Abstract
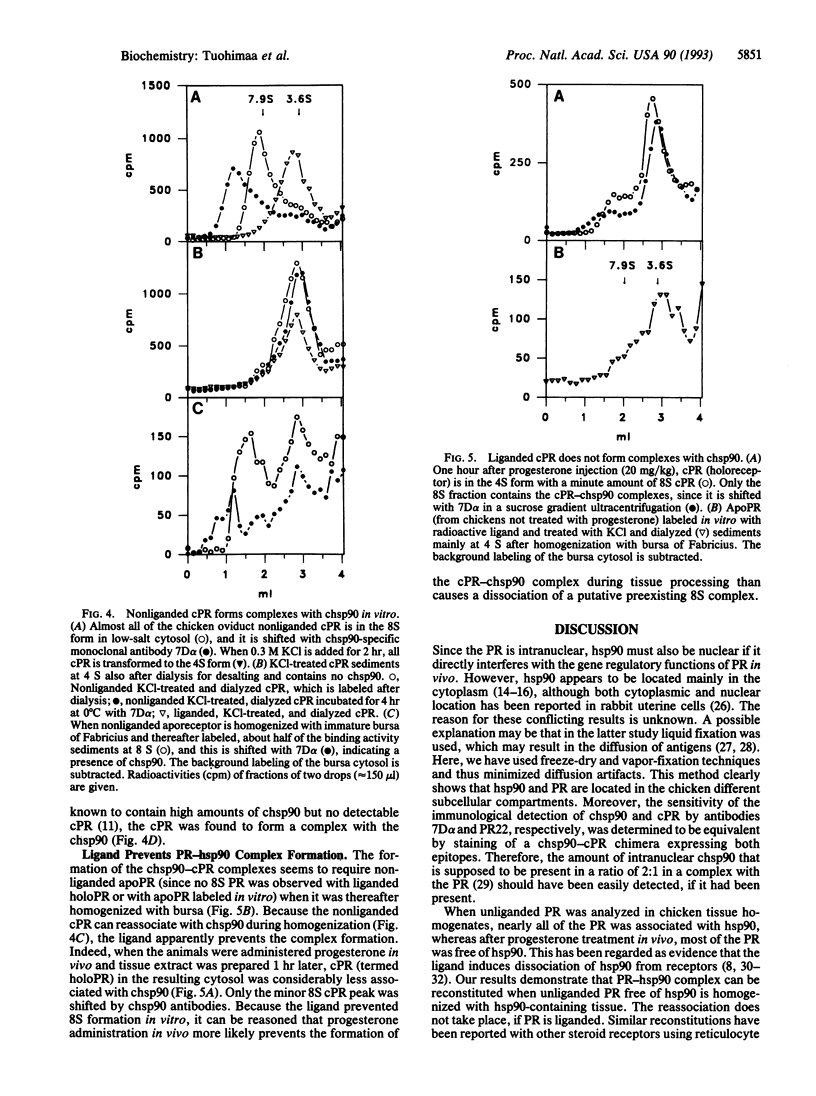
Heat shock protein 90 (hsp90) is associated with many steroid receptors in tissue homogenates. It is widely accepted that hsp90 regulates the binding of the receptor to the corresponding gene regulatory element. However there is no unequivocal evidence that steroid receptor-hsp90 complexes are present in the intact cells. We demonstrate here the absence of progesterone receptor (PR)-hsp90 complexes in intact target cell nuclei, using immunohistochemical and biochemical methods to determine the location and composition of the nonliganded (aporeceptor) and liganded (holoreceptor) PR complexes. In the chicken oviduct cells, both apo- and holoreceptors were nuclear, while hsp90 was exclusively cytoplasmic. When expressed transiently in HeLa cells, hsp90 was detected in the cytoplasm and PR was detected in the nucleus. Their location or staining intensity was not affected when they were coexpressed in the same cells. To confirm that the sensitivity of the immunohistochemical detection of hsp90 and PR did not differ significantly, a chimeric hsp90-PR was transiently expressed in HeLa cells. Both hsp90 and PR antigens of the chimera were detected in nuclei with the same intensity. In homogenates of the same tissue samples that were used for immunohistochemistry, the PR was complexed with hsp90. Hsp90-PR complexes were formed in vitro when immature bursa of Fabricius, known to contain high levels of hsp90, was homogenized in the presence of hsp90-free aporeceptor, while holoreceptor did not associate with hsp90. Our data show that nuclear PR is not complexed with hsp90 in vivo and suggest that the 8S-PR may be an in vitro artifact generated during tissue processing.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baulieu E. E. Antihormone-steroid hormonal activity, heat-shock protein hsp 90 and receptors. Horm Res. 1987;28(2-4):181–195. doi: 10.1159/000180943. [DOI] [PubMed] [Google Scholar]
- Baulieu E. E. Contragestion and other clinical applications of RU 486, an antiprogesterone at the receptor. Science. 1989 Sep 22;245(4924):1351–1357. doi: 10.1126/science.2781282. [DOI] [PubMed] [Google Scholar]
- Beato M. Gene regulation by steroid hormones. Cell. 1989 Feb 10;56(3):335–344. doi: 10.1016/0092-8674(89)90237-7. [DOI] [PubMed] [Google Scholar]
- Briggs R. C., Montiel M. M., Wojtkowiak Z. Nuclear localization of lactoferrin in the human granulocyte: Artifact incurred during slide preparation. J Histochem Cytochem. 1983 Sep;31(9):1157–1162. doi: 10.1177/31.9.6350439. [DOI] [PubMed] [Google Scholar]
- Carson-Jurica M. A., Schrader W. T., O'Malley B. W. Steroid receptor family: structure and functions. Endocr Rev. 1990 May;11(2):201–220. doi: 10.1210/edrv-11-2-201. [DOI] [PubMed] [Google Scholar]
- Catelli M. G., Binart N., Jung-Testas I., Renoir J. M., Baulieu E. E., Feramisco J. R., Welch W. J. The common 90-kd protein component of non-transformed '8S' steroid receptors is a heat-shock protein. EMBO J. 1985 Dec 1;4(12):3131–3135. doi: 10.1002/j.1460-2075.1985.tb04055.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans R. M. The steroid and thyroid hormone receptor superfamily. Science. 1988 May 13;240(4854):889–895. doi: 10.1126/science.3283939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasc J. M., Renoir J. M., Faber L. E., Delahaye F., Baulieu E. E. Nuclear localization of two steroid receptor-associated proteins, hsp90 and p59. Exp Cell Res. 1990 Feb;186(2):362–367. doi: 10.1016/0014-4827(90)90317-4. [DOI] [PubMed] [Google Scholar]
- Gasc J. M., Renoir J. M., Radanyi C., Joab I., Tuohimaa P., Baulieu E. E. Progesterone receptor in the chick oviduct: an immunohistochemical study with antibodies to distinct receptor components. J Cell Biol. 1984 Oct;99(4 Pt 1):1193–1201. doi: 10.1083/jcb.99.4.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green S., Chambon P. Nuclear receptors enhance our understanding of transcription regulation. Trends Genet. 1988 Nov;4(11):309–314. doi: 10.1016/0168-9525(88)90108-4. [DOI] [PubMed] [Google Scholar]
- Green S., Issemann I., Sheer E. A versatile in vivo and in vitro eukaryotic expression vector for protein engineering. Nucleic Acids Res. 1988 Jan 11;16(1):369–369. doi: 10.1093/nar/16.1.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grody W. W., Schrader W. T., O'Malley B. W. Activation, transformation, and subunit structure of steroid hormone receptors. Endocr Rev. 1982 Spring;3(2):141–163. doi: 10.1210/edrv-3-2-141. [DOI] [PubMed] [Google Scholar]
- Gronemeyer H., Turcotte B., Quirin-Stricker C., Bocquel M. T., Meyer M. E., Krozowski Z., Jeltsch J. M., Lerouge T., Garnier J. M., Chambon P. The chicken progesterone receptor: sequence, expression and functional analysis. EMBO J. 1987 Dec 20;6(13):3985–3994. doi: 10.1002/j.1460-2075.1987.tb02741.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groyer A., Schweizer-Groyer G., Cadepond F., Mariller M., Baulieu E. E. Antiglucocorticosteroid effects suggest why steroid hormone is required for receptors to bind DNA in vivo but not in vitro. Nature. 1987 Aug 13;328(6131):624–626. doi: 10.1038/328624a0. [DOI] [PubMed] [Google Scholar]
- Hightower L. E. Heat shock, stress proteins, chaperones, and proteotoxicity. Cell. 1991 Jul 26;66(2):191–197. doi: 10.1016/0092-8674(91)90611-2. [DOI] [PubMed] [Google Scholar]
- Inano K., Haino M., Iwasaki M., Ono N., Horigome T., Sugano H. Reconstitution of the 9 S estrogen receptor with heat shock protein 90. FEBS Lett. 1990 Jul 2;267(1):157–159. doi: 10.1016/0014-5793(90)80313-8. [DOI] [PubMed] [Google Scholar]
- Isola J. J. The effect of progesterone on the localization of progesterone receptors in the nuclei of chick oviduct cells. Cell Tissue Res. 1987 Aug;249(2):317–323. doi: 10.1007/BF00215514. [DOI] [PubMed] [Google Scholar]
- Isola J., Ylikomi T., Tuohimaa P. Nuclear origin of progesterone receptor of the chick oviduct cytosol. An immunoelectron microscopic study. Histochemistry. 1986;86(1):53–58. doi: 10.1007/BF00492345. [DOI] [PubMed] [Google Scholar]
- Joab I., Radanyi C., Renoir M., Buchou T., Catelli M. G., Binart N., Mester J., Baulieu E. E. Common non-hormone binding component in non-transformed chick oviduct receptors of four steroid hormones. 1984 Apr 26-May 2Nature. 308(5962):850–853. doi: 10.1038/308850a0. [DOI] [PubMed] [Google Scholar]
- King W. J., Greene G. L. Monoclonal antibodies localize oestrogen receptor in the nuclei of target cells. Nature. 1984 Feb 23;307(5953):745–747. doi: 10.1038/307745a0. [DOI] [PubMed] [Google Scholar]
- Klein-Hitpass L., Cato A. C., Henderson D., Ryffel G. U. Two types of antiprogestins identified by their differential action in transcriptionally active extracts from T47D cells. Nucleic Acids Res. 1991 Mar 25;19(6):1227–1234. doi: 10.1093/nar/19.6.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai B. T., Chin N. W., Stanek A. E., Keh W., Lanks K. W. Quantitation and intracellular localization of the 85K heat shock protein by using monoclonal and polyclonal antibodies. Mol Cell Biol. 1984 Dec;4(12):2802–2810. doi: 10.1128/mcb.4.12.2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer M. E., Pornon A., Ji J. W., Bocquel M. T., Chambon P., Gronemeyer H. Agonistic and antagonistic activities of RU486 on the functions of the human progesterone receptor. EMBO J. 1990 Dec;9(12):3923–3932. doi: 10.1002/j.1460-2075.1990.tb07613.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pekki A., Tuohimaa P. New freeze-dry and vapor fixation method for immunohistochemistry of soluble proteins: subcellular location of the progesterone receptor. J Histochem Cytochem. 1989 Aug;37(8):1207–1213. doi: 10.1177/37.8.2754251. [DOI] [PubMed] [Google Scholar]
- Perrot-Applanat M., Groyer-Picard M. T., Logeat F., Milgrom E. Ultrastructural localization of the progesterone receptor by an immunogold method: effect of hormone administration. J Cell Biol. 1986 Apr;102(4):1191–1199. doi: 10.1083/jcb.102.4.1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picard D., Khursheed B., Garabedian M. J., Fortin M. G., Lindquist S., Yamamoto K. R. Reduced levels of hsp90 compromise steroid receptor action in vivo. Nature. 1990 Nov 8;348(6297):166–168. doi: 10.1038/348166a0. [DOI] [PubMed] [Google Scholar]
- Pratt W. B. Interaction of hsp90 with steroid receptors: organizing some diverse observations and presenting the newest concepts. Mol Cell Endocrinol. 1990 Nov 12;74(1):C69–C76. doi: 10.1016/0303-7207(90)90198-h. [DOI] [PubMed] [Google Scholar]
- Radanyi C., Joab I., Renoir J. M., Richard-Foy H., Baulieu E. E. Monoclonal antibody to chicken oviduct progesterone receptor. Proc Natl Acad Sci U S A. 1983 May;80(10):2854–2858. doi: 10.1073/pnas.80.10.2854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renoir J. M., Mester J. Chick oviduct progesterone receptor: structure, immunology, function. Mol Cell Endocrinol. 1984 Aug;37(1):1–13. doi: 10.1016/0303-7207(84)90123-0. [DOI] [PubMed] [Google Scholar]
- Scherrer L. C., Dalman F. C., Massa E., Meshinchi S., Pratt W. B. Structural and functional reconstitution of the glucocorticoid receptor-hsp90 complex. J Biol Chem. 1990 Dec 15;265(35):21397–21400. [PubMed] [Google Scholar]
- Schuh S., Yonemoto W., Brugge J., Bauer V. J., Riehl R. M., Sullivan W. P., Toft D. O. A 90,000-dalton binding protein common to both steroid receptors and the Rous sarcoma virus transforming protein, pp60v-src. J Biol Chem. 1985 Nov 15;260(26):14292–14296. [PubMed] [Google Scholar]
- Smith D. F., Schowalter D. B., Kost S. L., Toft D. O. Reconstitution of progesterone receptor with heat shock proteins. Mol Endocrinol. 1990 Nov;4(11):1704–1711. doi: 10.1210/mend-4-11-1704. [DOI] [PubMed] [Google Scholar]
- Sullivan W. P., Beito T. G., Proper J., Krco C. J., Toft D. O. Preparation of monoclonal antibodies to the avian progesterone receptor. Endocrinology. 1986 Oct;119(4):1549–1557. doi: 10.1210/endo-119-4-1549. [DOI] [PubMed] [Google Scholar]
- Sullivan W. P., Vroman B. T., Bauer V. J., Puri R. K., Riehl R. M., Pearson G. R., Toft D. O. Isolation of steroid receptor binding protein from chicken oviduct and production of monoclonal antibodies. Biochemistry. 1985 Jul 16;24(15):4214–4222. doi: 10.1021/bi00336a060. [DOI] [PubMed] [Google Scholar]
- Tuohimaa P., Renoir J. M., Radanyi C., Mester J., Joab I., Buchou T., Baulieu E. E. Antibodies against highly purified B-subunit of the chick oviduct progesterone receptor. Biochem Biophys Res Commun. 1984 Mar 15;119(2):433–439. doi: 10.1016/s0006-291x(84)80267-3. [DOI] [PubMed] [Google Scholar]
- Wikström A. C., Bakke O., Okret S., Brönnegård M., Gustafsson J. A. Intracellular localization of the glucocorticoid receptor: evidence for cytoplasmic and nuclear localization. Endocrinology. 1987 Apr;120(4):1232–1242. doi: 10.1210/endo-120-4-1232. [DOI] [PubMed] [Google Scholar]
- Yamamoto K. R. Steroid receptor regulated transcription of specific genes and gene networks. Annu Rev Genet. 1985;19:209–252. doi: 10.1146/annurev.ge.19.120185.001233. [DOI] [PubMed] [Google Scholar]
- Ylikomi T. J., Isola J. J., Gasc J. M., Tuohimaa P. J. Sexual maturation-associated and estrogen-induced progesterone receptor expression in the bursa of Fabricius. J Immunol. 1987 May 15;138(10):3174–3178. [PubMed] [Google Scholar]
- Ylikomi T., Bocquel M. T., Berry M., Gronemeyer H., Chambon P. Cooperation of proto-signals for nuclear accumulation of estrogen and progesterone receptors. EMBO J. 1992 Oct;11(10):3681–3694. doi: 10.1002/j.1460-2075.1992.tb05453.x. [DOI] [PMC free article] [PubMed] [Google Scholar]