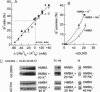
Abstract
The mechanism of action of polar/apolar inducers of cell differentiation, such as dimethyl sulfoxide and hexamethylene-bisacetamide, is still obscure. In this paper evidence is provided that their effects on murine erythroleukemia cells are modulated by various extracellular cations as a precise function of the cation effects on membrane surface potential. The interfacial effects of the inducers were directly measured on the charged electrode, showing that both dimethyl sulfoxide and hexamethylene-bisacetamide, at the effective concentrations for cell differentiation and within the physiological range of charge density, adsorb at the charged surface and produce a potential shift. A linear correlation was found between this shift and the inducer effects on cell differentiation. Besides offering a different interpretation of the mechanism of action of the inducers, these findings indicate that surface potential has a signaling function. They may also be relevant to cancer treatments based on tumor-cell commitment to terminal differentiation.
Full text
PDF
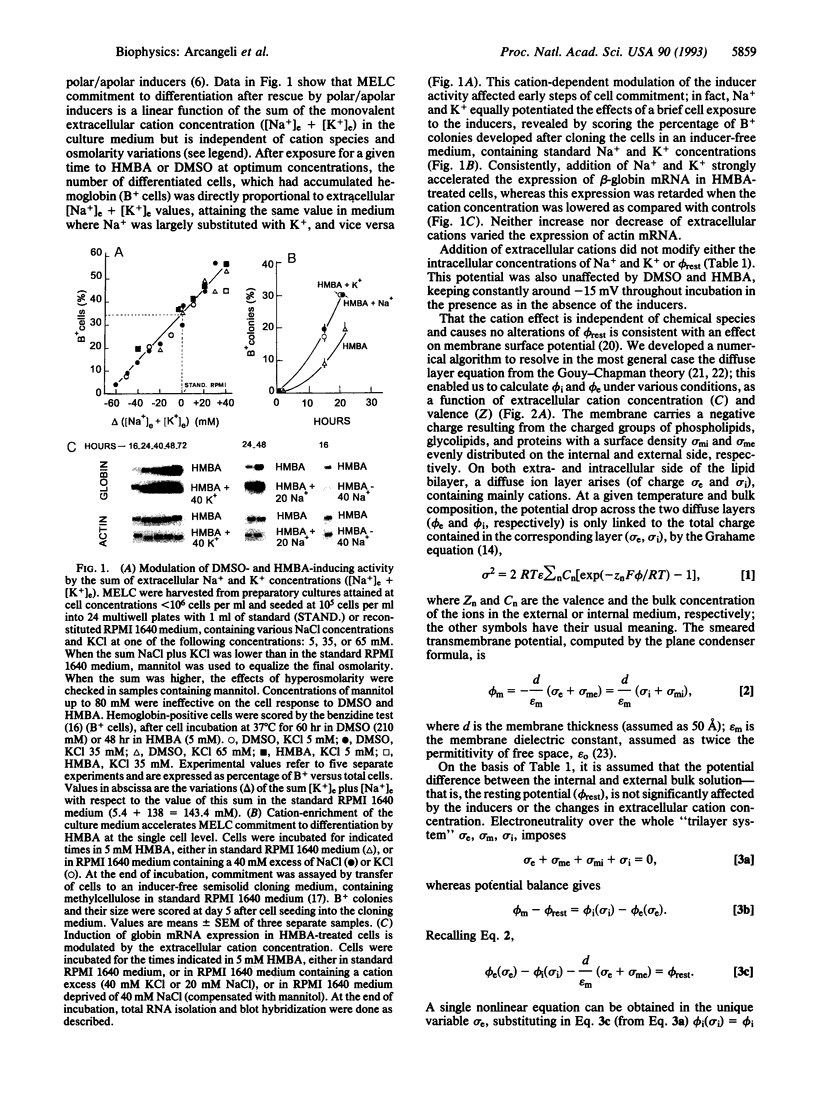
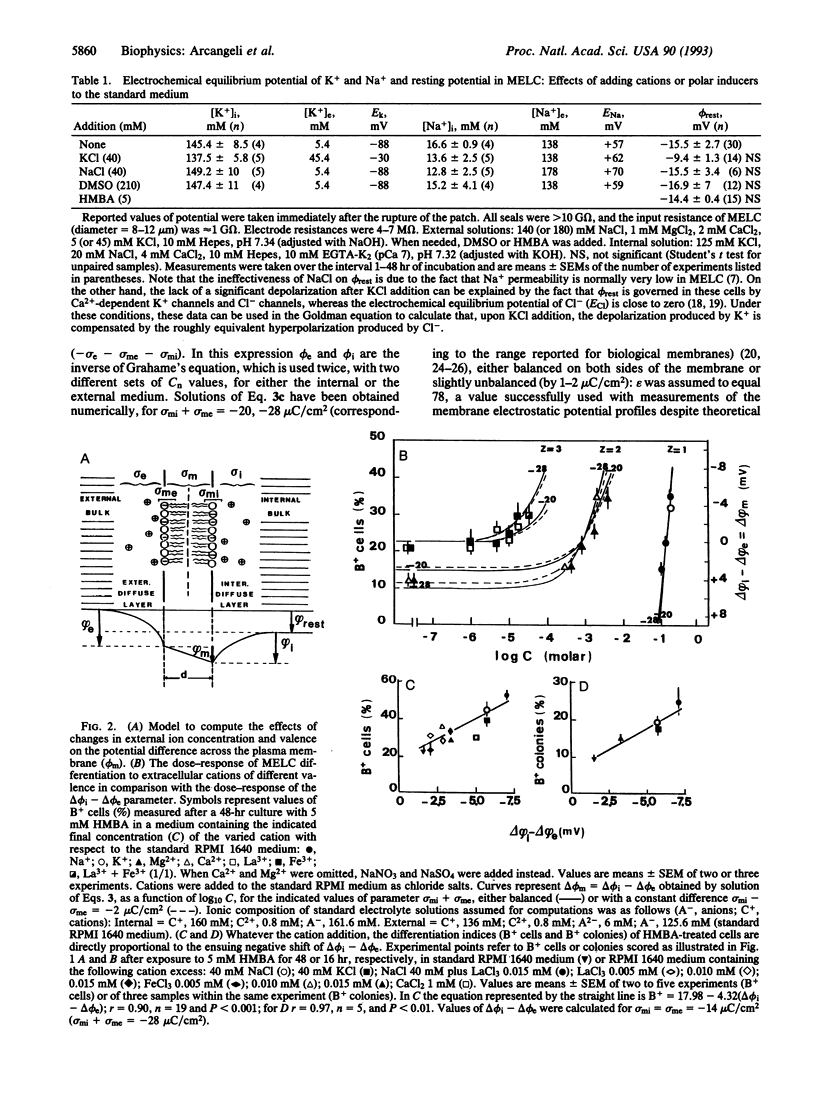
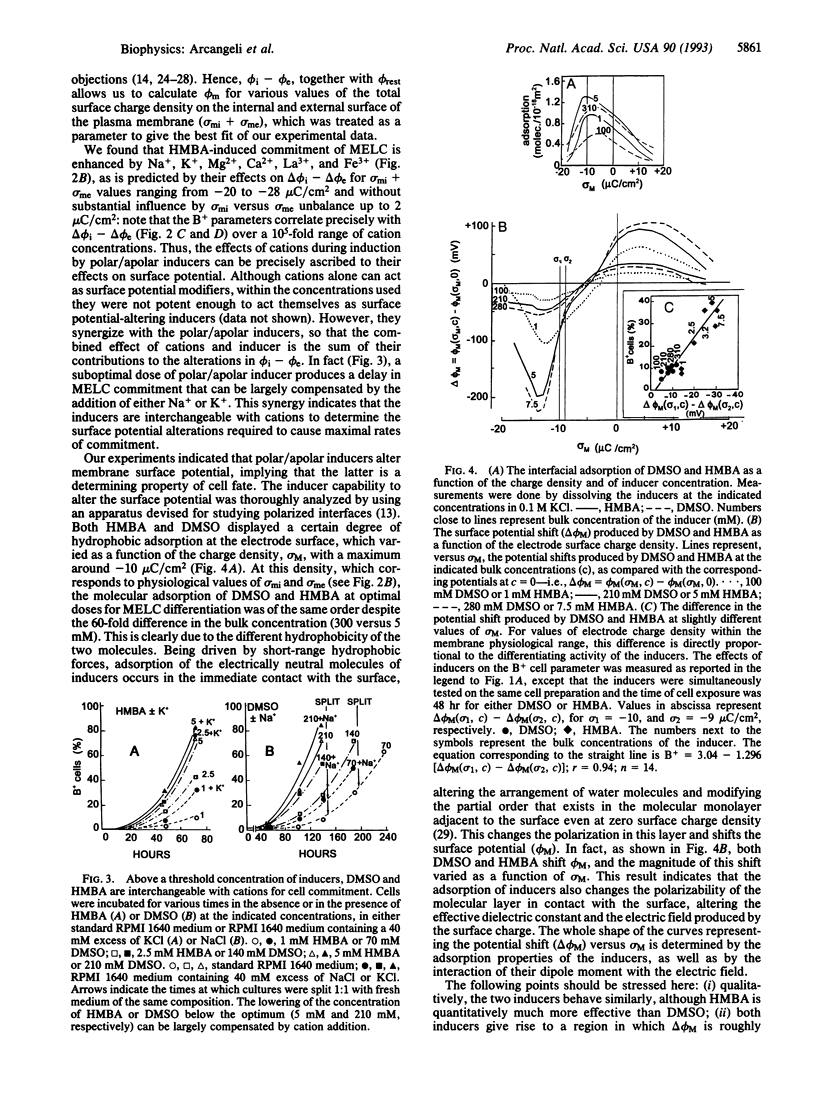

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alitalo K., Vaheri A. Pericellular matrix in malignant transformation. Adv Cancer Res. 1982;37:111–158. doi: 10.1016/s0065-230x(08)60883-0. [DOI] [PubMed] [Google Scholar]
- Arcangeli A., Del Bene M. R., Becchetti A., Wanke E., Olivotto M. Effects of inhibitors of ion-motive ATPases on the plasma membrane potential of murine erythroleukemia cells. J Membr Biol. 1992 Mar;126(2):123–136. doi: 10.1007/BF00231911. [DOI] [PubMed] [Google Scholar]
- Arcangeli A., Ricupero L., Olivotto M. Commitment to differentiation of murine erythroleukemia cells involves a modulated plasma membrane depolarization through Ca2+-activated K+ channels. J Cell Physiol. 1987 Sep;132(3):387–400. doi: 10.1002/jcp.1041320302. [DOI] [PubMed] [Google Scholar]
- Arcangeli A., Wanke E., Olivotto M., Camagni S., Ferroni A. Three types of ion channels are present on the plasma membrane of Friend erythroleukemia cells. Biochem Biophys Res Commun. 1987 Aug 14;146(3):1450–1457. doi: 10.1016/0006-291x(87)90812-6. [DOI] [PubMed] [Google Scholar]
- Binggeli R., Weinstein R. C. Membrane potentials and sodium channels: hypotheses for growth regulation and cancer formation based on changes in sodium channels and gap junctions. J Theor Biol. 1986 Dec 21;123(4):377–401. doi: 10.1016/s0022-5193(86)80209-0. [DOI] [PubMed] [Google Scholar]
- Breslow R., Jursic B., Yan Z. F., Friedman E., Leng L., Ngo L., Rifkind R. A., Marks P. A. Potent cytodifferentiating agents related to hexamethylenebisacetamide. Proc Natl Acad Sci U S A. 1991 Jul 1;88(13):5542–5546. doi: 10.1073/pnas.88.13.5542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown G. C. Electrostatic coupling between membrane proteins. FEBS Lett. 1990 Jan 15;260(1):1–5. doi: 10.1016/0014-5793(90)80051-j. [DOI] [PubMed] [Google Scholar]
- Chandler W. K., Hodgkin A. L., Meves H. The effect of changing the internal solution on sodium inactivation and related phenomena in giant axons. J Physiol. 1965 Oct;180(4):821–836. doi: 10.1113/jphysiol.1965.sp007733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelman G. M. Cell adhesion and the molecular processes of morphogenesis. Annu Rev Biochem. 1985;54:135–169. doi: 10.1146/annurev.bi.54.070185.001031. [DOI] [PubMed] [Google Scholar]
- FRANKENHAEUSER B., HODGKIN A. L. The action of calcium on the electrical properties of squid axons. J Physiol. 1957 Jul 11;137(2):218–244. doi: 10.1113/jphysiol.1957.sp005808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friend C., Scher W., Holland J. G., Sato T. Hemoglobin synthesis in murine virus-induced leukemic cells in vitro: stimulation of erythroid differentiation by dimethyl sulfoxide. Proc Natl Acad Sci U S A. 1971 Feb;68(2):378–382. doi: 10.1073/pnas.68.2.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grassi F., Lux H. D. Voltage-dependent GABA-induced modulation of calcium currents in chick sensory neurons. Neurosci Lett. 1989 Oct 23;105(1-2):113–119. doi: 10.1016/0304-3940(89)90021-9. [DOI] [PubMed] [Google Scholar]
- Groudine M., Weintraub H. Propagation of globin DNAase I-hypersensitive sites in absence of factors required for induction: a possible mechanism for determination. Cell. 1982 Aug;30(1):131–139. doi: 10.1016/0092-8674(82)90019-8. [DOI] [PubMed] [Google Scholar]
- Gurdon J. B. Embryonic induction--molecular prospects. Development. 1987 Mar;99(3):285–306. doi: 10.1242/dev.99.3.285. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Honig B. H., Hubbell W. L., Flewelling R. F. Electrostatic interactions in membranes and proteins. Annu Rev Biophys Biophys Chem. 1986;15:163–193. doi: 10.1146/annurev.bb.15.060186.001115. [DOI] [PubMed] [Google Scholar]
- Marks P. A., Sheffery M., Rifkind R. A. Induction of transformed cells to terminal differentiation and the modulation of gene expression. Cancer Res. 1987 Feb 1;47(3):659–666. [PubMed] [Google Scholar]
- McLaughlin S. The electrostatic properties of membranes. Annu Rev Biophys Biophys Chem. 1989;18:113–136. doi: 10.1146/annurev.bb.18.060189.000553. [DOI] [PubMed] [Google Scholar]
- McLaughlin S., Whitaker M. Cations that alter surface potentials of lipid bilayers increase the calcium requirement for exocytosis in sea urchin eggs. J Physiol. 1988 Feb;396:189–204. doi: 10.1113/jphysiol.1988.sp016958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melloni E., Pontremoli S., Michetti M., Sacco O., Cakiroglu A. G., Jackson J. F., Rifkind R. A., Marks P. A. Protein kinase C activity and hexamethylenebisacetamide-induced erythroleukemia cell differentiation. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5282–5286. doi: 10.1073/pnas.84.15.5282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosior M., McLaughlin S. Peptides that mimic the pseudosubstrate region of protein kinase C bind to acidic lipids in membranes. Biophys J. 1991 Jul;60(1):149–159. doi: 10.1016/S0006-3495(91)82038-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orkin S. H., Harosi F. I., Leder P. Differentiation in erythroleukemic cells and their somatic hybrids. Proc Natl Acad Sci U S A. 1975 Jan;72(1):98–102. doi: 10.1073/pnas.72.1.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsegian V. A. Possible modulation of reactions on the cell surface by changes in electrostatic potential that accompany cell contact. Ann N Y Acad Sci. 1974;238:362–371. doi: 10.1111/j.1749-6632.1974.tb26804.x. [DOI] [PubMed] [Google Scholar]
- Reuben R. C., Rifkind R. A., Marks P. A. Chemically induced murine erythroleukemic differentiation. Biochim Biophys Acta. 1980 Sep 22;605(3):325–346. doi: 10.1016/0304-419x(80)90015-3. [DOI] [PubMed] [Google Scholar]
- Rovera G., Bonaiuto J. The phenotypes of variant clones of Friend mouse erythroleukemic cells resistant to dimethyl sulfoxide. Cancer Res. 1976 Nov;36(11 Pt 1):4057–4061. [PubMed] [Google Scholar]
- Spremulli E. N., Dexter D. L. Polar solvents: a novel class of antineoplastic agents. J Clin Oncol. 1984 Mar;2(3):227–241. doi: 10.1200/JCO.1984.2.3.227. [DOI] [PubMed] [Google Scholar]
- Tsong T. Y., Astumian R. D. Electroconformational coupling: how membrane-bound ATPase transduces energy from dynamic electric fields. Annu Rev Physiol. 1988;50:273–290. doi: 10.1146/annurev.ph.50.030188.001421. [DOI] [PubMed] [Google Scholar]
- Tsong T. Y. Deciphering the language of cells. Trends Biochem Sci. 1989 Mar;14(3):89–92. doi: 10.1016/0968-0004(89)90127-8. [DOI] [PubMed] [Google Scholar]