Costs of meningococcal outbreaks comprising costs incurred during the disease response phase and the disease surveillance phase are substantial. The increasing occurrence of meningococcal cases in outbreak situations and the associated control costs should appropriately be considered in economic evaluations of vaccination programs in countries affected by these outbreaks. More studies documenting outbreak-control strategies in terms of costs and resource use are needed.
Keywords: caregiver costs, indirect costs, Latin American countries, Neisseriameningitidis, outbreaks
Abstract
Background. The impact of meningitis outbreaks is substantial. We aim to calculate the costs of meningococcal outbreaks in Brazil and Colombia from the healthcare system perspective.
Methods. A review of the literature was performed on costs associated with meningococcal outbreak in Latin America. Structured interviews capturing information about the use of resources, expenses allocated to treatment of infection, immunization campaigns, and response activities during the outbreak and disease surveillance pre- and postoutbreak were directed at local health authorities in Brazil and Colombia to foster a greater understanding of the economic impact of meningococcal outbreaks. All costs were expressed in 2014 US values.
Results. The Vila Brandina outbreak in Brazil reported 3 cases that were associated with a total investigation and outbreak management cost of $34 425 ($11 475 per notified case), representing 2.7 more than the annual gross domestic product per capita in Brazil. In contrast, the outbreak in Cartagena de Indias in Colombia reported 6 cases at a cost of the disease response phase of $735 or 9.5% of the annual gross domestic product per capita ($123 per notified case). For the disease surveillance phase, the costs ranged from $3935 (in Cartagena de Indias) to $6667 (in Vila Brandina). Serogroups B and C were responsible for the majority of meningococcal outbreaks reported in Brazil and Colombia.
Conclusions. Findings of this study underscore the importance of meningococcal disease in the region. Future research should focus on a more detailed investigation of costs of meningococcal outbreaks covering all phases of an outbreak.
Although most of the global risk of meningococcal outbreaks occurs in countries throughout the “meningitis belt” in sub-Saharan Africa (SSA) [1, 2], outbreaks of meningococcal disease have claimed many lives in countries of Latin America, particularly Brazil, Argentina, Chile, and Uruguay. In contrast to the “meningitis belt” in SSA where epidemics occur from December to April [3], outbreaks in countries of the region occur at irregular intervals throughout the year.
Serogroups B and C are currently responsible for the majority of the meningococcal outbreaks reported in the region. However, these serogroups are not the only ones contributing to outbreaks. The rapid emergence of serogroup W135 (associated with the ST-11 complex) in Argentina, Brazil, and Chile in recent years [3] has also contributed to outbreaks in countries of the region and demonstrates the unpredictable nature of meningococcal disease epidemiology. Outbreaks of serogroups B and C meningococcal disease, characterized by increased rates of disease among persons who live in the same community, occur frequently in Brazil. In contrast, outbreaks of meningococcal disease serogroup W135, reported in southern Brazil, Argentina and Chile [4], Cuba [5], and Dominican Republic [6], are characterized by small-scale outbreaks. Experts anticipate that these serotypes will continue to grow in incidence and become widespread in other countries of the region [4].
To understand the economic impact of meningococcal outbreaks, we performed a literature review focusing on meningococcal outbreaks in the region of Latin America and the Caribbean. We also quantified the costs associated with meningococcal outbreaks in Brazil and Colombia; we also compared the costs of these outbreaks to other outbreaks in the region. This study is the first study to present the costs of multiple meningococcal outbreaks in the region.
METHODS
Review of the Literature of Meningococcal Outbreaks
We performed a review of the literature to assess the cost of meningococcal outbreaks in selected countries of the region and the region of Africa. We conducted online literature searches using key electronic databases including all ages for the period 2000 to 2014 (Supplementary Annex 1). We reviewed data from all languages, concentrating on English, Spanish, and Portuguese. We used search terms relating to meningococcal disease and costs (Supplementary Annex 1). Eligible studies were selected according to the predetermined inclusion criteria, which considered the following: articles published in a peer-reviewed journal with a focus on meningococcal disease in the region of Latin America and the Caribbean and Africa; articles written in English, Portuguese or Spanish; and articles that focused specifically on an economic evaluation.
Estimating the Cost of Meningococcal Outbreaks in Selected Countries of the Region
We calculated the costs of 2 recent outbreaks in Brazil and Colombia from the healthcare system perspective to improve our understanding of the economic burden of meningococcal outbreaks. We chose to focus on these outbreaks because they are recent and information about them such as the number of cases reported, the population at risk, the geographical size of the outbreak, and costs incurred are available. We followed the World Health Organization definition of an outbreak as the occurrence of cases of disease in excess of what would normally be expected in a defined community, geographical area, or season [1].
We described and value all economic resources used to mitigate the outbreak and to treat the cases arising from it. Amongst resources consumed when an outbreak occurs, we distinguished medical from nonmedical resources. Medical resources were further divided into direct treatment costs (eg, hospital beds, doctor visits, medication) and direct control costs or costs from mitigation strategies to control the outbreak (health department investigations and announcements, purchase, and administration of prophylaxis, such as immune globulin injections and vaccine). Nonmedical resources represent indirect costs. These arise as a consequence of the outbreak interrupting the normal activities of everyday life and include the economic value of lost time at work, lost business opportunities, as well as lost time for household chores and leisure activities. All costs are expressed in 2014 US-dollar values.
For each of the outbreaks, we collected (1) demographic information of the cases and (2) information of the various phases of the outbreaks, including activities performed during the response phase (control of the outbreak) and the disease surveillance phase (disease monitoring). We performed interviews with structured standardized questionnaires to capture this information (Supplementary Annex 2).
Resource utilization and costs of control of the outbreak (disease response phase) and monitoring of disease (disease surveillance phase) were collected retrospectively. The interviews, administered to health authorities involved in the outbreak, captured information about the use of resources, expenses allocated to case management (eg, chemoprophylaxis), and the costs associated with immunization campaigns and response activities during the outbreak and disease surveillance after the outbreak.
The disease response phase is the time an outbreak is being controlled. Only cost inputs that are specific to the disease response phase were included. These include personnel, office supplies, gasoline consumption, rifampicin chemoprophylaxis, and vaccines. We were able to capture all these costs for the outbreak in Vila Brandina. The outbreak in Cartagena de India did not make available information about personnel costs and chemoprophylaxis costs, because serogroup B vaccination is currently unavailable in Colombia.
In contrast, disease surveillance is the period when the disease is monitored to identify isolated cases and when prevention measures (eg, routine immunization), and control measures (eg, chemoprophylaxis), are implemented on suspected meningococcal cases. Disease surveillance occurs throughout the year, independent of the occurrence of an outbreak. However, we were not able to quantify the cost of disease surveillance for the whole year due to time constraints and limited funds for the study. For the outbreak in Vila Brandina, we estimated the cost of disease surveillance for 3 months, whereas the cost of disease surveillance for the outbreak in Cartagena de India lasted only 18 days.
We reported on the total costs of an outbreak and the mean cost per outbreak case. These costs were calculated using the “ingredients” method, in which values of each input were based on its unit price and the quantity used weighted by the estimated percentage of the input used for the evaluated phase of the outbreak (eg, disease response phase, disease surveillance phase).
To determine the economic impact of the outbreak on the gross domestic product (GDP), we presented the costs as a percentage of the annual GDP per capita using World Bank estimates [7]. In Brazil and Colombia, the annual GDP per capita was 12 576 and 7763 during 2011 and 2012, respectively. We expressed costs in 2014 US dollars using the official exchange rate [8]. Cost data were analyzed using Microsoft Excel 2011 for Mac.
RESULTS
Review of the Literature of Meningococcal Outbreaks
Despite the sporadic report of meningococcal disease, the economic impact of such an outbreak can be substantial. To date, a limited number of studies describing the economic impact of meningococcal outbreaks have been published in Latin America and the Caribbean. We identified only 5 citations for review. Based on predefined inclusion and exclusion criteria, we abstracted the full text of 4 peer-reviewed studies that were considered relevant to this study.
Colombini et al [6] reported meningococcal outbreaks in 2 health districts in Burkina Faso in 2007 that resulted in 25 000 cases and 1700 related deaths with an estimated total direct cost of $3.5 million (in 2006 US dollars). This cost corresponded to 5% of the annual health expenditures in Burkina Faso. The total average cost incurred by each household per meningitis case was $90, corresponding to 34% of the GDP per capita. Households in which a person experienced sequelae incurred an additional cost of $25–$154 for rehabilitation (in 2006 US dollars). The majority of the households paid for all or a part of the meningitis care, which included medicines and physician consultations. The average loss of income among people with meningitis was $11.5 (in 2006 US dollars). People with meningitis who worked lost an average of 21 days, and those with meningitis attending school missed an average of 12 days. Likewise, households lost an average of $9.1 in assets (eg, crops, cattle) (in 2007 US dollars) [9]. The cost and impacts to the public health system of the same outbreak in Burkina Faso were estimated at a total societal cost of $9.4 million (or $0.69 per capita), with health system costs of $7.1 million (in 2006 US dollars) [10].
The cost of a more recent meningococcal outbreak in the Colombian Caribbean city of Cartagena de India [9] resulted in 6 cases with an average age of 4.6 years (standard deviation = 3.5). The estimated cost of control of the outbreak was 0.80 per 1000 inhabitants (or a total of $735 in 2011 US dollars). This corresponds to the disease response phase and includes personnel costs and costs of office supplies and chemoprophylaxis. The total cost of the disease surveillance phase of the outbreak (or monitoring costs) was estimated at $4.1 per 1000 inhabitants (or $3935 in 2011 US dollars). In addition to the costs of control and monitoring of meningococcal disease, the costs related to hospital care provided during the outbreak were $5.1 per 1000 (or $4921 in 2011 US dollars), contributing over 65% of the total cost for some patients [8]. All of the cases identified were reverse transcription-polymerase chain reaction positive for Neisseria meningitidis for serogroup B. More than half of the patients died during the outbreak.
Another study reported a community outbreak in Campinas, São Paulo, Brazil [11]. In this study, 9 reported cases were associated with a total investigation and outbreak management cost per case of $17 000 (in 2007 US dollars) [10]. The total cost to manage the outbreak was $142 755 (in 2007 US dollars). This included the costs of chemoprophylaxis and vaccination, the cost of transportation, and the costs of personnel. Control strategies accounted for more than 85% of the total costs. Total indirect costs resulted in over $1500 (in 2007 US dollars).
All 4 of these studies have study limitations in common. The primary study limitation relates to the narrow timeframe of the analyses. Although meningococcal outbreaks may be brief, the disease surveillance phase of an outbreak, the period in which disease cases are monitored, should be performed regularly and should be ongoing. Another limitation relates to the unavailability of certain medicines during an outbreak. The meningococcal outbreak in Colombia [9] occurred when there was no meningococcal vaccine available in the country. The absence of a vaccination program affects how the outbreak is controlled and results in an underestimation of the outbreak costs. Moreover, data collection during the outbreak was performed through medical chart reviews and administrative data, limiting the type of information about the outbreak. The inability to access information about a patient during an outbreak is also a limitation because not including exposed cases could underestimate the total costs of the outbreak. Another important limitation is that all studies consider only a small sample of people; people that have access to healthcare services. Therefore, the findings may not reflect the situation in the whole country. Other study limitations relate to the exclusion of costs borne for the treatment of an outbreak case. Treatment costs borne by families in less formal settings include treatment at home or by traditional healers. The studies that we reviewed did not ask questions about these types of costs.
Costs of Meningococcal Outbreaks in Brazil and Colombia
Meningococcal Outbreak in Vila Brandina, São Paulo, Brazil (2011)
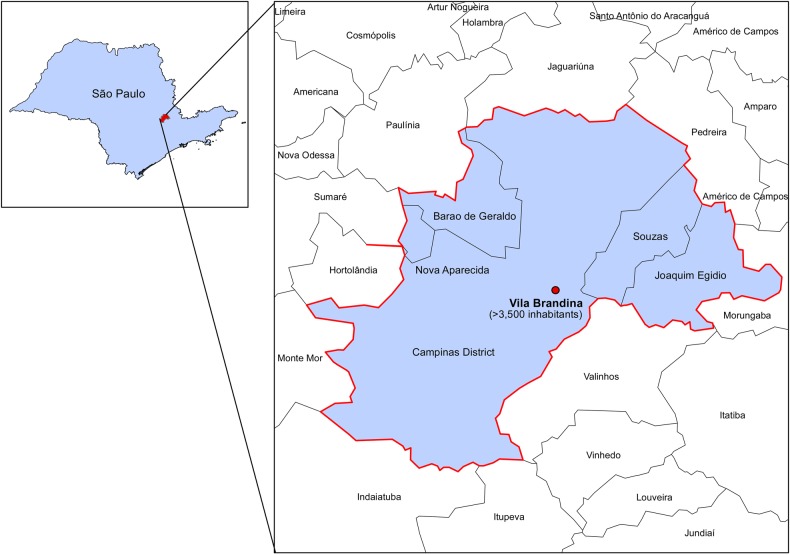
A meningococcal outbreak occurred in October 2011 in Vila Brandina, a community in the city of Campinas, São Paulo, Brazil (Figure 1). This outbreak was characterized by serogroup C outbreak based on results of the multilocus sequence typing performed. No other serotypes were identified during the outbreak. Vila Brandina is a shantytown located in the Southeast and in the city of Campinas, a municipality in São Paulo state with over 3500 inhabitants. With a population of approximately 1.1 million inhabitants, Campinas is one of the most populous cities in Brazil and the third most populous municipality in the state of São Paulo. The outbreak involved 3 meningococcal cases: 2 children (age 3 years) and an adult (age 36 years). One of the children and the adult were treated successfully whereas the other child died. Two of the identified cases had laboratory confirmation for serogroup C; 1 case was confirmed by clinical and epidemiological criteria. The index case was discovered late, during the investigation of the last 2 cases. In this study, we defined the disease response phase as the period in which new cases could arise from time of onset of symptoms to the last day of symptom onset and 10 days later (October 10, 2011 and October 26, 2011). The disease surveillance phase was defined as the period in which the cases and immediate contacts in the health clinics and in the community were monitored. For this outbreak, the disease surveillance phase was 1 month.
Figure 1.
Map of Vila Brandina, Campinas, São Paulo, Brazil, and area affected by the meningococcal outbreak.
The total cost associated with control of the outbreak in Vila Brandina outbreak was $34 425 ($11 475 per notified case). This represents 2.7 more than Brazil's GDP per capita level in 2011 (Table 1). The meningococcal C conjugate vaccine and personnel were the main contributors of this cost, with 60% and 34% of the total cost, respectively. The duration of the disease response phase was 27 days. This included the surveillance period that was used to establish the epidemiological end of the outbreak. The first case (an adult) had onset of symptoms on October 10, 2011, whereas the other 2 cases (both children) had onset of symptoms October 26, 2011 and October 27, 2011, respectively.
Table 1.
Cost of the 2011 Meningococcal Outbreak in Vila Brandina, City of Campinas, Municipality of São Paulo State (US Dollars 2014)
| Resources | Disease Response Phase (Control of the Outbreak)a | Disease Surveillance Phase (Monitoring of Disease Cases)b |
|---|---|---|
| Personnel | 11 715 | 3225 |
| Office supplies | 255 | NR |
| Gasoline consumption | 56 | 41 |
| Rifampicin chemoprophylaxis | 1304 | 54 |
| Meningococcal C conjugate vaccine | 20 652 | 3347 |
| Meningococcal polysaccharide vaccine | 443 | NR |
| Total | 34 425 | 6667 |
Abbreviation: NR, not reported.
a The duration of the disease response phase was 27 days.
b The duration of the disease surveillance phase was 30 days.
For the disease surveillance phase or the period when meningococcal disease is monitored, which lasted 30 days, the cost was estimated at $6667 ($2.67 per exposed case), which raised the total expenditure of the outbreak to 3 times Colombia's GPD per capita level for the same year. The cost of disease surveillance was attributed mainly to meningococcal C conjugate vaccine costs and personnel costs, with 50% and 48% of the total costs, respectively. Meningococcal C conjugate vaccine was given to all suspected meningococcal cases during surveillance.
Vaccines were also applied during the disease response (investigation and outbreak management) phase. These were the meningococcal polysaccharide vaccine for children ≥2 years and the meningococcal C conjugate vaccine for children ≤2 years of age. Before the outbreak and during surveillance, only meningococcal C conjugate vaccine was administered. During the outbreak, the Ministry of Health delivered both the meningococcal C and the meningococcal polysaccharide vaccines free of charge to the municipality of Campinas. A total of 2500 doses of the polysaccharide vaccine were received, and 1815 doses were applied during the outbreak and disease surveillance phase at a vaccine purchase price of $7.35 per dose (Brazilian Real [R$]13.79). The Ministry of Health in Brazil purchased the meningococcal C conjugate at $9.85 per dose (R$18.50) during the response phase. Two hundred doses of this vaccine were received, and 40 doses were applied to affected patients during this period.
Rifampicin chemoprophylaxis was given to immediate family members and other contacts (eg, neighbors) during the response and disease surveillance phases. The strategy of rifampicin chemoprophylaxis is generally implemented to immediate family members only, but, given the adverse living conditions in Vila Brandina, an expanded strategy of rifampicin chemoprophylaxis was adopted to include other exposed contacts. Cost related to communication and advocacy efforts during the outbreak were not considered. The costs of laboratory tests and training of personnel were not available; thus, they were not considered in the cost calculation of the investigation and management of the outbreak. No office supplies were considered in the cost estimation of disease surveillance, because information was not available.
Meningococcal Outbreak in Cartagena de Indias, Colombia (2012)
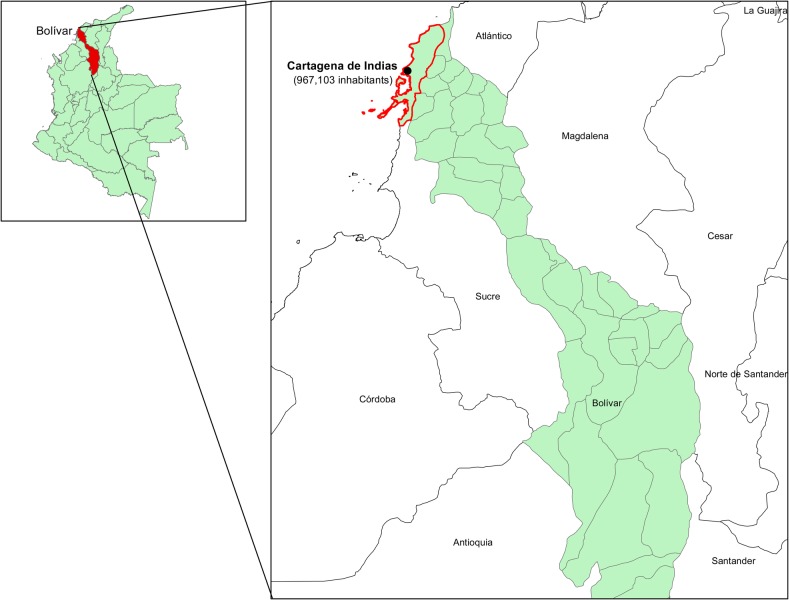
From February 21 to March 17, 2012, The District Health Department, in association with the “Napoleon Franco Pareja Children Hospital”, investigated a meningococcal outbreak in a poor neighborhood in southeast Cartagena de Indias, Colombia (Figure 2). With 967 103 inhabitants, Cartagena de Indias is a port city and popular beach destination and one of the most important tourist destinations in the region of Latin America, where 95.5% of the population lives in urban areas. Cartagena de Indias is the capital of 1 of the 8 departments that make up the Colombian Caribbean region, and it is also the fifth most populous city in Colombia. An estimated 5000 people were exposed to the 2012 outbreak. The timeframe of the study for the response phase was shorter in Cartagena than for the outbreak in Vila Brandina, with the confirmation date of the index case starting on February 21, 2012 and ending 7 days later on February 28, 2012.
Figure 2.
Map of Cartagena de Indias, Colombia, and area affected by the meningococcal outbreak.
Six cases of meningococcal disease (including 2 suspected cases) were identified in children in the total time of the outbreak. Three children died and 3 fully recovered. All N meningitidis isolates were identified as serogroup B. To stop the outbreak, chemoprophylaxis clinics were organized in the population at risk; 50 doses of antibiotics were administered to the close contacts and public health staff. Twenty children that were close contact to the index case received prophylaxis initially. In addition, 26 children that were secondary contacts received prophylaxis at school, in addition to 2 pediatricians and 2 nurses from the hospital exposed to the outbreak.
The 6 cases in the 2012 Cartagena de Indias outbreak were associated with a total investigation and outbreak management costs of $735 (Table 2), representing 9% of Colombia's annual GPD per capita for the same year. Of the total cost, 68% was related to personnel costs and 32% were due to chemoprophylaxis. The costs of the outbreak in Cartagena de Indias did not include all cost components (eg, vaccine costs, treatment costs, transportation costs) in the calculation.
Table 2.
Cost of the 2012 outbreak in Cartagena de Indias, Colombia (US Dollars 2014)a,b
| Resources | Disease Response Phase (Control of the Outbreak) | Disease Surveillance Phase (Monitoring of Disease Cases) |
|---|---|---|
| Personnel | $502.3 | $3921.9 |
| Office supplies | NR | $13.18 |
| Gasoline consumption | NR | NR |
| Chemoprophylaxis | $232.8 | NR |
| Meningococcal C conjugate vaccine | NR | NR |
| Meningococcal polysaccharide vaccine | NR | NR |
| Total | $735.1 | $3935.08 |
Abbreviation: NR, not reported.
a Based on the findings from Pinzón-Redondo et al [9].
b Based on 6 cases identified during the outbreak.
DISCUSSION
Only 1 unpublished study on the economic impact of a meningococcal outbreak in Brazil was found in the literature [11], and 1 study on costs of a recent meningococcal outbreak in Colombia [9] was also found. No other studies on the economic impact of meningococcal outbreaks in countries of the region are available. The costs of an outbreak are typically found to be substantially higher than estimates from sporadic cases (eg, cost-of-illness studies) and estimates used in cost-effectiveness analyses. This is largely due to costly outbreak-control measures. Postexposure surveillance and prophylaxis is a major cost factor.
The difference in costs for the response phase between the outbreak in Vila Brandina and the outbreak in Cartagena de Indias was due to the type of cost components included in the cost calculation. For the Cartagena outbreak, only personnel costs and chemoprophylaxis costs were estimated, whereas the Vila Brandina outbreak considered personnel, office supplies, gasoline consumption, chemoprophylaxis, and vaccination costs. There are marked differences between both countries with regard to the outbreak: year of the event, how the surveillance was carried out, and differences in the resources that both countries had or were available to deal with the outbreak. Differences in costs were also due to the number of outbreak cases, the area of exposure, the perspective under consideration, and management practices.
For the disease surveillance phase, the costs ranged from $3935 (Cartagena de India outbreak) to $6667 (Vila Brandina outbreak). For the Vila Brandina outbreak, the costs of disease surveillance were attributed mainly to meningococcal C conjugate vaccine costs and personnel costs, with 50% and 48% of the total costs, respectively. For the Cartagena de India outbreak, the costs of disease surveillance were mainly attributed to personnel costs. Vaccine costs were not included in the calculations of the outbreak in Colombia because the Cuban vaccine strategy, comprising the serogroup C capsular polysaccharide and the outer membrane vesicles of serogroup B meningococcus, was not available in Colombia at the time of the outbreak.
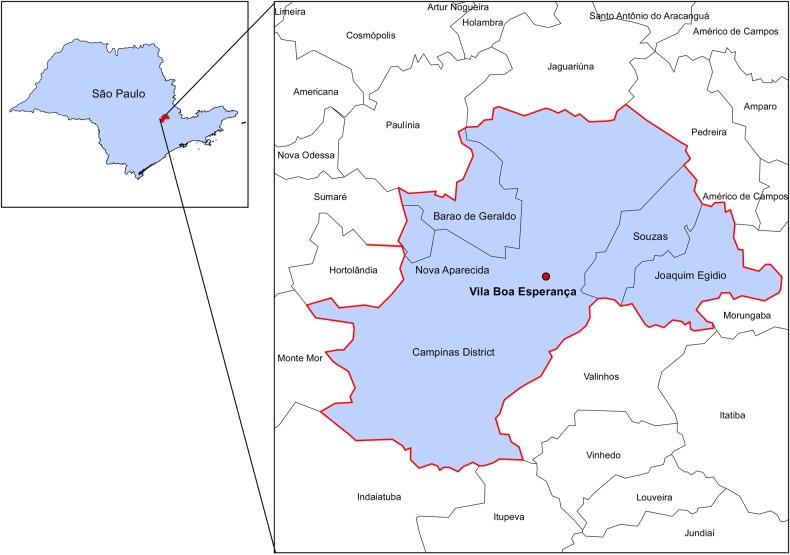
For the city of Campinas, the cost of the meningococcal disease outbreak to its health system was substantial ($34 425 or $11 475 per case). The largest contributor of the total cost was the reactive vaccination campaigns using meningococcal C conjugate vaccine and meningococcal polysaccharide vaccine. The highest cost was due to the vaccine itself. Personnel costs were also substantial accounting for 34% of the total cost of the outbreak. When compared with an earlier outbreak that took place in Vila Boa Esperança (Figure 3) in 2007 [10], the costs of the 2011 outbreak were lower ($34 425 vs $128 963) because there were fewer exposed cases and required fewer personnel to treat and vaccinate the exposed population (Table 3). The timeframe for each outbreak was also different; 10 days for the Vila Boa Esperança outbreak vs 27 days for the Vila Brandina outbreak. Nine cases were reported during the Vila Boa Esperança 2007 outbreak, 6 more cases than the 2011 outbreak. These cases were associated with a total investigation and outbreak management cost of $128 963 ($14 329 per case) (Table 2), more than 3 times the costs of the 2011 outbreak, which estimated the costs of the disease response phase at $34 425 ($11 475 per notified case).
Figure 3.
Map of Vila Boa Esperança, Campinas, São Paulo, Brazil, and area affected by the meningococcal outbreak.
Table 3.
Comparison of the 2007 and 2011 Meningococcal Outbreaks in City of Campinas, Municipality of São Paulo State (US Dollars 2014)
| Resources | 2007 Outbreaka,b (n = 9) (Disease Response Phase) | 2011 Outbreakc (n = 3) (Disease Response Phase) |
|---|---|---|
| Personnel | 51 051 | 11 715 |
| Office supplies | 8379 | 255 |
| Gasoline consumption | 465 | 56 |
| Rifampicin chemoprophylaxis | 63 | 1304 |
| Vaccinationd | 69 005 | 21 095 |
| Total | 128 963 | 34 425 |
a Based on the findings from Kemp et al [11].
b This corresponds to the 2007 outbreak in Vila Boa Esperança, Campinas, São Paulo.
c This corresponds to the 2011 outbreak in Vila Brandina, Campinas, São Paulo.
d Vaccination includes meningococcal C conjugate vaccine and meningococcal polysaccharide vaccine.
When out-of-pocket expenses and loss of productivity were considered, the 2007 outbreak was associated with a total investigation and outbreak management cost of $143 000 ($16 000 per notified case). For the 2011 outbreak, caregivers of the 3 outbreak cases were not interviewed because ethical approval was not obtained. As a result, no out-of-pocket expenditure and loss of productivity information was included in the cost calculation of the 2011 outbreak. More importantly, the cost of treating the outbreak cases was not estimated because access to patient charts was not permitted. This resulted in substantial underestimation of the costs for the 2011 outbreak.
The difference in cost between the 2 outbreaks was due to the number of cases involved the outbreaks, the size of the population, the area of exposure, and the management practices. In 2007, the conjugate vaccine was not available for routine immunization of exposed patients. The meningococcal C conjugate vaccine was introduced for routine immunization in 2011. For the latter outbreak, infants received 2 doses at 3 and 5 months with a booster dose at 12 months, whereas toddlers between 12 and 23 months of age received 1 dose. No catch-up campaigns in the older age groups were planned.
Moreover, only costs associated with the control of the outbreak (disease response phase) were measured in the 2007 outbreak, because disease surveillance was considered independent of the outbreak. In contrast to the costs associated with the 2011 outbreak, the costs associated with the earlier outbreak were estimated from the societal perspective.
The outbreaks described in the literature may be subject to publication bias because outbreaks with an exceptional impact may stimulate interest in economic analysis more than the “average outbreak”. Nevertheless, the estimates presented here give an indication of the upper and lower boundaries of such events, and the information they provide is likely to become more important in choosing cost-effective programs for the prevention of meningococcal disease.
The economic burden of a meningococcal outbreak goes beyond economic impact. Although this was not part of the analysis, it is important to note the tremendous impact to the communities and the country at large. Given the unpredictable nature of meningococcal outbreaks, most people are not prepared for containment measures. This raises important questions about the opportunity costs of meningococcal outbreaks from a societal perspective and, specifically, the impact that it has on the quality of life of individuals.
Calculating the costs associated with outbreaks is challenging because there are various entities bearing the cost of an outbreak, and it is not always clear who bears the cost of the outbreak. Moreover, the people and the programs involved in the control and prevention of an outbreak use the same inputs for other outbreaks, and it may be difficult to discern what inputs are attributed to the program. This problem is exacerbated by the limited data available on costs. Often, cost information is not reported or presented in the right format. Every outbreak is different in terms of the population exposed to the outbreak, the location, the number of cases exposed and the severity of the cases, and the duration of the outbreak. Actions to prevent and control the outbreak depend greatly on (1) the experience of the surveillance team and (2) the health system. The control strategy that is implemented also depends on the pressure that society exerts on the healthcare system to control the outbreak. Political pressure may lead to unnecessary control measures. It is important to note that the analysis did not include the capital costs generally associated with an outbreak or the indirect costs of an outbreak, providing only a partial picture of the cost of an outbreak.
The extent to which meningococcal outbreaks contribute to poverty, by way of catastrophic health expenditures, is unknown. When meningococcal outbreaks occur, the families of those impacted by the outbreak may have to use savings, sell property, or even take out loans to pay for their care. In addition, their time spent away from production may impact negatively on their revenues. In short, an outbreak can both increase expenditures and reduce incoming finances, and thereby drive families into poverty. The estimates in the present study provided only a partial picture of the cost of an outbreak, because it did not consider any of these cost and income components. Indirect costs comprising loss of caregiver income and missing school attendance for school-age children have been reported in earlier outbreaks [6, 10] as some of the most important components of a meningococcal outbreak. Cost associated with medicines, consultations, and other medical interventions, despite being free during an outbreak, may represent a substantial burden to households because treatment may be incurred by caregivers [6, 10]. Capital costs, however, may be low because outbreak-related equipment are generally borrowed from routine health activities. Only health systems costs were considered in the current analysis due to time constraints and limited data available. We did not consider costs borne by families for treatment of meningococcal disease in less formal settings (ie, treatment at home or by traditional healers) or costs in the private sector. Last but not least, the size of the outbreaks was small; however, the sizes are representative of outbreaks that occur in Brazil and Colombia and other countries of the region.
CONCLUSIONS
Future outbreaks may increase in size resulting in higher costs of outbreaks. The increasing occurrence of meningitis cases in outbreak situations and the associated control costs should appropriately be accounted for in economic evaluations of vaccination programs. To do this, more studies documenting such outbreak-control strategies in terms of costs and resource use are needed.
Supplementary Material
Supplementary material is available online at Open Forum Infectious Diseases (http://OpenForumInfectiousDiseases.oxfordjournals.org/).
Acknowledgments
We thank Cristina Garcia and the entire Adult Global Estimation of Disease Burden and Distribution of Serotype of Serious Pneumococcal and Meningococcal Disease team for sharing materials and experience from Johns Hopkins University's International Vaccine Access Center's Global Disease Burden project. We are grateful to Barbara B. Landreth (Centers for Disease Control and Prevention Public Health Library and Information Center) for assistance with the updated literature search and the retrieval of full text articles. We are also grateful to Claudia Lopes and Carlos Castañeda for assistance with data abstraction regarding the literature review. In addition, we thank Brigina Kemp, Alvaro Pérez Ihl, María Carrasquilla Sotomayor, Nelson Jose Alvis Zakzuk, and Rodrigo Nogueira Angerami for assistance with the data collection of the cost study. We also thank all regional researchers and economists who shared data and experience with us and the local physicians and parents who took part in the economic surveys.
This paper is dedicated to Dr. Ciro de Quadros for his guidance, vision, and support in fostering a greater understanding of the changing epidemiology and costs of meningococcal disease.
Financial support. This study was funded by Sabin Vaccine Institute.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1.World Health Organization, Geneva. Enhanced surveillance of epidemic meningococcal meningitis in Africa: a three-year experience. Wkly Epidemiol Rec 2005; 80:313–20. [PubMed] [Google Scholar]
- 2.Molesworth AM, Thomson MC, Connor SJ et al. . Where is the meningitis belt? Defining an area at risk of epidemic meningitis in Africa. Trans R Soc Trop Med Hyg 2002; 96:242–9. [DOI] [PubMed] [Google Scholar]
- 3.Sáfadi MA, de los Monteros LE, López EL et al. . The current situation of meningococcal disease in Latin America and recommendations for a new case definition from the Global Meningococcal Initiative. Expert Rev Vaccines 2013; 12:903–15. [DOI] [PubMed] [Google Scholar]
- 4.Osvaldo RC, Reynaldo JB, Carlota PC. [Enfermedad rneningocócica y Vamengoc BC en menores de un año Cuba, 1983 a 1991]. Rev Cubana Med Trop 1996; 48:34–9. [PubMed] [Google Scholar]
- 5.Secretaría de Estado de Salud Pública y Asistencia Social. Dirección General de Epidemiología. [Situación de la enfermedad meningocócica en Republica Dominicana]. Boletin Epidemiología 1997; 11:8–10. [Google Scholar]
- 6.Colombini A, Bationo F, Zongo S et al. . Costs for households and community perception of meningitis epidemics in Burkina Faso. Clin Infect Dis 2009; 49:1520–5. [DOI] [PubMed] [Google Scholar]
- 7.World Bank. World Development Indicators, 2011. Available at: http://data.worldbank.org/indicator/NY.GDP.PCAP.CD Accessed 15 May 2014.
- 8.International Monetary Fund. Currency Composition of Official Foreign Exchange Reserves. Washington, DC: International Monetary Fund; 2012. Available at: http://www.imf.org/external/np/sta/cofer/eng/index.htm Accessed 15 May 2014. [Google Scholar]
- 9.Pinzón-Redondo H, Coronell-Rodriguez W, Diaz-Martinez I et al. . Estimating costs associated with a community outbreak of meningococcal disease in a Colombian Caribbean city. J Health Population and Nutr 2014; 32:539–48. [PMC free article] [PubMed] [Google Scholar]
- 10.Colombini A, Badolo O, Gessner B et al. . Cost and impact of meningitis epidemics for the public health system in Burkina Faso. Vaccine 2011; 29:5474–80. [DOI] [PubMed] [Google Scholar]
- 11.Kemp B, Takemoto ML, Freitas AR et al. . Cost estimate for invasive meningococcal disease outbreak control – a study in Campinas, São Paulo. Abstract presented at the 28th ESPID Meeting Nice, France, 2010. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.