Summary
Prenatal exposure to valproic acid (VPA), an established antiepileptic drug, has been reported to impair postnatal cognitive function in children born to VPA-treated epileptic mothers. However, how these defects arise and how they can be overcome remain unknown. Using mice, we found that comparable postnatal cognitive functional impairment is very likely correlated to the untimely enhancement of embryonic neurogenesis, which led to depletion of the neural precursor cell pool and consequently a decreased level of adult neurogenesis in the hippocampus. Moreover, hippocampal neurons in the offspring of VPA-treated mice showed abnormal morphology and activity. Surprisingly, these impairments could be ameliorated by voluntary running. Our study suggests that although prenatal exposure to antiepileptic drugs such as VPA may have detrimental effects that persist until adulthood, these effects may be offset by a simple physical activity such as running.
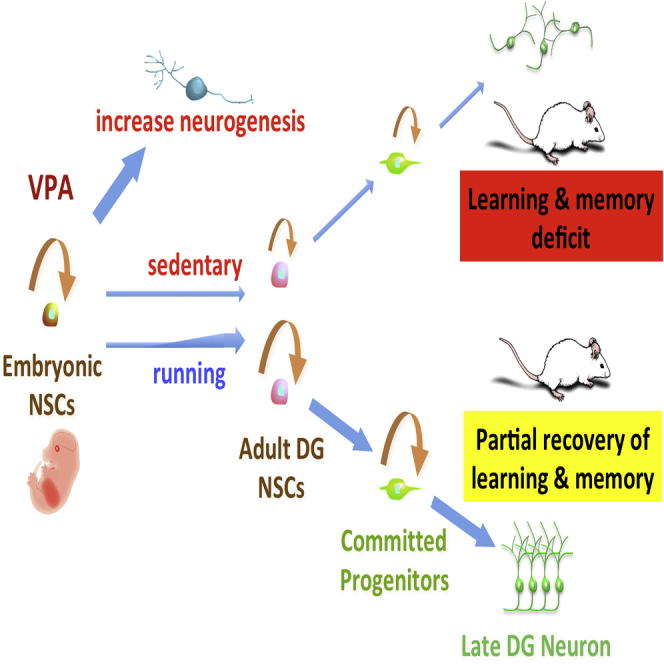
Graphical Abstract
Highlights
-
•
Prenatal VPA treatment caused an untimely enhancement of embryonic neurogenesis
-
•
Prenatal VPA treatment has the long-term effect of impairing adult neurogenesis
-
•
Reduced level of adult neurogenesis is associated with cognitive functional impairments
-
•
Voluntary running can ameliorate the persistent detrimental effects caused by VPA
Nakashima and colleagues show that prenatal VPA exposure in mice produced comparable postnatal cognitive functional impairments in children from epileptic mothers. VPA enhances embryonic neurogenesis, which then leads to depletion of the neural precursor cell pool, resulting in a decreased level of adult hippocampal neurogenesis. They further show that voluntary running could reinstate the neurogenesis and restore cognitive functions.
Introduction
Epilepsy is one of the most common neurological disorders in the world and is characterized by uncontrollable seizures (Chang and Lowenstein, 2003). Epilepsy can affect anyone, at any age, and there are an estimated 50 million afflicted people worldwide (Meinardi et al., 2001, Ngugi et al., 2010, Joint Epilepsy Council, 2011). The incidence of epilepsy is estimated to be higher than 0.5 cases per 1,000 of population per year (Sander, 2003). Around 30% of sufferers are women of childbearing age (Joint Epilepsy Council, 2011). During pregnancy, epileptic patients must balance the maternal and fetal risks associated with seizures against the potential teratogenicity of antiepileptic drugs (AEDs) (Battino and Tomson, 2007). Although it was recently reported that several commonly used AEDs could produce postnatal impairment of cognitive function if taken during pregnancy (Meador et al., 2009, Meador et al., 2011, Meador et al., 2012, Meador et al., 2013), the precise pathology underlying such impairment remains unknown, and effective treatments for affected children of epileptic mothers who took AEDs during their pregnancy are therefore currently unavailable.
Valproic acid (VPA [2-propylpentanoic acid]) is an established drug in the long-term treatment of epilepsy (Blaheta and Cinatl, 2002). Several studies have revealed that VPA can directly inhibit histone deacetylase (HDAC) activity and cause hyperacetylation of histones, thereby activating gene transcription (Göttlicher et al., 2001, Phiel et al., 2001). VPA significantly impairs postnatal cognitive function (Meador et al., 2009, Meador et al., 2011, Meador et al., 2012, Meador et al., 2013) and can lead to severe developmental defects if taken in early gestational stages (DiLiberti et al., 1984, Nau et al., 1991). We have previously shown, in several culture systems, that VPA enhances neurogenesis and drives neural precursor cells (NPCs) into the neuronal lineage over the glial lineage by a process involving HDAC inhibition (Hsieh et al., 2004, Abematsu et al., 2010, Juliandi et al., 2012). Here, we show that comparable postnatal cognitive functional impairment after prenatal VPA exposure in mice is caused by the untimely enhancement of embryonic neurogenesis, which leads to depletion of the NPCs pool and consequently a decreased level of adult neurogenesis in the hippocampus. We further show that hippocampal neurons in the offspring of VPA-treated mice have an abnormal morphology and activity. Nevertheless, these impairments can be alleviated by voluntary running.
Results
VPA Enhances Embryonic Neurogenesis and Alters Global Gene Expression through HDAC Inhibition
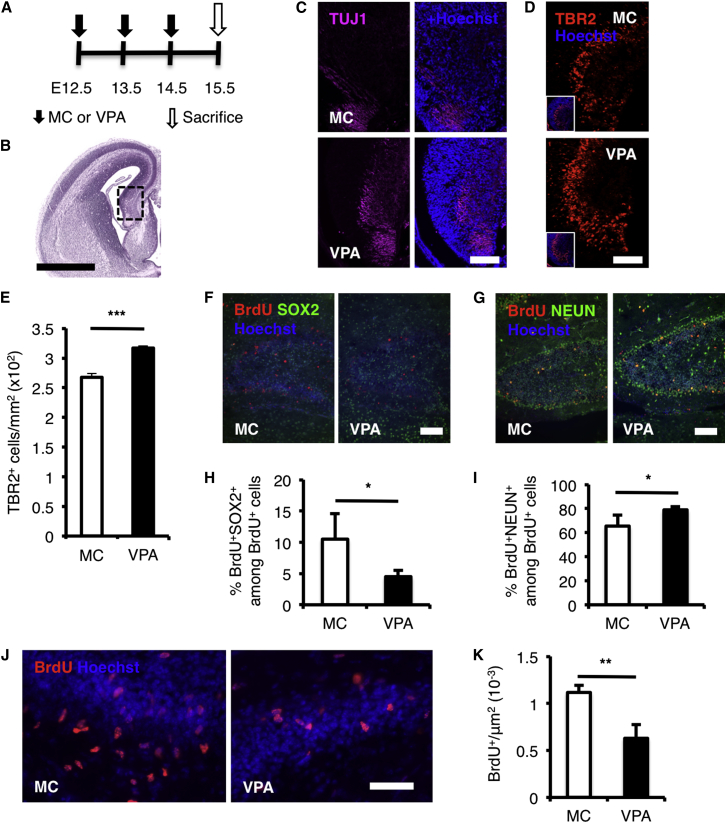
We orally administered VPA or vehicle (methylcellulose [MC]) to pregnant mice on embryonic day (E)12.5 to E14.5, a mid-gestational period when neurogenesis is prominent (Figure 1A). We found that VPA increased global histone acetylation (Figures S1B–S1D) and enhanced neurogenesis as shown by the increased thickness of TUJ1 stained region in the cortical hem of VPA-treated mice (Figure 1C) and increased production of TBR2-positive intermediate neuronal progenitors born in the dentate neuroepithelium (Figures 1D and 1E) in the developing mouse brain (Figures 1B and S1A; see Figures S1E–S1G for embryonic cortex). We also found that VPA depleted the NPC pool (Figures 1F and 1H, hippocampus; Figures S1E and S1F, cortex) and reduced the number of proliferating NPCs (Figures 1J and 1K, hippocampus; Figures S1H and S1I, cortex). These results were compatible with our previous in vitro observations (Hsieh et al., 2004, Abematsu et al., 2010, Juliandi et al., 2012). Moreover, we found that the fraction of cells that had exited the cell cycle (i.e., differentiated) was higher in the E15.5 forebrain of VPA-treated mice, as shown by an increased number of BrdU-retaining cells that were negative for the proliferation marker KI-67 compared to BrdU-retaining cells that were still KI-67-positive, after a single injection of BrdU on E14.5 to label proliferating cells (Figures S1J and S1K). These results show that VPA treatment enhances embryonic neurogenesis and reduces the pool of NPCs.
Figure 1.
Prenatal VPA Treatment Enhances Untimely Embryonic Neurogenesis
(A) Experimental timeline of prenatal VPA treatment. E, embryonic day.
(B) The region highlighted by the dashed black rectangle in E15.5 forebrain sections was used for analysis in (C) and (D). The image is modified from the Electronic Prenatal Mouse Brain Atlas. Scale bar, 1 mm.
(C) The thickness of the region stained by immature neuron marker β-tubulin isotype III (TUJ1; magenta) is increased in the cortical hem of VPA-treated mice. Scale bar, 100 μm.
(D) VPA treatment increases the production of TBR2+ intermediate neuronal progenitors (red) born in the dentate neuroepithelium. Scale bar, 100 μm.
(E) Quantification of the density of TBR2+ in (D).
(F–I) The proportion of E14.5 BrdU-labeled NPCs (red) expressing NPC marker SOX2 (green; F and H) is reduced, while the ones that had differentiated into NEUN+ neurons are increased at P7 hippocampus after embryonic VPA treatment (green; G and I). Scale bars, 100 μm.
(J and K) Embryonic VPA treatment reduces the number of highly proliferating NPCs (red) labeled by 30 min single-pulse BrdU injection in the P7 DG. See also Figure S1 for other immunostaining data in the cortex of embryonic forebrain.
MC, prenatal methylcellulose (vehicle); VPA, prenatal valproic acid. Data are represented as means. n = 3 for each group. Error bars indicate the SD. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, two-tailed t test. Scale bar, 50 μm. See also Figure S1.
Phenotypic changes should be generally induced by gene expression changes, and we wanted to examine whether VPA-induced gene expression change is due to its HDAC inhibiting property. To this end, we administered VPA or valpromide (VPM), an analog AED without HDAC-inhibiting activity, and performed transcriptome analyses at three time points after the final administration. We found that 3 hr after the last administration, global gene expression in the E14.5 telencephalon was changed substantially by VPA, but not by VPM (Figure S1L; GEO: GSE42904). This global change had almost completely disappeared in both cortex and hippocampus at E18.5 and in hippocampus at P84, although several genes still displayed differential expression levels (Figure S1L). We have found previously that VPM treatment increased neither global histone acetylation nor neurogenesis (Abematsu et al., 2010). Although VPA and VPM might have another pharmacological activity differences beside HDAC-inhibiting activity, taken together our results strongly suggest that VPA alters global gene expression mainly through its HDAC-inhibiting property, and this alteration is short-lived, being restricted mainly to the period when VPA was being given to the mice.
Prenatal VPA Treatment Has the Long-Term Effect of Impairing Adult Neurogenesis, Learning, and Memory
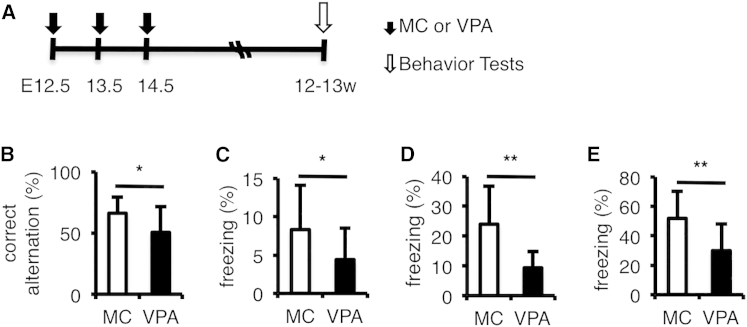
We next investigated whether the enhancement of neurogenesis and changes in gene expression caused by prenatal VPA exposure during the period of prominent neurogenesis could lead to postnatal impairment of cognitive function, as reported in humans (Meador et al., 2009, Meador et al., 2011, Meador et al., 2012, Meador et al., 2013). We conducted several behavioral tests on 12- to 13-week-old male mice (Figure 2A). Although the locomotor activity of VPA-treated mice declined, the decline rate was very slight (Tables S1 and S2) so that we could measure the emotional or cognitive behaviors. We found indeed that VPA-treated mice performed poorly mainly in tests that assessed learning and memory, such as Y-maze (Figure 2B) and contextual and cued fear associative tests (Figures 2C–2E, S4A, S4C, and S4E; Table S1), but not in the other tests (Table S1). VPA-treated mice have a lower correct-arm alternation in Y-maze. VPA-treated mice also have a lower freezing response in contextual and cued fear associative tests than MC-treated mice (control), despite the fact that both groups have similar fear response to foot shock during conditioning (Table S1), indicating that hippocampal-dependent learning and memory (Sarnyai et al., 2000, Van der Borght et al., 2007) are impaired in the VPA-treated mice.
Figure 2.
VPA-Treated Mice Perform Poorly in Learning and Memory Tests
(A) Experimental timeline of prenatal VPA treatment and postnatal behavior tests. E, embryonic day; w, weeks old.
(B) VPA-treated mice have a lower correct-arm alternation than MC-treated mice (control).
(C–E) VPA-treated mice have a lower freezing response than MC-treated mice (control) in conditioning (C; day 1), contextual (D; day 2), and cued fear associative tests (E; day 3). See also Figures S4A, S4C, and S4E for time course of freezing response and Table S1 for a summary of behavior data.
MC, prenatal methylcellulose (vehicle); VPA, prenatal valproic acid. Data are represented as means. n = 12 for each group. Error bars indicate the SD. ∗p < 0.05, ∗∗p < 0.01, two-tailed t test. See also Figure S4 and Table S1.
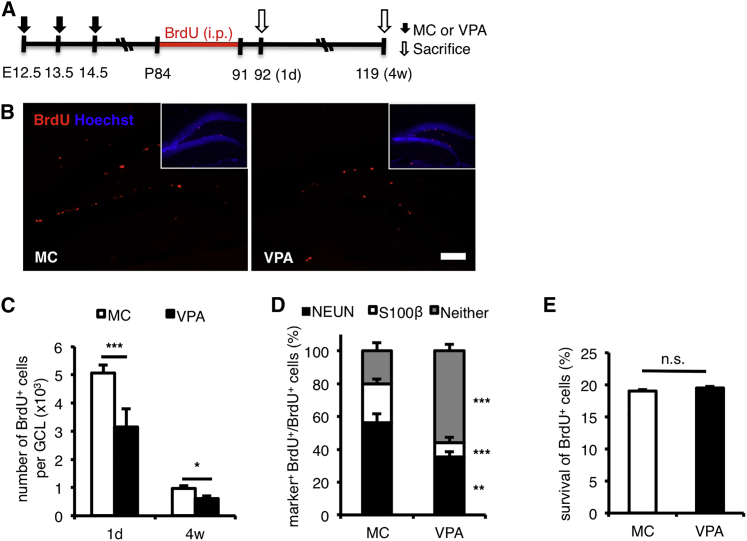
In light of these results, we decided to focus on adult NPCs in the hippocampal dentate gyrus (DG), as they have been shown to play a functional role in learning and memory processes by undergoing neurogenesis to generate adult-born neural cells (Zhao et al., 2008). We injected BrdU once a day for 7 days into 12-week-old mice to label proliferating NPCs in the DG and then sacrificed the mice 1 day (to assess cell proliferation) or 4 weeks (to assess cell survival and fate) after the last BrdU injection (Figure 3A). We found that the number of BrdU-retaining cells in VPA-treated mice was lower at both time points than that in MC-treated control mice (Figures 3B and 3C). We also found fewer proliferating KI-67-positive cells in VPA-treated mice (Figures S2A and S2B). When we traced the fate of BrdU-retaining cells 4 weeks after the last BrdU injection (Figure 3A), we found that a lower proportion of BrdU-retaining cells had differentiated into NEUN-positive neurons and S100β-positive astrocytes in VPA-treated mice than in MC-treated mice (Figure 3D). The possibility that more BrdU-retaining cells had died in VPA-treated mice during the 4-week period can be ruled out, because the survival rate of BrdU-retaining cells in these mice was similar to that in MC-treated mice (Figure 3E). These results imply that BrdU-retaining cells in VPA-treated mice either differentiated to another cell type(s) or differentiated more slowly than those in MC-treated mice. The latter explanation is more plausible, because we found that a higher proportion of BrdU-retaining cells in VPA-treated mice still expressed SOX2 (an NPC marker) and KI-67 (a proliferation marker) even 4 weeks after the last BrdU injection (Figures S2C–S2E). We also found that 1 day after the last BrdU injection, almost all BrdU-positive cells were still KI-67-positive in VPA-treated mice, whereas several BrdU-positive but KI-67-negative cells already existed in MC-treated mice (Figure S2F). We also examined amygdala and cortex, other brain regions that were suggested to play important roles in the regulation of memory and fear associative responses (LeDoux, 2003, van Strien et al., 2009). It has been shown previously that amygdala volume associates with differences in fear associative responses (Yang et al., 2008). However, we found that VPA-treated mice have a similar amygdala size to that of MC-treated mice, both at P7 and P84 (Figures S3A–S3D). We also found no significant difference in the expression level of cortical layer-specific genes such as Cux1, Satb2, and Ctip2 (Molyneaux et al., 2007) in the cortex of P84 VPA-treated mice (Figures S3E–S3G). Taken together, these results suggest that prenatal VPA treatment has the long-term effect of impairing adult neurogenesis and contribute to the poor performance of VPA-exposed mice in learning and memory tests.
Figure 3.
Prenatal VPA Treatment Has the Long-Term Effect on the Adult Neurogenesis
(A) Experimental timeline of prenatal VPA treatment and adult neurogenesis analysis. E, embryonic day; P, postnatal day; 1d, 1 day after the last intraperitoneal (i.p.) BrdU injection; 4w, 4 weeks after the last i.p. BrdU injection.
(B) Representative images of brain sections including the hippocampal DG stained for BrdU (red) and with Hoechst 33258 (blue),1 day after the last BrdU injection. See also Figure S2A for KI-67 staining.
(C) Quantification of BrdU+ cells in the granule cell layer (GCL), 1 day (1d; n = 8 for each group) and 4 weeks (4w; n = 8 for each group) after the last BrdU injection, shows a reduction of BrdU+ cells in the hippocampus. See also Figure S2B for quantification of KI-67+ cells in the GCL.
(D) NPC differentiation into NEUN+ neurons and S100β+ astrocytes is impaired in VPA-treated mice, as shown by a reduced proportion of marker-positive and BrdU+ cells among total BrdU+ cells at 4 weeks after the last BrdU injection (n = 4 for each group).
(E) BrdU+ cell survival is similar in VPA- and MC-treated mice. Quantification of BrdU+ cell survival in each group as a percentage of BrdU+ cells at 4w relative to BrdU+ cells at 1d (n = 8 for each group).
MC, prenatal methylcellulose (vehicle); VPA, prenatal valproic acid. Data are represented as means. Error bars indicate the SD. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, n.s., not significantly different, two-tailed t test. Scale bar, 100 μm. See also Figures S2 and S3.
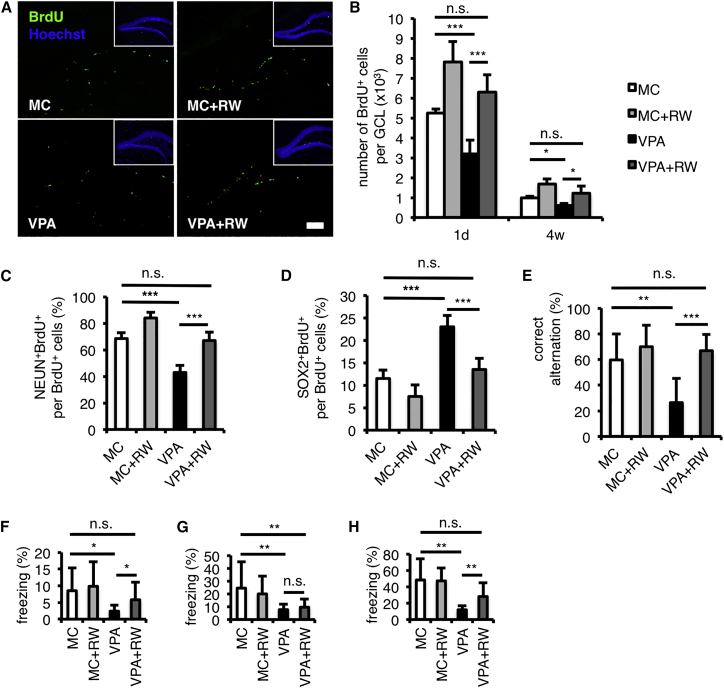
Voluntary Running Restores Learning and Memory Deficiencies in VPA-Treated Mice, Probably through Increased Neurogenesis that Yields Neurons with Normal Morphology
Voluntary running can increase adult neurogenesis in the hippocampal DG (van Praag et al., 1999a, van Praag et al., 1999b). We next provided the mice with a running wheel (RW) after they had weaned and repeated the same BrdU-injection experiment when they reached 12 weeks (P84; Figure 3A). We observed an increased number of BrdU-retaining cells in MC- and VPA-treated mice with the RW, both 1 day and 4 weeks after the last BrdU injection (Figures 4A and 4B). We also found an increased proportion of BrdU-retaining cells that had differentiated into NEUN-positive neurons and a reduced proportion of BrdU-retaining cells that still SOX2-positive in MC- and VPA-treated mice with the RW, 4 weeks after the last BrdU injection (Figures 3A, 4C, and 4D). Voluntary running also enabled VPA-treated mice to perform better in hippocampus-dependent learning and memory test, the correct alternation in Y-maze (F(1, 13) = 22.74, p < 0.001, one-way ANOVA), and this performance was not significantly different in comparison to MC-treated mice (Figure 4E; Table S2). Moreover, we found that voluntary running also led VPA-treated mice performing better in conditioning (F(1, 13) = 3.06, p = 0.10, one-way ANOVA) and cued fear tests (F(1, 13) = 6.80, p < 0.05, one-way ANOVA), although not in contextual fear test (F(1, 13) = 0.47, p = 0.50, one-way ANOVA). As each mouse showed similar normal fear response, the performance of VPA-treated mice after voluntary running in conditioning and cued fear tests, but not in contextual fear test, recovered to levels that were not significantly different from that of MC-treated mice (Figures 4F–4H, S4B, S4D, and S4F; Table S2). However, when we analyzed all experimental groups by two-way ANOVA with both prenatal treatment and postnatal activity as factors, the results did not show a significant effect of postnatal activity on the performance of mice in hippocampus-dependent learning and memory tests (Table S2). This is mainly because voluntary running in MC-treated mice made no significant contribution to the performance in hippocampus-dependent learning and memory tests (conditioning: F(1, 13) = 0.13, p = 0.72; contextual: F(1, 13) = 0.26, p = 0.62; cued: F(1, 13) = 0.01, p = 0.93; Y-maze: F(1, 13) = 1.17, p = 0.30; one-way ANOVA). In addition, we found no significant interaction between prenatal drug treatment and postnatal activity in relation to the behavior of mice, except for the correct alternation in Y-maze (F(1, 26) = 5.59, p < 0.05, two-way ANOVA; Table S2). Therefore, it seems likely that only a certain level of adult neurogenesis is required for mice to perform normally in these hippocampus-dependent learning and memory tests, so that MC-treated mice with or without a RW showed no significant differences in these tests even though MC-treated mice with a RW displayed higher levels of neurogenesis than those without one. However, because the level of adult neurogenesis in the VPA-treated mice was below this threshold, the mice performed poorly in these tests; voluntary running improved their performance, probably by increasing adult neurogenesis in the hippocampus to above the threshold level.
Figure 4.
Voluntary Running Restores Adult Neurogenesis and Cognitive Deficiencies of VPA-Treated Mice
(A) Representative images of brain sections including the hippocampal DG stained for BrdU (green) and with Hoechst 33258 (blue), 1 day after the last BrdU injection. See Figure 2A for the experimental timeline.
(B) Quantification of BrdU+ in the granule cell layer (GCL), 1 day (1d; n = 8 for each group) and 4 weeks (4w; n = 8 for each group) after the last BrdU injection, shows an increased number of BrdU+ cells in the hippocampus after voluntary running.
(C and D) Voluntary running recovers the proportion of BrdU+ cells that had differentiated into NEUN+ neurons (C) and the ones that still expressed SOX2 (D) among total BrdU+ cells at 4 weeks after the last BrdU injection (n = 4 for each group).
(E) Reduction of correct-arm alternation in Y-maze tests of VPA-treated mice is recovered by voluntary running (n = 7 for MC and VPA + RW; n = 8 for MC + RW and VPA).
(F–H) Voluntary running recovers the freezing response in conditioning (F; day 1) and in cued fear associative tests (H; day 3), but not in contextual fear associative tests (G; day 2; n = 7 for MC and VPA + RW; n = 8 for MC + RW and VPA). See also Figures S4B, S4D, and S4F for the time course of the freezing response and Table S2 for a summary of behavior data.
MC, prenatal methylcellulose (vehicle); MC + RW, prenatal methylcellulose and postnatal running; VPA, prenatal valproic acid; VPA + RW, prenatal valproic acid and postnatal running. Data are represented as means. Error bars indicate the SD. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, n.s., not significantly different, two-tailed t test. Scale bar, 100 μm. See also Figure S4 and Table S2.
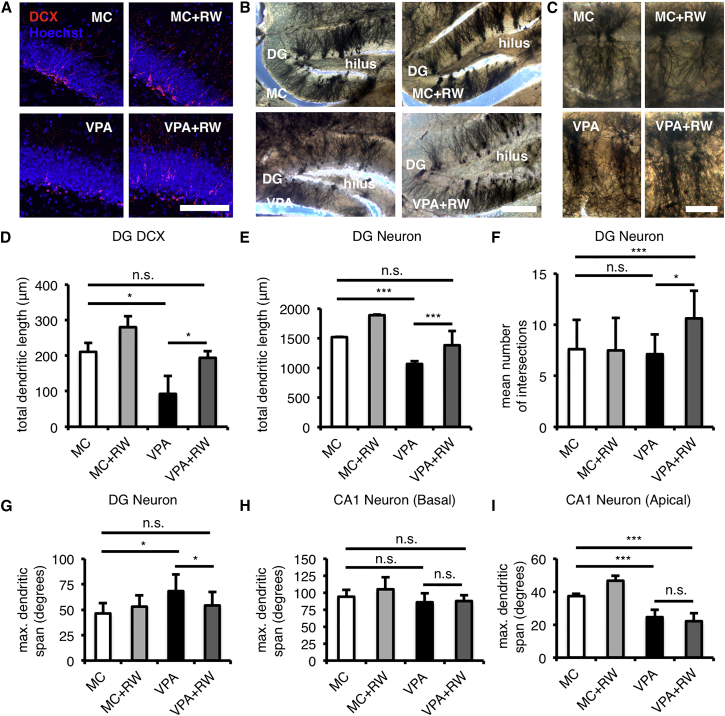
We next sought to determine whether adult neurogenesis in the DG of VPA-treated mice generated normal or abnormal neurons. We found that DCX-positive immature neurons (Figures 5A and S5A) and Golgi-stained mature neurons (Figures 5B and S5B) in the DG of VPA-treated mice displayed an abnormal morphology with shorter total dendritic length (Figures 5D and 5E) and fewer dendrite processes toward the molecular layer, as shown by a wider maximum dendritic span compared to MC-treated mice (Figure 5G). The dendritic complexity of Golgi-stained neurons in VPA-treated mice, however, was similar to that of MC-treated mice (Figures 5F and S5B). Surprisingly, this abnormal morphology could also be overcome by voluntary running (Figures 5A, 5B, 5D, 5E, 5G, S5A, and S5B). Taken together, these observations suggest that the restoration of learning and memory deficiencies in VPA-treated mice through running is involved in increased neurogenesis, which gives rise to neurons with normal morphology.
Figure 5.
Voluntary Running Restores Neuronal Morphology in VPA-Treated Mice
(A) Impaired morphology of DCX+ young neurons in the DG of VPA-treated mice is recovered by voluntary running (n = 4 for each group). Scale bar, 100 μm.
(B) Impaired morphology of Golgi-Cox stained neurons in the DG of VPA-treated mice is recovered by voluntary running (n = 3 for each group). Note that voluntary running recovered non-molecular layer-oriented dendrites in VPA-treated mice to molecular layer-oriented ones. Scale bar, 100 μm.
(C) Impaired morphology of Golgi-Cox stained neurons in the CA1 of VPA-treated mice is not recovered by voluntary running (n = 3 for each group). Note that the less-ramified and straighter apical dendrites in VPA-treated mice could not be recovered by voluntary running. Scale bar, 50 μm.
(D–F) Voluntary running recovers total dendritic length of DCX+ young neurons (D) and Golgi-Cox stained neurons (E) and increases dendritic complexity of Golgi-Cox stained neurons (F) in the DG of VPA-treated mice. See also Figures S5A and S5B for Sholl analysis.
(G–I) Abnormal dendritic span of DG neurons (G), but not of apical dendritic span of CA1 neurons (I), is recovered by voluntary running in VPA-treated mice, while basal dendrites of CA1 neurons show similar dendritic span across groups (H).
MC, prenatal methylcellulose (vehicle); MC + RW, prenatal methylcellulose and postnatal running; VPA, prenatal valproic acid; VPA + RW, prenatal valproic acid and postnatal running. Data are represented as means. Error bars indicate the SD. ∗p < 0.05, ∗∗∗p < 0.001, n.s., not significantly different, two-tailed t test. See also Figure S5.
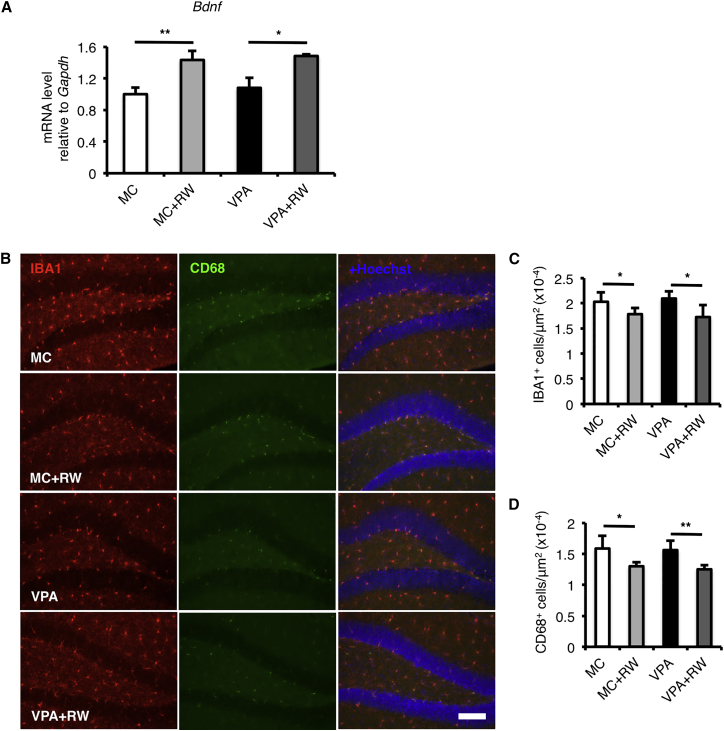
Several mechanisms have been proposed to be responsible for the voluntary running-induced increase in adult hippocampal neurogenesis. Voluntary running can induce expression of neurotrophic factors such as brain-derived neurotrophic factor (Bdnf) in the hippocampus (van Praag, 2009, Farmer et al., 2004), and Bdnf has been suggested to have an important role in adult neurogenesis, neuronal maturation, and dendrite arborization (Tolwani et al., 2002, Bekinschtein et al., 2011, Stranahan, 2011). Indeed, we found an increased level of Bdnf expression after running in both MC- and VPA-treated mice hippocampus (Figure 6A). Voluntary running has also been shown to reduce the number of microglia and its activation (Gebara et al., 2013, Kohman et al., 2013), and previous reports showed that microglia can suppress the adult hippocampal neurogenesis (Sierra et al., 2010, Vukovic et al., 2012, Matsuda et al., 2015). Interestingly, we found that the number of IBA1-positive microglia and CD68-positive-activated microglia was also decreased by voluntary running in both MC- and VPA-treated mice (Figures 6B–6D). Therefore, it is plausible that voluntary running helped VPA-treated mice through the increased adult hippocampal neurogenesis that was caused by the increase level of Bdnf expression and the reduction of microglia and its activated form in the hippocampus.
Figure 6.
Voluntary Running Increases the Bdnf Expression Level and Reduces Microglia and Activated Microglia in the Hippocampus
(A) The expression level of brain-derived neurotrophic factor (Bdnf) was increased by voluntary running in both MC- and VPA-treated mice.
(B–D) Voluntary running reduced the number of IBA1+ microglia (red; B and C) and CD68+-activated microglia (green; B and D) in both MC- and VPA-treated mice.
MC, prenatal methylcellulose (vehicle); MC + RW, prenatal methylcellulose and postnatal running; VPA, prenatal valproic acid; VPA + RW, prenatal valproic acid and postnatal running; Gapdh, glyceraldehyde 3-phosphate dehydrogenase. Data are represented as means. n = 3 for each group. Error bars indicate the SD. ∗p < 0.05, ∗∗p < 0.01, two-tailed t test. Scale bar, 100 μm.
Voluntary Running Cannot Mitigate Abnormal Neuronal Morphology or Function of the Hippocampal CA1 Region in VPA-Treated Mice
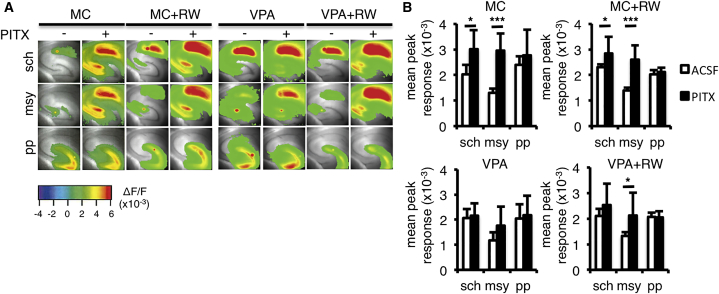
The freezing response in the contextual fear test after voluntary running by VPA-treated mice did not recover to the level displayed by MC-treated mice (Figures 4G and S4D; Table S2). It has been proposed that recall of contextual memories relies more on CA1 than other regions in the hippocampus (Hall et al., 2001). Indeed, we found that apical dendrite morphology was abnormal in CA1 neurons of VPA-treated mice, and this defect was not repaired by voluntary running (Figures 5C, 5H, and 5I). Moreover, when we examined the region-specific restoration by voluntary running of neuronal activity in the hippocampus, the basal neuronal responses upon electrical stimulation were not affected by VPA nor voluntary running in the three major synaptic connections in the hippocampus (CA3-CA1, Schaffer collateral afferent [sch]; DG-CA3, mossy fiber [msy]; EC-DG, perforant pathway [pp]) possibly due to some homeostatic balancing mechanism (Turrigiano and Nelson, 2004, Turrigiano, 2011). However, when the excitatory activity was measured, with the increase in activity caused by an inhibitor for GABAA receptor picrotoxin (PITX) application, the effect of VPA became apparent. That is, the PITX induced an increase in sch and msy in MC-treated mice, which was not seen in VPA-treated mice, suggesting the impairment of inhibitory action in VPA-mice. The voluntary running could restore the characteristics only in msy (Figure 7). These results may explain why the recovery of neurogenesis and neuronal morphology of the DG in VPA-treated mice could not ameliorate their poor performance in the contextual associative test. Previous studies have indicated that ablation of adult neurogenesis in the DG leads to defective performance in a contextual associative test (Saxe et al., 2006, Wojtowicz et al., 2008) (but see Shors et al., 2002), but enhanced neurogenesis or running was weakly related to the performance in the test (Wojtowicz et al., 2008).
Figure 7.
Voluntary Running Restores Neuronal Activity in VPA-Treated Mice
(A) Representative pseudocolor activity map images of brain slices including the hippocampus show that voluntary running can only recover the impairment of GABAA receptor-mediated inhibition in the mossy fiber pathway (msy) of VPA-treated mice, after treatment with the GABAA receptor channel antagonist picrotoxin (PITX) (n = 6 for MC, n = 9 for MC + RW, n = 7 for VPA, n = 8 for VPA + RW). Electrical stimulation was applied to Schaffer collateral afferents at the CA3/CA1 border of CA1 (sch); to the granule cell layer to stimulate the mossy fiber pathway (msy); and to the molecular layer of the upper blade in the DG (pp).
(B) Quantification of the neural response in artificial cerebrospinal fluid (ACSF), with (black bars) or without PITX (white bars; n = 6 for MC, n = 9 for MC + RW, n = 7 for VPA, n = 8 for VPA + RW). Note that although the augmentation of the neural response caused by GABAA receptor-mediated inhibition with PITX application seen in sch and msy was abolished in VPA-treated mice, voluntary running could restore the augmentation only in the msy.
MC, prenatal methylcellulose (vehicle); MC + RW, prenatal methylcellulose and postnatal running; VPA, prenatal valproic acid; VPA + RW, prenatal valproic acid and postnatal running. Data are represented as means. Error bars indicate the SD. ∗p < 0.05, ∗∗∗p < 0.001, two-tailed t test.
Discussion
The precise pathology underlying postnatal impairment of cognitive function in children of epileptic expectant mothers treated with VPA, commonly used AED, is unknown. Based on our findings in mice, we propose that the impairment is attributable to the untimely enhancement of embryonic neurogenesis, which leads to depletion of the NPC pool and consequently to a decreased level of postnatal neurogenesis in the hippocampus. Children of epileptic expectant mothers treated with VPA may also have hippocampal neurons with abnormal morphology and activity, as were observed in this study. Although prenatal exposure to AEDs such as VPA may have detrimental effects that persist until adulthood, we suggest that these effects could be mitigated by a simple physical activity such as running. Our results thus offer a straightforward strategy to help children born to epileptic mothers.
The cognitive deficits associated with prenatal VPA exposure might not due solely to the reduced neurogenesis with the abnormal neuronal morphology in the hippocampus, and there is a possibility that the low freezing responses in fear associative tests were contributed by the deficiency in amygdala, nociception, and/or motoric functions. Nevertheless, our data suggested that the reduced neurogenesis associated with the abnormal neuronal morphology in the hippocampus were very likely to be correlated with the observed cognitive deficits for several reasons. First, voluntary running is well known for its effect on enhancing both adult neurogenesis in the DG of the hippocampus and hippocampus-dependent learning and memory (Zhao et al., 2008), and this voluntary running could recover the cognitive deficit, if not all, in VPA-treated mice with reduced neurogenesis in the DG. Second, to the best of our knowledge, there are no reports to date that show a direct contribution of voluntary running to the enhancement of amygdala function that subsequently leads to an improvement in the cued fear response. Third, based on experiments that we have conducted, we could not find any significant differences in amygdala size and in the expression levels of cortical layer-specific genes of MC- and VPA-treated mice, with or without voluntary running. Fourth, total traveled distance in the open field, elevated plus and the Y-maze tests, and the number of light/dark transitions were not significantly different between MC- and VPA-treated mice, although in our earlier experiment some of these parameters showed modest differences. Moreover, in fear associative test, VPA-treated mice move similarly to MC-treated mice before the start of the tone (pre-tone), which indicate that motor deficiency is unlikely to be the main cause of low freezing responses in VPA-treated mice. Fifth, MC- and VPA-treated mice have similar basal nociceptive response and startle response to electric footshock during the conditioning for fear-associative test, thus it seems unlikely that VPA-treated mice have abnormal nociception and cannot sense the foot shock. Taking these facts into consideration, we therefore suggested that the reduced neurogenesis associated with the abnormal neuronal morphology in the hippocampus were very likely to be a critical cause of the observed cognitive deficits. However, we still cannot completely exclude the possibility that changes in other brain areas may also contribute to the deficits, warranting further future investigation.
We and others have shown previously that VPA treatment induces neuronal differentiation but suppresses glial differentiation of cultured multipotent NPCs (Hsieh et al., 2004, Balasubramaniyan et al., 2006, Murabe et al., 2007, Abematsu et al., 2010, Juliandi et al., 2012). We have now demonstrated that VPA also increases histone acetylation in the embryonic forebrain and induces neuronal differentiation of embryonic NPCs. Previous study have shown that VPA promotes neuronal differentiation by increasing histone H4 acetylation at proneural gene promoters (Yu et al., 2009). However, several studies have suggested that the activation of GSK-3β/β-catenin and/or ERK pathway is the main cause for the increase neurogenesis of NPCs by VPA (Yuan et al., 2001, Jung et al., 2008, Hao et al., 2004, Go et al., 2012). It has been suggested that VPA might have various cellular effects that will depend on the context of VPA usage and/or cell type and experimental design used in the study, which warrant further research to reveal the connection between these effects (Kostrouchová et al., 2007, Rosenberg, 2007).
We suggest that gene expression change caused by VPA is attributable mainly to its HDAC-inhibiting activity. To date, more than a dozen HDACs have been characterized and they are classified into at least three major groups. In particular, HDAC1 and HDAC2, belonging to the class I group, have been reported to regulate NPC differentiation (Sun et al., 2011). NPCs express high levels of HDAC1 and some of them also express low levels of HDAC2 (MacDonald and Roskams, 2008). Interestingly, as NPCs are committed to the neuronal lineage, expression of HDAC2 is upregulated while that of HDAC1 is downregulated and becomes undetectable in most post-mitotic neurons (MacDonald et al., 2005, MacDonald and Roskams, 2008); on the other hand, HDAC1 expression is sustained in the majority of cells in glial lineages (astrocytes and oligodendrocytes), in which HDAC2 is not detected (Shen et al., 2005, MacDonald and Roskams, 2008). Moreover, HDAC2, but not HDAC1, was found to inhibit astrocytic differentiation (Humphrey et al., 2008). Therefore, although VPA is capable of inhibiting both HDAC1 and HDAC2 (Kazantsev and Thompson, 2008), it is tempting to speculate that the main target of VPA in HDAC inhibition-mediated neuronal differentiation of NPCs is HDAC1. It will be of interest to explore this possibility in a future study.
Neurogenesis in the adult mammalian brain occurs throughout life and has been clearly demonstrated at two locations under physiological conditions: the SVZ of the lateral ventricle and the subgranular zone (SGZ) of the DG in the hippocampus (Alvarez-Buylla and Lim, 2004). Several studies have shown that hippocampal neurogenesis is regulated by both physiological and pathological activities at different stages, including (1) proliferation of NPCs, (2) fate determination and differentiation of NPCs, and (3) survival, maturation, and integration of newborn neurons (Zhao et al., 2008). Furthermore, each of these stages is subject to regulation by numerous intrinsic and extrinsic factors (Suh et al., 2009). Genetic and environmental factors that affect adult hippocampal neurogenesis also cause alteration in cognitive performance, suggesting roles for adult hippocampal neurogenesis in learning and memory (Zhao et al., 2008). Our results showed that VPA-treated mice have a decreased level of postnatal neurogenesis in the hippocampus, which correlates with their poor performance in learning and memory tests. We have shown here and elsewhere (Hsieh et al., 2004, Jessberger et al., 2007) that VPA can reduce the proliferation of NPCs, and this reduction, together with the enhancement of neurogenesis, probably led to the depletion of the NPC pool in VPA-treated mice. It is possible that this depletion caused a slower differentiation of the residual NPCs in order to maintain required number of NPC pool during life. This possibility is an interesting avenue to be explored in the future.
In accordance with previous studies (van Praag et al., 1999a, van Praag et al., 1999b), we found that voluntary running augments hippocampal neurogenesis of both MC- and VPA-treated mice, and it restores learning and memory deficiencies in VPA-treated mice. A previous report has shown the same restoration of decreased hippocampal neurogenesis and learning deficits in aged rodents by voluntary running (van Praag et al., 2005), although the precise molecular mechanisms responsible for voluntary running-induced neurogenesis remain undetermined (Deng et al., 2010). Here, we propose that at least the increase expression level of Bdnf, and the reduction of activated microglia may contribute to the restoration of impaired hippocampal neurogenesis and neuronal morphology in the DG of VPA-treated mice after voluntary running. However, future exploration is necessary to reveal the direct connection between the increase expression level of Bdnf and the reduction of microglia and its activated form in the hippocampus after voluntary running.
Experimental Procedures
Animal Treatment
All experiments were carried out according to institutional animal experimentation guidelines, which comply with the NIH Guide for Care and Use of Laboratory Animals. All efforts were made to minimize the number of animals used and their suffering. Pregnant C57BL/6 mice were individually housed in plastic breeding cages with free access to water and pellet diet in a 12-hr light-dark cycle. For a detailed description of groups and treatments, see the Supplemental Experimental Procedures.
Immunohistochemistry, Nissl, and Golgi Staining
Mice were anesthetized and perfused with PBS followed by 4% PFA in PBS. The brain was dissected, postfixed, and processed for immunohistochemistry. For Nissl staining, brain sections were defatted with xylene, hydrated through a graded ethanol series (100%, 95%, and 70%), and washed with water before stained with 0.2% thionin solution (pH 4.0). Sections were then dehydrated in water and a graded ethanol series (70%, 95%, and 100%), clear in xylene, and mounted with Entellan (Merck). For Golgi staining, the brain was removed from the skull without any perfusion and then sectioned (100 μm) on a cryostat. For a more detailed description and list of antibodies, see the Supplemental Experimental Procedures.
Measurement and Morphometrics
For a detailed description of cell count, volume measurement and cell/tissue morphometrics, see the Supplemental Experimental Procedures.
Gene Expression Analysis
For a detailed description of GeneChip and real-time qPCR procedures, see the Supplemental Experimental Procedures.
Behavioral Tests
Behavioral experiments were performed sequentially using male mice. For a more detailed description, see the Supplemental Experimental Procedures.
Voltage-Sensitive Dye Imaging
Experiment was done using hippocampal slices. For a more detailed description, see the Supplemental Experimental Procedures.
Statistics
Statistical analyses were performed by Student’s two-tailed t test (unpaired) and one-way or two-way ANOVA using R software (http://www.r-project.org) (n indicates individual mice).
Author Contributions
B.J. and K.N. conceived and designed the study. B.J., K. Tanemura, K.I., T.T., Y.F., M.O.I., N.M., and D.I. carried out the experiments. B.J., K. Tanemura, K.I., T.T., M.A., T.S., K. Tsujimura, M.N., and K.N. analyzed the data. J.K. supported the experiments. B.J. and K.N. wrote the manuscript.
Acknowledgments
We thank Y. Bessho, T. Matsui, Y. Nakahata, J. Kohyama, T. Takizawa, M. Namihira, S. Katada, and T. Imamura for valuable discussions. We also thank I. Smith for critical reading of the manuscript. We are very grateful to M. Tano for her excellent secretarial assistance and other laboratory members for discussion and technical help. This research was supported in part by the NAIST Global COE Program (Frontier Biosciences: Strategies for survival and adaptation in a changing global environment) from the Ministry of Education, Culture, Sports, Science and Technology of Japan (MEXT); a Grant-in-Aid for Scientific Research on Innovative Area: Neural Diversity and Neocortical Organization from MEXT; Health Sciences Research Grants from the Ministry of Health, Labour and Welfare, Japan; Core Research for Evolutional Science and Technology (CREST) from the Japan Science and Technology Corporation; and Research Fellowships for Young Scientists from the Japan Society for the Promotion of Science.
Published: November 19, 2015
Footnotes
This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
Supplemental Information includes Supplemental Experimental Procedures, five figures, and two tables and can be found with this article online at http://dx.doi.org/10.1016/j.stemcr.2015.10.012.
Accession Numbers
The accession number for the GeneChip data reported in this paper is GEO: GSE42904.
Supplemental Information
References
- Abematsu M., Tsujimura K., Yamano M., Saito M., Kohno K., Kohyama J., Namihira M., Komiya S., Nakashima K. Neurons derived from transplanted neural stem cells restore disrupted neuronal circuitry in a mouse model of spinal cord injury. J. Clin. Invest. 2010;120:3255–3266. doi: 10.1172/JCI42957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Buylla A., Lim D.A. For the long run: maintaining germinal niches in the adult brain. Neuron. 2004;41:683–686. doi: 10.1016/s0896-6273(04)00111-4. [DOI] [PubMed] [Google Scholar]
- Balasubramaniyan V., Boddeke E., Bakels R., Küst B., Kooistra S., Veneman A., Copray S. Effects of histone deacetylation inhibition on neuronal differentiation of embryonic mouse neural stem cells. Neuroscience. 2006;143:939–951. doi: 10.1016/j.neuroscience.2006.08.082. [DOI] [PubMed] [Google Scholar]
- Battino D., Tomson T. Management of epilepsy during pregnancy. Drugs. 2007;67:2727–2746. doi: 10.2165/00003495-200767180-00007. [DOI] [PubMed] [Google Scholar]
- Bekinschtein P., Oomen C.A., Saksida L.M., Bussey T.J. Effects of environmental enrichment and voluntary exercise on neurogenesis, learning and memory, and pattern separation: BDNF as a critical variable? Semin. Cell Dev. Biol. 2011;22:536–542. doi: 10.1016/j.semcdb.2011.07.002. [DOI] [PubMed] [Google Scholar]
- Blaheta R.A., Cinatl J., Jr. Anti-tumor mechanisms of valproate: a novel role for an old drug. Med. Res. Rev. 2002;22:492–511. doi: 10.1002/med.10017. [DOI] [PubMed] [Google Scholar]
- Chang B.S., Lowenstein D.H. Epilepsy. N. Engl. J. Med. 2003;349:1257–1266. doi: 10.1056/NEJMra022308. [DOI] [PubMed] [Google Scholar]
- Deng W., Aimone J.B., Gage F.H. New neurons and new memories: how does adult hippocampal neurogenesis affect learning and memory? Nat. Rev. Neurosci. 2010;11:339–350. doi: 10.1038/nrn2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiLiberti J.H., Farndon P.A., Dennis N.R., Curry C.J. The fetal valproate syndrome. Am. J. Med. Genet. 1984;19:473–481. doi: 10.1002/ajmg.1320190308. [DOI] [PubMed] [Google Scholar]
- Farmer J., Zhao X., van Praag H., Wodtke K., Gage F.H., Christie B.R. Effects of voluntary exercise on synaptic plasticity and gene expression in the dentate gyrus of adult male Sprague-Dawley rats in vivo. Neuroscience. 2004;124:71–79. doi: 10.1016/j.neuroscience.2003.09.029. [DOI] [PubMed] [Google Scholar]
- Gebara E., Sultan S., Kocher-Braissant J., Toni N. Adult hippocampal neurogenesis inversely correlates with microglia in conditions of voluntary running and aging. Front. Neurosci. 2013;7:145. doi: 10.3389/fnins.2013.00145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Go H.S., Kim K.C., Choi C.S., Jeon S.J., Kwon K.J., Han S.H., Lee J., Cheong J.H., Ryu J.H., Kim C.H. Prenatal exposure to valproic acid increases the neural progenitor cell pool and induces macrocephaly in rat brain via a mechanism involving the GSK-3β/β-catenin pathway. Neuropharmacology. 2012;63:1028–1041. doi: 10.1016/j.neuropharm.2012.07.028. [DOI] [PubMed] [Google Scholar]
- Göttlicher M., Minucci S., Zhu P., Krämer O.H., Schimpf A., Giavara S., Sleeman J.P., Lo Coco F., Nervi C., Pelicci P.G., Heinzel T. Valproic acid defines a novel class of HDAC inhibitors inducing differentiation of transformed cells. EMBO J. 2001;20:6969–6978. doi: 10.1093/emboj/20.24.6969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall J., Thomas K.L., Everitt B.J. Cellular imaging of zif268 expression in the hippocampus and amygdala during contextual and cued fear memory retrieval: selective activation of hippocampal CA1 neurons during the recall of contextual memories. J. Neurosci. 2001;21:2186–2193. doi: 10.1523/JNEUROSCI.21-06-02186.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao Y., Creson T., Zhang L., Li P., Du F., Yuan P., Gould T.D., Manji H.K., Chen G. Mood stabilizer valproate promotes ERK pathway-dependent cortical neuronal growth and neurogenesis. J. Neurosci. 2004;24:6590–6599. doi: 10.1523/JNEUROSCI.5747-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh J., Nakashima K., Kuwabara T., Mejia E., Gage F.H. Histone deacetylase inhibition-mediated neuronal differentiation of multipotent adult neural progenitor cells. Proc. Natl. Acad. Sci. USA. 2004;101:16659–16664. doi: 10.1073/pnas.0407643101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphrey G.W., Wang Y.H., Hirai T., Padmanabhan R., Panchision D.M., Newell L.F., McKay R.D., Howard B.H. Complementary roles for histone deacetylases 1, 2, and 3 in differentiation of pluripotent stem cells. Differentiation. 2008;76:348–356. doi: 10.1111/j.1432-0436.2007.00232.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessberger S., Nakashima K., Clemenson G.D., Jr., Mejia E., Mathews E., Ure K., Ogawa S., Sinton C.M., Gage F.H., Hsieh J. Epigenetic modulation of seizure-induced neurogenesis and cognitive decline. J. Neurosci. 2007;27:5967–5975. doi: 10.1523/JNEUROSCI.0110-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joint Epilepsy Council . Joint Epilepsy Council Publication; 2011. Epilepsy Prevalence, Incidence, and Other Statistic. [Google Scholar]
- Juliandi B., Abematsu M., Sanosaka T., Tsujimura K., Smith A., Nakashima K. Induction of superficial cortical layer neurons from mouse embryonic stem cells by valproic acid. Neurosci. Res. 2012;72:23–31. doi: 10.1016/j.neures.2011.09.012. [DOI] [PubMed] [Google Scholar]
- Jung G.A., Yoon J.Y., Moon B.S., Yang D.H., Kim H.Y., Lee S.H., Bryja V., Arenas E., Choi K.Y. Valproic acid induces differentiation and inhibition of proliferation in neural progenitor cells via the beta-catenin-Ras-ERK-p21Cip/WAF1 pathway. BMC Cell Biol. 2008;9:66. doi: 10.1186/1471-2121-9-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazantsev A.G., Thompson L.M. Therapeutic application of histone deacetylase inhibitors for central nervous system disorders. Nat. Rev. Drug Discov. 2008;7:854–868. doi: 10.1038/nrd2681. [DOI] [PubMed] [Google Scholar]
- Kohman R.A., Bhattacharya T.K., Wojcik E., Rhodes J.S. Exercise reduces activation of microglia isolated from hippocampus and brain of aged mice. J. Neuroinflammation. 2013;10:114. doi: 10.1186/1742-2094-10-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostrouchová M., Kostrouch Z., Kostrouchová M. Valproic acid, a molecular lead to multiple regulatory pathways. Folia Biol. (Praha) 2007;53:37–49. [PubMed] [Google Scholar]
- LeDoux J. The emotional brain, fear, and the amygdala. Cell. Mol. Neurobiol. 2003;23:727–738. doi: 10.1023/A:1025048802629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald J.L., Roskams A.J. Histone deacetylases 1 and 2 are expressed at distinct stages of neuro-glial development. Dev. Dyn. 2008;237:2256–2267. doi: 10.1002/dvdy.21626. [DOI] [PubMed] [Google Scholar]
- MacDonald J.L., Gin C.S., Roskams A.J. Stage-specific induction of DNA methyltransferases in olfactory receptor neuron development. Dev. Biol. 2005;288:461–473. doi: 10.1016/j.ydbio.2005.09.048. [DOI] [PubMed] [Google Scholar]
- Matsuda T., Murao N., Katano Y., Juliandi B., Kohyama J., Akira S., Kawai T., Nakashima K. TLR9 signalling in microglia attenuates seizure-induced aberrant neurogenesis in the adult hippocampus. Nat. Commun. 2015;6:6514. doi: 10.1038/ncomms7514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meador K.J., Baker G.A., Browning N., Clayton-Smith J., Combs-Cantrell D.T., Cohen M., Kalayjian L.A., Kanner A., Liporace J.D., Pennell P.B., NEAD Study Group Cognitive function at 3 years of age after fetal exposure to antiepileptic drugs. N. Engl. J. Med. 2009;360:1597–1605. doi: 10.1056/NEJMoa0803531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meador K.J., Baker G.A., Browning N., Cohen M.J., Clayton-Smith J., Kalayjian L.A., Kanner A., Liporace J.D., Pennell P.B., Privitera M., Loring D.W., NEAD Study Group Foetal antiepileptic drug exposure and verbal versus non-verbal abilities at three years of age. Brain. 2011;134:396–404. doi: 10.1093/brain/awq352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meador K.J., Baker G.A., Browning N., Cohen M.J., Bromley R.L., Clayton-Smith J., Kalayjian L.A., Kanner A., Liporace J.D., Pennell P.B., NEAD Study Group Effects of fetal antiepileptic drug exposure: outcomes at age 4.5 years. Neurology. 2012;78:1207–1214. doi: 10.1212/WNL.0b013e318250d824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meador K.J., Baker G.A., Browning N., Cohen M.J., Bromley R.L., Clayton-Smith J., Kalayjian L.A., Kanner A., Liporace J.D., Pennell P.B., NEAD Study Group Fetal antiepileptic drug exposure and cognitive outcomes at age 6 years (NEAD study): a prospective observational study. Lancet Neurol. 2013;12:244–252. doi: 10.1016/S1474-4422(12)70323-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinardi H., Scott R.A., Reis R., Sander J.W., ILAE Commission on the Developing World The treatment gap in epilepsy: the current situation and ways forward. Epilepsia. 2001;42:136–149. doi: 10.1046/j.1528-1157.2001.32800.x. [DOI] [PubMed] [Google Scholar]
- Molyneaux B.J., Arlotta P., Menezes J.R., Macklis J.D. Neuronal subtype specification in the cerebral cortex. Nat. Rev. Neurosci. 2007;8:427–437. doi: 10.1038/nrn2151. [DOI] [PubMed] [Google Scholar]
- Murabe M., Yamauchi J., Fujiwara Y., Hiroyama M., Sanbe A., Tanoue A. A novel embryotoxic estimation method of VPA using ES cells differentiation system. Biochem. Biophys. Res. Commun. 2007;352:164–169. doi: 10.1016/j.bbrc.2006.10.189. [DOI] [PubMed] [Google Scholar]
- Nau H., Hauck R.S., Ehlers K. Valproic acid-induced neural tube defects in mouse and human: aspects of chirality, alternative drug development, pharmacokinetics and possible mechanisms. Pharmacol. Toxicol. 1991;69:310–321. doi: 10.1111/j.1600-0773.1991.tb01303.x. [DOI] [PubMed] [Google Scholar]
- Ngugi A.K., Bottomley C., Kleinschmidt I., Sander J.W., Newton C.R. Estimation of the burden of active and life-time epilepsy: a meta-analytic approach. Epilepsia. 2010;51:883–890. doi: 10.1111/j.1528-1167.2009.02481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phiel C.J., Zhang F., Huang E.Y., Guenther M.G., Lazar M.A., Klein P.S. Histone deacetylase is a direct target of valproic acid, a potent anticonvulsant, mood stabilizer, and teratogen. J. Biol. Chem. 2001;276:36734–36741. doi: 10.1074/jbc.M101287200. [DOI] [PubMed] [Google Scholar]
- Rosenberg G. The mechanisms of action of valproate in neuropsychiatric disorders: can we see the forest for the trees? Cell. Mol. Life Sci. 2007;64:2090–2103. doi: 10.1007/s00018-007-7079-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sander J.W. The epidemiology of epilepsy revisited. Curr. Opin. Neurol. 2003;16:165–170. doi: 10.1097/01.wco.0000063766.15877.8e. [DOI] [PubMed] [Google Scholar]
- Sarnyai Z., Sibille E.L., Pavlides C., Fenster R.J., McEwen B.S., Tóth M. Impaired hippocampal-dependent learning and functional abnormalities in the hippocampus in mice lacking serotonin(1A) receptors. Proc. Natl. Acad. Sci. USA. 2000;97:14731–14736. doi: 10.1073/pnas.97.26.14731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxe M.D., Battaglia F., Wang J.W., Malleret G., David D.J., Monckton J.E., Garcia A.D., Sofroniew M.V., Kandel E.R., Santarelli L. Ablation of hippocampal neurogenesis impairs contextual fear conditioning and synaptic plasticity in the dentate gyrus. Proc. Natl. Acad. Sci. USA. 2006;103:17501–17506. doi: 10.1073/pnas.0607207103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen S., Li J., Casaccia-Bonnefil P. Histone modifications affect timing of oligodendrocyte progenitor differentiation in the developing rat brain. J. Cell Biol. 2005;169:577–589. doi: 10.1083/jcb.200412101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shors T.J., Townsend D.A., Zhao M., Kozorovitskiy Y., Gould E. Neurogenesis may relate to some but not all types of hippocampal-dependent learning. Hippocampus. 2002;12:578–584. doi: 10.1002/hipo.10103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierra A., Encinas J.M., Deudero J.J., Chancey J.H., Enikolopov G., Overstreet-Wadiche L.S., Tsirka S.E., Maletic-Savatic M. Microglia shape adult hippocampal neurogenesis through apoptosis-coupled phagocytosis. Cell Stem Cell. 2010;7:483–495. doi: 10.1016/j.stem.2010.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stranahan A.M. Physiological variability in brain-derived neurotrophic factor expression predicts dendritic spine density in the mouse dentate gyrus. Neurosci. Lett. 2011;495:60–62. doi: 10.1016/j.neulet.2011.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh H., Deng W., Gage F.H. Signaling in adult neurogenesis. Annu. Rev. Cell Dev. Biol. 2009;25:253–275. doi: 10.1146/annurev.cellbio.042308.113256. [DOI] [PubMed] [Google Scholar]
- Sun G., Fu C., Shen C., Shi Y. Histone deacetylases in neural stem cells and induced pluripotent stem cells. J. Biomed. Biotechnol. 2011;2011:835968. doi: 10.1155/2011/835968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolwani R.J., Buckmaster P.S., Varma S., Cosgaya J.M., Wu Y., Suri C., Shooter E.M. BDNF overexpression increases dendrite complexity in hippocampal dentate gyrus. Neuroscience. 2002;114:795–805. doi: 10.1016/s0306-4522(02)00301-9. [DOI] [PubMed] [Google Scholar]
- Turrigiano G. Too many cooks? Intrinsic and synaptic homeostatic mechanisms in cortical circuit refinement. Annu. Rev. Neurosci. 2011;34:89–103. doi: 10.1146/annurev-neuro-060909-153238. [DOI] [PubMed] [Google Scholar]
- Turrigiano G.G., Nelson S.B. Homeostatic plasticity in the developing nervous system. Nat. Rev. Neurosci. 2004;5:97–107. doi: 10.1038/nrn1327. [DOI] [PubMed] [Google Scholar]
- Van der Borght K., Havekes R., Bos T., Eggen B.J., Van der Zee E.A. Exercise improves memory acquisition and retrieval in the Y-maze task: relationship with hippocampal neurogenesis. Behav. Neurosci. 2007;121:324–334. doi: 10.1037/0735-7044.121.2.324. [DOI] [PubMed] [Google Scholar]
- van Praag H. Exercise and the brain: something to chew on. Trends Neurosci. 2009;32:283–290. doi: 10.1016/j.tins.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H., Christie B.R., Sejnowski T.J., Gage F.H. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc. Natl. Acad. Sci. USA. 1999;96:13427–13431. doi: 10.1073/pnas.96.23.13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H., Kempermann G., Gage F.H. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat. Neurosci. 1999;2:266–270. doi: 10.1038/6368. [DOI] [PubMed] [Google Scholar]
- van Praag H., Shubert T., Zhao C., Gage F.H. Exercise enhances learning and hippocampal neurogenesis in aged mice. J. Neurosci. 2005;25:8680–8685. doi: 10.1523/JNEUROSCI.1731-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Strien N.M., Cappaert N.L., Witter M.P. The anatomy of memory: an interactive overview of the parahippocampal-hippocampal network. Nat. Rev. Neurosci. 2009;10:272–282. doi: 10.1038/nrn2614. [DOI] [PubMed] [Google Scholar]
- Vukovic J., Colditz M.J., Blackmore D.G., Ruitenberg M.J., Bartlett P.F. Microglia modulate hippocampal neural precursor activity in response to exercise and aging. J. Neurosci. 2012;32:6435–6443. doi: 10.1523/JNEUROSCI.5925-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojtowicz J.M., Askew M.L., Winocur G. The effects of running and of inhibiting adult neurogenesis on learning and memory in rats. Eur. J. Neurosci. 2008;27:1494–1502. doi: 10.1111/j.1460-9568.2008.06128.x. [DOI] [PubMed] [Google Scholar]
- Yang R.J., Mozhui K., Karlsson R.M., Cameron H.A., Williams R.W., Holmes A. Variation in mouse basolateral amygdala volume is associated with differences in stress reactivity and fear learning. Neuropsychopharmacology. 2008;33:2595–2604. doi: 10.1038/sj.npp.1301665. [DOI] [PubMed] [Google Scholar]
- Yu I.T., Park J.Y., Kim S.H., Lee J.S., Kim Y.S., Son H. Valproic acid promotes neuronal differentiation by induction of proneural factors in association with H4 acetylation. Neuropharmacology. 2009;56:473–480. doi: 10.1016/j.neuropharm.2008.09.019. [DOI] [PubMed] [Google Scholar]
- Yuan P.X., Huang L.D., Jiang Y.M., Gutkind J.S., Manji H.K., Chen G. The mood stabilizer valproic acid activates mitogen-activated protein kinases and promotes neurite growth. J. Biol. Chem. 2001;276:31674–31683. doi: 10.1074/jbc.M104309200. [DOI] [PubMed] [Google Scholar]
- Zhao C., Deng W., Gage F.H. Mechanisms and functional implications of adult neurogenesis. Cell. 2008;132:645–660. doi: 10.1016/j.cell.2008.01.033. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.