Abstract
In the present study, the toxic effects of 1-octyl-3-methylimidazolium chloride ([Omim]Cl), 1-octyl-3-methylimidazolium bromide ([Omim]Br) and 1-octyl-3-methylimidazolium tetrafluoroborate ([Omim]BF4) in soil on Vicia faba (V. faba) seedlings at 0, 100, 200, 400, 600 and 800 mg kg−1 were assessed for the first time at the cellular and molecular level. Moreover, the toxicity of these three ionic liquids (ILs) was evaluated, and the influence of anions on the toxicity of the ILs was assessed. The results showed that even at 100 mg kg−1, the growth of V. faba seedlings was inhibited after exposure to the three ILs, and the inhibitory effect was enhanced with increasing concentrations of the three ILs. The level of reactive oxygen species (ROS) was increased after exposure to the three ILs, which resulted in lipid peroxidation, DNA damage and oxidative damage in the cells of the V. faba seedlings. In addition, the anion structure could influence the toxicity of ILs, and toxicity of the three tested ILs decreased in the following order: [Omim]BF4 > [Omim]Br > [Omim]Cl. Moreover, oxidative damage is the primary mechanism by which ILs exert toxic effects on crops, and ILs could reduce the agricultural productivity.
Ionic liquids (ILs) are a novel class of solvents that are entirely composed of ions at room temperature. ILs usually consist of large asymmetric organic cations, such as imidazolium, pyridinium, pyrrolidinium and quaternary ammonium, as well as small inorganic or organic anions, such as Cl−, Br− and BF4−1. ILs can serve as new types of multi-functional green solvents because of their unique physicochemical properties, such as negligible vapor pressure, good solubility, thermostabilization and recyclability1,2. Therefore, ILs have unique properties that can be useful in different fields, such as organic synthesis, catalysis, electrochemical and separation processes1,3. Currently, the commercial availability of ILs is increasing.
The increased interest in the use of ILs in industry will inevitably result in environmental exposure through various channels, such as accidental spills, leaching of landfill sites and via effluents or irrigation1. Although the application of ILs as green solvents has increased, relatively little is known about their toxicity. Studies have shown that ILs exhibit broad toxicity, and the toxicity of ILs is even greater than that of traditional organic solvents4,5. Therefore, we must consider the potential risks of this new chemical to people and the environment.
In recent years, research assessing the toxicity of ILs has been a very rapidly growing discipline. A considerable amount of data has been published on the toxicity of ILs in aquatic and terrestrial environments6,7,8,9. The toxicity has been evaluated on the basis of test systems at different levels of biological complexity, from molecules and cells to organisms representing different trophic levels. However, it is worth noting that previous studies examining the toxic effects of ILs on plants were primarily performed in nutrient solutions. In the natural environment, plants are mainly grown in soil. Moreover, soil represents a more complicated environment in which to study the toxic effects of ILs on plants, especially the influence of ILs on crops. Although the toxic effects of ILs in soil on terrestrial plants have been previously studied, the only endpoint assessed was growth inhibition10,11,12,13,14,15. Therefore, an investigation of the toxic effects of ILs in soil on plants at the cellular and molecular levels is important to achieve a comprehensive understanding of the toxicity of ILs and their influence on the agricultural environment.
Imidazolium-based ILs are the most widely used ILs16,17. The imidazolium-based ILs 1-octyl-3- methylimidazolium chloride ([Omim]Cl), 1-octyl-3-methylimidazolium bromide ([Omim]Br) and 1-octyl-3-methylimidazolium tetrafluoroborate ([Omim]BF4) have been previously used to study the toxic effects of ILs on organisms6,18,19. Additionally, V. faba is one of the primary agricultural crops in China and around the world20,21. Moreover, V. faba is a good environmental indicator organism; thus, it is widely used to study the toxic effects of chemicals22,23. With these factors in mind, we chose to study the toxic effects of the three imidazolium-based ILs in soil on V. faba seedlings at the cellular and molecular levels for the first time. The purpose of the present study was to evaluate the toxicity of the three ILs and to ascertain the influence of anions on the toxicity of ILs. Moreover, the present results could provide a theoretical basis for evaluating the environmental safety of ILs in soil and the influence of ILs on agricultural production.
Results
Effects of [Omim]Cl, [Omim]Br and [Omim]BF4 on the growth of V. faba seedlings
As shown in Fig. 1, the growth of V. faba seedlings was significantly inhibited after exposure to the three ILs, and this growth showed similar trends during the exposure period. Moreover, the inhibitory effect was enhanced with increasing concentrations of the three ILs. At the highest concentration (800 mg kg−1), the V. faba seedlings treated with the three ILs nearly stopped growing.
Figure 1.

Influence of [Omim]Cl (A), [Omim]Br (B) and [Omim]BF4 (C) at concentrations of 0, 100, 200, 400, 600 and 800 mg kg−1 on the growth of V. faba seedlings. Images were obtained on the 10th day of exposure.
As shown in Table 1, the shoot length, root length and dry weight significantly decreased with increasing concentrations of the three ILs. Significant differences in the shoot length, root length and dry weight were observed when the IL treatments were applied at 100 mg kg−1. The root length was inhibited more significantly than the shoot length and dry weight, and the inhibition induced by [Omim]BF4 was more obvious than that induced by [Omim]Cl and [Omim]Br. The roots of V. faba seedlings treated with [Omim]Cl and [Omim]Br stopped growing when these ILs were applied at 800 mg kg−1. Meanwhile, the roots of V. faba seedlings treated with [Omim]BF4 stopped growing when this IL was applied at 600 mg kg−1.
Table 1. Effects of [Omim]Cl, [Omim]Br and [Omim]BF4 on the shoot length, root length and dry weight of V. faba seedlings.
| IL | Concentration (mg kg−1) | Biomarkers |
||
|---|---|---|---|---|
| Shoot length (cm) | Root length (cm) | Dry weight (mg) | ||
| [Omim]Cl | 0 | 18.93 ± 0.39a | 9.67 ± 0.18a | 254.96 ± 13.50a |
| 100 | 17.94 ± 0.33b | 7.61 ± 0.28b | 232.03 ± 10.22b | |
| 200 | 15.45 ± 0.52c | 5.35 ± 0.43c | 194.57 ± 5.65c | |
| 400 | 11.27 ± 0.06d | 2.90 ± 0.23d | 131.28 ± 7.75d | |
| 600 | 8.12 ± 0.13e | 0.67 ± 0.12e | 94.67 ± 4.82e | |
| 800 | 5.77 ± 0.12f | 0f | 73.23 ± 4.26f | |
| [Omim]Br | 0 | 20.60 ± 0.45a | 10.62 ± 0.37a | 281.59 ± 17.87a |
| 100 | 18.79 ± 0.37b | 8.75 ± 0.47b | 247.49 ± 21.96b | |
| 200 | 15.89 ± 0.20c | 6.09 ± 0.19c | 205.40 ± 5.77c | |
| 400 | 11.79 ± 0.09d | 3.23 ± 0.29d | 139.05 ± 4.08d | |
| 600 | 7.63 ± 0.12e | 0.94 ± 0.18e | 97.67 ± 0.80e | |
| 800 | 5.28 ± 0.12f | 0f | 75.66 ± 2.36f | |
| [Omim]BF4 | 0 | 19.78 ± 0.27a | 10.47 ± 0.24a | 260.89 ± 9.16a |
| 100 | 16.32 ± 0.13b | 7.71 ± 0.23b | 209.83 ± 8.90b | |
| 200 | 13.53 ± 0.22c | 4.99 ± 0.15c | 165.87 ± 1.10c | |
| 400 | 9.32 ± 0.19d | 1.39 ± 0.15d | 113.59 ± 3.97d | |
| 600 | 6.47 ± 0.15e | 0e | 85.03 ± 2.10e | |
| 800 | 4.91 ± 0.05f | 0e | 62.07 ± 0.90f | |
Note: Different letters indicate significant differences between treatments at the p < 0.05 level.
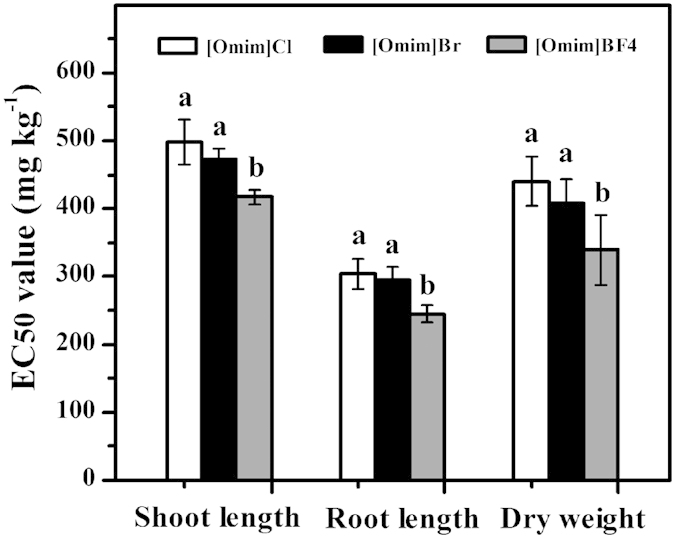
The 50% effective concentration (EC50) values of the three ILs with respect to their effects on shoot length, root length and dry weight are listed in Fig. 2. The EC50 value for root length was significantly lower than that for shoot length and dry weight. For shoot length, root length and dry weight, the EC50 values of [Omim]BF4 were significantly lower than those of [Omim]Cl and [Omim]Br. For shoot length, root length and dry weight, the EC50 values of [Omim]Cl were 498 mg kg−1, 304 mg kg−1 and 440 mg kg−1, respectively; the EC50 values of [Omim]Br were 473 mg kg−1, 295 mg kg−1 and 408 mg kg−1, respectively; and the EC50 values of [Omim]BF4 were 417 mg kg−1, 245 mg kg−1 and 339 mg kg−1, respectively.
Figure 2. The EC50 values of [Omim]Cl, [Omim]Br and [Omim]BF4 with respect to their effects on shoot length, root length and dry weight of V. faba seedlings.
The bars are the means ± standard error of three replicates. Different letters above the columns indicate significant differences (p < 0.05) between treatments as determined by the least significant difference (LSD) test.
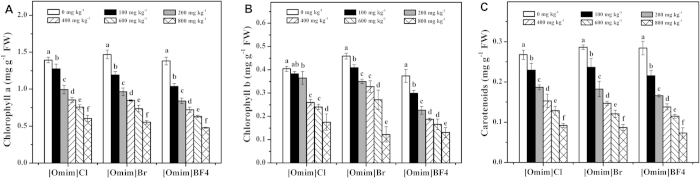
Effect of [Omim]Cl, [Omim]Br and [Omim]BF4 on the pigment content
As shown in Fig. 3, the chlorophyll a, chlorophyll b and carotenoid contents showed similar trends during the exposure period and were significantly reduced after exposure to the three ILs. The changes in chlorophyll a and carotenoids were more significant than the changes in chlorophyll b. In general, significant differences in chlorophyll a, chlorophyll b and carotenoids were observed when the ILs were applied at 100 mg kg−1.
Figure 3.
Effects of [Omim]Cl, [Omim]Br and [Omim]BF4 on the contents of chlorophyll a (A), chlorophyll b (B) and carotenoids (C) in V. faba seedling leaves. The bars are the means ± standard error of three replicates. Different letters above the columns indicate significant differences (p < 0.05) between treatments as determined by the least significant difference (LSD) test.
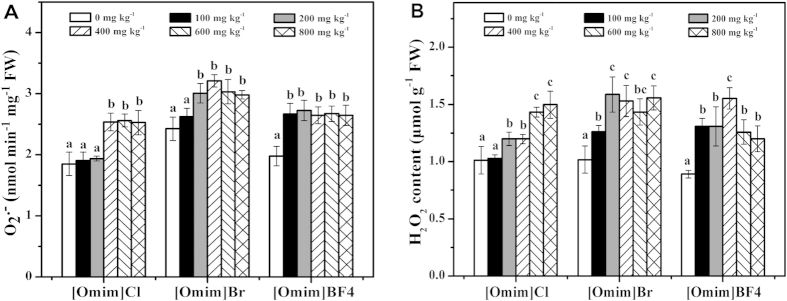
Effect of [Omim]Cl, [Omim]Br and [Omim]BF4 on the generation rate of superoxide free radical (O2 ·−) and the content of hydrogen peroxide (H2O2)
As shown in Fig. 4A, the O2·− generation rate was significantly increased after exposure to the three ILs. The significant differences induced by [Omim]Cl, [Omim]Br and [Omim]BF4 were first observed at 400 mg kg−1, 200 mg kg−1 and 100 mg kg−1, respectively.
Figure 4.
Effects of [Omim]Cl, [Omim]Br and [Omim]BF4 on the generation rate of O2− (A) and the content of H2O2 (B) in V. faba seedling leaves. The bars are the means ± standard error of three replicates. Different letters above the columns indicate significant differences (p < 0.05) between treatments as determined by the least significant difference (LSD) test.
As shown in Fig. 4B, the content of H2O2 followed a trend similar to that of the O2·− generation rate, as both H2O2 content and O2·− generation rate increased with increasing concentrations of the three ILs. The significant differences induced by [Omim]Cl, [Omim]Br and [Omim]BF4 were first observed at 200 mg kg−1, 100 mg kg−1 and 100 mg kg−1, respectively.
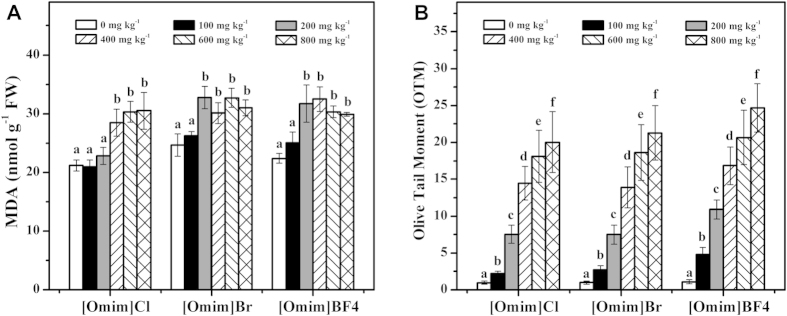
Effect of [Omim]Cl, [Omim]Br and [Omim]BF4 on the level of lipid peroxidation and the degree of DNA damage
The malondialdehyde (MDA) contents of the V. faba seedlings exposed to [Omim]Cl, [Omim]Br and [Omim]BF4 are listed in Fig. 5A. At 100 mg kg−1, the three ILs had little impact on the MDA content. The toxic effects of the three ILs were significantly enhanced at 400 mg kg−1, 200 mg kg−1 and 200 mg kg−1, respectively. Although the MDA content of V. faba seedlings exposed to 600 mg kg−1 and 800 mg kg−1 [Omim]BF4 treatments showed a decreasing trend during the exposure period, the MDA contents remained significantly higher than those in the control.
Figure 5.
Effects of [Omim]Cl, [Omim]Br and [Omim]BF4 on the level of lipid peroxidation (A) and the degree of DNA damage (B) in V. faba seedling leaves. The bars are the means ± standard error of three replicates. Different letters above the columns indicate significant differences (p < 0.05) between treatments as determined by the least significant difference (LSD) test.
As shown in Fig. 5B, the olive tail moment (OTM, the distance between the center of the head and the center of the tail and the percentage of total DNA in the tail) values were significantly enhanced after exposure to the three ILs, and significant differences were observed at 100 mg kg−1. At 100, 200, 400, 600 and 800 mg kg−1, the OTM values induced by [Omim]Cl were 2, 8, 15, 19 and 21 times higher, respectively, than the OTM value in the control; the OTM values induced by [Omim]Br were 2, 8, 14, 19 and 22 times higher, respectively, than the OTM value in the control; and the OTM values induced by [Omim]BF4 were 4, 10, 15, 19 and 22 times higher, respectively, than the OTM value in the control.
In addition, we studied the percentage of cells (n = 100) in different DNA damage classes. The present results showed that the cells in the control group were distributed in level I. When exposed to the three ILs at 100, 200 and 400 mg kg−1, the cells were primarily distributed in levels II, III and IV. When exposed to 600 and 800 mg kg−1 ILs, the cells were primarily distributed in level V.
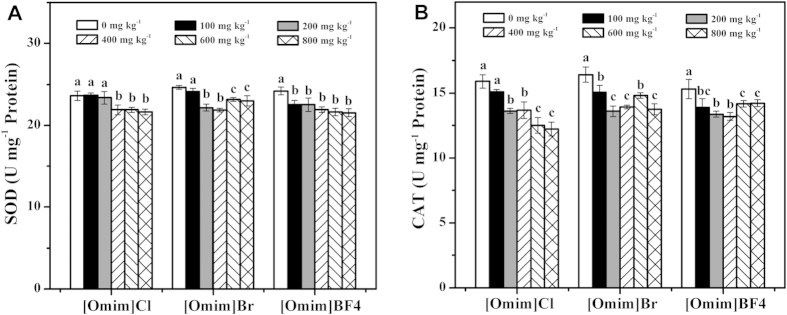
Effect of [Omim]Cl, [Omim]Br and [Omim]BF4 on superoxide dismutase (SOD) and catalase (CAT) activities
As shown in Fig. 6A, SOD activities were inhibited after exposure to the three ILs. In general, SOD activities decreased with increasing concentrations of the three ILs; however, this decrease was not observed for [Omim]Br at 600 and 800 mg kg−1. For [Omim]Cl, [Omim]Br and [Omim]BF4, significant differences were first observed at 400 mg kg−1, 200 mg kg−1 and 100 mg kg−1, respectively.
Figure 6.
Effects of [Omim]Cl, [Omim]Br and [Omim]BF4 on the activities of SOD (A) and CAT (B) in V. faba seedling leaves. The bars are the means ± standard error of three replicates. Different letters above the columns indicate significant differences (p < 0.05) between treatments as determined by the least significant difference (LSD) test.
As shown in Fig. 6B, CAT activity showed a trend similar to that of SOD activity, as both CAT and SOD activities decreased after exposure to the three ILs. CAT activity decreased with increasing the concentrations of [Omim]Cl. Although the CAT activity showed an upward trend after exposure to [Omim]Br at 600 mg kg−1, it was still lower than that in the control group. CAT activities decreased after exposure to [Omim]BF4 at 100, 200 and 400 mg kg−1; however, at 600 and 800 mg kg−1, CAT activities increased.
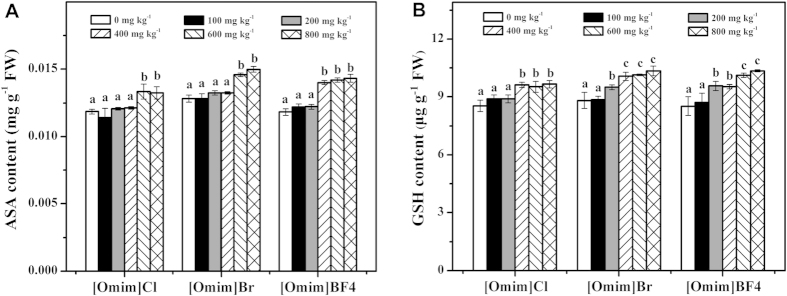
Effect of [Omim]Cl, [Omim]Br and [Omim]BF4 on ascorbic acid (ASA) and reduced glutathione (GSH) contents
As shown in Fig. 7A, ASA contents were enhanced after exposure to the three ILs. However, the three ILs had little influence on the ASA contents when applied at 100 and 200 mg kg−1. When [Omim]Cl and [Omim]Br were applied at 600 and 800 mg kg−1, the ASA contents were significantly enhanced. For [Omim]BF4, significant differences were first observed at 400 mg kg−1.
Figure 7.
Effects of [Omim]Cl, [Omim]Br and [Omim]BF4 on the contents of ASA (A) and GSH (B) in V. faba seedling leaves. The bars are the means ± standard error of three replicates. Different letters above the columns indicate significant differences (p < 0.05) between treatments as determined by the least significant difference (LSD) test.
As shown in Fig. 7B, the GSH content showed a trend similar to that of the ASA content, as both GSH and ASA contents were enhanced after exposure to the three ILs. For [Omim]Cl, the GSH contents remained stable at 400, 600 and 800 mg kg−1. For [Omim]Br and [Omim]BF4, the GSH contents increased as the concentrations increased. For [Omim]Cl, [Omim]Br and [Omim]BF4, significant differences were first observed at 400, 200 and 200 mg kg−1, respectively.
Discussion
When plants are exposed to stress conditions, the most obvious phenomenon is the inhibition of growth24. In the present study, the growth of V. faba seedlings was significantly inhibited after exposure to [Omim]Cl, [Omim]Br and [Omim]BF4. Although the inhibitory effect was not obvious at low concentrations, it was significantly enhanced with increasing concentrations of the three ILs. The behavior of ILs is similar to that of plant hormones or salts at low concentrations, as ILs in small amounts have a minimal influence on the growth of plants18,25. However, at high concentrations, ILs could have a more significant impact on the membrane system by increasing lipophilicity and uptake, resulting in higher internal concentrations11. Thus, ILs in excess inevitably affect the growth of plants. In addition, the root of V. faba seedlings were the most significantly affected organ in the present study. We believe that this result is caused by long-term direct contact with ILs, which results in the excessive accumulation of ILs in roots. Previous reports also showed that the growth of plants is inhibited after exposure to ILs, regardless of whether exposure occurs in soil or nutrient solution9,10,11,12,13,14,15,18,25,26. Therefore, we believe that the mechanism by which ILs exert toxicity in different media is consistent.
In the present study, there were no differences between the EC50 values of [Omim]Cl and [Omim]Br for shoot length, root length and dry weight. However, the EC50 value of [Omim]BF4 differed from that of [Omim]Cl and [Omim]Br for shoot length, root length and dry weight. A similar result was also reported by Bubalo et al.27, who studied the toxic effects of [C4mim][CH3CO2], [C4mim]BF4 and [C4mim]Br in solution on barley seedlings. The EC50 values of the three ILs ranged from 300 mg L−1 to 600 mg L−1 for shoot length and root length, and there were significant differences among the EC50 values of [C4mim][CH3CO2], [C4mim]BF4 and [C4mim]Br for shoot length, root length and dry weight. A similar result was also reported by Matzke et al.11, who studied the role of anion species in the toxicity of 1-alkyl-3-methylimidazolium ILs. Therefore, we believe that the structures of the anions could influence the toxicity of the ILs. In addition, based on the EC50 values of the three ILs in the present study, we believe that these ILs can be ordered based on their toxicity as follows: [Omim]BF4 > [Omim]Br > [Omim]Cl.
Photosynthesis is the basis of plant growth and development, and chlorophyll is an important pigment that captures light energy from the sun to produce glucose via photosynthesis28. Carotenoids are other important pigments that can transfer energy to chlorophyll29. In the present study, the contents of chlorophyll a, chlorophyll b and carotenoids were significantly decreased after exposure to the three ILs. Therefore, we believe that the three ILs affected the photosynthesis of V. faba seedlings, resulting in an inhibitory effect on growth. Previous studies also reported that ILs could inhibit photosynthesis by inhibiting the activity of the Hill reaction, which is the first step of photosynthesis18. In addition, studies have shown that carotenoids belong to a group of lipophilic antioxidants and can prevent the formation of reactive oxygen species (ROS) to protect the photosynthetic apparatus29,30. Therefore, we believe that the decrease in carotenoid content is associated with the increased ROS level. Similar results were also reported by Liu et al.9, Liu et al.26 and Bubalo et al.27 who studied the toxic effects of ILs on plants.
Various environmental stresses can disrupt the balance of cellular homeostasis in plants, resulting in increased accumulation of ROS, including O2·− and H2O228,29,31. In the present study, the O2·− generation rate and the H2O2 content were enhanced after exposure to the three ILs, indicating that the balance of generation and scavenging of ROS in V. faba seedlings was disrupted by the three ILs. In addition, the ROS level in [Omim]Br and [Omim]BF4 treatments stopped increasing at high concentrations, which may be caused by the defense of antioxidant system. Kumar et al.32,33 studied the toxic effects of ILs on green seaweed and found that the O2·− generation rate and the H2O2 content were increased after exposure to ILs. Similar results were also reported by Liu et al.9, Bubalo et al.27 and Zhang et al.34 when they studied the toxic effects of ILs on plants.
Excess ROS are highly reactive and toxic, which can cause damage to proteins, lipids and DNA29,35. MDA is the byproduct of lipid peroxidation and reflects the degree of plant sensitivity to ROS-induced oxidation36. In the present study, the MDA content was also increased after exposure to the three ILs, which indicated that the three ILs caused lipid peroxidation in the cells of V. faba seedlings. Similar results were also obtained by Liu et al.9, Liu et al.26, Bubalo et al.27 and Zhang et al.34 in their studies of the toxic effects of ILs on plants.
DNA is a nucleic acid that carries cellular genetic information. Comet assay was used to detect the degree of DNA damage, which indicates whether organisms have suffered genotoxic effects due to environmental stress37. Studies have shown that cellular DNA is damaged by excess ROS under various environmental stresses, such as high salt, ultraviolet radiation or exposure to toxic contaminants23,38,39. In the present study, the OTM values increased significantly after the V. faba seedlings were exposed to the three ILs. Moreover, the degree of DNA damage also increased with increasing concentrations of the three ILs. Therefore, we believe that the three ILs were genotoxic to the cells of V. faba seedlings. In addition, although the ROS level stopped increasing at high concentrations, the OTM values still increased. The reason is due to DNA repair occurs more slowly than DNA damage. The reduced ROS level resulted in the increase rate of OTM observed at concentrations ranging from 400 to 800 mg kg−1 was lower than that observed at concentrations ranging from 100 to 400 mg kg−1. The DNA of plants was seriously damaged by ILs also reported by Kumar et al.32,33, who studied the toxic effects of ILs on green seaweed.
Antioxidant enzymes such as SOD and CAT play an important role in scavenging the excess ROS in plant cells. SOD is considered to be the first line of defense against ROS, as it can catalyze the conversion of O2·− to O2 and H2O240. Nonetheless, H2O2 is still harmful to cells, and it must be scavenged by other antioxidant enzymes, such as CAT. In the present study, SOD and CAT activities were inhibited after exposure to the three ILs, resulting in increases in the O2·− generation rate and the H2O2 content. The SOD and CAT activities decreased after exposure to ILs was also reported by Liu et al.18, who studied the toxic effects of [Omim]Cl on rice seedlings.
Under environmental stress, the content of antioxidant compounds, such as ASA and GSH, also changed in response to oxidative stress. ASA is considered to be a powerful antioxidant compound due to its ability to donate electrons in a number of enzymatic and nonenzymatic reactions41. GSH can detoxify xenobiotic and prevent oxidative damage from ROS42. Moreover, ASA and GSH have a synergistic effect in the elimination of O2·− and H2O2 via the ASA-GSH cycle. In the present study, although the activities of antioxidant enzymes were inhibited, it is likely that antioxidant compounds played an important role in removing excess ROS. Therefore, the contents of ASA and GSH in V. faba seedling leaves were increased after exposure to the three ILs, especially at 600 and 800 mg kg−1. Accordingly, the ROS level stopped increasing at high concentration treatments. Similar changes in the contents of antioxidant compounds after exposure to ILs were also reported by Ma et al.19 and Yu et al.43 in their studies of the toxic effects of [Omim]Br on Daphnia magna and snails.
The relationship between these biomarkers was listed in Fig. 8. In the present study, the ROS level in V. faba seedlings was significantly increased after exposure to [Omim]Cl, [Omim]Br and [Omim]BF4. Excess ROS caused damage to the cells of V. faba seedlings, which resulted in lipid peroxidation, DNA damage and oxidative damage. Additionally, the excess ROS caused a decrease in pigment contents, which influenced photosynthesis and inhibited the growth of V. faba seedlings. On the other hand, the excess ROS was scavenged by antioxidant enzymes and antioxidant compounds, resulting in the ROS level return to normal. However, if the ROS level reaches a death threshold after exposure to high concentrations of ILs, it can not only result in damage to cells but also trigger death signaling44. Therefore, we believe that oxidative damage is the main mechanism by which ILs exert toxicity and that ILs can reduce agricultural productivity. Moreover, previous studies examining the toxic effects of ILs on plants also reported that ILs could inhibit plant growth and cause oxidative stress in plant cells9,25,26,27,32,33,34.
Figure 8. The relationship between these biomarkers used in the present study.
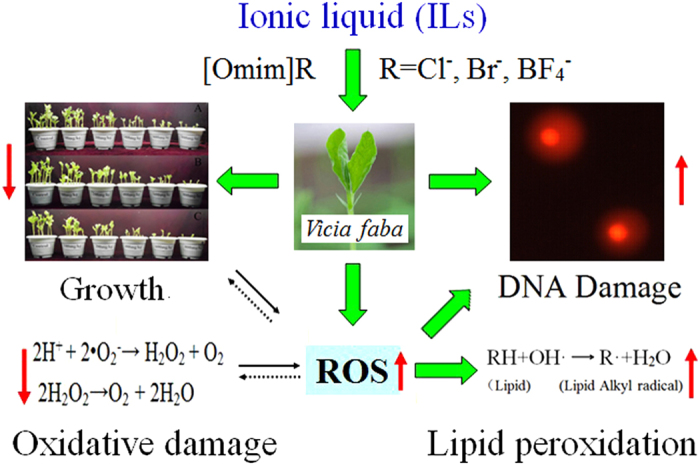
The letter R represents the anions Cl−, Br− and BF4−.
Materials and Methods
Materials
The ILs 1-octyl-3-methylimidazolium chloride, 1-octyl-3-methylimidazolium bromide and 1-octyl-3-methylimidazolium tetrafluoroborate ([Omim]Cl, [Omim]Br and [Omim]BF4; CAS NO. 64697-40-1, 61545-99-1 and 244193-52-0, respectively, all 99% purity) were obtained from Chengjie Chemical Co. Ltd. (Shanghai, China). The structure of the tested ILs is listed in Fig. 9.
Figure 9. The structure of the ILs used in the present study.
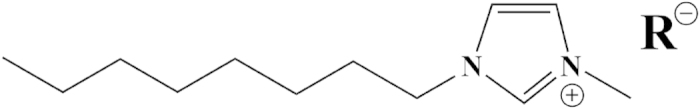
The letter R represents the anion (i.e., Cl, Br or BF4).
The V. faba seeds were purchased from the College of Life Science, Shandong Agricultural University (Taian, China).
The tested soil was taken from the test field of Shandong Agricultural University (Taian, China) and sampled from a depth of 2-20 cm. The soil was filtered through a 2-mm sieve and then naturally air-dried in the laboratory. The physical and chemical characteristics of the tested soil were measured according to the method described by Wang et al.45. The organic matter content, available potassium content, organic nitrogen content, available phosphorus content, maximum field capacity and pH of the soil were 17.6 ± 1.1 g kg−1, 125.7 ± 7.4 mg kg−1, 132.3 ± 9.6 mg kg−1, 18.4 ± 1.6 mg kg−1, 18.5 ± 1.4% and 7.6 ± 0.4, respectively.
Experimental design
Until now, no reports have focused on the toxic effects of [Omim]Cl, [Omim]Br and [Omim]BF4 in soil on plants. Therefore, the following concentrations were established to study the effects of these three ILs on the growth of V. faba seedlings: 0, 0.1, 1, 5, 10, 50, 100, 200, 400, 600, 800 and 1000 mg kg−1. The results of preliminary experiments showed that the growth conditions of V. faba seedlings at 0, 0.1, 1, 5, 10, 50 and 100 mg kg−1 were consistent. However, the V. faba seedlings stopped growing at 1000 mg kg−1. Therefore, based on these preliminary experiments, the concentrations used for testing were 0, 100, 200, 400, 600 and 800 mg kg−1.
A total of 8 g of each IL was dissolved in 500 mL of deionized water. Subsequently, the appropriate amounts of [Omim]Cl, [Omim]Br and [Omim]BF4 solutions and deionized water were added to 1000 g of soil such that the moisture of the soil was controlled at 60%. For each IL, eighteen pots were prepared and divided into six groups according to the IL concentration. Next, the soil was mixed to sufficiently homogenize the [Omim]Cl, [Omim]Br and [Omim]BF4 and was transferred into pots.
V. faba seeds were selected and surface sterilized in 30% sodium hypochlorite solution for 10 min, they were then thoroughly washed with distilled water. The sterilized seeds were soaked in distilled water for 24 h, and the water was replaced every 8 h. Then, ten seeds that were approximately the same size were selected and sown at a maximum depth of 1 cm from the surface. The seeds were vertically inverted in the pots, with the endosperm of the seeds facing downward. The V. faba seeds were then cultivated in a temperature-controlled greenhouse at 22°C for 14 h/day and 18°C for 10 h/night. The light intensity in the greenhouse was 200 μmol m−2 s−1, and the relative humidity of the soil was monitored by weighing every second day and controlled at 50–60%. On the 10th day of cultivation, the V. faba seedlings were randomly sampled for the various analyses.
Determination of shoot length, root length and dry weight
On the 10th day of cultivation, five V. faba seedlings from each replicate were randomly selected to measure the shoot length and root length according to the method of Wang et al.25. Subsequently, these V. faba seedlings were oven-dried at 105°C for 15 min and then at 65°C for 48 h to measure the dry weight using a 1/10,000 analytical balance9.
Determination of pigment content
The chlorophyll a, chlorophyll b and carotenoid contents were measured according to the method described by Meloni et al.46. Fresh leaves (0.1 g) were randomly collected from a V. faba seedling in each replicate, and then the leaves were placed in a 15-mL centrifuge tube. Then, 10 mL of 80% acetone was added, and the tube was placed in the dark for 40 h. The pigment was extracted into the acetone, and the absorbance values of the resulting extracts were measured at 470 nm, 646 nm and 663 nm using an ultraviolet/visible spectrophotometer (Shimadzu, UV-2550). The results are expressed as mg g−1 fresh weight (FW) of seedlings.
The generation rate of O2 ·−
The O2·− generation rate was measured based on the method of Qiu et al.47 by monitoring nitrite formation. Fresh leaves (0.25 g) of V. faba seedlings from each replicate were ground with 2.5 mL of 50 mM phosphate buffer (pH 7.8) containing 1 mM ethylenediamine tetraacetic acid (EDTA) and 1% (w/v) polyvinylpyrrolidone (PVP). The extract was centrifuged at 10,800 rpm for 10 min, and subsequently, 50 mM phosphate buffer (0.5 mL, pH 7.8) and 1 mM hydroxylamine hydrochloride (1 mL) were added to the supernatant (0.5 mL). The mixture was incubated in a water bath at 25°C. Approximately 1 h later, 7 mM α-naphthylamine (1 mL) and 17 mM sulfanilamide (1 mL) were added to the sample, and the sample was incubated at 25°C for 20 min. The absorbance was then measured at 530 nm using an ultraviolet/visible spectrophotometer (Shimadzu, UV-2550). An NO2− standard curve was used to calculate the generation rate of O2·−.
Determination of the H2O2 content
The H2O2 content was determined according to the method of Qiu et al.47. For each replicate, 0.25 g of fresh leaves were homogenized in 2.5 mL of 0.1% (w/v) trichloroacetic acid (TCA) and centrifuged at 13,200 rpm for 15 min at 4°C. Then, 1 mL of the supernatant was added to 1 mL of 100 mM phosphate buffer (pH 7.0) and 2 mL of 1 M potassium iodide (KI). The absorbance of the mixture was measured at 390 nm. The H2O2 content was calculated based on a standard curve and is expressed as μmol g−1 FW.
Measurement of lipid peroxidation
The lipid peroxidation level was determined by measuring the amount of MDA according to the method of Song et al.48. MDA was extracted from V. faba seedling leaves with 0.1% TCA solution, and then the sample was centrifuged at 10,800 rpm for 20 min. Next, an equal volume of 0.5% (w/v) thiobarbituric acid (TBA) in 20% TCA was added to the supernatant. The sample was incubated in a water bath (95°C) for 30 min, and then the samples were placed in an ice bath to stop the reaction. Approximately 10 min later, the mixture was centrifuged at 10,800 rpm for 15 min, and the absorbance of the mixture was measured at 532 nm and 600 nm. The concentration of MDA is expressed as nmol g−1 FW.
Comet assay
A comet assay was performed according to the method of Song et al.23. The extraction buffer (pH 7.5) containing 10 mM MgSO4, 50 mM KCl, 5 mM 4-hydroxyethyl piperazine ethyl sulfonic acid (HEPES), 0.3 mM dithiothreitol (DTT) and 0.25% (v/v) Triton X-100 was prepared according to the method of Lee and Lin49. Nuclei from V. faba seedling leaf cells were collected in 250 μL of cold extraction buffer using a new razor blade. Then, 100 μL of 0.8% normal melting agarose was pipetted onto microscope slides, one side of which was fully frosted. After solidification on ice, the second layer (40 μL of nuclear suspension solution mixed with 40 μL of 1% low-melting-point agarose) and third layer (50 μL of 0.5% low-melting-point agarose) were successively pipetted onto the microscope slides. The microscope slides were incubated in electrophoresis buffer (pH > 13), which contained 300 mM NaOH and 1 mM ethylenediamine tetraacetic acid disodium salt (Na2-EDTA), for 20 min. Subsequently, the slides were electrophoresed at 25 V (300 mA) for 15 min. Following electrophoresis, the slides were neutralized in 0.4 M Tris buffer (pH 7.5) for 15 min, and then stained with 30 μL of ethidium bromide (EB; 13 μg mL−1) for 15 min. Finally, the microscope slides were analyzed using a computerized image analysis system attached to a fluorescence microscope (Olympus, BX71). The OTM was used to quantify the extent of DNA damage.
Determination of antioxidant enzyme activities
Fresh V. faba seedling leaves (0.5 g) were homogenized in 5 mL of extraction solution containing 50 mM ice-cold phosphate buffer (pH 7.8), 1 mM EDTA and 1% PVP. The sample was centrifuged at 13,200 rpm for 20 min at 4°C, and subsequently, the supernatant was used to assay protein content as well as SOD and CAT activities.
Protein content was measured according to the method of Bradford50 and standardized to bovine serum albumin. The absorbance was measured at 595 nm.
SOD activity was determined according to the method of Song et al.23. The reaction system (3 mL) contained 50 mM phosphate buffer (pH 7.8), 750 μM NBT, 130 mM methionine, 20 μM riboflavin, 0.1 mM EDTA, and 50 μL of the enzyme extract. After illumination at 5000 lx for 15 min, the absorbance of the mixture was measured at 560 nm using an ultraviolet/visible spectrophotometer (Shimadzu, UV-2550).
CAT activity was measured according to the method of Song et al.48. The reaction system (3 mL) contained 100 mM potassium phosphate buffer (pH 7.0), 20 mM H2O2 and 0.1 mL of the enzyme extract. The decrease in absorbance at 240 nm was recorded for 1 min using an ultraviolet/visible spectrophotometer (Shimadzu, UV-2550).
Determination of antioxidant compound contents
Fresh V. faba seedling leaves (0.5 g) were homogenized in 5 mL of 5% TCA and centrifuged at 13,200 rpm for 10 min at 4°C. The supernatant was used to assay ASA and GSH contents.
The ASA content was measured according to the method of Shen et al.51. The reaction system (3 mL) contained 200 μL of the supernatant, 100 mM potassium phosphate buffer (pH 7.4), 44% H3PO4, 10% TCA (w/v), 4% 2,2-dipyridyl (w/v), 3% FeCl3 (w/v) and deionized water. The absorbance of the mixture was measured at 525 nm after a 1-h incubation at 37°C.
The GSH content was measured according to the method of Qiu et al.47. The reaction system (3 mL) contained 200 μL of the supernatant, 100 mM potassium phosphate buffer (pH 7.7) and 2.51 mg mL−1 5,5′-dithiobis (2-nitrobenzoicacid) (DTNB). The absorbance of the mixture was measured at 412 nm after a 5-min incubation at 30°C.
Statistical analysis
All analyses were performed in triplicate, and the results are presented as the mean ± SD. The data were analyzed using SPSS software (version 17.0, SPSS Inc.). One-way analysis of variance (ANOVA) was performed on all data and probit analysis was used to calculate the EC50 values. Differences among the treatments were assessed using the least significant difference (LSD) test at the p < 0.05 level. The Comet Assay Software Project (CASP) was used to analyze the comet images. The cells (n = 100) were categorized into grades of damage based on the percentage of tail DNA according to the method of Anderson et al.52 as follows: I, zero or minimal (<10%) tail DNA; II, low level of damage (10–25%); III, intermediate level of damage (25–50%); IV, high level of damage (50–75%); and V, extreme damage (>75%).
Additional Information
How to cite this article: Liu, T. et al. Biochemical toxicity and DNA damage of imidazolium-based ionic liquid with different anions in soil on Vicia faba seedlings. Sci. Rep. 5, 18444; doi: 10.1038/srep18444 (2015).
Acknowledgments
The present study was supported by grants from the National Natural Science Foundation of China [Nos. 21277083 and 21377075].
Footnotes
Author Contributions T.L. performed the experiments, analyzed the data and wrote the manuscript. L.S.Z. and J.H.W. designed the study. J.W., J.Z., X.S. and C.Z. contributed materials and reagents.
References
- Bubalo M. C., Radošević K., Redovniković I. R., Halambek J. & Srček V. G. A brief overview of the potential environmental hazards of ionic liquids. Ecotox. Environ. Saf. 99, 1–12 (2014). [DOI] [PubMed] [Google Scholar]
- Deetlefs M. & Seddon K. R. Assessing the greenness of some typical laboratory ionic liquid preparations. Green Chem. 12, 17–30 (2010). [Google Scholar]
- Fajardo O. Y., Bresme F., Kornyshev A. A. & Urbakh M. Electrotunable lubricity with ionic liquid nanoscale films. Sci. Rep. 5, 7698 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samori C., Pasteris A., Galletti P. & Tagliavini E. Acute toxicity of oxygenated and nonoxygenated imidazolium-based ionic liquids to Daphnia magna and Vibrio fischeri. Environ. Toxicol. Chem. 26, 2379–2382 (2007). [DOI] [PubMed] [Google Scholar]
- Ventura S. P. et al. Designing ionic liquids: the chemical structure role in the toxicity. Ecotoxicology 22, 1–12 (2013). [DOI] [PubMed] [Google Scholar]
- Yu M., Li S. M., Li X. Y., Zhang B. J. & Wang J. J. Acute effects of 1-octyl-3-ethylimidazolium bromide ionic liquid on the antioxidant enzyme system of mouse liver. Ecotox Environ Safe. 71, 903–908 (2008). [DOI] [PubMed] [Google Scholar]
- Samorì C. Ionic liquids and their biological effects towards microorganisms. Curr. Org. Chem. 15, 1888–1904 (2011). [Google Scholar]
- Dong M. et al. Toxic effects of 1-decyl-3-methylimidazolium bromide ionic liquid on the antioxidant enzyme system and DNA in zebrafish (Danio rerio) livers. Chemosphere 91, 1107–1112 (2013). [DOI] [PubMed] [Google Scholar]
- Liu T. et al. Effects of the ionic liquid 1-octyl-3-methylimidazolium hexafluorophosphate on the growth of wheat seedlings. Environ. Sci. Pollut. R. 21, 3936–3945 (2014). [DOI] [PubMed] [Google Scholar]
- Jastorff B. et al. Progress in evaluation of risk potential of ionic liquids-basis for an eco-design of sustainable products. Green Chem. 7, 362–372 (2005). [Google Scholar]
- Matzke M. et al. The influence of anion species on the toxicity of 1-alkyl-3-methylimidazolium ionic liquids observed in an (eco) toxicological test battery. Green Chem. 9, 1198–1207 (2007). [Google Scholar]
- Matzke M., Stolte S., Arning J., Uebers U. & Filsera J. Imidazolium based ionic liquids in soils: effects of the side chain length on wheat (Triticum aestivum) and cress (Lepidium sativum) as affected by different clays and organic matter. Green Chem. 10, 584–591 (2008). [Google Scholar]
- Matzke M., Stolte S., Arning J., Uebers U. & Filsera J. Ionic liquids in soils: effects of different anion species of imidazolium based ionic liquids on wheat (Triticum aestivum) as affected by different clay minerals and clay concentrations. Ecotoxicology 18, 197–203 (2009). [DOI] [PubMed] [Google Scholar]
- Studzińska S. & Buszewski B. Study of toxicity of imidazolium ionic liquids to watercress (Lepidium sativum L.). Anal. Bioanal. Chem. 393, 983–990 (2009). [DOI] [PubMed] [Google Scholar]
- Biczak R., Pawłowska B., Bałczewski P. & Rychter P. The role of the anion in the toxicity of imidazolium ionic liquids. J. Hazard. Mater. 274, 181–190 (2014). [DOI] [PubMed] [Google Scholar]
- Latała A., Stepnowski P., Ne˛dzi M. & Mrozi W. Marine toxicity assessment of imidazolium ionic liquids: acute effects on the Baltic algae Oocystis submarina and Cyclotella meneghiniana. Aquat. Toxicol. 73, 91–98 (2005). [DOI] [PubMed] [Google Scholar]
- Zhang S. D., Lefebvre H., Tessier M. & Fradet A. Influence of brønsted acid ionic liquid structure on hydroxyacid polyesterification. Green Chem. 13, 2786–2793 (2011). [Google Scholar]
- Liu H. J., Zhang S. X., Hu X. N. & Chen C. D. Phytotoxicity and oxidative stress effect of 1-octyl-3-methylimidazolium chloride ionic liquid on rice seedlings. Environ. Pollut. 181, 242–249 (2013). [DOI] [PubMed] [Google Scholar]
- Ma J. G., Dong X. Y., Fang Q., Li X. Y. & Wang J. J. Toxicity of imidazolium-based ionic liquids on Physa acuta and the snail antioxidant stress response. J. Biochem. Mol. Toxic. 28, 69–75 (2014). [DOI] [PubMed] [Google Scholar]
- Rubiales D. Faba beans in sustainable agriculture. Field Crops Res. 115, 201–202 (2010). [Google Scholar]
- Jensen E. S., Peoples M. B. & Hauggaard-Nielsen H. Faba bean in cropping systems. Field Crops Res. 115, 203–216 (2010). [Google Scholar]
- Wang C. R. et al. Oxidative stress, defense response, and early biomarkers for lead-contaminated soil in Vicia faba seedlings. Environ. Toxicol. Chem. 27, 970–977 (2008). [DOI] [PubMed] [Google Scholar]
- Song Y. et al. Effects of atrazine on DNA damage and antioxidative enzymes in Vicia faba, Environ. Toxicol. Chem. 28, 1059–1062 (2009). [DOI] [PubMed] [Google Scholar]
- Blancaflor E. B., Jones D. L. & Gilroy S. Alterations in the cytoskeleton accompany aluminum-induced growth inhibition and morphological changes in primary roots of maize. Plant Physiol. 118, 159–172 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L. S. et al. Effect of 1-butyl-3-methylimidazolium tetrafluoroborate on the wheat (Triticumaestivum L.) seedlings. Environ Toxicol. 24, 296–303 (2009). [DOI] [PubMed] [Google Scholar]
- Liu P. et al. Toxic effects of 1-methyl-3-octyl-imidazolium bromide on the wheat seedlings. J. Environ. Sci. 22, 1974–1979 (2010). [DOI] [PubMed] [Google Scholar]
- Bubalo M. C. et al. Imidiazolium based ionic liquids: Effects of different anions and alkyl chains lengths on the barley seedlings. Ecotox. Environ. Safe. 101, 116–123 (2014). [DOI] [PubMed] [Google Scholar]
- Huang C. J. et al. Effects of concentrations of sodium chloride on photosynthesis, antioxidative enzymes, growth and fiber yield of hybrid ramie. Plant Physiol. Biochem. 76, 86–93 (2014). [DOI] [PubMed] [Google Scholar]
- Gill S. S. & Tuteja N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Bioch. 48, 909–930 (2010). [DOI] [PubMed] [Google Scholar]
- Sharma P., Jha A. B., Dubey R. S. & Pessarakli M. Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J. Bot. 2012, 1–26 (2012). [Google Scholar]
- Liu M. et al. Potent effects of dioscin against obesity in mice. Sci. Rep. 5, 7973 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar M., Trivedi N., Reddy C. R. & Jha B. Toxic effects of imidazolium ionic liquids on the green seaweed Ulva lactuca: oxidative stress and DNA damage. Chem. Res. Toxicol. 24, 1882–1890 (2011). [DOI] [PubMed] [Google Scholar]
- Kumar M., Trivedi N., Reddy C. R. & Jha B. The ameliorating effect of Acadian marine plant extract against ionic liquids-induced oxidative stress and DNA damage in marine macroalga Ulva lactuca. J. Appl. Phycol. 25, 369–378 (2013). [Google Scholar]
- Zhang B., Li X., Chen D. & Wang J. Effects of 1-octyl-3- methylimidazolium bromide on the antioxidant system of Lemna minor. Protoplasma 250, 103–110 (2013). [DOI] [PubMed] [Google Scholar]
- Lu L. Y., Ou N. & Lu Q. B. Antioxidant induces DNA damage, cell death and mutagenicity in human lung and skin normal cells. Sci. Rep. 3, 3169 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Lopez U. et al. The oxidative stress caused by salinity in two barley cultivars is mitigated by elevated CO2. Plant Physiol. 135, 29–42 (2009). [DOI] [PubMed] [Google Scholar]
- Ventura L. et al. Single cell gel electrophoresis (comet) assay with plants: research on DNA repair and ecogenotoxicity testing. Chemosphere 92, 1–9 (2013). [DOI] [PubMed] [Google Scholar]
- Ghosh M., Bandyopadhyay M. & Mukherjee A. Genotoxicity oftitanium dioxide (TiO2) nanoparticles at two trophic levels: plant and human lymphocytes. Chemosphere 81, 1253–1262 (2010). [DOI] [PubMed] [Google Scholar]
- El-Yazbi A. F. & Loppnow G. R. Detecting UV-induced nucleic- acid damage. Trac-Trend. Anal. Chem. 61, 83–91 (2014). [Google Scholar]
- Foyer C. H. & Shigeoka S. Understanding oxidative stress and antioxidant functions to enhance photosynthesis. Plant Physiol. 155, 93–100 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler G. L., Jones M. A. & Smirnoff N. The biosynthetic pathway of vitamin C in higher plants. Nature, 393, 365–369 (1998). [DOI] [PubMed] [Google Scholar]
- Tausz M., Sircelj H. & Grill D. The glutathione system as a stress marker in plant ecophysiology: is a stress-response concept valid? J. Exp. Bot. 55, 1955–1962 (2004). [DOI] [PubMed] [Google Scholar]
- Yu M. et al. Effects of the 1-alkyl-3-methylimidazolium bromide ionic liquids on the antioxidant defense system of Daphnia magna. Ecotoxicol. Environ. Safe. 72, 1798–1804 (2009). [DOI] [PubMed] [Google Scholar]
- Zhu C., Hu W., Wu H. & Hu X. No evident dose-response relationship between cellular ROS level and its cytotoxicity–a paradoxical issue in ROS-based cancer therapy. Sci. Rep. 4, 5029 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B., Liu G. B., Xue S. & Zhu B. B. Changes in soil physico-chemical andmicrobiological properties during natural succession on abandoned farmlandin the Loess Plateau. Environ. Earth Sci. 62, 915–925 (2011). [Google Scholar]
- Meloni D. A., Oliva M. A., Martinez C. A. & Cambraia J. Photosynthesis and activity of superoxide dismutase, peroxidase and glutathione reductase in cotton under salt stress. Environ. Exp. Bot. 49, 69–76 (2003). [Google Scholar]
- Qiu Z. B., Liu X., Tian X. J. & Yue M. Effects of CO2 laser pretreatment on drought stress resistance in wheat. J. Photoch. Photobio. B 90, 17–25 (2008). [DOI] [PubMed] [Google Scholar]
- Song N. H., Yin X. L., Chen G. F. & Yang H. Biological responses of wheat (Triticum aestivum) plants to the herbicide chlorotoluron in soils. Chemosphere 68, 1779–1787 (2007). [DOI] [PubMed] [Google Scholar]
- Lee H. C. & Lin T. Y. Isolation of plant nuclei suitable for flow cytometry from recalcitrant tissue by use of a filtration column. Plant Mol. Biol. Rep. 23, 53–58 (2005). [Google Scholar]
- Bradford M. M. A rapid sensitive method for the quantification of microgramquantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72, 248–254 (1976). [DOI] [PubMed] [Google Scholar]
- Shen C. H., Krishnamurthy R. & Yeh K. W. Decreased L-ascorbate content mediating bolting is mainly regulated by the galacturonate pathway in Oncidium. Plant Cell Physiol. 50, 935–946 (2009). [DOI] [PubMed] [Google Scholar]
- Anderson D., Yu T. W., Phillips B. J. & Schmezer P. The effects of various antioxidants and other modifying agents on oxygen-radicalgenerated DNA damage in human lymphocytes in the comet assay. Mutat. Res. 307, 261–271 (1994). [DOI] [PubMed] [Google Scholar]