong cytoplasmic positivity (40X). (C) Progesterone receptor demonstrated strong and diffuse nuclear positivity (40X). (D) Estrogen receptor revealed focal nuclear positivity (40X).
Immunohistochemistry revealed strong and diffuse cytoplasmic positivity for smooth muscle actin (SMA). Provided with the patient's history of a uterine leiomyoma, immunohistochemical stains for estrogen receptor (ER) and progesterone receptor (PR) were performed. The cells demonstrated strong and diffuse nuclear positivity for PR, focal nuclear positivity for ER and negative immunostaining for human melanoma black-45 (HMB-45) (Fig. 2).
The initial differential diagnosis included primary lung smooth muscle proliferations, which encompass pulmonary hamartoma, lymphangioleiomyomatosis (LAM), leiomyoma, and leiomyosarcoma, as well as smooth muscle metastases from distant sites. Immunoreactivity of the spindle cells for desmin and smooth muscle actin confirmed smooth muscle differentiation, while the presence of estrogen and progesterone receptors suggested derivation from the female genital tract. Given the histopathological findings, she was diagnosed with benign metastasizing leiomyomatosis (BML). Her postoperative period was uncomplicated and she was discharged home on post-operative day (POD) 2. Currently, she remains asymptomatic and is receiving leuprolide acetate 3.75 mg intramuscular monthly.
3. Discussion
Benign metastasizing leiomyomatosis (BML) is a rare disease that typically occurs in women of reproductive age with a history of uterine leiomyomata who have been treated with hysterectomy. Most often the patients are asymptomatic with pulmonary nodules found incidentally on chest imaging. If patients present with symptoms, they are non-specific and include cough, dyspnea, and chest pain. The time interval between hysterectomy and diagnosis of BML is variable. In a review of 74 cases, the mean interval was 10 years, with a range from 3 to 20 years [4]. In our patient, BML was diagnosed within 1 year of her hysterectomy.
Radiographic findings most often reveal solitary or multiple well-circumscribed nodules, ranging from a few millimeters to several centimeters. There is endobronchial and pleural sparing. On repeat imaging, the nodules can remain unchanged, increase or decrease in size, or become cavitary or cystic, as was seen in our patient [2] In patients with multiple, bilateral pulmonary cysts, BML can have similar radiographic appearance to lymphangioleiomyomatosis (LAM).
The combined morphologic and immunophenotypic profile help delineate BML and LAM. As in our patient, the smooth muscle proliferations are distributed in a peribronchial pattern, as opposed to around the bronchial lymphatics and interlobular septa and pleura as seen in LAM. The tumor cells in BML are negative for HMB-45 while those in LAM are positive, confirming melanocytic differentiation as a member of the perivascular epithelioid cell tumor (PEComa) family [5].
The pathogenesis of BML has yet to be defined. The presence of hormone receptors and susceptibility to antihormonal therapy suggests Müllerian origin. Hypotheses include hormone-sensitive in-situ proliferation of smooth muscle bundles, hematogenous spread of benign smooth muscle cells from uterine leiomyoma, or surgically induced hematogenous spread during hysterectomy [6]. Surgical seeding intraoperatively seems less likely, based on cases in which BML was identified on preoperative imaging prior to hysterectomy [7]. Some investigators have suggested that the lesions represent a low-grade, slow-growing and multifocal leiomyosarcoma. However, histologic examination consistently fails to show features of malignancy including an increased mitotic rate, necrosis and cytologic atypia [6].
At present, no consensus guidelines exist for the treatment of BML. Current therapies include antiestrogen management with salpingo-oophorectomy, gonadotropin-releasing hormone (GnRH) analog, or conservative management with observation and surveillance for progression [2]. Overall, prognosis is favorable. In one case series of 10 patients, median survival was 94 months (range between 7 and 101 months), and only one patient died of BML-related complications [8].
Although rare, BML should be included in the differential diagnosis of reproductive-age women who have undergone hysterectomy for uterine leiomyoma and subsequently present with multiple, pulmonary nodules or cysts. Confirming smooth muscle origin and estrogen and progesterone receptor positivity via immunohistochemistry remains paramount to establishing the diagnosis.
Funding
None.
Conflicts of interest
None.
Disclosures
None.
Acknowledgment
None.
References
- 1.Steiner P. Metastasizing fibroleiomyoma of the uterus: a report of a case and review of the literature. Am. J. Pathol. 1939;15:89–109. [PMC free article] [PubMed] [Google Scholar]
- 2.Jautzke G., Muller E., Thalmann U. Immunohistological detection of estrogen and progesterone receptors in multiple and well-differentiated leiomyomatous lung tumors in women with uterine leiomyomas (so-called benign metastasizing leiomyomas): a report on 5 cases. Path Res. Prac. 1996;192(3):215–223. doi: 10.1016/S0344-0338(96)80224-X. [DOI] [PubMed] [Google Scholar]
- 3.Richards J.C., Lynch D.A., Chung J.H. Cystic and nodular lung disease. Clin. Chest Med. 2015;36(2):299–312. doi: 10.1016/j.ccm.2015.02.011. [DOI] [PubMed] [Google Scholar]
- 4.Ki E.Y., Hwang S.J., Lee K.H. Benign metastasizing leiomyoma of the lung. World J. Surg. Oncol. 2013;11:279. doi: 10.1186/1477-7819-11-279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pitts S., Oberstein E.M., Glassberg M.K. Benign metastasizing leiomyoma and lymphangioleiomyomatosis: sex-specific diseases? Clin. Chest Med. 2004;25(2):343–360. doi: 10.1016/j.ccm.2004.01.014. [DOI] [PubMed] [Google Scholar]
- 6.Patton K.T., Cheng L., Papavero V., Blum M.G., Yeldandi A.V., Adley B.P. Benign metastasizing leiomyoma: clonality, telomere length and clinicopathologic analysis. Mod. Pathol. 2006;19(1):719–727. doi: 10.1038/modpathol.3800504. [DOI] [PubMed] [Google Scholar]
- 7.Rege A.S., Snyder J.A., Scott W.J. Benign metastasizing leiomyoma: a rare cause of multiple pulmonary nodules. ATS. 2012;93(6):e149–e151. doi: 10.1016/j.athoracsur.2011.12.047. [DOI] [PubMed] [Google Scholar]
- 8.Kayser K., Zink S., Schneider T. Benign metastasizing leiomyoma of the uterus: documentation of clinical, immunohistochemical and lectin-histochemical data of ten cases. Virchows Arch. 2000;437(3):284–292. doi: 10.1007/s004280000207. [DOI] [PubMed] [Google Scholar]