Summary
The recruitment of endogenous adult neural stem cells for brain repair is a promising regenerative therapeutic strategy. This strategy involves stimulation of multiple stages of adult neural stem cell development, including proliferation, self-renewal, and differentiation. Currently, there is a lack of a single therapeutic approach that can act on these multiple stages of adult neural stem cell development to enhance neural regeneration. Here we show that metformin, an FDA-approved diabetes drug, promotes proliferation, self-renewal, and differentiation of adult neural precursors (NPCs). Specifically, we show that metformin enhances adult NPC proliferation and self-renewal dependent upon the p53 family member and transcription factor TAp73, while it promotes neuronal differentiation of these cells by activating the AMPK-aPKC-CBP pathway. Thus, metformin represents an optimal candidate neuro-regenerative agent that is capable of not only expanding the adult NPC population but also subsequently driving them toward neuronal differentiation by activating two distinct molecular pathways.
Graphical Abstract
Highlights
-
•
Metformin enhances neural precursor proliferation/self-renewal and differentiation
-
•
Metformin requires TAp73 to promote neural precursor proliferation/self-renewal
-
•
Metformin activates the aPKC-CBP pathway to induce neural precursor differentiation
-
•
AMPK stimulates neuronal differentiation by activating the aPKC-CBP pathway
Wang and colleagues used both adult neural precursor cell culture and BrdU in vivo labeling to show that metformin acts on multiple stages of adult neural precursor development including proliferation, self-renewal, and differentiation. Specifically, metformin requires TAp73 gene expression to promote neural precursor proliferation/self-renewal, while metformin activates the aPKC-CBP pathway to induce neural precursor differentiation.
Introduction
The observation of adult neural stem cells in the mammalian brain (Imayoshi et al., 2008, Reynolds and Weiss, 1992, Zhao et al., 2008) suggested that these stem cells could be mobilized for the repair of the injured or degenerating brain. A growing body of literature shows that adult neural stem cells are recruited in response to neural injury or degeneration, representing an attempt at endogenous repair (Kernie and Parent, 2010, Mitchell et al., 2004). However, this level of endogenous repair was not sufficient to repair the damaged brain. Thus, extensive efforts are underway to harness endogenous neural precursor cells (NPCs) as a novel regenerative therapeutic strategy to treat neural injury or brain degeneration. The recruitment of endogenous adult NPCs involves stimulation of multiple stages of adult NPC development, including proliferation, self-renewal, and differentiation. Thus, an optimal regenerative strategy would stimulate both proliferation/self-renewal and neuronal differentiation in order to generate sufficient numbers of new neurons to replace those lost after brain injury or degeneration.
Metformin, an FDA (Food and Drug Administration)-approved diabetes drug, was recently shown to promote adult neurogenesis under both physiological and pathological conditions in vivo (Liu et al., 2014, Jin et al., 2014, Wang et al., 2012). However, metformin has multiple molecular actions (Pernicova and Korbonits, 2014), and it is still not clear which ones are important for its neural effects. For example, metformin activates atypical protein kinase C (aPKC)-mediated CREB-binding protein (CBP) phosphorylation to regulate gluconeogenic gene expression in liver cells and enhance embryonic murine and human NPC differentiation (He et al., 2009, Wang et al., 2012). Moreover, metformin increases the levels of the p53 family member transcription factor TAp73 in cancer cells (Engelmann et al., 2015, Rosenbluth et al., 2008), and TAp73 is essential for adult NPC self-renewal and proliferation (Fujitani et al., 2010), suggesting that this protein might also be important for metformin’s effects in the brain.
Here, we show that metformin treatment enhances both proliferation/self-renewal and neuronal differentiation of adult NPCs by activating two different molecular pathways. Metformin increases adult NPC proliferation/self-renewal via TAp73 while it promotes neuronal differentiation by activating the AMPK-aPKC-CBP pathway. Thus, metformin represents an optimal neuroregenerative agent to recruit endogenous neural stem cells to replace the loss of neural cells after brain injury and degeneration.
Results
Metformin Enhances Proliferation/Self-Renewal and Neuronal Differentiation of Adult NPCs
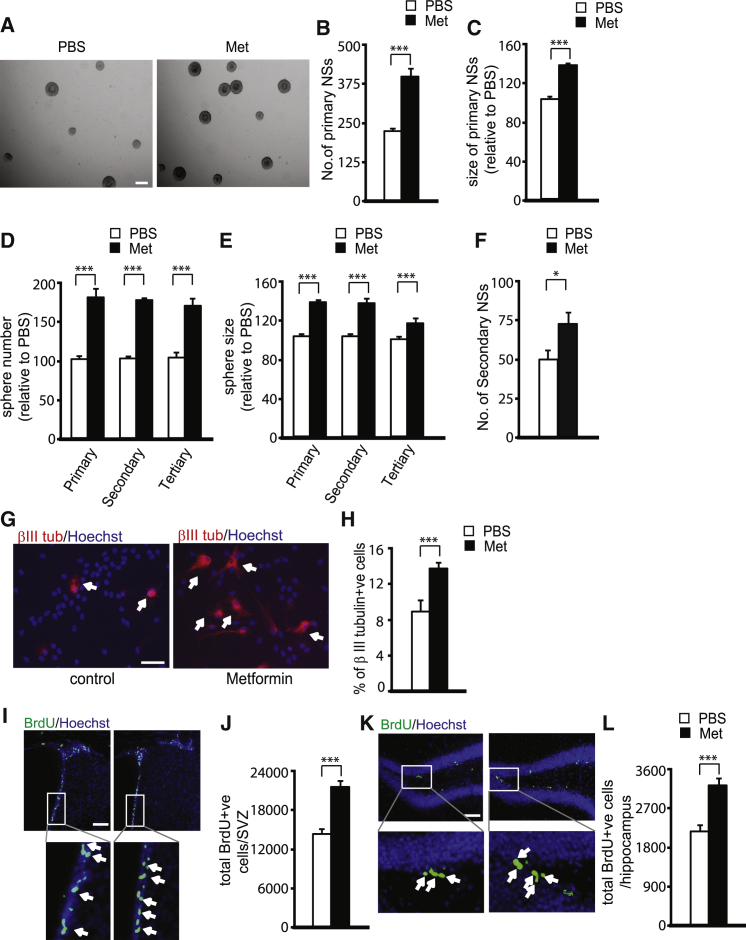
Previously, we showed that metformin, an FDA-approved diabetes drug, promotes embryonic murine and human NPC differentiation in culture and adult neurogenesis in vivo (Wang et al., 2012). To ask whether metformin acts on multiple stages of adult neural precursor development to increase adult murine neurogenesis in vivo, we used adult subventricular zone (SVZ) neurosphere cultures as a model system to assess functional roles of metformin in regulating the proliferation, self-renewal, and neuronal differentiation of adult NPCs. First, we examined primary neurosphere formation. Metformin (500 nM), added to freshly isolated SVZ NPCs for 6 days, robustly increased the number and size of primary neurospheres, indicators of self-renewal and proliferation, respectively (Figures 1A–1C). Metformin treatment of primary neurospheres also enhanced the number and size of secondary and tertiary neurospheres that were passaged every 4 days in the absence of metformin (Figures 1D and 1E), indicating that transient metformin treatment produces sustained NPC self-renewal and proliferation responses. Second, we treated dissociated NPCs derived from primary neurospheres with metformin (1 μM) for 7 days. Metformin treatment significantly increased the number of secondary neurospheres (Figure 1F), again indicating augmented self-renewing ability. Third, metformin significantly enhanced neuronal differentiation (Figures 1G and 1H), as we have previously published for embryonic NPCs (Wang et al., 2012). Finally, we examined the effect of metformin on adult NPC survival. Monolayer-cultured adult NPCs were treated with metformin (500 nM or 1 μM) for 2 days and assessed for cleaved caspase-3 by immunostaining and condensed nuclei. NPCs underwent apoptosis at a relatively low rate (∼5%) in both vehicle (PBS) and metformin-treated cultures at 500 nM, while apoptosis was decreased to ∼3% by 1 μM metformin (Figure S1). Therefore, inhibition of apoptosis was most likely not a major contributor to the robust increase in the number and size of adult neurospheres induced by metformin treatment.
Figure 1.
Metformin Enhances the Self-Renewal and Neuronal Differentiation of Adult Neural Precursors
(A–C) Primary neurospheres cultured from the adult SVZ were grown in the presence or absence of 500 nM metformin (Met) and quantified 6 days later, as pictured in (A). Scale bar, 200 μm. (B) Quantification of primary neurosphere (NS) number following metformin or PBS exposure. ∗∗∗p ≤ 0.001 (n = 5 for each group). (C) Quantification of the neurosphere diameter following metformin or PBS exposure, as in (A). ∗∗∗p ≤ 0.001 (pooled data from five independent experiments).
(D and E) Primary neurospheres were passaged either once (secondary) or twice (tertiary) into untreated media, and the number (D) and diameter (E) of spheres was quantified 4 days later. ∗∗∗p ≤ 0.001 (n = 4 for each group).
(F) Quantitative analysis of number of secondary neurospheres in the absence and presence of metformin (1 μM). ∗p < 0.05 (pooled data from four independent experiments).
(G) Immunofluorescence images of βIII-tubulin (βIII tub)-positive neurons generated from SVZ adult neurospheres in the absence and presence of metformin. Arrows denote βIII-tubulin-positive cells. Scale bar, 50 μm.
(H) Quantitative analysis of the percentage of βIII-tubulin-positive (β III tubulin+ive) neurons generated in the absence and presence of metformin (200 nM), as shown in (G). ∗∗∗p < 0.001 (pooled data from four independent experiments).
(I–L) In (I) and (K), fluorescence photomicrographs are shown of coronal SVZ (I) and SGZ (K) sections from 3-month-old WT mice that received PBS or metformin daily by injection (i.p., 200 mg/kg) for 7 days, followed by a single BrdU (100 mg/kg) injection 24 hr before. Sections were stained for BrdU (green) and counterstained with Hoechst 33258 (blue). Scale bar, 100 μm. (J and L) Quantitative analysis of total number of BrdU-positive (BrdU+ve) precursor cells within the SVZ (J) and SGZ (L) regions, determined from sections as shown in (I) and (K). ∗∗∗p < 0.001 (pooled data from three independent experiments). Arrows denote BrdU-positive cells.
Error bars indicate SEM.
See also Figure S1.
We performed similar experiments in vivo, treating adult mice with metformin intraperitoneally (i.p.; 200 mg/kg) for 7 days, followed by a single bromodeoxyuridine (BrdU) injection (i.p., 100 mg/kg to mark proliferating NPCs). Mice were sacrificed 24 hr later to assess the total number of BrdU-positive cells, which represent cycling NPCs. Metformin treatment in vivo significantly expanded the BrdU-positive NPC populations in the SVZ (Figures 1I and 1J), consistent with the SVZ neurosphere data. We also assessed the number of BrdU-positive cells in the subgranular zone (SGZ) of the dentate gyrus, the other major neurogenic region in the adult brain. As seen in the SVZ, metformin increased the total relative number of BrdU-positive cells in the SGZ (Figures 1K and 1L). Thus, metformin promotes multiple stages of adult NPC development including proliferation, self-renewal, and neuronal differentiation.
TAp73 Is Required for Metformin-Enhanced Proliferation and Self-Renewal of Adult NPCs
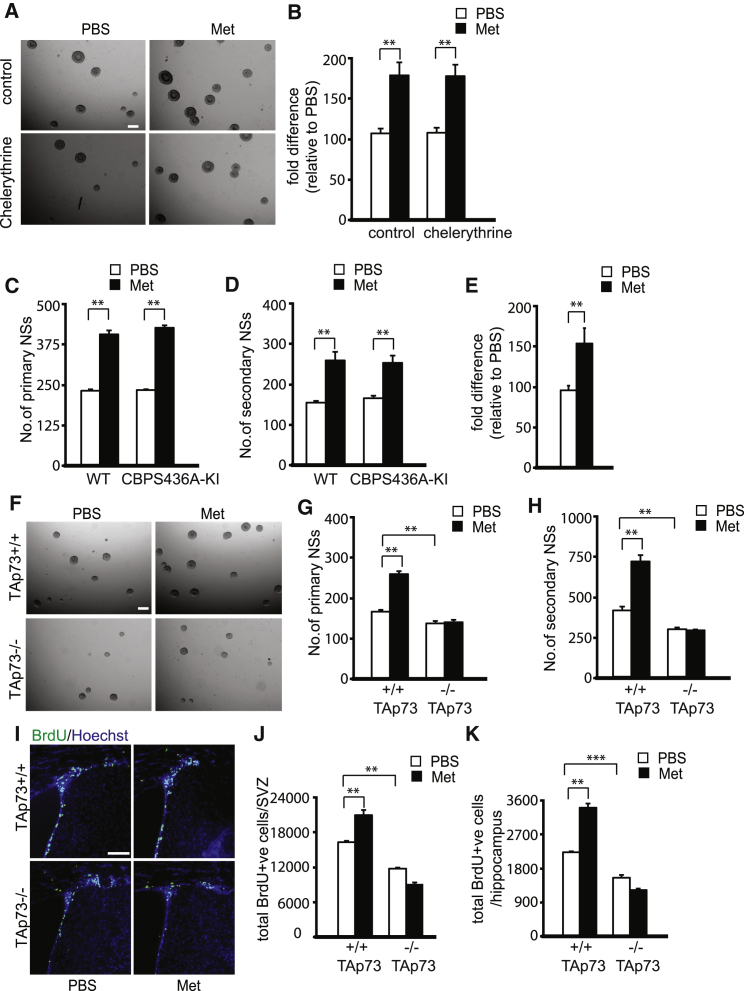
Based on our previous work showing that aPKC-mediated CBP phosphorylation at S436 is required for metformin-stimulated differentiation of embryonic NPCs, we asked whether this pathway was also involved in metformin-induced proliferation and/or self-renewal of adult NPCs. To do this, we performed adult neurosphere culture of wild-type (WT) mice in the presence of a pan-PKC inhibitor, chelerythrine, and adult neurosphere culture from a phospho-mutant CBPS436A knockin (CBPS436A-KI) mouse strain, in which the aPKC-CBP pathway is deficient due to the change of the serine (S) 436 residue to alanine (A) in the aPKC phosphorylation site of the CBP allele (Zhou et al., 2004). Quantification showed that chelerythrine did not block the increase in neurosphere formation induced by metformin (Figures 2A and 2B). Consistent with these data, metformin enhanced the number of primary and secondary neurospheres cultured from adult CBPS436A-KI as well as WT mice (Figures 2C and 2D).
Figure 2.
TAp73 Is Required for Metformin-Induced Self-Renewal and Proliferation of Adult Neural Precursors
(A and B) Primary neurospheres (NSs) were cultured from the SVZ of adult mice (3 months old) in the presence or absence of metformin (Met) and treated with the pan-PKC inhibitor, chelerythrine. (B) Quantification of primary neurospheres following 6 days in culture was done based on representative micrographs shown in (A). Scale bar, 200 μm. ∗∗p ≤ 0.01 (pooled data from four independent experiments).
(C) The SVZ of 3-month-old WT and CBPS436A-KI mice was dissected and cultured, and primary neurosphere formation was quantified 6 days later in the presence and absence of metformin. ∗∗p ≤ 0.01. (pooled data from three independent experiments).
(D) Metformin- or PBS-treated primary neurospheres (as in A) from both genotypes were passaged into untreated media, and the number of secondary neurospheres was quantified 4 days later. ∗∗p ≤ 0.01. (pooled data from three independent experiments).
(E) qRT-PCR for TAp73 mRNA performed on RNA extracted from primary neurospheres grown in the presence or absence of metformin. ∗∗p ≤ 0.01 (n = 4 for each group).
(F–H) Primary neurospheres were cultured from the SVZ of 3-month-old TAp73+/+ and TAp73−/− mice in either the presence or absence of 500 nM metformin. Scale bar, 200 μm. (G) Quantification of primary neurospheres from both genotypes following metformin exposure was done based on representative micrographs shown in (F). ∗∗p ≤ 0.01 (pooled data from four independent experiments). (H) Primary neurospheres cultured with or without metformin (as in A) from both genotypes were passaged into untreated media, and secondary neurospheres were counted 4 days later. ∗∗p ≤ 0.01 (pooled data from four independent experiments).
(I) Confocal micrographs of representative coronal sections through the lateral ventricles of metformin- or PBS-injected TAp73+/+ and TAp73−/− mice, stained for BrdU (green) 24 hr after BrdU injection. Sections are counterstained for Hoechst 33258 (blue). Scale bar, 100 μm.
(J and K) Quantification of the total number of BrdU-positive (BrdU+ve) cells in the SVZ (J) and SGZ (K) of metformin- or PBS-treated mice of both genotypes, as pictured in (I). ∗∗∗p ≤ 0.001; ∗∗p ≤ 0.01 (pooled data from three independent experiments).
Error bars indicate SEM.
These data suggest that metformin must act through a second pathway to enhance the proliferation and self-renewal of adult NPCs. In this regard, in cancer cells, metformin increases TAp73 levels (Engelmann et al., 2015, Rosenbluth et al., 2008), and TAp73 is essential for adult NPC self-renewal and proliferation (Fujitani et al., 2010). Therefore, we asked whether metformin might act via TAp73 to promote NPC self-renewal and proliferation. First, we determined whether metformin increased TAp73 mRNA levels in NPCs as it does in cancer cells. qRT-PCR of primary adult neurosphere mRNA showed that TAp73 expression levels were significantly increased by metformin treatment (Figure 2E). Next, we investigated whether TAp73 was responsible for the metformin-induced increase in NPC proliferation and self-renewal. To assess this, we isolated adult SVZ neurospheres from mice where TAp73 is specifically knocked out (Tomasini et al., 2008) and treated them for 6 days with 500 nM metformin. Metformin enhanced the number of neurosphere-initiating cells cultured from the TAp73+/+ but not TAp73−/− SVZ (Figures 2F and 2G). Moreover, when these spheres were passaged, the TAp73−/− cells gave rise to fewer neurospheres, as previously published (Fujitani et al., 2010), and these numbers did not increase with metformin treatment (Figure 2H). To determine whether TAp73 was also required for metformin-mediated NPC self-renewal and proliferation in vivo, we injected adult TAp73−/− mice with metformin and BrdU, as described earlier, and the number of BrdU-positive SVZ cells was quantified. This analysis showed that there were fewer BrdU-positive cells in the SVZ of adult TAp73−/− mice as compared to TAp73+/+ mice (Figures 2I and 2J). Additionally, the number of BrdU-positive cells in TAp73−/− mice was unaffected by metformin administration, whereas BrdU-positive cells increased in TAp73+/+ mice treated with metformin. Similar results were obtained when we analyzed the other neurogenic zone of the same mice, the dentate gyrus SGZ (Figure 2K). Thus, TAp73 is required for the metformin-mediated enhancement of adult NPC proliferation and self-renewal.
The AMPK-aPKC-CBP Pathway Mediates the Metformin-Induced Increase in Neuronal Differentiation of Adult NPCs and Not Self-Renewal or Proliferation
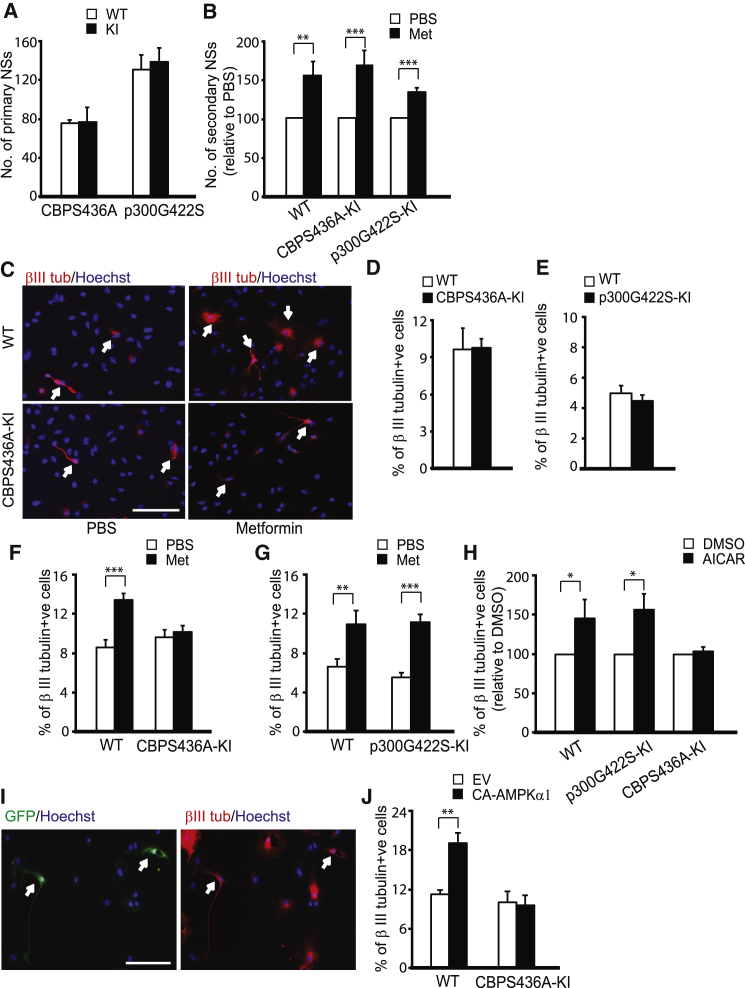
We previously demonstrated that metformin promoted the differentiation of embryonic NPCs and that this was inhibited by genetic knockdown of either aPKCs or CBP (Wang et al., 2012). To ask whether this same pathway was essential for the differentiation of adult NPCs, we took advantage of two knockin mouse models that specifically target the aPKC-CBP pathway; the phospho-mutant CBPS436A-KI mouse and a phospho-competent p300G422S knockin mouse (p300G422S-KI). p300G422S encodes a mutationally generated aPKC phosphorylation site in the CBP homolog p300, which normally lacks this aPKC site (He et al., 2009, He et al., 2012). While the CBPS436A mouse encodes a loss-of-function mutation in the aPKC-CBP pathway, the p300G422S mouse functioned as an additional WT control in our experiments.
To determine the effect of aPKC-CBP signaling on the proliferation, self-renewal, and differentiation of adult NPCs, we cultured and quantified neurospheres from the SVZ of adult mice of these different genotypes. The number and size of neurospheres were similar in all cases (Figures 3A and 3B), suggesting that the aPKC-CBP pathway is not required for NPC self-renewal or proliferation. Then, we differentiated these neurospheres in the presence or absence of metformin and quantified the number of neurons that were generated by immunostaining for βIII-tubulin (Figure 3C). In the absence of metformin, CBPS436A-KI and p300G422S-KI neurospheres generated similar numbers of newborn neurons relative to WT controls (Figures 3C–3E). In contrast, metformin did not increase neuronal differentiation from CBPS436A-KI neurospheres as it did from control (Figure 3F) or p300G422S-KI neurospheres (Figure 3G). Thus, metformin requires the aPKC-CBP pathway to enhance the genesis of neurons from adult NPCs.
Figure 3.
Metformin Enhances Neuronal Differentiation of Adult Neural Precursors by Activating the aPKC-CBP Pathway
(A) Quantification of primary neurosphere (NS) number cultured from 3-month-old CBPS436A-KI and p300G422S-KI (KI) mice (pooled data from four independent experiments).
(B) Quantification of the secondary neurosphere number from CBPS436A-KI and p300G422S-KI mice in the absence and presence of metformin (Met). ∗∗p < 0.01; ∗∗∗p < 0.001 (pooled data from four independent experiments).
(C) Immunofluorescence images of βIII-tubulin-positive neurons generated from SVZ WT and CBPS436A-KI neurospheres in the absence and presence of metformin. Arrows denote βIII-tubulin-positive cells. Scale bar, 20 μm.
(D and E) Quantitative analysis of the percentage of βIII-tubulin-positive (β III tubulin+ve) neurons generated from CBPS436A-KI neurospheres (D) and p300G422S-KI neurospheres (E), determined as shown in (C). (pooled data from four independent experiments).
(F and G) Quantitative analysis of the percentage of βIII-tubulin (βIII tub)-positive neurons generated from SVZ WT and CBPS436A-KI (F) and p300G422S (G) neurospheres in the absence and presence of metformin (200 nM). ∗∗∗p < 0.001; ∗∗p < 0.01 (pooled data from four independent experiments).
(H) Quantitative analysis of the percentage of βIII-tubulin-positive neurons generated from SVZ WT, CBPS436A-KI, and p300G422S-KI neurospheres in the absence and presence of AICAR (500 nM). ∗p < 0.05 (pooled data from four independent experiments).
(I) Immunofluorescence images of GFP-positive, βIII-tubulin-positive neurons generated from SVZ WT neurospheres transfected with CA-AMPKα1. Arrows denote GFP-positive, βIII-tubulin-positive cells. Scale bar, 20 μm.
(J) Quantification of the percentage of transfected, βIII-tubulin-positive neurons generated from SVZ WT and CBPS436A-KI neurospheres. EV, empty vector, CA-AMPKα1, constitutively active AMPK α1 subunit. ∗∗p < 0.01 (pooled data from four independent experiments).
Error bars indicate SEM.
To determine whether the aPKC-CBP pathway was activated by the metformin target AMPK, we first asked whether a second AMPK activator, AICAR, had similar effects as metformin. Quantification showed that 500 nM AICAR increased the proportion of newborn neurons in WT and p300G422S-KI cultures (Figure 3H), while it had no effect on the CBPS436A-KI cultures (Figure 3H). Second, we overexpressed a constitutively active AMPK alpha1 subunit (CA-AMPKα1) in cultured adult NPCs and observed an increased in the proportion of newborn neurons in WT but not CBPS436A-KI cultures (Figures 3I and 3J). Thus, AMPK activation is sufficient to enhance neurogenesis, and this requires the aPKC-CBP pathway.
Discussion
The data presented here support four major conclusions. First, metformin treatment enhances the proliferation, self-renewal, and neuronal differentiation of adult NPCs. Second, metformin treatment stimulates TAp73 gene expression in adult NPCs, and TAp73 is required for the metformin-induced proliferation and self-renewal of adult NPCs. Third, metformin requires the aPKC-mediated CBP S436 phosphorylation to induce neuronal differentiation of adult NPCs. Finally, AMPK activation stimulates adult NPC neuronal differentiation by activating the aPKC-CBP pathway.
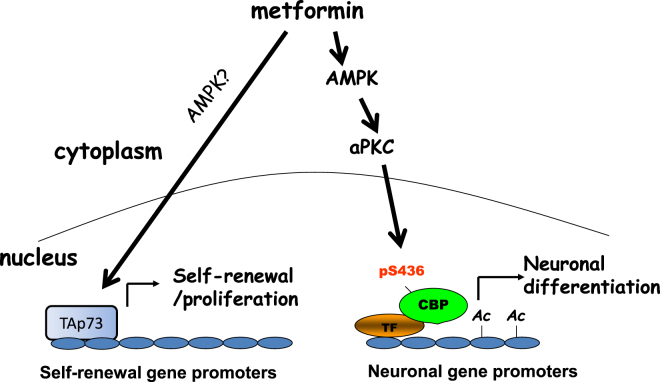
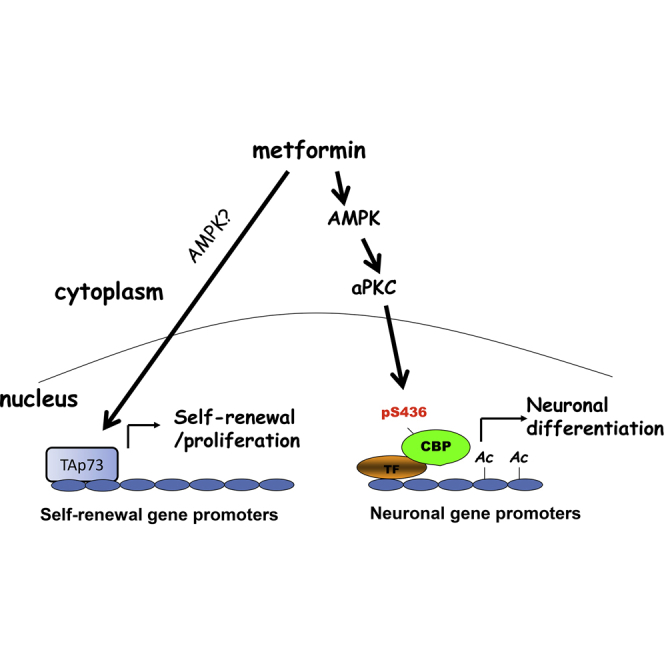
We previously showed that metformin treatment enhances murine adult neurogenesis in vivo without depleting the endogenous NPC pool (Wang et al., 2012). This finding has led to studies to repurpose metformin as a neuroregenerative agent to treat the injured or degenerating brain (Liu et al., 2014, Jin et al., 2014). Our findings here provide insights into the molecular mechanisms that regulate the dual functional roles of metformin in modulating adult NPC biology. Metformin treatment acts on two distinct molecular pathways to promote proliferation/regeneration and differentiation of adult NPCs, respectively. Our previous study identified TAp73 as an essential protein for adult NPC self-renewal (Fujitani et al., 2010). Here, we show that TAp73 is required for metformin to promote the proliferation and self-renewal of adult NPCs and to expand the NPC population in vivo. p73 is known to enhance the expression of self-renewal genes such as Sox2 and Notch family members in embryonic neurospheres (Talos et al., 2010). TAp73 also is a known tumor suppressor (Irwin et al., 2003), so the metformin-mediated increase in its activity should not increase the possibility of tumorigenesis. The second pathway regulated by metformin is one that we previously identified in NPCs, the aPKC-CBP pathway (Wang et al., 2012). This pathway is likely not involved in metformin-induced NPC proliferation and self-renewal but is essential for metformin-induced neuronal differentiation. Moreover, we found that AMPK is an upstream kinase that stimulates the aPKC-CBP pathway to promote neuronal differentiation. Although the germline AMPK β subunit knockout mice have been shown to have perturbed embryonic NPC biology (Dasgupta and Milbrandt, 2009), our study is the first to assess the role of AMPK activity in adult NPC fate decisions. Together, these results indicate that metformin activates two signaling pathways in NPCs: a TAp73 pathway that mediates self-renewal and proliferation and an AMPK-aPKC-CBP pathway that is required for neuronal differentiation (Figure 4).
Figure 4.
Model Describing Two Distinct Molecular Pathways Mediating Metformin-Induced Proliferation/Self-Renewal and Neuronal Differentiation
TF, transcription factors; Ac, acetylation.
One interesting finding in this study is that initial treatment of metformin in primary neurospheres not only increases the number and size of primary neurospheres but also enhances the number and size of secondary and tertiary neurospheres cultured in the absence of metformin. These data imply that metformin could be used ex vivo to expand exogenous neural stem cells before transplantation and that these neural stem cells will continually expand in vivo after transplantation. Therefore, metformin might be an ideal preconditioning reagent to improve neuroregenerative efficiency of transplanted exogenous neural stem cells in vivo for the treatment of brain injury and neurodegenerative diseases.
Our study also demonstrates that the aPKC-mediated single phosphorylation of CBP serves as a signaling sensor that responds to micro-environmental changes to fine-tune neuronal differentiation of adult NPCs. This CBP phosphorylation may directly alter CBP histone acetyltransferase activity or chromatin binding. Identification of downstream targets of the aPKC-CBP pathway at both genomic and epigenomic levels will be the subject of our future work.
Experimental Procedures
Animals
All animal use was approved by the animal care committees of the Hospital for Sick Children and the University of Ottawa in accordance with the Canadian Council of Animal Care policies. TAp73−/−, CBPS436A, and p300G422S mice were maintained on a 12-hr/12-hr light/dark cycle with ad libitum access to food and water.
BrdU Labeling
To quantify proliferating precursors in the SVZ and SGZ, adult mice were injected i.p. with a single dose of BrdU (Sigma-Aldrich) at 100 mg/kg. Animals were sacrificed 24 hr later via sodium pentobarbital overdose and transcardially perfused with PBS followed by 4% paraformaldehyde. Brains were processed for immunostaining as previously described (Wang et al., 2012), which is detailed in the Supplemental Experimental Procedures.
Neurosphere Culture and Transfection
For adult SVZ cultures, the subependyma of the lateral ventricles was dissected out as previously described (Fujitani et al., 2010). Cell density and viability were determined via trypan blue exclusion and a hemocytometer. Freshly isolated cells were then plated at a density of ten cells per microliter in six-well (2 ml per well) ultra-low attachment plates (Corning) in serum-free medium (SFM) containing 20 ng/ml epidermal growth factor (EGF; Sigma-Aldrich), 10 ng/μl fibroblast growth factor 2 (FGF2; Sigma-Aldrich), 1× B27 (Becton Dickinson; BD), and 2 μg/ml heparin (Sigma-Aldrich). Primary neurospheres were counted on day 6 after dissection, with only spheres composed of over 50 cells being counted. Sphere diameter was quantified using Northern Eclipse software (Empix), by drawing a line through the length of each sphere at 6 days of culture. To assess self-renewal, neurospheres were passaged either once or twice by mechanical dissociation, passed through a 40-μm cell strainer, plated at a density of two cells per microliter, and counted 4 days later. All neurosphere experiments were performed in triplicate wells, and results are representative of at least three independent experiments. For the differentiation assay, individual neurospheres were collected, gently triturated, and plated onto poly-L-ornithine (Sigma)- and laminin (BD)-coated wells in the presence of 10% FBS with SFM for 7 days. To assess NPC apoptosis, secondary neurospheres were grouped, gently triturated, and plated onto poly-L-Ornithine (Sigma) and laminin (BD)-coated wells in the presence of SFM containing 20 ng/ml EGF, 10 ng/μl FGF2, 1× B27, and 2 μg/ml heparin for 2 days. Metformin stock solution (1 mM) was made in PBS, AICAR stock solution (5 mM) was made in DMSO, and chelerythrine stock solution (1 mM) was made in DMSO. All these drugs were added to the cultures at various stages of cultures as indicated. For transfection, detailed information was described in Supplemental Experimental Procedures.
qRT-PCR
qRT-PCR was performed as previously described (Cancino et al., 2015). Detailed information was described in Supplemental Experimental Procedures.
Immunocytochemistry, Microscopy, and Quantification
Detailed information is described in Supplemental Experimental Procedures.
Author Contributions
M.P.F. performed adult neurosphere assays and BrdU in vivo labeling; K.H. performed adult neurosphere assays and differentiation assays; L.H. and F.W. generated CBPS436A-KI and p300G422S-KI mouse strains; and M.F., K.H., F.D.M., D.R.K., and J.W. contributed to experimental design, data interpretation, and writing the paper.
Acknowledgments
This work was supported by the J.P. Bickell Foundation, by OHRI internal start-up funding to J.W., and by a Canadian Institutes of Health research grant to F.D.M. and D.R.K. F.D.M. and D.R.K are Canada Research Chairs, and F.D.M. is a Howard Hughes Medical Institute International Research Scholar.
Published: November 19, 2015
Footnotes
This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
Supplemental Information includes Supplemental Experimental Procedures and one figure and can be found with this article online at http://dx.doi.org/10.1016/j.stemcr.2015.10.014.
Supplemental Information
References
- Cancino G.I., Fatt M.P., Miller F.D., Kaplan D.R. Conditional ablation of p63 indicates that it is essential for embryonic development of the central nervous system. Cell Cycle. 2015 doi: 10.1080/15384101.2015.1087618. Published online September 11, 2015. PMID: 26359534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasgupta B., Milbrandt J. AMP-activated protein kinase phosphorylates retinoblastoma protein to control mammalian brain development. Dev. Cell. 2009;16:256–270. doi: 10.1016/j.devcel.2009.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelmann D., Meier C., Alla V., Pützer B.M. A balancing act: orchestrating amino-truncated and full-length p73 variants as decisive factors in cancer progression. Oncogene. 2015;34:4287–4299. doi: 10.1038/onc.2014.365. [DOI] [PubMed] [Google Scholar]
- Fujitani M., Cancino G.I., Dugani C.B., Weaver I.C., Gauthier-Fisher A., Paquin A., Mak T.W., Wojtowicz M.J., Miller F.D., Kaplan D.R. TAp73 acts via the bHLH Hey2 to promote long-term maintenance of neural precursors. Curr. Biol. 2010;20:2058–2065. doi: 10.1016/j.cub.2010.10.029. [DOI] [PubMed] [Google Scholar]
- He L., Sabet A., Djedjos S., Miller R., Sun X., Hussain M.A., Radovick S., Wondisford F.E. Metformin and insulin suppress hepatic gluconeogenesis through phosphorylation of CREB binding protein. Cell. 2009;137:635–646. doi: 10.1016/j.cell.2009.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He L., Naik K., Meng S., Cao J., Sidhaye A.R., Ma A., Radovick S., Wondisford F.E. Transcriptional co-activator p300 maintains basal hepatic gluconeogenesis. J. Biol. Chem. 2012;287:32069–32077. doi: 10.1074/jbc.M112.385864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imayoshi I., Sakamoto M., Ohtsuka T., Takao K., Miyakawa T., Yamaguchi M., Mori K., Ikeda T., Itohara S., Kageyama R. Roles of continuous neurogenesis in the structural and functional integrity of the adult forebrain. Nat. Neurosci. 2008;11:1153–1161. doi: 10.1038/nn.2185. [DOI] [PubMed] [Google Scholar]
- Irwin M.S., Kondo K., Marin M.C., Cheng L.S., Hahn W.C., Kaelin W.G., Jr. Chemosensitivity linked to p73 function. Cancer Cell. 2003;3:403–410. doi: 10.1016/s1535-6108(03)00078-3. [DOI] [PubMed] [Google Scholar]
- Jin Q., Cheng J., Liu Y., Wu J., Wang X., Wei S., Zhou X., Qin Z., Jia J., Zhen X. Improvement of functional recovery by chronic metformin treatment is associated with enhanced alternative activation of microglia/macrophages and increased angiogenesis and neurogenesis following experimental stroke. Brain Behav. Immun. 2014;40:131–142. doi: 10.1016/j.bbi.2014.03.003. [DOI] [PubMed] [Google Scholar]
- Kernie S.G., Parent J.M. Forebrain neurogenesis after focal Ischemic and traumatic brain injury. Neurobiol. Dis. 2010;37:267–274. doi: 10.1016/j.nbd.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Tang G., Zhang Z., Wang Y., Yang G.Y. Metformin promotes focal angiogenesis and neurogenesis in mice following middle cerebral artery occlusion. Neurosci. Lett. 2014;579:46–51. doi: 10.1016/j.neulet.2014.07.006. [DOI] [PubMed] [Google Scholar]
- Mitchell B.D., Emsley J.G., Magavi S.S., Arlotta P., Macklis J.D. Constitutive and induced neurogenesis in the adult mammalian brain: manipulation of endogenous precursors toward CNS repair. Dev. Neurosci. 2004;26:101–117. doi: 10.1159/000082131. [DOI] [PubMed] [Google Scholar]
- Pernicova I., Korbonits M. Metformin--mode of action and clinical implications for diabetes and cancer. Nat. Rev. Endocrinol. 2014;10:143–156. doi: 10.1038/nrendo.2013.256. [DOI] [PubMed] [Google Scholar]
- Reynolds B.A., Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science. 1992;255:1707–1710. doi: 10.1126/science.1553558. [DOI] [PubMed] [Google Scholar]
- Rosenbluth J.M., Mays D.J., Pino M.F., Tang L.J., Pietenpol J.A. A gene signature-based approach identifies mTOR as a regulator of p73. Mol. Cell. Biol. 2008;28:5951–5964. doi: 10.1128/MCB.00305-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talos F., Abraham A., Vaseva A.V., Holembowski L., Tsirka S.E., Scheel A., Bode D., Dobbelstein M., Brück W., Moll U.M. p73 is an essential regulator of neural stem cell maintenance in embryonal and adult CNS neurogenesis. Cell Death Differ. 2010;17:1816–1829. doi: 10.1038/cdd.2010.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasini R., Tsuchihara K., Wilhelm M., Fujitani M., Rufini A., Cheung C.C., Khan F., Itie-Youten A., Wakeham A., Tsao M.S. TAp73 knockout shows genomic instability with infertility and tumor suppressor functions. Genes Dev. 2008;22:2677–2691. doi: 10.1101/gad.1695308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Gallagher D., DeVito L.M., Cancino G.I., Tsui D., He L., Keller G.M., Frankland P.W., Kaplan D.R., Miller F.D. Metformin activates an atypical PKC-CBP pathway to promote neurogenesis and enhance spatial memory formation. Cell Stem Cell. 2012;11:23–35. doi: 10.1016/j.stem.2012.03.016. [DOI] [PubMed] [Google Scholar]
- Zhao C., Deng W., Gage F.H. Mechanisms and functional implications of adult neurogenesis. Cell. 2008;132:645–660. doi: 10.1016/j.cell.2008.01.033. [DOI] [PubMed] [Google Scholar]
- Zhou X.Y., Shibusawa N., Naik K., Porras D., Temple K., Ou H., Kaihara K., Roe M.W., Brady M.J., Wondisford F.E. Insulin regulation of hepatic gluconeogenesis through phosphorylation of CREB-binding protein. Nat. Med. 2004;10:633–637. doi: 10.1038/nm1050. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.