Summary
The CNS contains many diverse neuronal subtypes, and most neurological diseases target specific subtypes. However, the mechanism of neuronal subtype specificity of disease phenotypes remains elusive. Although in vitro disease models employing human pluripotent stem cells (PSCs) have great potential to clarify the association of neuronal subtypes with disease, it is currently difficult to compare various PSC-derived subtypes. This is due to the limited number of subtypes whose induction is established, and different cultivation protocols for each subtype. Here, we report a culture system to control the regional identity of PSC-derived neurons along the anteroposterior (A-P) and dorsoventral (D-V) axes. This system was successfully used to obtain various neuronal subtypes based on the same protocol. Furthermore, we reproduced subtype-specific phenotypes of amyotrophic lateral sclerosis (ALS) and Alzheimer’s disease (AD) by comparing the obtained subtypes. Therefore, our culture system provides new opportunities for modeling neurological diseases with PSCs.
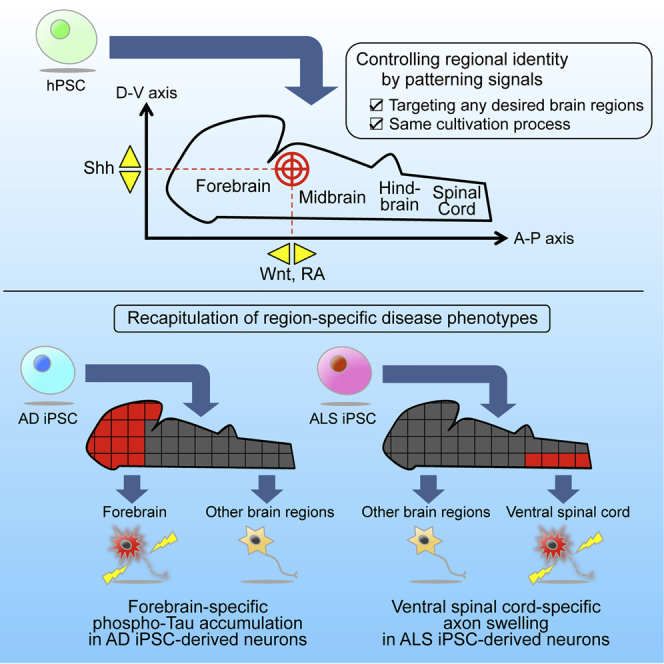
Graphical Abstract
Highlights
-
•
The regional identity of PSC-derived neurons can be controlled precisely
-
•
Phenotypes between different neuronal subtypes were compared successfully
-
•
Neuronal subtype-specific phenotypes of ALS and AD were reproduced in vitro
-
•
A novel tool is offered to study subtype specificity of disease phenotypes
Okano, Akamatsu, and colleagues established a culture system to control the regional identity of PSC-derived neurons. This system was used to obtain a variety of neuronal subtypes based on the same protocol. By comparing these subtypes, neurological disease phenotypes were reproduced in vitro in a subtype-specific manner. Therefore, this system is useful for the study of subtype specificity of disease phenotypes.
Introduction
Modeling human diseases with pluripotent stem cells (PSCs), including embryonic stem cells (ESCs) and induced PSCs (iPSCs), has remarkable potential to generate new insights into understanding disease pathogenesis and to open up new avenues for effective therapies. In particular, modeling neurological diseases is of great interest given that it is difficult to obtain patient-derived neural cells or tissues because of the limited accessibility to the brain. Indeed, ESCs and iPSCs derived from patients have been used to study several neurological diseases, including amyotrophic lateral sclerosis (ALS; Dimos et al., 2008, Egawa et al., 2012), Alzheimer’s disease (AD; Israel et al., 2012, Kondo et al., 2013, Yagi et al., 2011), Parkinson’s disease (Devine et al., 2011, Imaizumi et al., 2012, Nguyen et al., 2011), schizophrenia (Brennand et al., 2011, Bundo et al., 2014, Hook et al., 2014), epilepsy (Higurashi et al., 2013, Jiao et al., 2013, Liu et al., 2013), and Rett syndrome (Andoh-Noda et al., 2015, Marchetto et al., 2010). Because most neurological diseases affect one or more specific lesion area(s), PSCs were differentiated into corresponding neuronal subtypes in such studies (Imaizumi and Okano, 2014, Marchetto and Gage, 2012, Mattis and Svendsen, 2011, Okano and Yamanaka, 2014). However, these approaches cannot procure the mechanism of subtype specificity of disease phenotypes; that is, why some neuronal subtypes are selectively damaged whereas others evade pathogenesis.
To clarify the mechanism of subtype specificity, it is necessary to compare phenotypes between disease-susceptible and -insusceptible neuronal subtypes. Nonetheless, this approach has not been developed to date for two reasons. First, only a few neuronal subtypes can be successfully induced from PSCs. Second, each previously reported protocol to induce specific subtypes varies in its cultivation procedures, giving rise to variability between protocols in efficiency, maturity, and culture-afflicted stress and preventing a direct comparison across different neuronal subtypes.
Here we addressed these problems by focusing on control of the regional identity of PSC-derived neural progenitors and neurons. The developing neural tube is subdivided into distinct regions along the anteroposterior (A-P) and dorsoventral (D-V) axes, and each region produces a specific subtype of neurons (Kiecker and Lumsden, 2012). Namely, neuronal subtype specification in the neural tube is determined in a region-specific manner. If the regional identity of PSC-derived neurons could be regulated at will based on the same protocol, then any desired subtypes could be induced with the same efficiency and under the same culture conditions. A protocol permitting such regulation would enable the reproduction of disease phenotypes in any given brain region and also the comparison of phenotypes between different neuronal subtypes.
In this study, we report a robust method to control the regional identity of PSC-derived neural progenitors and neurons based on a single protocol. To do so, Wnt, retinoic acid (RA), and sonic hedgehog (Shh) signaling were modulated to manipulate the A-P and D-V identities of neural progenitors. Regional identity was maintained throughout neural differentiation, generating a variety of neuronal subtypes, including cortical projection neurons, cortical interneurons, midbrain dopaminergic neurons, hindbrain serotonergic neurons, spinal cord sensory interneurons, and spinal cord motor neurons. Finally, we compared these neuronal subtypes and detected ventral spinal cord-specific neurite swellings in ALS iPSCs and forebrain-specific accumulation of phosphorylated tau (p-tau) in AD iPSCs. Therefore, this culture system could be a useful tool to approach the mechanism of subtype specificity of neurological disease phenotypes.
Results
Strategy for Defining the Regional Identity of PSC-Derived Neural Progenitors
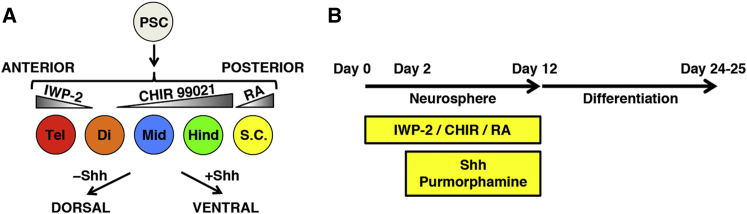
Because Wnt, RA, and Shh signaling are involved in the formation of the A-P and D-V axes during neural development (Briscoe and Ericson, 2001, Marshall et al., 1992, Nordström et al., 2002), we explored the possibility that these patterning signaling molecules might have analogous effects in neural cells differentiated from PSCs. We hypothesized that the A-P identity (ranging from the telencephalon to the spinal cord) can be controlled by regulating Wnt and RA signaling and that the D-V identity can be controlled similarly by regulating Shh signaling (Figure 1A). To evaluate this hypothesis, we induced neural progenitors derived from ESCs (KhES-1; Suemori et al., 2006) by using the neurosphere culture system (modified from Chaddah et al., 2012) and treated the cells with modulators of these patterning signaling pathways during neurosphere formation (Figure 1B). The modulators included the porcupine inhibitor IWP-2 (a Wnt antagonist; Chen et al., 2009), the glycogen synthase kinase (GSK) 3β inhibitor CHIR99021 (a Wnt agonist, referred to hereafter as CHIR; Ring et al., 2003), RA, Shh protein, and the Smo receptor agonist purmorphamine (a Shh agonist; Sinha and Chen, 2006). Although the diameter of the neurospheres was increased by treatment with CHIR and RA, each of the experimental conditions was able to generate neurospheres (Figures S1A and S1B). Moreover, the patterning factors did not influence the number of neurospheres generated (Figure S1C), and the neural progenitor marker NESTIN was expressed similarly under all conditions (Figure S1D). These results indicate that neural induction is unaffected by Wnt, RA, or Shh signaling.
Figure 1.
Strategy for Defining the Regional Identity of PSC-Derived Neural Progenitors
(A) Schematic of the strategy employed for the manipulation of regional identity. Wnt signaling was modulated by using the porcupine inhibitor IWP-2 and the GSK3β inhibitor CHIR. Tel, telencephalon; Di, diencephalon; Mid, midbrain; Hind, hindbrain; S.C., spinal cord.
(B) Overview of the culture protocol. Patterning factors (IWP-2, CHIR, RA, Shh, and purmorphamine) were added during neurosphere formation.
Modulation of the A-P Identity of PSC-Derived Neural Progenitors by Wnt and RA Signaling
We next examined the expression of A-P markers (Figure 2A) in ESC-derived neurospheres by qRT-PCR (Figure 2B). IWP-2-treated neurospheres expressed high levels of the forebrain markers FOXG1 and SIX3 (Oliver et al., 1995, Xuan et al., 1995). The expression levels of the forebrain/midbrain markers OTX1 and OTX2 (Simeone et al., 1992) were highest in untreated neurospheres. The low expression level of OTX1 in IWP-2-treated neurospheres agrees with the observation that this gene is not expressed in the anterior-most region of the forebrain (Shimamura et al., 1997). EN1, which is expressed in the midbrain and the anterior hindbrain (Hanks et al., 1995), was highly expressed in neurospheres treated with CHIR at concentrations of 0.5 and 1 μM. HOXB4 and HOXC4, markers of the posterior hindbrain and the spinal cord (Hunt et al., 1991), were expressed primarily in cultures exposed to 3 μM CHIR, and their expression levels were enhanced further by the addition of RA. These results indicate that the posteriorization of neural progenitors is driven by activation of the Wnt and RA signaling pathways.
Figure 2.
A-P Patterning by Modifying Wnt and RA Signaling
(A) Expression of A-P markers in the neural tube in vivo.
(B) qRT-PCR analysis of neurospheres for A-P marker expression (n = 3 independent experiments, mean ± SEM). Activation of Wnt and RA signaling posteriorized the neurospheres, whereas blockade of Wnt signaling anteriorized the neurospheres.
(C) Immunocytochemical analysis of neurospheres for A-P markers. The frequency of neurospheres containing immunopositive cells is shown as the percentage of total neurospheres (n = 3 independent experiments, mean ± SEM). A-P patterning was also confirmed at the protein level. Scale bar, 50 μm).
See also Table S1.
The regulation of marker expression correlating with the A-P axis was also confirmed by immunocytochemical analysis for the FOXG1, OTX1, and HOXB4 proteins (Figure 2C). Furthermore, we confirmed the robustness of our protocol by using the 201B7 and 253G1 iPSC lines (Nakagawa et al., 2008, Takahashi et al., 2007). In both of these iPSC lines, the expression levels of the A-P markers in the neurospheres were similar to those in ESC-derived neurospheres (Figure S2). These results suggest that the A-P identity of PSC-derived neural progenitors can be controlled by IWP-2, CHIR, and RA during neurosphere formation.
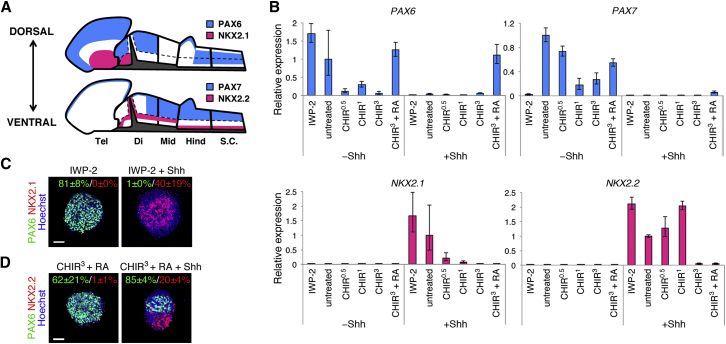
Control of the D-V Identity of PSC-Derived Neural Progenitors by Shh Signaling
In addition to the A-P identity, we next attempted to control the D-V identity by modulating Shh signaling. We hypothesized that neural progenitors derived from PSCs are dorsalized in the absence of Shh and that the activation of Shh signaling ventralizes neural progenitors with no influence on the A-P identity. To address this hypothesis, we assessed the expression levels of D-V makers in neurospheres by qRT-PCR analysis with the addition of Shh protein and purmorphamine (termed the “+Shh” group) (Figures 3A and 3B). We used these two Shh activators simultaneously because a previous report has demonstrated that co-treatment with Shh protein and purmorphamine was greatly superior to high concentrations of either of the two alone (Maroof et al., 2013). Other neurospheres received no exogenous ventralizing factors (termed the “−Shh” group).
Figure 3.
D-V Patterning by Treatment with Shh
(A) Expression of D-V markers in the neural tube. The dashed line indicates the alar-basal boundary.
(B) qRT-PCR analysis of neurospheres for D-V marker expression (n = 3 independent experiments, mean ± SEM). Neurospheres not treated with Shh showed a dorsal identity, whereas Shh treatment ventralized the neurospheres.
(C and D) Immunocytochemical analysis of neurospheres for PAX6/NKX2.1 (C) and PAX6/NKX2.2 (D). The frequency of neurospheres containing immunopositive cells is shown as the percentage of total neurospheres (n = 3 independent experiments, mean ± SEM). D-V patterning was also confirmed at the protein level. Scale bars, 50 μm.
See also Table S1.
The D-V markers PAX6, PAX7, NKX2.1, and NKX2.2 are differentially expressed in vivo according to A-P and D-V positions (Figure 3A). PAX6 is expressed dorsally in the forebrain, and the expression pattern also covers both the dorsal and the ventral portions of the posterior hindbrain and the spinal cord (Ericson et al., 1997, Osumi et al., 1997, Takahashi and Osumi, 2002, Walther and Gruss, 1991). In contrast, the midbrain and the anterior hindbrain lack PAX6 expression (with the exception of the rhombic lip) (Engelkamp et al., 1999, Schwarz et al., 1999, Swanson et al., 2005, Yamasaki et al., 2001). PAX7 is expressed throughout the dorsal part of the neural tube except for the anterior forebrain, where this gene is expressed only in the small dorsal-most region (Matsunaga et al., 2001). NKX2.1 marks the ventral forebrain (Qiu et al., 1998), whereas NKX2.2 is expressed ventrally throughout the A-P axis (Shimamura et al., 1995). Consistent with the in vivo expression patterns of these genes, PAX6 was highly expressed in neurospheres with forebrain characteristics (i.e., IWP-2-treated and untreated neurospheres) in the −Shh group as well as those with the characteristics of the posterior hindbrain and the spinal cord (i.e., CHIR3+RA-treated neurospheres). Only the latter maintained PAX6 expression after Shh activation. Meanwhile, PAX7 was upregulated in the −Shh group, except under the IWP-2-treated condition, whereas PAX7 expression was low under all conditions in the +Shh group. NKX2.1 was expressed primarily in neurospheres with forebrain characteristics (IWP-2-treated and untreated) in the +Shh group. The expression of NKX2.2 was generally high in the +Shh group. The relatively low expression level of NKX2.2 in neurospheres with posterior characteristics in the +Shh group agrees with the fact that NKX2.2 expression is initially only seen in the anterior part of the neural tube and then gradually extends to the spinal cord (Shimamura et al., 1995).
The control of the D-V axis shown by qRT-PCR analysis was also confirmed by immunocytochemical analysis for the PAX6, NKX2.1, and NKX2.2 proteins (Figures 3C and 3D). Moreover, D-V regulation was reproduced readily in the iPSC lines (Figure S2). Our results indicate that the D-V identity of PSC-derived neural progenitors can be controlled without perturbing the A-P identity.
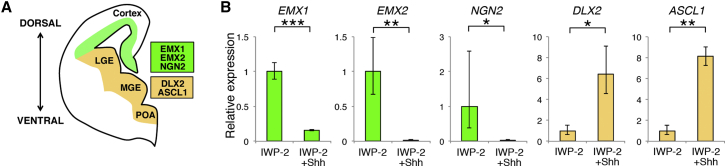
Recapitulation of Telencephalic D-V Patterning
To further confirm the D-V control, we examined the expression of telencephalon-specific D-V markers (Figure 4A). EMX1/2 and DLX2 are confined to the dorsal and the ventral telencephalon, respectively (with the exception of small diencephalic regions) (Porteus et al., 1991, Simeone et al., 1992). Although the proneural genes NGN2 and ASCL1 are expressed in various regions of the neural tube, a D-V bias in their expression is clearly observed in the telencephalon (Fode et al., 2000). EMX1/2 and NGN2 were significantly upregulated in IWP-2-treated neurospheres without Shh activation. In contrast, the expression of DLX2 and ASCL1 was increased by Shh activation. These data provide additional evidence for the D-V control of PSC-derived neural progenitors.
Figure 4.
Telencephalic D-V Patterning
(A) Expression of D-V markers in the telencephalon. MGE, medial ganglionic eminence; LGE, lateral ganglionic eminence; POA, preoptic area.
(B) qRT-PCR analysis of neurospheres for telencephalic D-V marker expression (n = 3−4 independent experiments; mean ± SEM; ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001; Student’s t test).
Differentiation of Neural Progenitors into Specific Neuronal Subtypes in Accordance with Regional Identity
To investigate whether the regional identity of neural progenitors was retained upon differentiation into neurons, neurospheres were subjected to a neural differentiation protocol (Figure 1B). Our protocol predominantly produced neurons rather than astrocytes or oligodendrocytes (Figure S3A). We examined the expression of synapse markers and the intracellular calcium level during electrical field stimulation (Figures S3B–S3D). These results indicate that neurons derived by our protocol were functional regardless of the experimental conditions.
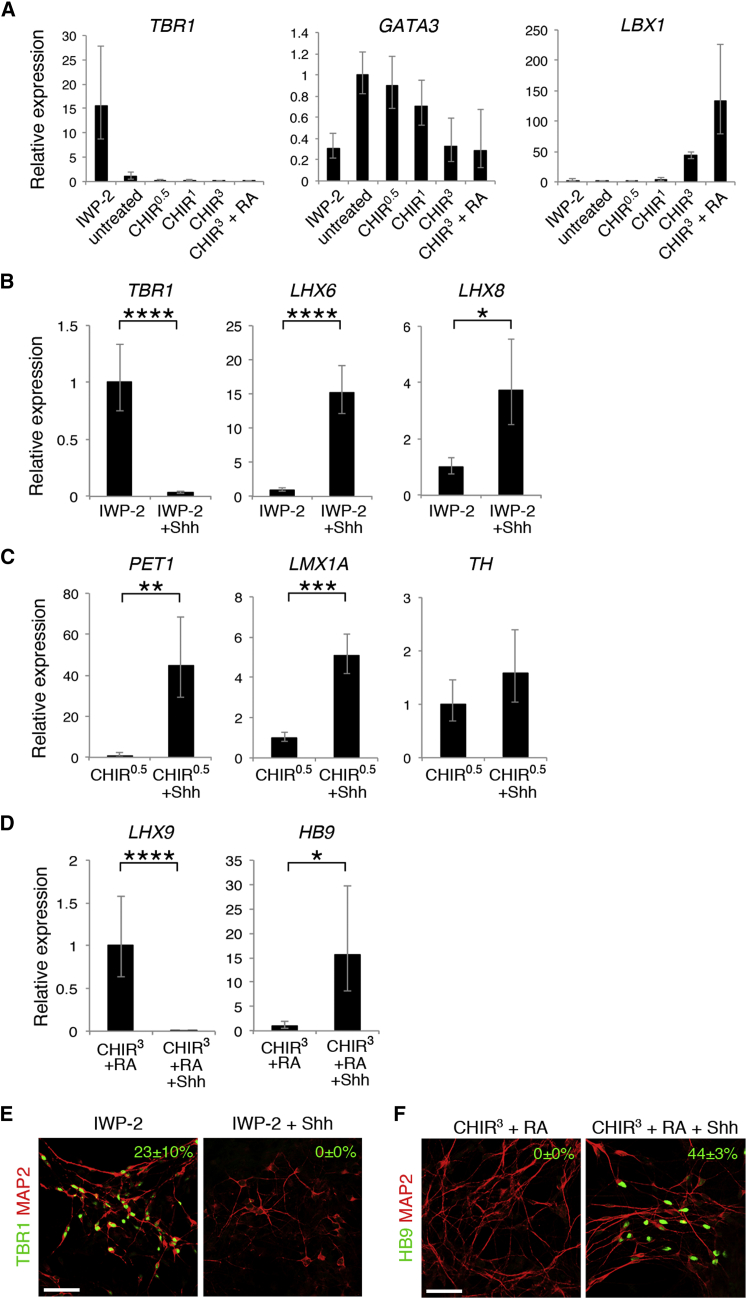
We determined the A-P identity of neurosphere-differentiated neurons (Figure 5A). Neurons generated from IWP-2-treated neurospheres expressed high levels of the forebrain cortical neuronal marker TBR1 (Bulfone et al., 1995). GATA3, expressed from the diencephalon to the hindbrain (George et al., 1994), was upregulated at low CHIR concentrations (0−1 μM). LBX1, which marks dorsal neurons in the hindbrain and the spinal cord (Jagla et al., 1995), was highly expressed in neurons differentiated from CHIR3+RA-treated neurospheres. These results imply that neural progenitors retain their A-P identity in differentiated neuronal cultures.
Figure 5.
Generation of Region-Specific Neuronal Subtypes
(A) qRT-PCR analysis of neurosphere-derived neurons for TBR1, GATA3, and LBX1 expression (n = 4−5 independent experiments, mean ± SEM). A-P patterning was maintained throughout neuronal differentiation in vitro.
(B−D) qRT-PCR analysis of neurosphere-derived neurons for the expression of neuronal subtype markers expressed in the forebrain (B), the mid/hindbrain (C), and the spinal cord (D) (n = 5−7 independent experiments; mean ± SEM; ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001; Student’s t test). Neurospheres differentiated into specific neuronal subtypes in accordance with their regional identity.
(E and F) Immunocytochemical analysis of neurosphere-derived neurons for TBR1 (E) and HB9 (F). The frequency of immunopositive cells is shown as the percentage of MAP2+ neurons (n = 3 independent experiments, mean ± SEM). Scale bars, 50 μm.
See also Table S1.
We next examined the D-V identity of differentiated neurons (Figures 5B–5F). In the telencephalon, TBR1+ cortical neurons are born dorsally, whereas the ventral area produces cortical interneurons, which is marked by LHX6 and LHX8 expression (Marín and Rubenstein, 2001). As expected, the expression level of TBR1 was elevated in neurons generated from IWP-2-treated neurospheres and decreased by activation of Shh signaling (Figures 5B and 5E). In contrast, LHX6 and LHX8 were significantly upregulated by Shh activation (Figure 5B). Dopaminergic and serotonergic neurons are selectively derived from the ventral portion of the midbrain and the hindbrain, respectively (Goridis and Rohrer, 2002). Although the gene expression of the dopamine-synthesizing enzyme TH did not change significantly, that of LMX1A and FOXA2, master transcriptional regulators of dopaminergic neuronal differentiation (Andersson et al., 2006, Ferri et al., 2007), was upregulated markedly by Shh activation under the 0.5 μM CHIR-treated condition (Figure 5C; Figure S4A). We also detected high expression of the serotonergic neuronal marker PET1 (Hendricks et al., 1999) concomitantly with a small population of cells that were immunopositive for serotonin (Figure 5C; Figure S4C). Furthermore, VMAT2 and AADC, markers for both dopaminergic and serotonergic neurons (Flames and Hobert, 2011), were upregulated (Figure S4A). LHX9, which marks d1 neurons in the dorsal spinal cord (Liem et al., 1997), was highly expressed in neurons generated from CHIR3+RA-treated neurospheres, whereas the expression of HB9, which marks motor neurons in the ventral spinal cord (Arber et al., 1999), was elevated by ventralization via Shh activation (Figures 5D and 5F). CHT, an additional spinal cord motor neuron marker that is essential for the uptake of choline into motor neurons (Okuda et al., 2000), was also upregulated (Figure S4B).
Finally, we tested whether neural crest-derived peripheral neurons were induced. The peripheral neuron marker Peripherin (Escurat et al., 1990) was not detected under almost all conditions. However, approximately 20% of neurons were immunopositive for this marker under the CHIR3+RA-treated and CHIR3+RA+Shh-treated conditions. Under the CHIR3+RA-treated condition, BRN3A, an additional peripheral neuron marker (Fedtsova and Turner, 1995), was also expressed in a portion of Peripherin+ neurons (Figure S4D). Given that neural crest cells originate from the dorsal-most region of the neural tube (Knecht and Bronner-Fraser, 2002), these results are consistent with their dorsal identity. Under the CHIR3+RA+Shh-treated condition, however, Peripherin+ neurons expressed the motor neuron marker SMI-32 (Carriedo et al., 1996). This result is consistent with the fact that Peripherin is expressed not only in peripheral neurons but also in spinal cord motor neurons (Escurat et al., 1990). In summary, both the A-P and D-V identities acquired in the neural progenitor stage were maintained throughout neuronal differentiation in culture, and neural progenitors were able to differentiate into specific neuronal subtypes in accordance with their regional identities.
Reproduction of Subtype-Specific Disease Phenotypes In Vitro
In most neurological diseases, specific neuronal subtypes are selectively impaired. For example, spinal cord motor neurons are selectively damaged in ALS (Bruijn et al., 2004), and forebrain cortical neurons are preferentially affected in AD (Braak and Braak, 1991, Morrison and Hof, 1997, Thal et al., 2002). Although this subtype specificity probably plays an important role in pathology, its mechanism remains to be elucidated. Particularly in PSC-based disease models, most previous reports have focused only on the disease-susceptible neuronal subtype, and other aspects of subtype specificity have yet to be explored.
Our first aim was to test whether disease subtype specificity can be recapitulated in vitro by using PSCs. We derived neurons from disease-specific iPSC lines via our protocol described above. Here we used ALS and AD iPSC lines carrying TARDBPM337V and PSEN1A246E/PSEN2N141I mutations, respectively (Egawa et al., 2012, Yagi et al., 2011), and the control of regional identity was confirmed in neurospheres derived from each iPSC line (Figure S5).
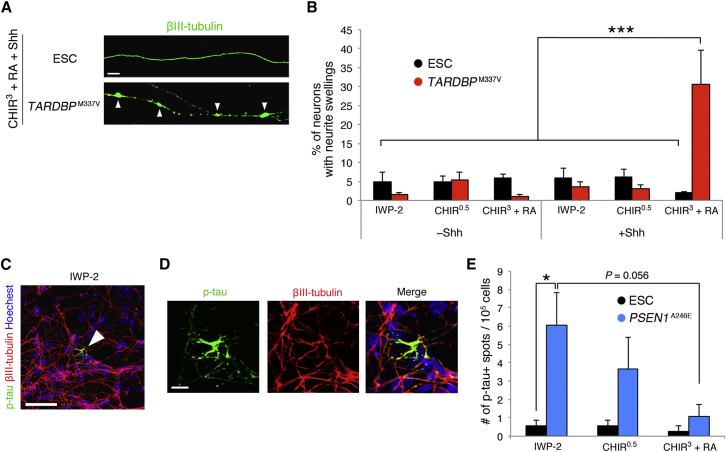
In the ALS iPSC-derived neurons, neurite swellings were observed preferentially in neurons with the characteristics of the ventral spinal cord generated from CHIR3+RA+Shh-treated neurospheres (Figures 6A and 6B). In contrast, neurons with other regional identities rarely exhibited swellings. Because neurite swellings (or “spheroids”) are a feature of neuronal cell dysfunction in ALS (Delisle and Carpenter, 1984), these observations suggest that ventral spinal cord-specific phenotypes of ALS were recapitulated successfully in our system. Next, we tried to demonstrate motor neuron specificity among the ventral spinal cord subtypes by labeling motor neurons with the fluorescence protein (Venus) reporter under control of the HB9 promoter. However, the cells showed fluorescence labeling of only the soma and proximal neurites, probably because of weak intracellular diffusion of the Venus protein. Consequently, we failed to identify neurite swellings distal to the soma in HB9-Venus-positive neurons (data not shown). Therefore, currently, we cannot eliminate the possibility that subtypes other than motor neurons in the ventral spinal cord were also affected by neurite swellings. Indeed, several recent reports indicate that spinal cord interneurons are implicated in ALS pathology (reviewed in Turner and Kiernan, 2012).
Figure 6.
Reproduction of Subtype-Specific Disease Phenotypes In Vitro
(A) Representative images of neurites from PSC-derived neurons with the characteristics of the ventral spinal cord (CHIR3+RA+Shh-treated). ALS iPSC-derived neurons exhibited swollen neurites (arrowheads). Scale bar, 10 μm.
(B) Quantification of neurons with neurite swellings (n = 3 independent experiments, mean ± SEM, ∗∗∗p < 0.001, ANOVA with Tukey’s test). ALS iPSC-derived neurons with the characteristics of the ventral spinal cord were remarkably damaged.
(C) Immunocytochemical analysis of AD iPSC-derived neurons with forebrain characteristics (IWP-2-treated). Accumulation of p-tau was observed in the AD iPSC cultures (arrowhead). Scale bar, 50 μm.
(D) Higher-magnification images of the immunoreactive p-tau+ spots shown in (C). Scale bar, 10 μm.
(E) Quantification of p-tau+ spots (n = 3 independent experiments, mean ± SEM, ∗p < 0.05, ANOVA with Tukey’s test). Accumulation of p-tau was increased in AD iPSC-derived neurons with forebrain characteristics.
In AD iPSC-derived neurons, we found spot-like accumulations of p-tau (Figures 6C and 6D). The number of p-tau+ spots increased in neurons with forebrain characteristics generated from IWP-2-treated neurospheres compared with the CHIR3+RA condition corresponding to the spinal cord (Figure 6E; Figure S6A). To eliminate the possibility that the addition of CHIR during the neurosphere stage led to inhibition of GSK3β-mediated tau phosphorylation in the differentiation stage, we next measured GSK3β activity in differentiated neurons by quantifying nuclear β-catenin intensity. We confirmed that β-catenin intensity remained relatively constant under all conditions and that residual CHIR had no effect on tau phosphorylation in the differentiation stage (Figure S6B). These results indicate that a characteristic feature of AD pathology, i.e., p-tau accumulation, was reproduced in a region-specific manner. Taken together, our findings show that the subtype specificity of neurological disease phenotypes can be modeled in vitro by defining the regional identity of PSC-derived neural progenitors and neurons.
Discussion
In this study, we established a culture system for controlling the regional identity of PSC-derived neural progenitors and neurons based on an identical protocol. Simple treatment with three factors, IWP-2, CHIR, and RA, effectively patterned the A-P identity, ranging from the telencephalon to the spinal cord. In addition, Shh activation converted the D-V identity from dorsal into ventral without perturbing the A-P identity. Furthermore, subtype-specific ALS and AD phenotypes were reproduced in vitro by using the described protocol.
Our findings are consistent with previous reports demonstrating that the graded signaling activities of Wnt/RA and Shh establish the A-P and D-V axes in vivo. Also noteworthy is that our study shows that this model for early neural patterning, which is proposed mostly from work on Xenopus, chicks, and mice, can be applied to human development. Because in vivo experiments of the human embryo are ethically challenging and technically difficult, our culture system now provides novel opportunities to uncover the mechanism of neural patterning in humans.
A remaining issue regarding our present culture system is the ability to recapitulate the environment after regional specification. In some neuronal subtypes, additional signals are needed for further specification, maturation, and maintenance. When cells migrate from their birthplace or if they project to distant targets, they often receive such signals from cells with different regional identity. Such additional signals cannot be recapitulated in our present culture system. In the case of midbrain dopaminergic neurons, for example, transforming growth factor β (TGF-β) expressed in their projection targets is necessary for their maintenance (Poulsen et al., 1994). Dopaminergic neurons in our culture did not express late-stage markers such as PITX3 and DAT, probably because of the lack of such additional signals (data not shown). In addition to treatment with signaling modulators, co-culturing with cells of the destination of migration or cells of the projection targets would be a promising approach to overcome this issue.
Specific brain regions are preferentially damaged in most neurological diseases, whereas regions other than the lesioned area remain relatively unaffected. For this reason, the differentiation of patient-derived iPSCs into specific neuronal subtypes representing the lesion site is a valuable approach for modeling human neurological diseases (Imaizumi and Okano, 2014, Okano and Yamanaka, 2014). The current investigation reproduced p-tau accumulation as an AD phenotype by endowing neurons with forebrain characteristics. On the other hand, a previous report using the same iPSC clones did not detect such a phenotype, probably because of the induction of inappropriate subtypes (Yagi et al., 2011). These results highlight the importance of correct neuronal subtype specification in reproducing neurological disease phenotypes with PSCs.
Some investigators have reported the directed differentiation of PSCs into a limited number of neuronal subtypes, including midbrain dopaminergic neurons and spinal cord motor neurons (Kriks et al., 2011, Li et al., 2005). Given that each differentiation protocol is overly optimized for a particular subtype, the protocol in question cannot be readily applied to additional neuronal subtypes. Nevertheless, our culture system theoretically enables the differentiation of PSCs into all neuronal subtypes, making it possible to efficiently generate subtypes whose induction from PSCs has been difficult.
Also, as emphasized above, our culture system is based on the same protocol to obtain neuronal subtypes with equivalent efficiency and under the same general culture conditions (with the exception of the modulation of different signaling cascades). Therefore, our method allows comparative analysis of a variety of neuronal subtypes, whereas earlier investigations on disease modeling have focused only on selectively affected subtypes. Moreover, other investigators have reported that the modulation of Wnt signaling can direct the regional identity of PSC-derived neurons in a manner similar to that observed in our culture system. Nevertheless, a parallel analysis of disease phenotypes between neurons with different regional identities was not performed in these studies (Kirkeby et al., 2012, Moya et al., 2014). To fully understand the mechanism of disease pathology and progression, one must investigate not only the manner in which specific neuronal subtypes are affected but also how other subtypes remain unaffected. In the case of genetic diseases, neural subtype specificity of disease phenotypes is not necessarily accounted for by the expression pattern of the responsible genes. For example, some mutations in TARDBP, which is globally expressed across the entire brain, cause motor neuron-selective degeneration (Kabashi et al., 2008, Kabashi et al., 2010, Sephton et al., 2010). Similarly, although PSEN1 and PSEN2 show widespread expression in the brain, their mutations predominantly affect forebrain neurons (Rogaev et al., 1995, Sherrington et al., 1995). However, the mechanism of neuronal subtype specificity of these disease phenotypes is unclear. Our culture system may permit investigations toward filling in the gaps in this knowledge base.
We do not yet know the mechanism of neuronal subtype specificity of various neurological disease phenotypes. Identification of factors enforcing pathogenesis in disease-susceptible subtypes or evading pathogenesis in insusceptible subtypes will help to clarify this. Regardless, our culture system will undoubtedly allow new insights into modeling neurological diseases with PSCs.
Experimental Procedures
Culture of Undifferentiated ESCs and iPSCs
The human ESC (hESC) line KhES-1 (Suemori et al., 2006), the control human iPSC (hiPSC) lines 201B7 and 253G1 (Nakagawa et al., 2008, Takahashi et al., 2007), the ALS iPSC line A3411 (Egawa et al., 2012), and the AD iPSC lines PS1-2 and PS2-1 (Yagi et al., 2011) were cultured on mitomycin C-treated SNL murine fibroblast feeder cells in standard hESC medium (DMEM/F12, Sigma) containing 20% KnockOut serum (KSR) replacement (Life Technologies), 0.1 mM non-essential amino acids (Sigma), 0.1 mM 2-mercaptoethanol (Sigma), and 4 ng/ml fibroblast growth factor 2 (FGF-2) (PeproTech)) in an atmosphere containing 3% CO2.
hESCs were used in accordance with the guidelines regarding the utilization of hESCs with approval from the Ministry of Education, Culture, Sports, Science, and Technology (MEXT) of Japan and the Keio University School of Medicine Ethics Committee. All experimental procedures for iPSCs derived from patients were approved by the Keio University School of Medicine Ethics Committee (approval no. 20080016).
Neuronal Induction
Neuronal induction of hESCs/iPSCs was performed as described previously (Chaddah et al., 2012), with slight modifications. Briefly, hESCs/iPSCs were pretreated for 6 days with 3 μM SB431542 (Tocris), 150 nM LDN193189 (StemRD), and 3 μM CHIR99021 (Stemgent). They were then dissociated and seeded at a density of 10 cells/μl in medium hormone mix (MHM) (Okada et al., 2004, Okada et al., 2008, Shimazaki et al., 2001) with selected growth factors and inhibitors under conditions of 4% O2/5% CO2. The growth factors and inhibitors included 10 ng/ml human leukemia inhibitory factor (LIF) (Nacalai Tesque), 20 ng/ml FGF-2, 1× B27 supplement (Invitrogen), 2 μM SB431542, and 10 μM Y-27632 (Calbiochem).
Defining the day on which neurosphere culture was started as day 0, the following additives were included in the neurosphere culture: 2 μM IWP-2 (Sigma), 0.5–3 μM CHIR99021, and 1 μM RA (Sigma) from days 0–12 and 100 ng/ml Shh-C24II (R&D Systems) and 1 μM purmorphamine (Calbiochem) from days 2–12. On day 12, neurospheres were replated en bloc on dishes coated with poly-ornithine and laminin and cultured under conditions of 5% CO2. The medium was changed to MHM supplemented with 1× B27 and 1 μM DAPT (Sigma). DAPT was excluded in the AD phenotype experiments.
qRT-PCR
Total RNA was isolated with the RNeasy mini kit (QIAGEN) with DNase I treatment, and cDNA was prepared by using a ReverTraAce qPCR RT kit (Toyobo). The qRT-PCR analysis was performed with SYBR premix Ex TaqII (Takara Bio) on a ViiA 7 real-time PCR system (Applied Biosystems). Values were normalized to ACTB. Reactions were carried out in duplicate, and data were analyzed by using the comparative (ΔΔCt) method. Relative expression levels are presented as geometric means ± geometric SEM. The primer sets used in these experiments are listed in Table S2.
Immunocytochemistry
Cells were fixed with 4% paraformaldehyde for 15 min at room temperature and then washed three times with PBS. After incubating with blocking buffer (PBS containing 5% normal fetal bovine serum and 0.3% Triton X-100) for 1 hr at room temperature, the cells were incubated overnight at 4°C with primary antibodies diluted with blocking buffer. We used blocking buffer without Triton X-100 for O4 staining and buffer with 0.03% Triton X-100 for Synaptotagmin and VAMP2 staining. Details of the primary antibodies and the dilution conditions are listed in Table S3. The cells were again washed three times with PBS and incubated with secondary antibodies conjugated with Alexa Fluor 488, Alexa Fluor 555, or Alexa Fluor 647 (Life Technologies) and Hoechst33342 (Dojindo Laboratories) for 1 hr at room temperature. After washing three times with PBS and once with distilled water, samples were mounted on slides and examined by using a LSM-710 confocal laser-scanning microscope (Carl Zeiss). For anti-NKX2.2 antibody staining, biotinylated secondary antibodies (Jackson ImmunoResearch Laboratories) were used after exposure to 1% H2O2 for 30 min at room temperature to inactivate endogenous peroxidase. The signals were then enhanced by using the Vectastain ABC kit (Vector Laboratories), followed by the TSA fluorescence system (PerkinElmer).
Author Contributions
K. Imaizumi, W.A., and H.O. conceived and designed the project. K. Imaizumi and T.S. performed the experiments. K. Ibata and M.Y. performed the calcium imaging assays. K. Imaizumi and K.F. established the nuclear β-catenin intensity assay. K. Imaizumi, W.A., and H.O. interpreted the data and wrote the manuscript.
Acknowledgments
We are grateful to Y. Okada (Aichi Medical University) and T. Matsumoto (Ajinomoto) for technical assistance, helpful advice, and discussions; T. Abe (Keio University) for statistical analysis; and all members of the H.O. laboratory for encouragement and kind support. We also thank Y. Okada for the HB9-Venus reporter, M. Itakura (Kitasato University) for antibodies, N. Nakatsuji and H. Suemori (Kyoto University) for hESC clones, S. Yamanaka and M. Nakagawa (Kyoto University) for hiPSC clones (253G1 and 201B7), H. Inoue (Kyoto University) for ALS iPSC clones, and N. Suzuki and D. Ito (Keio University) for AD iPSC clones. This work was supported by funding from the Project for the Realization of Regenerative Medicine and Support for Core Institutes for iPS Cell Research from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (MEXT) (to H.O. and W.A.); the Research Center Network for Realization Research Centers/Projects of Regenerative Medicine (the Program for Intractable Disease Research utilizing disease-specific iPS Cells) from the Japan Science and Technology Agency (JST) and the Japan Agency for Medical Research and Development (AMED) (to H.O.); Scientific Research on Innovative Area, a MEXT Grant-in-Aid Project FY2014-2018 “Brain Protein Aging and Dementia Control” (to H.O.); New Energy and Industrial Technology Development Organization (to H.O. and W.A.); and the Keio University Medical Science Fund (to K. Imaizumi). H.O. is a paid scientific advisory board member for SanBio Co., Ltd.
Published: November 5, 2015
Footnotes
This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
Supplemental Information includes Supplemental Experimental Procedures, six figures, and three tables and can be found with this article online at http://dx.doi.org/10.1016/j.stemcr.2015.10.005.
Contributor Information
Wado Akamatsu, Email: awado@juntendo.ac.jp.
Hideyuki Okano, Email: hidokano@a2.keio.jp.
Supplemental Information
References
- Andersson E., Tryggvason U., Deng Q., Friling S., Alekseenko Z., Robert B., Perlmann T., Ericson J. Identification of intrinsic determinants of midbrain dopamine neurons. Cell. 2006;124:393–405. doi: 10.1016/j.cell.2005.10.037. [DOI] [PubMed] [Google Scholar]
- Andoh-Noda T., Akamatsu W., Miyake K., Matsumoto T., Yamaguchi R., Sanosaka T., Okada Y., Kobayashi T., Ohyama M., Nakashima K. Differentiation of multipotent neural stem cells derived from Rett syndrome patients is biased toward the astrocytic lineage. Mol. Brain. 2015;8:31. doi: 10.1186/s13041-015-0121-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arber S., Han B., Mendelsohn M., Smith M., Jessell T.M., Sockanathan S. Requirement for the homeobox gene Hb9 in the consolidation of motor neuron identity. Neuron. 1999;23:659–674. doi: 10.1016/s0896-6273(01)80026-x. [DOI] [PubMed] [Google Scholar]
- Braak H., Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- Brennand K.J., Simone A., Jou J., Gelboin-Burkhart C., Tran N., Sangar S., Li Y., Mu Y., Chen G., Yu D. Modelling schizophrenia using human induced pluripotent stem cells. Nature. 2011;473:221–225. doi: 10.1038/nature09915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briscoe J., Ericson J. Specification of neuronal fates in the ventral neural tube. Curr. Opin. Neurobiol. 2001;11:43–49. doi: 10.1016/s0959-4388(00)00172-0. [DOI] [PubMed] [Google Scholar]
- Bruijn L.I., Miller T.M., Cleveland D.W. Unraveling the mechanisms involved in motor neuron degeneration in ALS. Annu. Rev. Neurosci. 2004;27:723–749. doi: 10.1146/annurev.neuro.27.070203.144244. [DOI] [PubMed] [Google Scholar]
- Bulfone A., Smiga S.M., Shimamura K., Peterson A., Puelles L., Rubenstein J.L. T-brain-1: a homolog of Brachyury whose expression defines molecularly distinct domains within the cerebral cortex. Neuron. 1995;15:63–78. doi: 10.1016/0896-6273(95)90065-9. [DOI] [PubMed] [Google Scholar]
- Bundo M., Toyoshima M., Okada Y., Akamatsu W., Ueda J., Nemoto-Miyauchi T., Sunaga F., Toritsuka M., Ikawa D., Kakita A. Increased l1 retrotransposition in the neuronal genome in schizophrenia. Neuron. 2014;81:306–313. doi: 10.1016/j.neuron.2013.10.053. [DOI] [PubMed] [Google Scholar]
- Carriedo S.G., Yin H.Z., Weiss J.H. Motor neurons are selectively vulnerable to AMPA/kainate receptor-mediated injury in vitro. J. Neurosci. 1996;16:4069–4079. doi: 10.1523/JNEUROSCI.16-13-04069.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaddah R., Arntfield M., Runciman S., Clarke L., van der Kooy D. Clonal neural stem cells from human embryonic stem cell colonies. J. Neurosci. 2012;32:7771–7781. doi: 10.1523/JNEUROSCI.3286-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen B., Dodge M.E., Tang W., Lu J., Ma Z., Fan C.-W., Wei S., Hao W., Kilgore J., Williams N.S. Small molecule-mediated disruption of Wnt-dependent signaling in tissue regeneration and cancer. Nat. Chem. Biol. 2009;5:100–107. doi: 10.1038/nchembio.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delisle M.B., Carpenter S. Neurofibrillary axonal swellings and amyotrophic lateral sclerosis. J. Neurol. Sci. 1984;63:241–250. doi: 10.1016/0022-510x(84)90199-0. [DOI] [PubMed] [Google Scholar]
- Devine M.J., Ryten M., Vodicka P., Thomson A.J., Burdon T., Houlden H., Cavaleri F., Nagano M., Drummond N.J., Taanman J.-W. Parkinson’s disease induced pluripotent stem cells with triplication of the α-synuclein locus. Nat. Commun. 2011;2:440. doi: 10.1038/ncomms1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimos J.T., Rodolfa K.T., Niakan K.K., Weisenthal L.M., Mitsumoto H., Chung W., Croft G.F., Saphier G., Leibel R., Goland R. Induced pluripotent stem cells generated from patients with ALS can be differentiated into motor neurons. Science. 2008;321:1218–1221. doi: 10.1126/science.1158799. [DOI] [PubMed] [Google Scholar]
- Egawa N., Kitaoka S., Tsukita K., Naitoh M., Takahashi K., Yamamoto T., Adachi F., Kondo T., Okita K., Asaka I. Drug screening for ALS using patient-specific induced pluripotent stem cells. Sci. Transl. Med. 2012;4:145ra104. doi: 10.1126/scitranslmed.3004052. [DOI] [PubMed] [Google Scholar]
- Engelkamp D., Rashbass P., Seawright A., van Heyningen V. Role of Pax6 in development of the cerebellar system. Development. 1999;126:3585–3596. doi: 10.1242/dev.126.16.3585. [DOI] [PubMed] [Google Scholar]
- Ericson J., Rashbass P., Schedl A., Brenner-Morton S., Kawakami A., van Heyningen V., Jessell T.M., Briscoe J. Pax6 controls progenitor cell identity and neuronal fate in response to graded Shh signaling. Cell. 1997;90:169–180. doi: 10.1016/s0092-8674(00)80323-2. [DOI] [PubMed] [Google Scholar]
- Escurat M., Djabali K., Gumpel M., Gros F., Portier M.M. Differential expression of two neuronal intermediate-filament proteins, peripherin and the low-molecular-mass neurofilament protein (NF-L), during the development of the rat. J. Neurosci. 1990;10:764–784. doi: 10.1523/JNEUROSCI.10-03-00764.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedtsova N.G., Turner E.E. Brn-3.0 expression identifies early post-mitotic CNS neurons and sensory neural precursors. Mech. Dev. 1995;53:291–304. doi: 10.1016/0925-4773(95)00435-1. [DOI] [PubMed] [Google Scholar]
- Ferri A.L.M., Lin W., Mavromatakis Y.E., Wang J.C., Sasaki H., Whitsett J.A., Ang S.L. Foxa1 and Foxa2 regulate multiple phases of midbrain dopaminergic neuron development in a dosage-dependent manner. Development. 2007;134:2761–2769. doi: 10.1242/dev.000141. [DOI] [PubMed] [Google Scholar]
- Flames N., Hobert O. Transcriptional control of the terminal fate of monoaminergic neurons. Annu. Rev. Neurosci. 2011;34:153–184. doi: 10.1146/annurev-neuro-061010-113824. [DOI] [PubMed] [Google Scholar]
- Fode C., Ma Q., Casarosa S., Ang S.L., Anderson D.J., Guillemot F. A role for neural determination genes in specifying the dorsoventral identity of telencephalic neurons. Genes Dev. 2000;14:67–80. [PMC free article] [PubMed] [Google Scholar]
- George K.M., Leonard M.W., Roth M.E., Lieuw K.H., Kioussis D., Grosveld F., Engel J.D. Embryonic expression and cloning of the murine GATA-3 gene. Development. 1994;120:2673–2686. doi: 10.1242/dev.120.9.2673. [DOI] [PubMed] [Google Scholar]
- Goridis C., Rohrer H. Specification of catecholaminergic and serotonergic neurons. Nat. Rev. Neurosci. 2002;3:531–541. doi: 10.1038/nrn871. [DOI] [PubMed] [Google Scholar]
- Hanks M., Wurst W., Anson-Cartwright L., Auerbach A.B., Joyner A.L. Rescue of the En-1 mutant phenotype by replacement of En-1 with En-2. Science. 1995;269:679–682. doi: 10.1126/science.7624797. [DOI] [PubMed] [Google Scholar]
- Hendricks T., Francis N., Fyodorov D., Deneris E.S. The ETS domain factor Pet-1 is an early and precise marker of central serotonin neurons and interacts with a conserved element in serotonergic genes. J. Neurosci. 1999;19:10348–10356. doi: 10.1523/JNEUROSCI.19-23-10348.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higurashi N., Uchida T., Lossin C., Misumi Y., Okada Y., Akamatsu W., Imaizumi Y., Zhang B., Nabeshima K., Mori M.X. A human Dravet syndrome model from patient induced pluripotent stem cells. Mol. Brain. 2013;6:19. doi: 10.1186/1756-6606-6-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hook V., Brennand K.J., Kim Y., Toneff T., Funkelstein L., Lee K.C., Ziegler M., Gage F.H. Human iPSC neurons display activity-dependent neurotransmitter secretion: aberrant catecholamine levels in schizophrenia neurons. Stem Cell Reports. 2014;3:531–538. doi: 10.1016/j.stemcr.2014.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt P., Gulisano M., Cook M., Sham M.H., Faiella A., Wilkinson D., Boncinelli E., Krumlauf R. A distinct Hox code for the branchial region of the vertebrate head. Nature. 1991;353:861–864. doi: 10.1038/353861a0. [DOI] [PubMed] [Google Scholar]
- Imaizumi Y., Okano H. Modeling human neurological disorders with induced pluripotent stem cells. J. Neurochem. 2014;129:388–399. doi: 10.1111/jnc.12625. [DOI] [PubMed] [Google Scholar]
- Imaizumi Y., Okada Y., Akamatsu W., Koike M., Kuzumaki N., Hayakawa H., Nihira T., Kobayashi T., Ohyama M., Sato S. Mitochondrial dysfunction associated with increased oxidative stress and α-synuclein accumulation in PARK2 iPSC-derived neurons and postmortem brain tissue. Mol. Brain. 2012;5:35. doi: 10.1186/1756-6606-5-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Israel M.A., Yuan S.H., Bardy C., Reyna S.M., Mu Y., Herrera C., Hefferan M.P., Van Gorp S., Nazor K.L., Boscolo F.S. Probing sporadic and familial Alzheimer’s disease using induced pluripotent stem cells. Nature. 2012;482:216–220. doi: 10.1038/nature10821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagla K., Dollé P., Mattei M.G., Jagla T., Schuhbaur B., Dretzen G., Bellard F., Bellard M. Mouse Lbx1 and human LBX1 define a novel mammalian homeobox gene family related to the Drosophila lady bird genes. Mech. Dev. 1995;53:345–356. doi: 10.1016/0925-4773(95)00450-5. [DOI] [PubMed] [Google Scholar]
- Jiao J., Yang Y., Shi Y., Chen J., Gao R., Fan Y., Yao H., Liao W., Sun X.F., Gao S. Modeling Dravet syndrome using induced pluripotent stem cells (iPSCs) and directly converted neurons. Hum. Mol. Genet. 2013;22:4241–4252. doi: 10.1093/hmg/ddt275. [DOI] [PubMed] [Google Scholar]
- Kabashi E., Valdmanis P.N., Dion P., Spiegelman D., McConkey B.J., Vande Velde C., Bouchard J.-P., Lacomblez L., Pochigaeva K., Salachas F. TARDBP mutations in individuals with sporadic and familial amyotrophic lateral sclerosis. Nat. Genet. 2008;40:572–574. doi: 10.1038/ng.132. [DOI] [PubMed] [Google Scholar]
- Kabashi E., Lin L., Tradewell M.L., Dion P.A., Bercier V., Bourgouin P., Rochefort D., Bel Hadj S., Durham H.D., Vande Velde C. Gain and loss of function of ALS-related mutations of TARDBP (TDP-43) cause motor deficits in vivo. Hum. Mol. Genet. 2010;19:671–683. doi: 10.1093/hmg/ddp534. [DOI] [PubMed] [Google Scholar]
- Kiecker C., Lumsden A. The role of organizers in patterning the nervous system. Annu. Rev. Neurosci. 2012;35:347–367. doi: 10.1146/annurev-neuro-062111-150543. [DOI] [PubMed] [Google Scholar]
- Kirkeby A., Grealish S., Wolf D.A., Nelander J., Wood J., Lundblad M., Lindvall O., Parmar M. Generation of regionally specified neural progenitors and functional neurons from human embryonic stem cells under defined conditions. Cell Rep. 2012;1:703–714. doi: 10.1016/j.celrep.2012.04.009. [DOI] [PubMed] [Google Scholar]
- Knecht A.K., Bronner-Fraser M. Induction of the neural crest: a multigene process. Nat. Rev. Genet. 2002;3:453–461. doi: 10.1038/nrg819. [DOI] [PubMed] [Google Scholar]
- Kondo T., Asai M., Tsukita K., Kutoku Y., Ohsawa Y., Sunada Y., Imamura K., Egawa N., Yahata N., Okita K. Modeling Alzheimer’s disease with iPSCs reveals stress phenotypes associated with intracellular Aβ and differential drug responsiveness. Cell Stem Cell. 2013;12:487–496. doi: 10.1016/j.stem.2013.01.009. [DOI] [PubMed] [Google Scholar]
- Kriks S., Shim J.-W., Piao J., Ganat Y.M., Wakeman D.R., Xie Z., Carrillo-Reid L., Auyeung G., Antonacci C., Buch A. Dopamine neurons derived from human ES cells efficiently engraft in animal models of Parkinson’s disease. Nature. 2011;480:547–551. doi: 10.1038/nature10648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X.J., Du Z.W., Zarnowska E.D., Pankratz M., Hansen L.O., Pearce R.A., Zhang S.C. Specification of motoneurons from human embryonic stem cells. Nat. Biotechnol. 2005;23:215–221. doi: 10.1038/nbt1063. [DOI] [PubMed] [Google Scholar]
- Liem K.F., Jr., Tremml G., Jessell T.M. A role for the roof plate and its resident TGFbeta-related proteins in neuronal patterning in the dorsal spinal cord. Cell. 1997;91:127–138. doi: 10.1016/s0092-8674(01)80015-5. [DOI] [PubMed] [Google Scholar]
- Liu Y., Lopez-Santiago L.F., Yuan Y., Jones J.M., Zhang H., O’Malley H.A., Patino G.A., O’Brien J.E., Rusconi R., Gupta A. Dravet syndrome patient-derived neurons suggest a novel epilepsy mechanism. Ann. Neurol. 2013;74:128–139. doi: 10.1002/ana.23897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchetto M.C., Gage F.H. Modeling brain disease in a dish: really? Cell Stem Cell. 2012;10:642–645. doi: 10.1016/j.stem.2012.05.008. [DOI] [PubMed] [Google Scholar]
- Marchetto M.C.N., Carromeu C., Acab A., Yu D., Yeo G.W., Mu Y., Chen G., Gage F.H., Muotri A.R. A model for neural development and treatment of Rett syndrome using human induced pluripotent stem cells. Cell. 2010;143:527–539. doi: 10.1016/j.cell.2010.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marín O., Rubenstein J.L. A long, remarkable journey: tangential migration in the telencephalon. Nat. Rev. Neurosci. 2001;2:780–790. doi: 10.1038/35097509. [DOI] [PubMed] [Google Scholar]
- Maroof A.M., Keros S., Tyson J.A., Ying S.W., Ganat Y.M., Merkle F.T., Liu B., Goulburn A., Stanley E.G., Elefanty A.G. Directed differentiation and functional maturation of cortical interneurons from human embryonic stem cells. Cell Stem Cell. 2013;12:559–572. doi: 10.1016/j.stem.2013.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall H., Nonchev S., Sham M.H., Muchamore I., Lumsden A., Krumlauf R. Retinoic acid alters hindbrain Hox code and induces transformation of rhombomeres 2/3 into a 4/5 identity. Nature. 1992;360:737–741. doi: 10.1038/360737a0. [DOI] [PubMed] [Google Scholar]
- Matsunaga E., Araki I., Nakamura H. Role of Pax3/7 in the tectum regionalization. Development. 2001;128:4069–4077. doi: 10.1242/dev.128.20.4069. [DOI] [PubMed] [Google Scholar]
- Mattis V.B., Svendsen C.N. Induced pluripotent stem cells: a new revolution for clinical neurology? Lancet Neurol. 2011;10:383–394. doi: 10.1016/S1474-4422(11)70022-9. [DOI] [PubMed] [Google Scholar]
- Morrison J.H., Hof P.R. Life and death of neurons in the aging brain. Science. 1997;278:412–419. doi: 10.1126/science.278.5337.412. [DOI] [PubMed] [Google Scholar]
- Moya N., Cutts J., Gaasterland T., Willert K., Brafman D.A. Endogenous WNT signaling regulates hPSC-derived neural progenitor cell heterogeneity and specifies their regional identity. Stem Cell Reports. 2014;3:1015–1028. doi: 10.1016/j.stemcr.2014.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa M., Koyanagi M., Tanabe K., Takahashi K., Ichisaka T., Aoi T., Okita K., Mochiduki Y., Takizawa N., Yamanaka S. Generation of induced pluripotent stem cells without Myc from mouse and human fibroblasts. Nat. Biotechnol. 2008;26:101–106. doi: 10.1038/nbt1374. [DOI] [PubMed] [Google Scholar]
- Nguyen H.N., Byers B., Cord B., Shcheglovitov A., Byrne J., Gujar P., Kee K., Schüle B., Dolmetsch R.E., Langston W. LRRK2 mutant iPSC-derived DA neurons demonstrate increased susceptibility to oxidative stress. Cell Stem Cell. 2011;8:267–280. doi: 10.1016/j.stem.2011.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordström U., Jessell T.M., Edlund T. Progressive induction of caudal neural character by graded Wnt signaling. Nat. Neurosci. 2002;5:525–532. doi: 10.1038/nn0602-854. [DOI] [PubMed] [Google Scholar]
- Okada Y., Shimazaki T., Sobue G., Okano H. Retinoic-acid-concentration-dependent acquisition of neural cell identity during in vitro differentiation of mouse embryonic stem cells. Dev. Biol. 2004;275:124–142. doi: 10.1016/j.ydbio.2004.07.038. [DOI] [PubMed] [Google Scholar]
- Okada Y., Matsumoto A., Shimazaki T., Enoki R., Koizumi A., Ishii S., Itoyama Y., Sobue G., Okano H. Spatiotemporal recapitulation of central nervous system development by murine embryonic stem cell-derived neural stem/progenitor cells. Stem Cells. 2008;26:3086–3098. doi: 10.1634/stemcells.2008-0293. [DOI] [PubMed] [Google Scholar]
- Okano H., Yamanaka S. iPS cell technologies: significance and applications to CNS regeneration and disease. Mol. Brain. 2014;7:22. doi: 10.1186/1756-6606-7-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuda T., Haga T., Kanai Y., Endou H., Ishihara T., Katsura I. Identification and characterization of the high-affinity choline transporter. Nat. Neurosci. 2000;3:120–125. doi: 10.1038/72059. [DOI] [PubMed] [Google Scholar]
- Oliver G., Mailhos A., Wehr R., Copeland N.G., Jenkins N.A., Gruss P. Six3, a murine homologue of the sine oculis gene, demarcates the most anterior border of the developing neural plate and is expressed during eye development. Development. 1995;121:4045–4055. doi: 10.1242/dev.121.12.4045. [DOI] [PubMed] [Google Scholar]
- Osumi N., Hirota A., Ohuchi H., Nakafuku M., Iimura T., Kuratani S., Fujiwara M., Noji S., Eto K. Pax-6 is involved in the specification of hindbrain motor neuron subtype. Development. 1997;124:2961–2972. doi: 10.1242/dev.124.15.2961. [DOI] [PubMed] [Google Scholar]
- Porteus M.H., Bulfone A., Ciaranello R.D., Rubenstein J.L. Isolation and characterization of a novel cDNA clone encoding a homeodomain that is developmentally regulated in the ventral forebrain. Neuron. 1991;7:221–229. doi: 10.1016/0896-6273(91)90260-7. [DOI] [PubMed] [Google Scholar]
- Poulsen K.T., Armanini M.P., Klein R.D., Hynes M.A., Phillips H.S., Rosenthal A. TGF beta 2 and TGF beta 3 are potent survival factors for midbrain dopaminergic neurons. Neuron. 1994;13:1245–1252. doi: 10.1016/0896-6273(94)90062-0. [DOI] [PubMed] [Google Scholar]
- Qiu M., Shimamura K., Sussel L., Chen S., Rubenstein J.L.R. Control of anteroposterior and dorsoventral domains of Nkx-6.1 gene expression relative to other Nkx genes during vertebrate CNS development. Mech. Dev. 1998;72:77–88. doi: 10.1016/s0925-4773(98)00018-5. [DOI] [PubMed] [Google Scholar]
- Ring D.B., Johnson K.W., Henriksen E.J., Nuss J.M., Goff D., Kinnick T.R., Ma S.T., Reeder J.W., Samuels I., Slabiak T. Selective glycogen synthase kinase 3 inhibitors potentiate insulin activation of glucose transport and utilization in vitro and in vivo. Diabetes. 2003;52:588–595. doi: 10.2337/diabetes.52.3.588. [DOI] [PubMed] [Google Scholar]
- Rogaev E.I., Sherrington R., Rogaeva E.A., Levesque G., Ikeda M., Liang Y., Chi H., Lin C., Holman K., Tsuda T. Familial Alzheimer’s disease in kindreds with missense mutations in a gene on chromosome 1 related to the Alzheimer’s disease type 3 gene. Nature. 1995;376:775–778. doi: 10.1038/376775a0. [DOI] [PubMed] [Google Scholar]
- Schwarz M., Alvarez-Bolado G., Dressler G., Urbánek P., Busslinger M., Gruss P. Pax2/5 and Pax6 subdivide the early neural tube into three domains. Mech. Dev. 1999;82:29–39. doi: 10.1016/s0925-4773(99)00005-2. [DOI] [PubMed] [Google Scholar]
- Sephton C.F., Good S.K., Atkin S., Dewey C.M., Mayer P., 3rd, Herz J., Yu G. TDP-43 is a developmentally regulated protein essential for early embryonic development. J. Biol. Chem. 2010;285:6826–6834. doi: 10.1074/jbc.M109.061846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherrington R., Rogaev E.I., Liang Y., Rogaeva E.A., Levesque G., Ikeda M., Chi H., Lin C., Li G., Holman K. Cloning of a gene bearing missense mutations in early-onset familial Alzheimer’s disease. Nature. 1995;375:754–760. doi: 10.1038/375754a0. [DOI] [PubMed] [Google Scholar]
- Shimamura K., Hartigan D.J., Martinez S., Puelles L., Rubenstein J.L. Longitudinal organization of the anterior neural plate and neural tube. Development. 1995;121:3923–3933. doi: 10.1242/dev.121.12.3923. [DOI] [PubMed] [Google Scholar]
- Shimamura K., Martinez S., Puelles L., Rubenstein J.L. Patterns of gene expression in the neural plate and neural tube subdivide the embryonic forebrain into transverse and longitudinal domains. Dev. Neurosci. 1997;19:88–96. doi: 10.1159/000111190. [DOI] [PubMed] [Google Scholar]
- Shimazaki T., Shingo T., Weiss S. The ciliary neurotrophic factor/leukemia inhibitory factor/gp130 receptor complex operates in the maintenance of mammalian forebrain neural stem cells. J. Neurosci. 2001;21:7642–7653. doi: 10.1523/JNEUROSCI.21-19-07642.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simeone A., Acampora D., Gulisano M., Stornaiuolo A., Boncinelli E. Nested expression domains of four homeobox genes in developing rostral brain. Nature. 1992;358:687–690. doi: 10.1038/358687a0. [DOI] [PubMed] [Google Scholar]
- Sinha S., Chen J.K. Purmorphamine activates the Hedgehog pathway by targeting Smoothened. Nat. Chem. Biol. 2006;2:29–30. doi: 10.1038/nchembio753. [DOI] [PubMed] [Google Scholar]
- Suemori H., Yasuchika K., Hasegawa K., Fujioka T., Tsuneyoshi N., Nakatsuji N. Efficient establishment of human embryonic stem cell lines and long-term maintenance with stable karyotype by enzymatic bulk passage. Biochem. Biophys. Res. Commun. 2006;345:926–932. doi: 10.1016/j.bbrc.2006.04.135. [DOI] [PubMed] [Google Scholar]
- Swanson D.J., Tong Y., Goldowitz D. Disruption of cerebellar granule cell development in the Pax6 mutant, Sey mouse. Brain Res. Dev. Brain Res. 2005;160:176–193. doi: 10.1016/j.devbrainres.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Takahashi M., Osumi N. Pax6 regulates specification of ventral neurone subtypes in the hindbrain by establishing progenitor domains. Development. 2002;129:1327–1338. doi: 10.1242/dev.129.6.1327. [DOI] [PubMed] [Google Scholar]
- Takahashi K., Tanabe K., Ohnuki M., Narita M., Ichisaka T., Tomoda K., Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- Thal D.R., Rüb U., Orantes M., Braak H. Phases of A beta-deposition in the human brain and its relevance for the development of AD. Neurology. 2002;58:1791–1800. doi: 10.1212/wnl.58.12.1791. [DOI] [PubMed] [Google Scholar]
- Turner M.R., Kiernan M.C. Does interneuronal dysfunction contribute to neurodegeneration in amyotrophic lateral sclerosis? Amyotroph. Lateral Scler. 2012;13:245–250. doi: 10.3109/17482968.2011.636050. [DOI] [PubMed] [Google Scholar]
- Walther C., Gruss P. Pax-6, a murine paired box gene, is expressed in the developing CNS. Development. 1991;113:1435–1449. doi: 10.1242/dev.113.4.1435. [DOI] [PubMed] [Google Scholar]
- Xuan S., Baptista C.A., Balas G., Tao W., Soares V.C., Lai E. Winged helix transcription factor BF-1 is essential for the development of the cerebral hemispheres. Neuron. 1995;14:1141–1152. doi: 10.1016/0896-6273(95)90262-7. [DOI] [PubMed] [Google Scholar]
- Yagi T., Ito D., Okada Y., Akamatsu W., Nihei Y., Yoshizaki T., Yamanaka S., Okano H., Suzuki N. Modeling familial Alzheimer’s disease with induced pluripotent stem cells. Hum. Mol. Genet. 2011;20:4530–4539. doi: 10.1093/hmg/ddr394. [DOI] [PubMed] [Google Scholar]
- Yamasaki T., Kawaji K., Ono K., Bito H., Hirano T., Osumi N., Kengaku M. Pax6 regulates granule cell polarization during parallel fiber formation in the developing cerebellum. Development. 2001;128:3133–3144. doi: 10.1242/dev.128.16.3133. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.