Abstract
Treatment 2.0 is an initiative launched by UNAIDS and WHO in 2011 to catalyze the next phase of treatment scale-up for HIV. The initiative defines strategic activities in 5 key areas, drugs, diagnostics, commodity costs, service delivery and community engagement in an effort to simplify treatment, expand access and maximize program efficiency. For adults, many of these activities have already been turned into treatment policies. The recent WHO recommendation to use a universal first line regimen regardless of gender, pregnancy and TB status is a treatment simplification very much in line with Treatment 2.0. But despite that fact that Treatment 2.0 encompasses all people living with HIV, we have not seen the same evolution in policy development for children. In this paper we discuss how Treatment 2.0 principles can be adapted for the pediatric population. There are several intrinsic challenges. The need for distinct treatment regimens in children of different ages makes it hard to define a one size fits all approach. In addition, the fact that many providers are reluctant to treat children without the advice of specialists can hamper decentralization of service delivery. But at the same time, there are opportunities that can be availed now and in the future to scale up pediatric treatment along the lines of Treatment 2.0. We examine each of the five pillars of Treatment 2.0 from a pediatric perspective and present eight specific action points that would result in simplification of pediatric treatment and scale up of HIV services for children.
Introduction
Despite successes in the prevention of mother-to-child transmission (MTCT), about 900 children become infected with HIV each day, 90% in sub-Saharan Africa. Without antiretroviral treatment (ART), only half of all HIV-infected children survive to age 2 [1,2]. However, as of 2011, it is estimated that of the 2 million children throughout the world in need of ART, only one in three are receiving it, well below the estimated 58% for adults [3]. Several factors contribute to this treatment gap. To start with, access to timely early infant diagnosis (EID) is limited. Of the 22 priority countries in the Global Plan to Eliminate MTCT, four have been able to reach EID coverage of 60%, but globally only 30–35% of HIV-exposed infants (HEIs) have access to EID in the first 2 months of life [3]. Moreover, access to diagnosis and identifying a child as infected does not translate to ART access. Linkages between EID services [usually within prevention of mother-to-child transmission (PMTCT) programs] and ART services are very weak, resulting in high loss-to-follow-up and delayed treatment initiation (see Retention and Linkage to care article in this series) [4]. Apart from EID, there are few opportunities for HIV testing in children and adolescents whether infected perinatally or through behavioral risks, and so HIV infection often goes unrecognized until late in the course of illness when mortality is high [5]. Even after the ambitious goal of eliminating new infections in children is achieved, there will still be millions of children living with HIV who need care. Interventions to increase access to HIV diagnosis and treatment for children and adolescents are, and will continue to be, urgently needed.
In 2011, the WHO and UNAIDS launched the Treatment 2.0 Initiative which aims to expand treatment, improve efficiency and ensure sustainability of the global response to HIV for both adults and children. Treatment 2.0 comprises five key pillars: simplification of ART regimens and harmonization of regimens across age groups; access to point of care (PoC) diagnosis and monitoring; reduced costs of treatment; service delivery adapted to the needs of the population and community mobilization [6]. The newly published WHO Consolidated ARV guidelines, promote a Treatment 2.0 approach for adults. ART choices are simplified to two preferred once-daily regimens, and the approach to treatment is harmonized across diverse adult populations [7]. However, the situation for children is more complex. The choice of treatment is partly determined by age, and several different regimens are recommended because of limited pediatric data to compare one regimen against another and safety concerns with some drugs in younger children. At the same time the new guidance does make some recommendations (such as universal access to ART for all children <5 years), which are more in line with the intent of Treatment 2.0 for children.
In this paper, we discuss what elements are needed for developing a Treatment 2.0 strategy for children across each of the 5 pillars.
Pillar 1: antiretroviral drugs and regimens
The current situation
The 2013 WHO guidelines call for different ART regimens in children under and over three, based on clinical trial data which show that protease inhibitor based ART with lopinavir/ritonavir (LPVr) is associated with better outcomes than nonnucleoside reverse transcriptase inhibitor (NNRTI) based ART in children under 3 years of age [7,8]. However, in practice, the current LPVr formulation (a liquid that requires cold chain transport, is poorly palatable and contains 42% alcohol and 15% propylene glycol) is very difficult for programs in resource-limited settings to use. In addition, LPV/r solution is not recommended for neonates before a gestational age of 42 weeks and a postnatal age of at least 14 days [9]. The anticipated LPV/r granule formulation will address some of the challenges associated with the liquid, and one manufacturer has already filed dossiers with the US Food and Drug Administration (FDA). This granule formulation feeds into the work being done on modular fixed-dose sachets or capsules that contain nucleoside reverse transcriptase inhibitors (NRTIs) combined with LPV/r- a potentially valuable approach to simplifying pediatric HIV treatment in line with the principles of Treatment 2.0 [10].
Looking to the future
Pediatric formulations that offer for children what is available for adults that is, the convenience of well tolerated, once-a-day regimens in fixed-dose combinations (FDCs), are urgently needed. The ideal situation would be one in which the preferred pediatric regimens are the same as the preferred adult regimens. At present however, this is not possible. Adult preferred regimens all contain tenofovir (TDF), which although approved for use in children remains controversial due to safety concerns and the need to monitor for bone toxicity [11]. As a result, WHO guidelines recommend TDF-based ART only as an alternative option, for children between 3 and 10 years of age. National programs may therefore be reluctant to adopt the use of TDF in children, and manufacturers reluctant to make pediatric TDF-containing FDCs because of low demand. More data on the safety of TDF in young children is needed in order to encourage uptake.
Dolutegravir (DLG), an integrase inhibitor, was recently approved by FDA for adults and children age 12 and over [12]. DLG in combination with two once daily NRTIs (such as TDF, abacavir (ABC), lamivudine (3TC) or emtricitabine (FTC)) may offer a highly potent and well tolerated first-line treatment for both adults and children. Adult studies have shown the drug to be effective and well tolerated, and clinical trials of DLG in children are under way [13]. If DLG-based regimens are effective and well tolerated in both infants as well as older children, there is a possibility that future iterations of the WHO guidelines could recommend a single DLG-based regimen for all children and adults, thus simplifying implementation and coalescing demand.
Securing the pediatric antiretroviral market
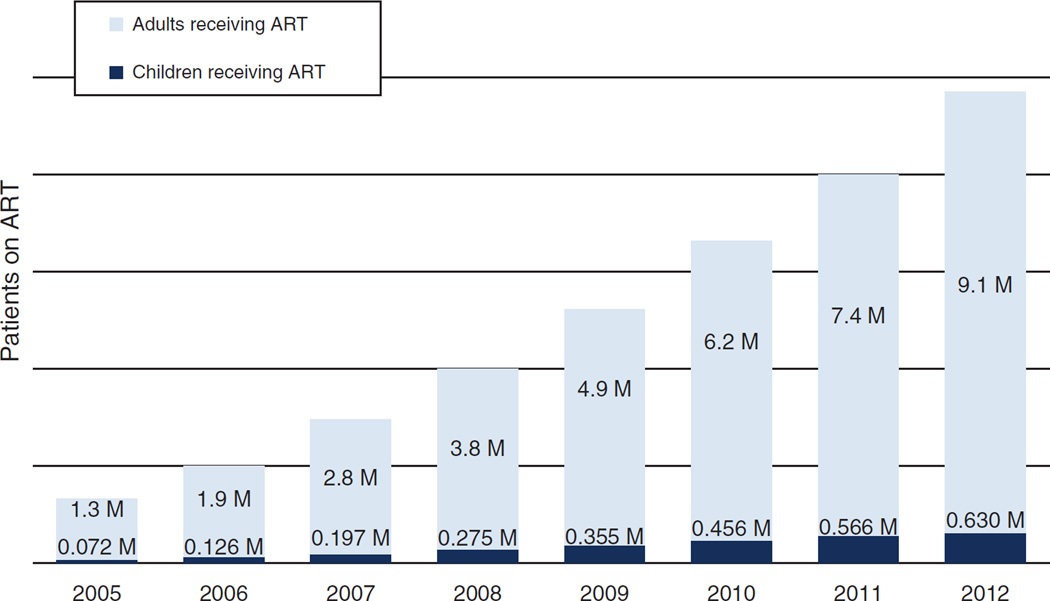
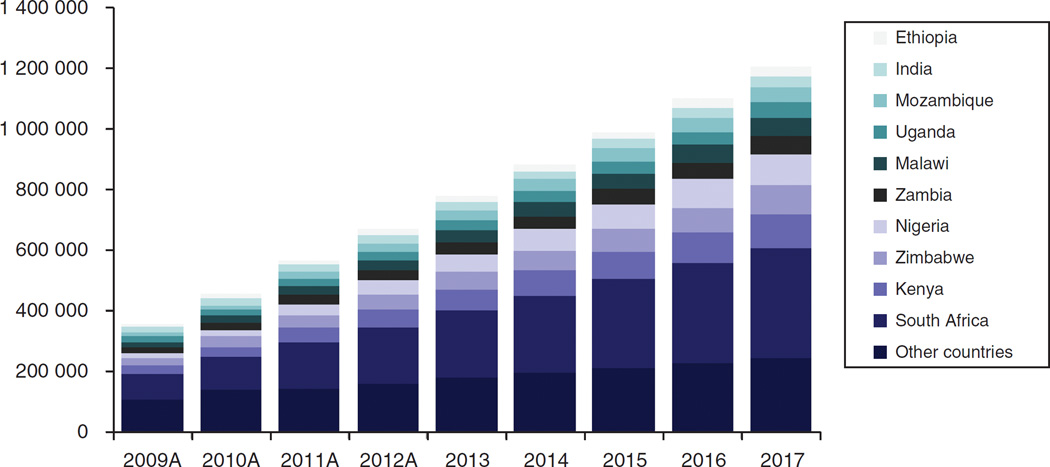
Demand for pediatric ARVs is significantly smaller than that for adults, and with the increasing focus on elimination of new HIV infections among children, there is an assumption that the pediatric ARV market will dwindle [14]. Although this may be true in the long term, in the short to medium term, there is room for growth. Recent data from the 22 countries that are part of the Global Plan suggest that only 34% of children in need receive ART (half of the adult rate), and children account for only 7% of all individuals on ART (Fig. 1). If universal access was achieved, pediatric ARV volumes would increase three-fold [15]. Estimates for the growth of the pediatric ARV market suggest that at the very least, demand will double over the next 4 years (Fig. 2).
Fig. 1. Patients on antiretroviral therapy (ART) in millions.
Source: WHO TUAPR 2011, (pub November 2011) for historical data; WHO/UNAIDS Global update on HIV treatment 2013,(pub June 2013) for 2011 and 2012 statistics on 22 countries within the Global Plan.
Fig. 2. Expected growth of pediatric antiretroviral therapy (ART) patients.
Source: 2009–2011 actual patient numbers provided by WHO TUAPR; 2012 patient numbers extrapolated using linear patient growth of WHO/UNAIDS reported 2011 figures (Courtesy CHAI).
Despite this growth potential, the pediatric ARV marketplace is fragile. The commercial life span of pediatric ARV formulations is short because of the rapid pace at which clinical recommendations evolve. At the same time, there is pressure to keep prices low which means that manufacturers struggle to recover their investment. Between 2006 and 2009, a large portfolio of child friendly ARV formulations were developed and brought to market, but now many of these are often unavailable because of insufficient demand to sustain batch production. The problem of short life span is compounded by market fragmentation. The spread of formulations across different regimens and age/weight bands means that each product becomes a low-volume product. WHO guidelines provide recommendations on the drug regimens to be used for ART in children. However, other than promoting the use of FDCs, they do not offer guidance on product selection.
In 2011, The Inter-Agency Task Team on prevention and treatment of HIV infection in pregnant women, mothers and their children (IATT) released recommendations on pediatric ARV product selection for national uptake. This is a list of 41 pediatric products, which are divided into ‘optimal’, ‘for limited use’ and ‘nonessential’ formulations [16]. Of the 41 products reviewed by the IATT, 15 formulations are listed as ‘optimal’, 11 as ‘limited use’ to allow transition to optimal products and 13 as ‘nonessential.’
Products were selected as optimal based on the principles of the WHO Essential Medicines List for Children. Since the IATT formulary was published in 2011 there has been a decline in procurement of limited use and nonessential products. As we look towards implementing future Treatment 2.0 options for children, consolidation of pediatric ARV demand around a minimal set of optimal products is essential to maintain high product volumes and ensure that manufacturers remain invested in pediatric formulation development. A revision of the IATT formulary is expected in 2013.
Pillar 2: diagnostics
Improved access to laboratory testing for HIV – especiallytopoint-of-care (PoC) and other simplified technologies, is necessary for ART scale-up. For older children (>18 months), testing needs are fully harmonized with adults [7]. Diagnosis can be made using the same rapid HIV-antibody tests, and now that the WHO guidelines have removed the need for CD4 percentages in children, immunological staging can be done using the same CD4 absolute count assays. For toxicity monitoring, all patients regardless of age, need access to the same hematology and chemistry tests – although interpretation of results may differ according to age-related norms. The one critical exception is EID. This can only be done using assays to detect viral nucleic acids (DNA, RNA or total nucleic acid) or viral proteins (p24antigen). These assays are technologically complex and typically only available at a few laboratories in each country [17]. And yet access to EID is absolutely essential to identify and treat children less than 18 months who have the highest mortality of all individuals with HIV (see EID article in this series).
Transport of dried blood spot specimens (which are easy to collect, safe to transport and stable at room temperature), has enabled significant scale up of EID services by linking a small number of central laboratories to a large number of sample collection sites at low-level health facilities, but needs remain [18,19]. Currently, only 30–35% of all HEI have access to timely diagnosis in the first 2 months of life [3]. Furthermore, delays in the return of results are common and sometimes exceed several months contributing to low retention and high mortality [20,21]. Use of SMS printers to facilitate EID results return has significantly improved turnaround time and should be implemented widely, but there is an urgent need to find innovative ways to close the access gap [22].
Emerging PoC platforms for EID provide an alternative to centralized testing that makes EID testing feasible even in primary health facilities or mobile clinics [23–25]. These PoC tests are expected to be simple to use (including by nonlaboratory staff) and can deliver rapid results (within 2 h). Some PoC platforms have built-in wireless networking capacity, so that test information including where the test was done and what the result was, can be uploaded instantly into a remote database. As yet, no PoC EID test is available commercially, but use of PoC might improve health outcomes in several ways:
Access to EID would likely increase – especially for remote, rural or hard-to-teach communities
‘While-you-wait’ results may save time and money for patients, increase efficiency due to fewer ‘wasted’ tests that are never collected, reduce loss-to-follow-up and improve early ART initiation rates
PoC instruments can be placed at any entry point within a facility – for example EID testing can be brought to the labor ward, enabling identification of HIV-infected infants at birth
PoC technologies facilitate task-sharing, allowing work load and resources to be re-distributed and health facilities to become more self-sufficient
Automated data collection will enable sophisticated real-time mapping of tests used and results obtained
Strengthening centralized EID and establishing PoC EID are not mutually exclusive but rather complementary strategies to achieve the goals of Treatment 2.0 for children. Countries will need to strike a balance between these two approaches recognizing the relative benefits of each [26]. For example, centralized testing may be better than PoC for large facilities which have high sample volumes, optimized turnaround time and already high retention rates. Conversely, PoC may be preferable in smaller clinics in which sample transport is costly and unreliable and retention rates are low. Implementation of PoC EID tests may require revision of the testing algorithm to define for example how to confirm positive PoC test results before treatment is initiated" confirmatory.
Virologic testing technologies whether laboratory-based or PoC are increasingly moving towards a single platform for both infant diagnosis and viral load monitoring [23]. This will ensure that as viral load testing becomes the norm for ART monitoring, access to EID is automatically expanded to a greater numbers of sites. Laboratory services in general face systemic challenges including poor supply chain management of reagents, insufficient maintenance of equipment, frequent personnel turnover and inadequate quality assurance [27]. Even as PoC tests are rolled out, it is important to maintain a strong laboratory infrastructure. A comprehensive Treatment 2.0 laboratory strategy for children should include general laboratory systems strengthening, optimization of centralized testing and appropriate and selective deployment of decentralized PoC testing to provide high quality testing services to all children in need.
Pillar 3: reducing costs
On a dosage basis, prices of pediatric ARV products tend to be higher than their adult equivalents (See Costing article in this series). Research on pediatric ARV pricing trends and cost drivers is limited but higher pricing per mg of drug is typically attributed to fixed costs such as packaging and the need for more costly manufacturing technologies to produce the formulations necessary to address the challenges of administering ARVs to children such as syrups or sprinkles [28–30]. Funding from UNITAID provided over $317 million from 2006 to 2011to catalyse growth in pediatric HIV commodities; the Global Fund and PEPFAR have also made significant investments in both EID services and delivery of pediatric ART [31]. However, the global sum of donor support for HIV has remained essentially unchanged since 2008 [32]. As a result, the pressure on National Programs to do more with the same resources will increase. The challenge for program managers is two-fold: How can budgets support a doubling or trebling of pediatric patients in the short-term to fill current treatment gaps and what innovative pricing mechanisms are available to sustain that level of service; and once demand goes down as infections are prevented, how will a declining market affect pricing and supply?
A healthy market with a range of comparable competitor prices will find an equilibrium that provides a profitable business for suppliers and value to consumers. In such a market, volume is a negotiating lever for buyers, enabling them to pressure suppliers to drive prices down. An example is the generic first line ARV drug market, where major funders/buyers such as PEPFAR, the Global Fund and the Government of South Africa – with important support from market influencers such as WHO, UNAIDS, USFDA, UNICEF and CHAI, have driven prices for adult treatment from over $10 000 per person per year in 2000 down to $120 to $150 [28,30]. Premium products such as second-line ARVs, are niche markets but still profitable for some suppliers as they command higher prices due to difficulty of manufacture and low volumes.
In the absence of policy action, pediatric ARVs may acquire the characteristics of premium products with few suppliers and increasing prices. Currently, two essential pediatric ARV products have only one quality-certified manufacturer highlighting the difficulty in engaging suppliers in this market. Furthermore, fragmented demand across more than 100 countries, many with very small order volumes, affects supply security – the on-going availability of products at affordable prices.
Treatment 2.0 concepts of demand consolidation around a limited set of products and pooled procurement across countries may help to control prices and ensure supply security, while still providing a commercial return to suppliers [33–35]. The success of the UNITAID Pediatric Program illustrates the value of both these strategies. Pooled volumes across many countries and consolidated demand around a small number of FDCs (rather than multiple syrup formulations) resulted in more accurate global demand forecasting and predictable orders, which enabled suppliers to plan production and offer both lower prices and consistent supply. As of 2011, a leading pediatric regimen cost US$130 per child per year, down from US$252 in 2006 [31]. Pricing transparency via mechanisms such as the WHO Global Price Reporting Mechanism is a key ingredient for success [36].
In the longer term, the HIV-infected pediatric population will decline due to reduced mother-to-child transmission and as infected children grow into adulthood. It may become necessary to explore further options to sustain the pediatric market, such as global consolidation of orders and supply channels or an element of premium pricing to encourage suppliers to stay in a less attractive business. This potential increase in the unit cost of pediatric ARVs will need to be recognized and planned for by funders and international agencies to ensure future availability. In addition, the global community may need to encourage or negotiate with manufacturers to either subsidize or donate pediatric ARVs to secure access.
In the meantime, actions taken by countries in-line with Treatment 2.0 recommended policies can promote and secure pricing today and in the near-term, enabling countries to increase value for money, further scale-up the number of children accessing treatment and stabilize the pediatric ARV market, incentivizing suppliers to invest and innovate further to develop clinically needed products.
Pillar 4: service delivery
Optimizing HIV service delivery is a key element of the Treatment 2.0 framework. Pediatric HIV services are delivered along the spectrum of care, from diagnosis, through treatment, to retention. A rift at any point impacts the effectiveness of the whole, but at present these elements are poorly connected. Diagnosis of HIV in infants and children takes place in several settings, including primary care and Maternal Child Health (MCH) clinics (See UNICEF article in this series). By contrast, care and treatment of HIV is typically only available in secondary and tertiary centers at ART clinics, which are mainly focused on adults with small numbers of children on treatment. In most Lower Middle Income Countries (LMICs), at least three different programs – ART services, Sexual and Reproductive Health and MCH programs – are involved in the health sector response to HIV. Frequently, the required political commitment to ensure that they are coordinated to achieve an effective integrated service is lacking. In addition, many women relocate to their maternal homes for delivery so there may be a geographical disconnect between sites where mothers and children are diagnosed, and where they need to come for ongoing treatment and care. The multitude of service delivery locations that a caregiver must negotiate belies the fact that HIV infection is a family issue; children mostly acquire it from their mothers, and many women or men acquire it from their partners. Where there is one index case, several members of the family may be in need of HIV services.
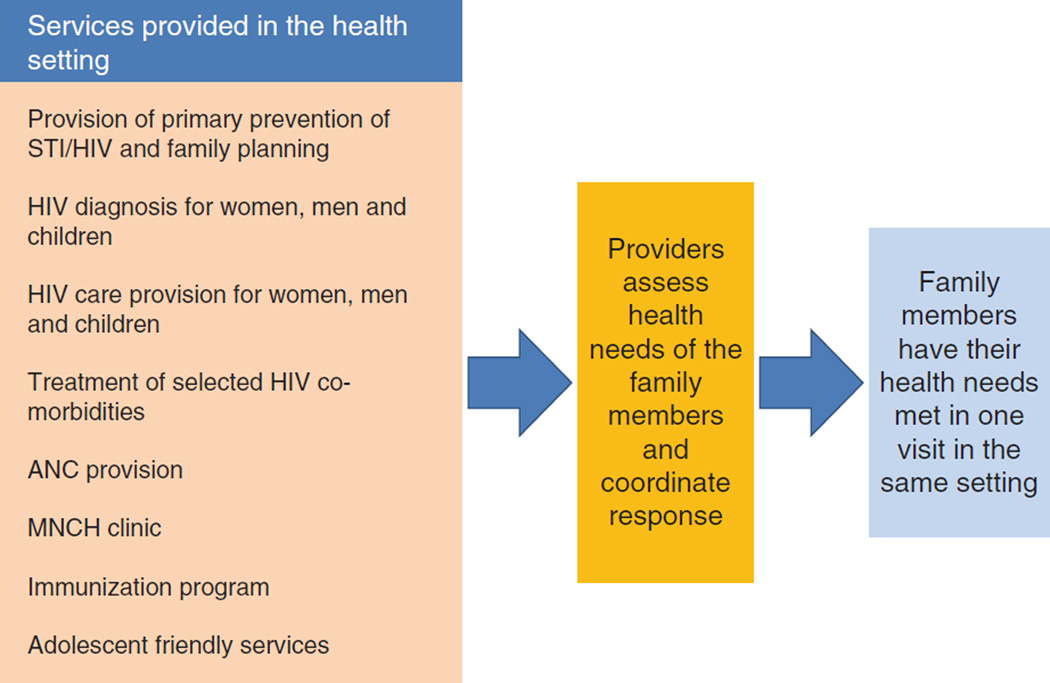
For HIV-infected women and children other types of health services are also needed, such as antenatal care (ANC), and immunizations (Fig. 3). Nutrition support and promotion of breast feeding is especially important, as healthy infant feeding practices play a major role in the survival of both HIV-exposed and infected children (see article on infant feeding in this series). Fragmentation of services places a significant burden on families and often results in loss to follow-up, but it also poses challenges for the program. When service delivery is fragmented, stock-outs of drugs and diagnostics are more likely and it is more difficult to collect and record program data.
Fig. 3. Fragmentation in care provision for selected interventions in a family with one or more members living with HIV.
ANC, antenatal clinic; ART, antiretroviral therapy; L & D, labor and delivery; MNCH, mothers, neonatal and child health.
One potential solution is to co-locate services by decentralizing ART to primary care facilities - a practice that is now formally recommended by WHO. This is now common for adults, but decentralization of pediatric care has lagged behind for several reasons including the limited number of health workers trained to provide pediatric HIV care, the lack of pediatric diagnostics and the need to stock pharmacies with many additional formulations. The lack of trained providers is especially important as children are often perceived as ‘too complicated’ and difficult to treat. Some pioneer programs have overcome these challenges and demonstrated excellent outcomes when pediatric HIV care was decentralized to primary care settings in a family care approach encouraging providers and clients to bring children into care [37–44] (Fig. 4). A large study performed in five sub-Saharan African countries showed that pediatric ART coverage increased and there was a suggestion of reduced mortality and improved retention in care when services were decentralized [39]. Further, a recent systematic review found that outcomes of ART care when provided to children by nurses was equivalent to that provided by doctors and specialised staff, at least for children who are not critically ill [40].
Fig. 4. Family centered provision of health services for HIV: a comprehensive and coordinated approach addresses the HIV and general health needs of all members of the family.
ANC, antenatal clinic; ART, antiretroviral therapy; MNCH, mothers, neonatal and child health; STI, sexual transmitted infection.
Service models for decentralization and integration may vary depending on several factors, including the availability of human and other resources and the prevalence of HIV. In high burden settings, integration of interventions for pediatric HIV diagnosis and care into MCH services such as immunization and well child visits should be actively promoted. By contrast, in low-burden settings, sparsely populated areas will have very few pediatric HIV clients and integration of pediatric HIV care across the entire health service may compromise the quality of care since some providers will have very little experience with HIV-infected or exposed children. It may be more appropriate to adopt a hybrid model where services are decentralized to a certain point, but in remote or rural communities, providers are trained how to recognize and diagnose HIV in children, and then refer them to regional or central facilities for more specialized services.
Because of the paucity of pediatricians in most developing countries, task sharing must accompany decentralization and integration of pediatric HIV care and treatment. Several countries such as South Africa and Swaziland provide formal training and certification to nurses to initiate and manage ART. These training programs have been modified to include pediatric HIV. The WHO Integrated Management of Childhood Illness (IMCI) training materials now include a pediatric HIV component and Tanzania, Zambia and Zimbabwe are implementing IMCI-based learning approaches for pediatric HIV, which combine face-to-face with distant learning modules in order to reach more providers [45]. Pediatric HIV training can be combined with interventions such as mentoring and site supervision and providing access to specialist providers for consultation in order to ensure that service quality is maintained. Although full versions of national pediatric HIV guidelines should be available as a resource in ART clinics that serve children, these documents are often too large and complex for the needs of the front-line primary care health worker managing children with HIV along-side several other clinical responsibilities. A smaller ‘pocket reference’, designed to be carried by health workers, which has the clinical essentials summarized can improve service delivery. Other job-aides such as testing algorithms, dosing tables and ART eligibility checklists can be produced in the form of desk charts or wall posters to reinforce good pediatric practice.
Pillar 5: involving our communities
Involving the community in the rollout of HIV services is recognized as an essential determinant of acceptability and serves as a model for other public health interventions. Traditional roles such as expert clients or peer counselors have expanded to wider and more complex participation, including the full engagement of people living with HIV (PLHIV) in decision-making processes. Global networks of PLHIV [46,47], are now represented in national and international fora and in the recent WHO guidelines, HIV program managers are recommended to include civil society stakeholders in all aspects of planning and service delivery [7]. However, involvement of the community in pediatric care has not been as comprehensive as for adults and pregnant women.
There are some successful models such as the Zvandiri Programme, in Zimbabwe founded in 2004 with the goal of providing psychosocial support to HIV-infected children and adolescents through a community-led support network [48,49]. The program is linked to ART centers and seeks to promote adherence and well being by offering life skills training, outreach to children who have been lost to follow-up and mental health counseling. Zvandiri reaches over 20 communities and has demonstrated retention rates of over 85%.
Zimbabwe’s PlayCenter Program is another example [50]. These are rural community centers, staffed by health workers and volunteers. They provide health, nutritional, psychosocial support and a play space for HIV-infected and -affected young children who are identified by community mobilizers. A new initiative now also offers mother-baby support groups for young mothers
While these examples of community engagement are encouraging and often extraordinary, they remain limited by scale and scope. In order to fully harness the strengths of the community in supporting children with HIV, community initiatives must form part of the national response and should be integrated with similar interventions for adults and pregnant women. At the international level, global PLHIV alliances should advocate for this type of expansion and integration as part of their mandate. At the country level, National Programs should support existing community organizations to identify locally appropriate ways of incorporating child and adolescent focused initiatives into their work. It is essential to involve HIV-affected children and youth in this process. Adult community members – especially those with specific professional skills, are important but inclusion of the young people who will access such initiatives is equally so. For example, HIV-infected adolescents who have received training in counselling, are uniquely suited to provide support to their peers in order to enhance retention and adherence. Selected proposed interventions for community participation and other specific loci for integration are identified as follows:
Building off existing programs for community PMTCT support to create linkages between EID, well child and pediatric ART services in order to minimize loss to follow up of mothers and infants across the PMTCT cascade [51].
Raising community awareness around case identification and PITC for infants, children and adolescents.
Providing community-based diagnosis of children and adolescents.
Expanding models of community adherence support such as self-forming groups of PLHIV to refill joint ARV prescriptions to address the needs of children and adolescents as well as adults [52].
Peer support programs expanded to HIV-affected youth.
As child and adolescent community initiatives expand, two important challenges must be addressed to ensure long-term success. The first relates to the sustainability of community interventions, which generally are poorly institutionalized and funded by donor grants rather than national budgets. In some cases, this has led to a lack of continuity and a perception among beneficiaries that such interventions are inevitably short-lived. Consistency and quality are critical elements to maintain in order for patients to value a community service and these can be best ensured when such services are locally managed, nationally owned and publicly funded. Health planners at national level should develop innovative approaches to provide support for proven community interventions and mainstream them into the health system.
The second challenge is that many community interventions still depend on voluntary unpaid work, which is disempowering and diminishes the valuable contribution of PLHIV to the program. This is especially true for Expert Client programs in which PLHIV who work with ART programs often receive no financial support or a minimal monthly stipend. Where programs provide a salary, outcomes are excellent. The Partners in Health Rwanda Community-HIV program, which uses lay community health workers to provide directly observed-therapy and other services has demonstrated long-term improvements in both retention (>90%) and mortality (<6%) on ART [53]. The Paediatric AIDS Treatment for Africa (PATA) expert client initiative supports 200 dedicated child focused expert clients across 49 clinics. Each clinic receives $260 monthly to employ PLHIV for task-sharing activities and community outreach. The initiative has resulted in improvements in clinic flow, better retention, stronger referral linkages and more efficient teamwork [54]. As national programs decentralize HIV care and treatment to the primary level, this type of task sharing is essential and should be made a routine component of service delivery.
Conclusion
The pillars of Treatment 2.0 represent a broad framework to simplify and streamline treatment and integrate HIV services into all layers of the health system. The overarching goal is to reach universal access to ART, despite declining levels of donor support. For adults, this is tenable and in many programs, elements of Treatment 2.0 are already well established. For children however, ART scale up has been slow and universal access remains elusive. Furthermore, with global attention focused on pediatric HIV prevention, and new WHO guidelines setting a high bar for optimal management of HIV-infected children, there is a real risk that pediatric treatment scale-up may decline. In this article, we propose eight pediatric Treatment 2.0 interventions, which we believe are necessary to reverse this trend.
Secure the pediatric ARV market through coordinated global purchasing and strategic product selection by national programs to minimize market fragmentation, maximize volume discounts and increase supply security.
Ensure that LPV/r granules are rapidly brought to market once approved as these will fill a critical formulation gap for infants. However, in the interim, work with other options – including LPV/r syrup and NVP-based FDCs. Increasing the momentum of infant ART scale up is critical, and although LPV/r-based therapy is ideal, NVP-based therapy is far better than no therapy at all.
Promote rapid, high-quality research to better define the risks and benefits of TDF as a drug for children and innovate newer drugs and regimens that could truly harmonize ART for patients of all ages.
Scale up both centralized and PoC infant diagnosis and strengthen the overall laboratory system to better serve the needs of children.
Emphasize task sharing for pediatric ART and develop rapid and robust plans to decentralize services and integrate with adult and maternal programs.
Implement routine approaches to testing infants and children in high burden settings.
Improve information management systems to ensure that children don’t fall through the cracks – especially along the PMTCT continuum.
Enhancing community participation in the response to pediatric HIV. The active role of communities in identifying children early, supporting referral and retention into care, ensuring equity in access to services and fighting stigma needs to be defined and supported.
Acknowledgements
The authors gratefully acknowledge the valuable contributions of the following individuals in preparing this manuscript
Trevor Peter
Bevin O’Neil
Anisa Ghadrshenas
David Mabirizi
Sydney Alison Kraemer
Julie Barzilay
Footnotes
Conflicts of interest
There are no conflicts of interest.
Disclaimer: The findings and conclusions in this article are those of the authors and do not necessarily represent the official position of the World Health Organization or the U.S. government including the U.S. Centers for Disease Control and Prevention and Agency for Toxic Substances Disease Registry and the United States Agency for International Development. The authors acknowledge the support of UNICEF and the Canadian International Development Agency (CIDA) whose financial assistance made this series possible and the U.S. President’s Emergency Plan for AIDS Relief for support of contributing staff time.
References
- 1.UNAIDS. 2012 Global Report on the Global AIDS Epidemic. [Accessed 20 August 2013];2012 http://www.unaids.org/en/media/unaids/contentassets/documents/epidemiology/2012/gr2012/20121120_UNAIDS_Global_Report_2012_en.pdf.
- 2.Newell ML, Brahmbhatt H, Ghys PD. Child mortality and HIV in Africa: a review. Lancet. 2004;18(Suppl 2):S27–S34. doi: 10.1097/00002030-200406002-00004. [DOI] [PubMed] [Google Scholar]
- 3.UNAIDS. 2013 Progress report on the global plan towards the elimination of new HIV infections among children by 2015 and keeping their mothers alive. [Accessed 20 August 2013];2013 http://www.unaids.org/en/media/unaids/contentassets/documents/unaidspublication/2013/20130625_progress_global_plan_en.pdf.
- 4.Chatterjee A, Tripathi S, Gass R, Hamunime N, Panha S, Kiyaga C, et al. Implementing services for Early Infant Diagnosis (EID) of HIV: a comparative descriptive analysis of national programs in four countries. BMC Public Health. 2011;11:553. doi: 10.1186/1471-2458-11-553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.UNICEF and WHO-IST/ESA. A rapid assessment of paediatric HIV care and treatment in four countries: Swaziland, Tanzania, Uganda, and Zimbabwe, 2012. [Accessed 20 August 2013]; [Unpublished draft report].
- 6.Hirnschall G, Schwartlander B. Treatment 2.0: catalysing the next phase of scale-up. Lancet. 2011;378:209–211. doi: 10.1016/S0140-6736(11)60247-X. [DOI] [PubMed] [Google Scholar]
- 7.WHO. 2013 Consolidated Guidelines on the Use of Antiretroviral Drugs for Treating and Preventing HIV Infectino: Recommendations for a public health approach. [Accessed 20 August 2013];2013 Jun 30; http://www.who.int/hiv/pub/guidelines/arv2013/download/en/index.html. [PubMed]
- 8.Violari A, Paed FC, Lindsey J, Hughes M, Mujuru H, Barlow-Mosha L, Kamthunzi P, et al. Nevirapine versus ritonavir-boosted lopinavir for HIV-infected children. N Engl J Med. 2012;366:2380–2389. doi: 10.1056/NEJMoa1113249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Food and Drug Administration. Full Prescribing Information: KALETRA. [Accessed 20 August 2013];2013 http://www.accessdata.fda.gov/drugsatfda_docs/label/2013/021251s045,021906s038lbl.pdf.
- 10.Drugs for Neglected Diseases initiative. Two ‘4 – in-1’ LPV/r-based fixed-dose combinations. [Accessed 20 August 2013];2013 Jun; http://www.dndi.org/diseases-projects/portfolio/two-4-in-1-lpv-r-based-fixed-dose-combinations.html. [Google Scholar]
- 11.Food and Drug Administration. Full Prescribing Information: VIREAD. [Accessed 20 August 2013];2013 http://www.accessdata.fda.gov/drugsatfda_docs/label/2013/021356s047,022577s004lbl.pdf.
- 12.ViiV Healthcare announces U.S. approval of Tivicay® (dolutegravir) for the treatment of HIV-1. [Accessed 20 August 2013];2013 Aug 12; http://www.viivhealthcare.com/media/press-releases/2013/august/viiv-healthcare-announces-us-approval-of-tivicay(-dolutegravir-for-the-treatment-of-hiv-1.aspx. [Google Scholar]
- 13.Cahn P, Pozniak AL, Mingrone H, Shuldyakov A, Brites C, Andrade-Villanueva JF, et al. Dolutegravir versus raltegravir in antiretroviral-experienced, integrase-inhibitor-naive adults with HIV: week 48 results from the randomised, double-blind, noninferiority SAILING study. Lancet. 2013;382:700–708. doi: 10.1016/S0140-6736(13)61221-0. [DOI] [PubMed] [Google Scholar]
- 14.UNAIDS: Joint United Nations Programme on HIV/AIDS. Countdown to zero: Global plan towards the elimination of new HIV infections among children by 2015 and keeping their mothers alive. [Accessed 30 July 2013];2011 http://www.unaids.org/en/media/unaids/contentassets/documents/unaidspublication/2011/20110609_jc2137_global-plan-elimination-hiv-children_en.pdf.
- 15.WHO/UNICEF/UNAIDS global update on HIV treatment 2013: Results, impact and opportunities. [Accessed 20 August 2013]; http://apps.who.int/iris/bitstream/10665/85326/1/9789241505734_eng.pdf.
- 16.WHO: The Interagency Task Team on prevention and treatment of HIV infection in pregnant women, mothers and their children (IATT) [Accessed 20 August 2013];Report of the Meeting of the Paediatric Working Group: Developing an Optimized list of Paediatric ARV Formulations. 2011 May 5; http://www.who.int/hiv/pub/meetingreports/iatt_meeting.pdf.
- 17.Stevens W, Sherman G, Downing R, Parsons LM, Ou C-Y, Crowley S, et al. Role of the laboratory in ensuring global access to ARV treatment for hiv-infected children: consensus statement on the performance of laboratory assays for early infant diagnosis. Open AIDS J. 2008;2:28–36. doi: 10.2174/1874613600802010017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sherman GG, Stevens G, Jones SA, Horsfield P, Stevens WS. Dried blood spots improve access to HIV diagnosis and care for infants in low-resource settings. J Acquir Immune Defic Syndr. 2005;38:615–617. doi: 10.1097/01.qai.0000143604.71857.5d. [DOI] [PubMed] [Google Scholar]
- 19.Stevens W, Erasmus E, Moloi M, Taleng T, Sarang S. Performance of a Novel Human Immunodeficiency Virus (HIV) Type 1 total nucleic acid-based real-time PCR assay using whole blood and dried blood spots for diagnosis of HIV in infants. J Clin Microbiol. 2008;46:3941–3945. doi: 10.1128/JCM.00754-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hsiao N-Y, Stinson K, Myer L. Linkage of HIV-infected infants from diagnosis to antiretroviral therapy services across the Western Cape, South Africa. PLoS ONE. 2013;8:e55308. doi: 10.1371/journal.pone.0055308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nuwagaba-Biribonwoha H, Werq-Semo B, Abdallah A, Cunningham A, Gamaliel JG, Mtunga S, et al. Introducing a multisite program for early diagnosis of HIV infection among HIV-exposed infants in Tanzania. BMC Pediatr. 2010;10:44. doi: 10.1186/1471-2431-10-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sabo U, Onotu D, Miral M, et al. SMS printer pilot programme for early infant diagnosis (EID) of HIV exposed infants for PMTCT in Kano State, Nigeria.; 6th IAS Conference on HIV Pathogenesis and Treatment; Abstract no. CDC036. [Google Scholar]
- 23.Murtagh M. UNITAID Technical Report. UNITAID; 2012. [Accessed 20 August 2013]. HIV/AIDS Diagnostics Landscape. Accessed May 8, 2013 at http://www.unitaid.eu/images/marketdynamics/publications/UNITAID-HIV_Diagnostics_Landscape-2nd_edition.pdf. [Google Scholar]
- 24.Parpia ZA, Elghanian R, Nabatiyan A, Hardie DR, Kelso DM. p24 antigen rapid test for diagnosis of acute pediatric HIV infection. J Acquir Immune Defic Syndr. 2010;55:413–419. doi: 10.1097/QAI.0b013e3181f1afbc. [DOI] [PubMed] [Google Scholar]
- 25.Boyle DS, Lehman DA, Lillis L, Peterson D, Singhal M, Armes N, et al. Rapid detection of HIV-1 proviral DNA for early infant diagnosis using recombinase polymerase amplification. MBio. 2013;4 doi: 10.1128/mBio.00135-13. doi:pii: e00135-13. 10.1128/mBio.00135-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schito ML, Peter TF, Cavanaugh S, et al. Opportunities and challenges for cost-efficient implementation of new point-of-care diagnostics for HIV and tuberculosis. J Infect Dis. 2012;205(Suppl 2):S169–S180. doi: 10.1093/infdis/jis044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nkengasong JN, Nsubuga P, Nwanyanwu O, Gershy-Damet GM, Roscigno G, Bulterys M, et al. Laboratory systems and services are critical in global health: time to end the neglect? Am J Clin Pathol. 2010;134:368–373. doi: 10.1309/AJCPMPSINQ9BRMU6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Supply Chain Management Systems. [Accessed 20 August 2013]; http://scms.pfscm.org/scms/ecatalog/arvs. [Google Scholar]
- 29.Clinton Health Access Initiative. ARV Ceiling Price List. [Accessed 20 August 2013];2013 May; http://www.clintonhealthaccess.org/files/CHAI%20ARV%20Ceiling%20Price%202013.pdf. [Google Scholar]
- 30.Waning B, Diedrichsen El, Jambert E, Barnighausen T, Li Y, Pouw M, et al. The global pediatric antiretroviral market: analyses of product availability and utilization reveal challenges for development of pediatric formulations and HIV/AIDS treatment in children. [Accessed 20 August 2013];BMC Pediatr. 2010 10:74. doi: 10.1186/1471-2431-10-74. http://www.biomedcentral.com/1471-2431/10/74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.UNITAID. Paediatric HIV/AIDS Project: Create the market for child-friendly HIV medicines. [Accessed 20 August 2013];2012 Jun; http://www.unitaid.eu/en/paediatrics. [Google Scholar]
- 32.Kaiser Family Foundation. International AIDS Assistance from Donor Governments: Commitments & Disbursements, 2002–2011. 2013 May [Google Scholar]
- 33.Burnett F, et al. Reducing costs through regional pooled procurement. Essential Drugs Monitor. 2003;32:7–8. http://apps.who.int/medicinedocs/pdf/s4940e/s4940e.pdf. [Google Scholar]
- 34.WHO. Meeting report. Geneva: WHO; 2007. Multicountry Regional Pooled Procurement of Medicines. http://apps.who.int/medicinedocs/documents/s14862e/s14862e.pdf. [Google Scholar]
- 35.Holmes C, Coggin W, Jamieson D, Mihm H, Granich R, Savio P. Use of generic antiretroviral agents and cost savings in PEPFAR treatment programs. [Accessed 20 August 2013];JAMA. 2010 304:313–320. doi: 10.1001/jama.2010.993. http://jama.jamanetwork.com/searchresults.aspx?q=Use%20of%20Generic%20Antiretroviral%20Agents%20and%20Cost%20Savings%20in%20PEPFAR%20Treatment%20Programs&t=&p=1&s=1&c=0. [DOI] [PubMed] [Google Scholar]
- 36.WHO. Global Price Reporting Mechanism. [Accessed 20 August 2013]; http://www.who.int/hiv/amds/gprm/en/ and http://apps.who.int/hiv/amds/price/hdd/.
- 37.Bolton-Moore C, Mubiana-Mbewe M, Cantrell R, Chintu N, Stringer E, Chi B, et al. Clinical outcomes and CD4 cell response in children receiving antiretroviral therapy at primary healthcare facilities in Zambia. JAMA. 2007;298:1888–1899. doi: 10.1001/jama.298.16.1888. [DOI] [PubMed] [Google Scholar]
- 38.Janssen N, Ndirangu J, Newell ML, Bland RM. Successful paediatric HIV treatment in rural primary care in Africa. Arch Dis Child. 2010;95:414–421. doi: 10.1136/adc.2009.169367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fayorsey RN, Saito S, Carter R, Gusmao E, Frederix K, Koech-Keter E, et al. Decentralization of pediatric HIV care and treatment in Five sub-Saharan African countries. J Acquir Immune Defic Syndr. 2013;62:e124–e130. doi: 10.1097/QAI.0b013e3182869558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Penazzato M, Davies M-A, Apollo T, Negussie E, Ford N. Task shifting for the delivery of paediatric antiretroviral treatment: a systematic review. JAIDS. 2013 doi: 10.1097/QAI.0000000000000024. Published ahead of print. [DOI] [PubMed] [Google Scholar]
- 41.Betancourt T, Abrams E, McBain R, Smith-Fawzi M. Family-centred approaches to the prevention of mother to child transmission of HIV. J Int AIDS S. 2010;13(Suppl 2):S2. doi: 10.1186/1758-2652-13-S2-S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Byakika-Tusiime J, Crane J, Oyugi J, Ragland K, Kawuma A, Musoke P, et al. Longitudinal antiretroviral adherence in HIV+ Ugandan parents and their children initiating HAART in the MTCT-Plus family treatment model: role of depression in declining adherence over time. [Accessed 20 August 2013];AIDS Behav. 2009 13(Suppl 1):82–91. doi: 10.1007/s10461-009-9546-x. [DOI] [PubMed] [Google Scholar]
- 43.Abrams EJ, Myer L, Rosenfield A, El-Sadr WM. Prevention of mother-to-child transmission services as a gateway to family-based human immunodeficiency virus care and treatment in resource-limited settings: rationale and international experiences. Am J Obstet Gynecol. 2007;197(3 Suppl):S101–S106. doi: 10.1016/j.ajog.2007.03.068. [DOI] [PubMed] [Google Scholar]
- 44.Geddes R, Knight S, Reid S, Giddy J, Esterhuizen T, Roberts C. Prevention of mother-to-child transmission of HIV programme: low vertical transmission in KwaZulu-Natal, South Africa. S Afr Med J. 2008;98:458–462. [PubMed] [Google Scholar]
- 45.WHO/UNICEF. IMCI Complementary course on HIV/AIDS. 2006 http://www.who.int/maternal_child_adolescent/documents/9241594373/en/.
- 46.National Networks of PLHIV: HIV Leadership Through Accountability. [Accessed 20 August 2013];2013 http://www.hivleadership.org/who-weare/national-networks.html. [Google Scholar]
- 47.Global Network of People Living with HIV. [Accessed 20 August 2013]; http://www.gnpplus.net/. [Google Scholar]
- 48.Sharer M, Andrew F. Transitioning of care and other services for adolescents living with HIV in sub-Saharan Africa. Arlington, VA: USAID’s AIDS Support and Technical Assistance Resources, AIDSTAR-One; 2012. [Google Scholar]
- 49.Southern Africa HIV and AIDS Information Dissemination Service. Our Children, Our Future: Zimbabwean Good Practices Responding to the Needs of Orphans and Vulnerable Children. 2010 Available at: http://www.safaids.net/content/our-children-our-future-zimbabwean-good-practices-responding-needs-or-phans-and-vulnerable-ch. [Google Scholar]
- 50.Patel D, Matyanga P, Nyamundaya T, Chimedza D, Webb K, Engelsmann B. Facilitating HIV testing, care and treatment for orphans and vulnerable children aged five years and younger through community-based early childhood development play-centres in rural Zimbabwe. J Int AIDS Soc. 2012;15(Suppl 2):17404. doi: 10.7448/IAS.15.4.17404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kim MH, Ahmed S, Buck WC, Preidis G, Hosseinipour MC, Bhalakia A, et al. The Tingatheprogramme: a pilot intervention using community health workers to create a continuum of care in the prevention of mother to child transmission of HIV (PMTCT) cascade of services in Malawi. J Int AIDS Soc. 2012;15(Suppl 2):17389. doi: 10.7448/IAS.15.4.17389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Decroo T, Tefler B, Biot M, Maïkéré J, Dezembro S, Cumba LI, et al. Distribution of antiretroviral treatment through self-forming groups of patients in Tete Province, Mozambique. J Acquir Immune Defic Syndr. 2011;56 doi: 10.1097/QAI.0b013e3182055138. [DOI] [PubMed] [Google Scholar]
- 53.Rich ML, Miller AC, Niyigena PRN, Franke MF, Niyonzima JB, Socci A, et al. Excellent clinical outcomes and high retention in care among adults in a community-based HIV treatment program in rural Rwanda. JAIDS March. 2012;59:e35–e42. doi: 10.1097/QAI.0b013e31824476c4. [DOI] [PubMed] [Google Scholar]
- 54.Pediatric AIDS Treatment for Africa. [Accessed 20 August 2013]; http://www.teampata.org/project_expert.asp. [Google Scholar]