Abstract
Background and aims. The aim of this study was to evaluate the effect of dilution and curing methods of an etch-and-rinse adhesive and a self-etching primer from the same manufacturer at early exposure time on cytotoxicity of primary human gingival fibroblasts.
Materials and methods. Primary human gingival fibroblasts were exposed to different dilutions of Adper Single Bond (ASB) and Adper Prompt L-Pop (APL) (3M ESPE, USA). They were evaluated in unpolymerized mode for 20 s, 5 min and 24 h and in polymerized mode for 24 h and 48 h. Cytotoxicity was evaluated using three cytotoxic tests (MTT, cell counting and DNA condensation). Data was analyzed by a one-way ANOVA and Post Hoc Tukey HSD test.
Results. Cytotoxicity tests revealed that unpolymerized APL was more cytotoxic compared to ASB after 20 s (P<0.05). By increasing the time to 5 min and 24 h, ASB was more cytotoxic than APL with lower dilutions. Polymerized ASB was more toxic than APL.
Conclusion. Both adhesives were cytotoxic in different dilutions, times and curing modes. Cytotoxicity of the unpolymerized self-etching primer (APL) was more than etch-and-rinse adhesive (ASB) in 20 s, which is important clinically and dentists should be aware of the harmful effects and try to minimize it by curing and rinsing soon after composite resin insertion. ASB was more cytotoxic at 5 min and 24h.
Keywords: Adhesive, cytotoxicity, fibroblasts
Introduction
Dentin bonding agents (DBAs) have been introduced for more than two decades to reduce microleakage across the restoration‒tooth interface, increase the retention of the restoration and improve bond strength.1,2 Several types of dentin adhesives have been introduced, but current products are categorized as two major groups: etch-and-rinse and self-etch systems.3 Compared to the etch-and-rinse strategy, which uses a separate etching step, self-etching adhesives use an acidic primer with a pH lower than that of phosphoric acid, which makes their application easier and shorter in time.2,3 Based on the pH, self-etch adhesives are categorized as mild, moderate and strong, which could result in inflammatory responses in pulpal, gingival and neural cells.4-8
Studies regarding the cytotoxicity of DBAs revealed that elutes from adhesives may exert potentially harmful effects on the gingival cells and may lead to mild to severe inflammatory reactions, cell changes and cytotoxicity.9-11 Some investigations have shown contact dermatitis, lichenoid reactions, sensitivity reactions, inflammation, necrosis of oral mucosa, parakeratosis and hyperkeratosis of mucosal epidermis and nebulous discoloration as side effects of the application of dentin adhesives.12-14
Various factors are responsible for the cytotoxic effects of DBAs, such as chemical ingredients, dilution, application time, curing mode and acidity.9,10,15 Among the different chemicals, some are cytotoxic. BISGMA, TEGDMA, 2-hydroxyethylmetacrylate (HEMA), ethylene glycol (EG) and initiators (e.g. camphorquinone) are common constituents of adhesive systems, which are cytotoxic to gingival fibroblasts.15-17
Since self-etch adhesives are not fully dispersed during bonding process and cavity restoration with composite resin, there is concern about their contact time with the gingiva. Kaga et al18suggested that contact with uncured primers and adhesives should be minimized. In this regard, long exposure of the acidic part of an aggressive self-etch adhesive without rinsing during its application, along with the chemical composition, might affect gingival fibroblasts.
Since most studies with DBAs have evaluated their cytotoxicity on mouse fibroblasts with only one or two cytotoxic tests and a few variables in this area,19-22 and mostly in 24 hours and after 24 hours,9-11,15-17, the current study focused on evaluation of the cytotoxicity of dentin adhesives at early exposure time of cells (20 s and 5 min) with three different tests: MTT assay is one of the sensitive tests to detect cellular damage according to mitochondrial dehydrogenase activities of cells;23,24 DNA condensation, which could also be considered a genotoxicity test, is associated with cell death and detection of apoptotic cells.25 Cell counting is a simple, inexpensive, convenient way to define the cytotoxic effect of a material,26 which is not capable of evaluating toxicity as the only test. The null hypothesis was that the self-etch adhesive could be more cytotoxic than two-step etch-and-rinse adhesive due to its higher acidity. It was also assumed that the cytotoxicity of both adhesives would increase with longer exposure time, lower dilutions and when they are unpolymerized.
Materials and Methods
Cell Culture
Primary human gingival fibroblasts (HGFs) were cultured by using the tissue explant technique. Inflammation-free HGF cells were obtained from surgical operations of impacted third molar teeth as part of a research project (NO.130/4891-1382) approved by the Medical Committee of the Research Department of Tehran University of Medical Sciences to work on humans as participants. Cells were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM) (Biochrom AG, Germany) supplemented with 10% fetal bovine serum (Sigma, USA). Cultures were maintained at 37°C and 5% CO2.
Test Materials and Preparation
This part of the study was similar to that of a study by Banava et al.26 The materials tested in this study are listed in 1. The cytotoxicity of two adhesive materials from the same company (3M ESPE, USA) named Adper Single Bond (ASB), an etch-and-rinse single bottle adhesive, and Adper Prompt L-Pop (APL), a self-etching adhesive, were evaluated at different times, with dilutions and in polymerized and unpolymerized modes. In the unpolymerized mode, the adhesives were dissolved in 96º ethanol and diluted serially with phosphate-buffered serum (PBS) and added to culture medium to obtain various dilutions (10-7 to 10-1). The ethanol concentration was not higher than 1% for each dilution, which is considered as non-toxic for HGF cultures.
Table 1. Principal components of the DBAs tested .
| Material (code) | Ingredients | Manufacturer | Batch numbers |
| Adper Single Bond (ASB) | BIS-GMA(5-35%) ,HEMA (5-25%), MMPAS (5-15%), UDMA,GDMA (2-25%), Ethanol (20-60%),Aqua (2-8%), cam-phoroquinone, Polyacrylic acid | 3M ESPE USA | N275505 |
| Adper Prompt L-Pop (APL) | Methacrylated phosphoric esters, Bis-GMA, camphoroquinone ,Stabilizers,Water, HEMA, HEMA phosphates,Methacrylate modi-fied PAA | 3M ESPE USA | Liquid A: D 17733 |
| Liquid B: 211713 |
In the polymerized mode, 10 µL of each DBA was applied centrally on a sterile glass slide and cured for 10 s with a halogen light-curing unit with a light intensity of 400 mW/cm2 (Dentamerica, CA, USA) at a distance of 1 mm. After polymerization, each test specimen was eluted in 2 mL of DMEM for 48 h in a 5% CO2 air atmosphere. Then the extracted media were passed through an 0.22-µm filter and these elutes were subsequently diluted with the culture medium at 1:2, 1:4, 1:6, 1:8, 1:10, (and 1:1000 just for ASB) ratios.
Exposure to Test Materials
The cells were diluted in fresh complete media and seeded in proper tissue culture plates, depending on the assay. After 24 h of culture, the cells were treated with 10-1 to 10-7 dilutions of unpolymerized adhesives for 20 s, 300 s (5 min) and 24 h, and different dilutions (1:4,1:6 ,1:8) of extracts of cured adhesives were treated for 24 h and 48 h. Negative control group was considered for each subsequent experiment.
Cytotoxicity Assays
Three cytotoxicity tests were used to evaluate the cytotoxicity of the mentioned DBAs.
MTT Assay
In this assay, 6×10-3 HGFs were cultured in DMEM in 96-well culture plates. At the end of each treatment, MTT dye was added to the wells. The plates were incubated for 3 h and formazan crystals were dissolved in dimethyl sulfoxide (Merck, Darmstadt, Germany). The optical density was measured at 570 nm using a microplate reader (Anthos Reader 2001, Salzburg, Austria). The percentage of cell viability was calculated as % control.
DNA Condensation Assay
Since the chromatin condensation is a prominent hallmark of apoptosis, the apoptotic cells were recognized in this assay on the basis of nuclear condensation using Hoechst 33258 staining (Sigma, UK). To conduct DNA condensation assay, HGFs were plated at 1×105 cells per well in slide chambers (Nuns, Naperville, IL, USA). At the end of each treatment, treated and untreated (control) cells were fixed with cold methanol-acetone 1:1 (v/v) for 15 min, then washed and incubated with Hoechst 33258 staining (1 µg/mL) in the dark for 20 min. The condensation was evaluated under a fluorescence microscope (Olympus BX50F) equipped with a 3-CCD color video camera (Sony DXC) (Japan). All the experiments were performed in triplicate and repeated at three different times (n=3).
Cell Counting Assay
HGFs were plated 3×104 per well in 24-well culture plates. After removing the medium at the end of each treatment, adherent HGFs were collected by trypsinization and counted using Trypan Blue Exclusion assay, and the ratio of viable cells was calculated.
Data Analysis
Statistical analyses were presented as means ± standard errors. The significance of the results obtained from the control and treated groups were statistically analyzed by one-way ANOVA and HSD Tukey tests. P-values less than 0.05 were considered statistically significant.
Results
The cytotoxicity results of Adper Single Bond (ASB) and Adper prompt L-Pop (APL) are presented in Figures 1and 2and2.
Figure 1.
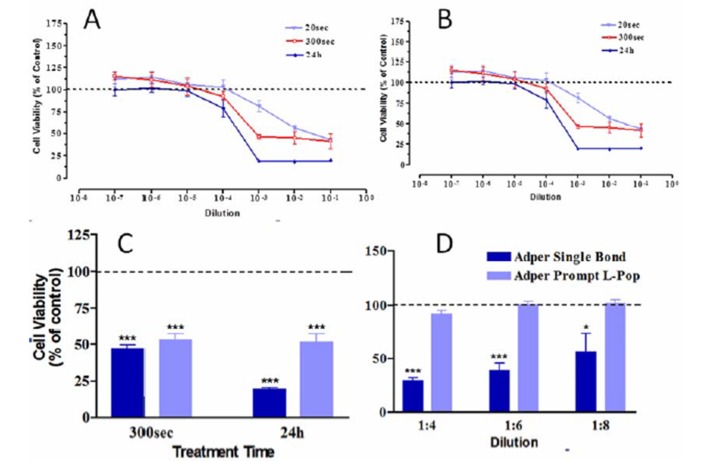
Effect of unpolymerized ASB & APL at various dilutions and different treatment times onHGF using MTT assay. (A) Effect of unpolymerized ASB. (B) Effect of unpolymerized APL. (C) Comparison of unpolymerized ASB and APL effects at 10-3 dilution with MTT assay. The results represents as means ± SE of three independent experiments (*** P < 0.001). Comparison of polymerized ASB and APL effects at various dilutions after 24 hrs of exposure using MTT test (D). The results represent as means ± SE of three independent experiments (* P < 0.05, *** P < 0.001).
Figure 2.
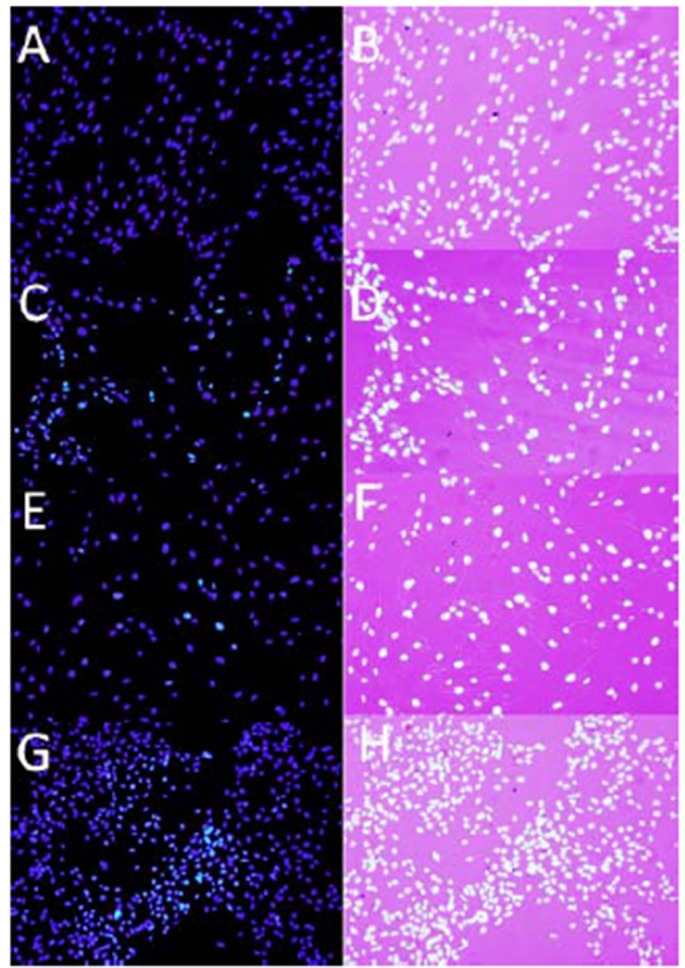
Hoechst staining of HGF following exposure to both adhesives for 24 hrs. HGF cells were treated with the (A,B) control medium , (C,D) unpolymerized APL at 10 -3dilution, (E,F) polymerized APL at 1:4 dilution. (G,H) cured ASB A, C, E and G were taken using fluorescence microscope. In B, D, F and H fluorescent images are superimposed on light microscope images of the same field. Apoptotic cells are indicated with arrows (Magnification ×20).
Table 2. Cytotoxic effect of polymerized ASB & APL was assessed using the cell counting assay at various dilutions on HGF. Values were expressed as mean percentage ± SE of cell mortality compared with control. N.D.: Not Determined .
| Dentin Bonding Agent | Dilution | Exposure Time | |
| 24 hrs | 48 hrs | ||
| Adper Single Bond (ASB) | 1:2 | 100 | 100 |
| 1:4 | 100 | 100 | |
| 1:6 | 40.80 ( 5.40 ) | 63.23 ( 1.13 ) | |
| 1:8 | 19.86 ( 3.29 ) | 53.83 ( 2.13 ) | |
| Adper Prompt L-pop (APL) | 1:2 | 100 | 100 |
| 1:4 | 54.83 ( 3.45 ) | 55.33 ( 1.03 ) | |
| 1:6 | 30.43 ( 1.72 ) | 34.83 ( 0.93 ) | |
| 1:8 | N.D | N.D | |
MTT assay
The results of the MTT assay are summarized in figures. Figure (1A) shows the cytotoxicity of uncured ASB after 20 s of application. The toxicity increased from 18.3% at 10-3 to 42.5% and 56.7%at 10-2 and 10-1dilutions, respectively, which was significantly higher than in the control group for 10-2 and 10-1 dilutions (P<0.001). By increasing exposure time to 300 s and 24h, the cytotoxic dilution changed to 10-3 and 10-5,respectively, and cell death exceeded 50% and 80%. Statistical analysis revealed that compared to 20s, ASB was significantly more cytotoxic in 10-3dilution after 300s (P<0.001) and in 10-3and 10-2 dilutions after 24 h (P<0.05). There were no significant differences in the cytotoxicity of ASB with an increase in time from 300 s to 24 h (P>0.05).
Figure (1B) shows the cytotoxicity of APL after 20 s. Cytotoxicity occurred at 10-3 dilution and increased to 49% in 10-2. After 300 s and 24 h the cytotoxic dilution moved to 10-4 and cell death reached 61% and 73%, respectively. APL was more cytotoxic in 10-3 dilution at 20 s. There was no significant difference between cytotoxicity at 300 s and 24 h (P>0.05).
Figure (1C) shows the comparison of cytotoxicity of unpolymerized ASB and APL. In this mode, APL was more cytotoxic than ASB after 20 s at 10-2 dilution (P<0.05). By increasing the exposure time to 300 s and 24 h, the first cytotoxic dilution changed to 10-3 and ASB was more cytotoxic than APL. Figure (1D) shows the results of MTT test of cured adhesives. Polymerized ASB was more toxic than polymerized APL at all dilutions.
Cell Counting Test
The results of cell counting assay in cured mode confirmed that cured ASB was more toxic than cured APL (Table 2).
DNA Condensation Test
The results of DNA condensation revealed the presence of apoptosis in less than 5% of total death in both cured and uncured adhesives (Figure 2).
Discussion
Biocompatibility is one of the important properties of dental materials, and adhesives are no exception.27 Several studies have reported the cytotoxicity of dentin bonding agents on pulpal and gingival cells.26-29 Contact of these materials with gingival tissue during adhesive restoration could cause harmful effects on gingival cells. These materials have bioactive ingredients which could result in their cytotoxicity.10-16 In the present study, the effect of two current adhesives was evaluated on human gingival fibroblasts. This cell line is the best cell type to predict the cytotoxicity of a material in clinical situations in comparison with mouse and bovine fibroblasts which might be more sensitive to some and could over-estimate the effect.30
Usually one or two tests are used to evaluate the cytotoxicity of a material, but in the present study, three tests were assigned to provide a complete and in-depth assessment of the cytotoxic effect. MTT assay should be one of the main tests in these kinds of studies because it shows cell viability in both cured and uncured modes.31 Cell counting cannot be the only test because of technical problems such as precipitation of the uncured adhesives which causes incomplete evaluation. DNA condensation which is a confirmation test for cell death and detection of apoptotic cells also could not be the sole test in a study because it just reveals the apoptotic cells. In this regard, this study showed complete assessment of the cytotoxicity of the tested adhesives.
The present study is consistent with previous studies, revealing that both adhesives are cytotoxic like other dentin adhesives after 24 h;27,28 the main difference between this study and others is the evaluation of the early exposure time of uncured adhesive after 20 s and 5 min, which has not been done before and most studies have evaluated the effects of cured adhesives in 24 h and after several days while in clinical situations adhesive materials may contact the gingival cells from the very first moment after application until the finishing and polishing of composite resin and rinsing with water. During this time uncured adhesives have enough time to contact and affect the gingiva. In this study, unpolymerized Adper Prompt L-Pop was more cytotoxic than Adper Single Bond in 20 s, which is clinically important. MTT assay showed that the longer the contact time of both adhesives in unpolymerized and polymerized mode the more the toxicity with lower dilutions. Kubia et al20 also showed that curing makes adhesives less cytotoxic than when unpolymerized, but they studied the effects after 24 h. Curing of an adhesive polymerized resins significantly diminishes the amount of free monomers and substantially reduces the potential of noxious stimuli.20,32 Since cured resins are not fully polymerized, they will also degrade with time and could release stimulants which could cause toxicitylater.20 Leakage of unpolymerized monomers of an adhesive after curing and adjacent to the gingiva could also result in cytotoxicity.33 The results of DNA condensation showed that the main mechanism of cell death was not apoptosis. These results were similar to the previous study which revealed that 85-99% of cell death was due to necrosis.34 It seems that for a definite consideration of cell death, other studies need to be conducted to evaluate the different types of adhesives chemically to reveal the main reason of cell death to make appropriate changes in future adhesives.
Although cell counting test showed the same results as MTT assay, it was unable to show the effect of unpolymerized adhesives due to technical problems. It seems that this test could not be used as the only cytotoxic assay in a study and should be accompanied by other tests to provide more detailed findings or ignored at all.
The results of the current study revealed that the toxicity is dose-dependent. Hashieh et al19 showed that all the dilutions of dentin bonding agents (from 10-8to 10-1), including ASB, are toxic in 24 hours on L-929 fibroblasts and with increasing the dilution, cytotoxicity will decrease. In the present study ASB was cytotoxic at 10-4in 5 min and 24 h. This difference could probably be due to the different cell type evaluated in that study, which seems to be more sensitive than human gingival fibroblasts that better represents clinical consequences.
As mentioned earlier, there are various factors responsible for the cytotoxicity of dentin adhesives.9,10,14The different components in dentin adhesives have different levels of cytotoxicity.15,17 Rathanasathien et al35 suggested that knowing the individual cytotoxicity of the components is not adequate when testing the cytotoxicity of DBAs that release multiple components.Bis-GMA, dimethoxybenzoine (DMBZ), urethane-di-methacrylate (UDMA),N,N-dimethyltetradecylamine (DMDTA), 2-hydroxy-ethyl-methyacrylate (HEMA) have severe cytotoxicity on fibroblasts, whereas dimethyl-p-toluidine (DMPT), benzyl-methacrylate (BEMA)and camphorquinone (CQ) showed mild cytotoxicity.36Non-irradiated CQ induces oxidative stress, DNA damage and cytotoxicity as well in primary HGF.37 Previous studies demonstrated that components of an adhesive may have a synergistic effect on the overall toxicity19 and confirmed that BIS-GMA is more cytotoxic than UDMA, TEGDMA and much more than HEMA, but interaction of two or more components together could decrease or increase the cytotoxicity of the combination.37 Kusdemir et al36 suggested that the type of methacrylate in the adhesive is an important contributing factor. They evaluated the cytotoxicity of six different self-etch adhesive with different methacrylate monomers and concluded that the BISGMA-based adhesives are more cytotoxic than UDMA-, TEGDMA- and HEMA-containing adhesives.36
Hydrophobic monomers, such as BIS-GMA, TEGDMA and UDMA, can leak from polymerized resins and cause side effects. Hydrophilic monomers such as HEMA are cytotoxic but the cytotoxicity is less than that of BIS-GMA and UDMA.14,36 However, if they are mixed with HEMA, the diffusion will easily occur, resulting in more cytotoxicity.38
The cytotoxicity of both adhesives in this study could be related to their methacrylate monomers as well as the CQ incorporated as photoinitiator as it has been shown to be not only a cytotoxic agent,39,40 but also a mutagen.41 ASB has more methacrylate monomers than APL. It contains BIS-GMA, MMPAS, UDMA, GDMA, and HEMA, which could be one of the reasons for its cytotoxicity. Adper Prompt L-Pop is a HEMA-based aggressive DBA according to its pH, i.e. 0.9–1.0.2 It has BIS-GMA, methacrylate-modified polyacrylic acid and HEMA phosphates that maybe caused less toxicity than ASB. It seems that there are other factors than the acidity of the primer in APL, which is responsible for its cytotoxicity.
Future in vitro and in vivo studies are needed to evaluate the replacement of the toxic ingredients of dentin adhesives by more biocompatible components.
According to the present study, it is suggested that dentists be cautious during adhesive restorations and avoid the contact of the adhesives with the gingival tissues, especially with the use of self-etch types with higher acidity at the very first seconds to prevent possible harmful effects. Application of a rubber dam or a Teflon ribbon on the gingiva to protect it from the effects of the adhesives could be useful. If contact occurs, the gingiva should be rinsed with copious amounts of water very soon after restoring the cavity, removing the additional adhesive from the cavity by brush applicator, not by air stream to avoid spreading it to gingiva, and using a high-quality light source to produce a higher degree of conversion of the monomers.
Conclusions
Within the limitations of the present study, the following could be concluded:
- The sensitivity of cytotoxicity to HGF depends on the materials tested, dilution, time of exposure and curing mode.
- Adper Prompt L-Pop was more cytotoxic than Adper Single Bond in unpolymerized mode at 20 s of exposure to human gingival fibroblast cells, which is important clinically.
- Cured Adper Prompt L-Pop had less cytotoxicity than Adper Single Bond in 24-48 h.
- By increasing the exposure time from 20 s to 24 h, lower dilutions of adhesives induced cytotoxicity.
- The cytotoxicity caused by adhesives in unpolymerized mode was significantly higher than polymerized mode, which should be considered in clinical application of these materials.
- It is wise to isolate the gingiva properly during adhesive restorations, especially the self-etch ones, and rinse the area immediately after composite resin polymerization to reduce the harmful effects of the uncured adhesives.
References
- 1.Garcia-Godoy F, Donly KJ. Dentin/enamel adhesives in pediatric dentistry. Pediatr Dent. 2002;24:462–4. [PubMed] [Google Scholar]
- 2.Perdigao J. New developments in dental adhesion. Dent Clin North Am. 2007;51:333–57. doi: 10.1016/j.cden.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 3.Swift EJ, Jr Jr. Dentin/enamel adhesives: review of the literature. Pediatr Dent. 2002;24:456–61. [PubMed] [Google Scholar]
- 4.Accorinte Mde L, Loguercio AD, Reis A, Muench A, de Araujo VC. Response of human pulp capped with a bonding agent after bleeding control with hemostatic agents. Oper Dent. 2005;30:147–55. [PubMed] [Google Scholar]
- 5.Cehreli ZC, Turgut M, Olmez S, Dagdeviren A, Atilla P. Short term human primary pulpal response after direct pulp capping with fourth-generation dentin adhesives. Clin Pediatr Dent. 2000;25:65–71. doi: 10.17796/jcpd.25.1.01m8g5033h867q63. [DOI] [PubMed] [Google Scholar]
- 6.Hanks CT, Strawn SE, Wataha JC, Craig RG. Cytotoxic effects of resin components on cultured mammalian fibroblasts. J Dent Res. 1991;70:1450–5. doi: 10.1177/00220345910700111201. [DOI] [PubMed] [Google Scholar]
- 7.Saghiri MA, Asgar K, Daliri M, Lotfi M, Delvarani A, Mehrvarzfar P. et al. Morphological behavior and attachment of p19 neural cells to root-end filling materials. Scanning. 2010;32:369–74. doi: 10.1002/sca.20209. [DOI] [PubMed] [Google Scholar]
- 8.Tsuneda Y, Hayakawa T, Yamamoto H, Ikemi T, Nemoto K. A histopathological study of direct pulp capping with adhesive resins. Oper Dent. 1995;20:223–9. [PubMed] [Google Scholar]
- 9.Grobler SR, Olivier A, Moodley D, van WKTW. Cytotoxicity of two concentrations of a dentine bonding agent on mouse 3T3 and human pulp fibroblast cell-lines. SADJ. 2004;59:368–70. [PubMed] [Google Scholar]
- 10.Sengun A, Buyukbas S, Hakki SS. Cytotoxic effects of dental desensitizers on human gingival fibroblasts. J Biomed Mater Res B. 2006;78:131–7. doi: 10.1002/jbm.b.30464. [DOI] [PubMed] [Google Scholar]
- 11.Teixeira HM, Do Nascimento AB, Hebling J, De Souza Costa CA. In vivo evaluation of the biocompatibility of three current bonding agents. J Oral Rehabil. 2006;33:542–50. doi: 10.1111/j.1365-2842.2005.01591.x. [DOI] [PubMed] [Google Scholar]
- 12.Geurtsen W, Spahl W, Muller K, Leyhausen G. Aqueous extracts from dentin adhesives contain cytotoxic chemicals. J Biomed Mater Res. 1999;48:772–7. doi: 10.1002/(sici)1097-4636(1999)48:6%3C772::aid-jbm2%3E3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 13.Reichl FX, Simon S, Esters M, Seiss M, Kehe K, Kleinsasser N. et al. Cytotoxicity of dental composite (co)monomers and the amalgam component Hg(2+) in human gingival fibroblasts. Arch Toxicol. 2006;80:465–72. doi: 10.1007/s00204-006-0073-5. [DOI] [PubMed] [Google Scholar]
- 14.Goldberg M. In vitro and in vivo studies on the toxicity of dental resin components: a review. Clin Oral Investig. 2008;12:1–8. doi: 10.1007/s00784-007-0162-8. [DOI] [PubMed] [Google Scholar]
- 15.Grobler SR, Oliver A, Moodley D, Van Wyk Kotze TJ. Cytotoxicity of recent dentin bonding agents on mouse fibroblast cells. Quintessence Int. 2008;39:511–6. [PubMed] [Google Scholar]
- 16.Falconi M, Ortolani M, Teti G, Zago M, Orsini G, Selan L. et al. Suppression of procollagen {alpha}1 type 1 by long-term low-dose exposure to 2-hydroxyethylmethacrylate in human gingival fibroblasts in vitro. Int J Toxicol. 2010;29:523–31. doi: 10.1177/1091581810375003. [DOI] [PubMed] [Google Scholar]
- 17. Rodriguez IA, Lopez-Gonzalez G, Rodriguez MA, Campos-Sanchez F, Alaminos M. Biological evaluation of 2-hydroxyethylmethacrylate (HEMA) toxicity in human gingival fibroblasts with histochemical X-ray microanalysis. J Adhes Dent 13:375-81. [DOI] [PubMed]
- 18.Kaga M, Noda M, Ferracane JL, Nakamura W, Oguchi H, Sano H. The in vitro cytotoxicity of eluates from dentin bonding resins and their effect on tyrosine phosphorylation of L929 cells. Dent Mater. 2001;17:333–9. doi: 10.1016/s0109-5641(00)00091-9. [DOI] [PubMed] [Google Scholar]
- 19.Hashieh IA, Cosset A, Franquin JC, Camps J. In vitro cytotoxicity of one-step dentin bonding systems. J Endod. 1999;25:89–92. doi: 10.1016/s0099-2399(99)80003-x. [DOI] [PubMed] [Google Scholar]
- 20.Koliniotou-Koubia E, Dionysopoulos P, Koulaouzidou EA, Kortsaris AH, Papadogiannis Y. In vitro cytotoxicity of six dentin bonding agents. J Oral Rehabil. 2001;28:971–5. doi: 10.1046/j.1365-2842.2001.00769.x. [DOI] [PubMed] [Google Scholar]
- 21.Tuncer S, Demirci M, Schweikl H, Erguven M, Bilir A, Kara Tuncer A. Inhibition of cell survival, viability and proliferation by dentin adhesives after direct and indirect exposure in vitro. Clin Oral Investig. 2012;16:1635–46. doi: 10.1007/s00784-011-0669-x. [DOI] [PubMed] [Google Scholar]
- 22.Vajrabhaya LO, Pasasuk A, Harnirattisai C. Cytotoxicity evaluation of single component dentin bonding agents. Oper Dent. 2003;28:440–4. [PubMed] [Google Scholar]
- 23.Hanelt M, Gareis M, Kollarczik B. Cytotoxicity of mycotoxins evaluated by the MTT-cell culture assay. Mycopathologia. 1994;128:167–74. doi: 10.1007/bf01138479. [DOI] [PubMed] [Google Scholar]
- 24.Ulukaya E, Ozdikicioglu F, Oral AY, Demirci M. The MTT assay yields a relatively lower result of growth inhibition than the ATP assay depending on the chemotherapeutic drugs tested. Toxicol In Vitro. 2008;22:232–9. doi: 10.1016/j.tiv.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 25.El-Kholany NR, Abielhassan MH, Elembaby AE, Maria OM. Apoptotic effect of different self-etch dental adhesives on odontoblasts in cell cultures. Arch Oral Biol. 2012;57:775–83. doi: 10.1016/j.archoralbio.2011.11.019. [DOI] [PubMed] [Google Scholar]
- 26.Banava S, Najibfard K, Ghahremani MH, Ostad SN. Cytotoxic effect of a dentin bonding agent: AdheSE. J Dent Teh. 2007;20:59–70. [Google Scholar]
- 27.Koulaouzidou EA, Helvatjoglu-Antoniades M, Palaghias G, Karanika-Kouma A, Antoniades D. Cytotoxicity of dental adhesives in vitro. Eur J Dent. 2009;3:3–9. [PMC free article] [PubMed] [Google Scholar]
- 28.Koulaouzidou EA, Helvatjoglou-Antoniades M, Palaghias G, Antoniades D. Effect of dual-cured adhesive resin cements on cell proliferation of pulp and human fibroblasts. Am J Dent. 2009;22:273–77. [PubMed] [Google Scholar]
- 29.Koulaouzidou EA, Papazisis KT, Yiannaki E, Palaghias G, Helvatjoglu-Antoniades M. Effects of dentin bonding agents on the cell cycle of fibroblasts. J Endod. 2009;35:275–9. doi: 10.1016/j.joen.2008.11.013. [DOI] [PubMed] [Google Scholar]
- 30.Eldeniz AU, Mustafa K, Orstavik D, Dahl JE. Cytotoxicity of new resin-, calcium hydroxide- and silicone-based root canal sealers on fibroblasts derived from human gingiva and L929 cell lines. Int Endod J. 2007;40:329–37. doi: 10.1111/j.1365-2591.2007.01211.x. [DOI] [PubMed] [Google Scholar]
- 31.de Gomes PS, Figueiral MH, Fernandes MHR, Scully C. Cytotoxicity of denture adhesives. Clin Oral Investig. 2011;15:885–93. doi: 10.1007/s00784-010-0464-0. [DOI] [PubMed] [Google Scholar]
- 32.Ergun G, Egilmez F, Uctasli MB, Yilmaz S. Effect of light curing type on cytotoxicity of dentine-bonding agents. Int Endod J. 2007;40:216–23. doi: 10.1111/j.1365-2591.2007.01225.x. [DOI] [PubMed] [Google Scholar]
- 33.Poskus LT, Lima RSMS, Lima IR, Guimarães JGA, Silva EM, Granjeiro JM. Cytotoxicity of current adhesive systems: in vitro testing on cell culture of L929 and balb/c 3T3 fibroblasts. Revista Odonto Ciência. 2009;24:129–34. [Google Scholar]
- 34.Roll EB, Dahl JE, Runningen G, Morisbak E. In vitro cell death induced by irradiation and chemicals relevant for dental applications; dose-response and potentiation effects. Eur J Oral Sci. 2004;112:273–9. doi: 10.1111/j.1600-0722.2004.00128.x. [DOI] [PubMed] [Google Scholar]
- 35.Ratanasathien S, Wataha JC, Hanks CT, Dennison JB. Cytotoxic interactive effects of dentin bonding components on mouse fibroblasts. J Dent Res. 1995;74:1602–6. doi: 10.1177/00220345950740091601. [DOI] [PubMed] [Google Scholar]
- 36. Kusdemir M, Gunal S, Ozer F, Imazato S, Izutani N, Ebisu S, et al. Evaluation of cytotoxic effects of six self-etching adhesives with direct and indirect contact tests. Dent Mater J. 2011 Nov 25. [Epub ahead of print] [DOI] [PubMed]
- 37.Volk J, Leyhausen G, Dogan S, Geurtsen W. Additive effects of TEGDMA and hydrogenperoxide on the cellular glutathione content of human gingival fibroblasts. Dent Mater. 2007;23:921–6. doi: 10.1016/j.dental.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 38.Hanks CT, Wataha JC, Sun Z. In vitro models of biocompatibility: a review. Dent Mater. 1996;12:186–93. doi: 10.1016/s0109-5641(96)80020-0. [DOI] [PubMed] [Google Scholar]
- 39.Gurpinar A, Onur MA, Cehreli ZC, Tasman F. Cytotoxicity of Two-step Self-etching Primer/Adhesives on L929 Cells. J Bioact. and Compat Polym. 2006;8:55–69. [Google Scholar]
- 40.Geursten W, Spahl W, Muller K, Leyhausen G. Variability of cytotoxicity and leaching of substances from five dentin adhesives. J Biomed Mater Res. 1999;48:772–7. doi: 10.1002/(sici)1097-4636(1999)48:6<772::aid-jbm2>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 41.Heil J, Reifferscheid G, Waldmann P, Leyhausen G, Geurtsen W. Genotoxicity of dental materials. Mutat Res. 1996;368:181–94. doi: 10.1016/s0165-1218(96)90060-9. [DOI] [PubMed] [Google Scholar]