Summary
Although SOX2+ stem cells are present in the postnatal pituitary gland, how they are regulated molecularly and whether they are required for pituitary functions remain unresolved questions. Using a conditional knockout animal model, here we demonstrate that ablation of the canonical Notch signaling in the embryonic pituitary gland leads to progressive depletion of the SOX2+ stem cells and hypoplastic gland. Furthermore, we show that the SOX2+ stem cells initially play a significant role in contributing to postnatal pituitary gland expansion by self-renewal and differentiating into distinct lineages in the immediate postnatal period. However, we found that within several weeks postpartum, the SOX2+ stem cells switch to an essentially dormant state and are no longer required for homeostasis/tissue adaptation. Our results present a dynamic tissue homeostatic model in which stem cells provide an initial contribution to the growth of the neonatal pituitary gland, whereas the mature gland can be maintained in a stem cell-independent fashion.
Graphical Abstract
Highlights
-
•
Notch signaling is necessary to maintain Sox2+ stem cells in the pituitary gland
-
•
Sox2+ cells and differentiated cells contribute to postnatal pituitary expansion
-
•
Sox2+ stem cells prove to be dispensable for adult pituitary gland homeostasis
-
•
Differentiated cells retain mitotic capacity and respond to physiological demands
In this article, Rosenfeld, Zhu, and colleagues demonstrate that Notch signaling is necessary to maintain Sox2+ stem cells in the pituitary gland. Sox2+ cells make a contribution to immediate postnatal expansion by proliferation and differentiation. However, they are not essential for adult pituitary gland homeostasis. Differentiated cells are capable of proliferation and respond to physiological demands.
Introduction
The pituitary gland plays a fundamental role in regulating a wide variety of physiological functions, including growth, lactation, stress response, reproduction, and metabolism. These complex functions are regulated by six distinct hormone-producing cell types distinguished by the different hormones they synthesize and secrete, including corticotropes secreting adrenocorticotrophic hormone (ACTH), thyrotropes secreting thyroid-stimulating hormone (TSH), somatotropes secreting growth hormone (GH), lactotropes secreting prolactin (PRL), gonadotropes secreting luteinizing hormone (LH) and follicle-stimulating hormone (FSH), and melanotropes secreting melanocyte-stimulating hormone (MSH). During pituitary organogenesis, these lineages emerge in a stereotypical spatio-temporal pattern from a common ectodermal primordium, Rathke’s pouch (RP). Extensive studies in model systems have demonstrated that multiple signaling pathways, transcription factors, and cofactors define the genetic hierarchy that controls embryonic pituitary development (Davis et al., 2011, Kelberman et al., 2009, Zhu et al., 2007).
We and others have shown previously that the evolutionarily conserved Notch signaling pathway plays an important role in early embryonic pituitary development (Kita et al., 2007, Raetzman et al., 2004, Raetzman et al., 2007, Zhu et al., 2006). Delta/Notch signaling, mediated by the critical transcription factor RBP-J, acts to prevent progenitor cells in the RP from premature differentiation through Hes1, one of the downstream target genes of the Notch pathway. It also controls the competence of progenitor cells by maintaining expression of the Prop1 gene, which encodes a pituitary-specific, paired-like homeodomain transcription factor necessary for the commitment of the PIT1 lineage of three cell types—somatotropes, thyrotropes, and lactotropes. In the absence of canonical Notch signaling, resulting from deletion of the Rbp-J gene at embryonic day (E) 10.5 in the RP using Pitx1-Cre transgenic mice, the progenitors adopt an early-born corticotrope cell fate at the expense of the late-arising PIT1 lineage (Kita et al., 2007, Raetzman et al., 2007, Zhu et al., 2006). Interestingly, the proliferating progenitors, residing in the periluminal region, are still present at the end of embryonic development in the mutant pituitary gland (Zhu et al., 2006). However, the mutant animals died of cleft palate shortly after birth because of broad expression of Pitx1-Cre in the oral ectoderm (unpublished data), leaving an open question regarding whether continued Notch signaling is required to maintain these pituitary progenitors in the postnatal period. Recently, it has been suggested that Notch signaling is required for progenitor maintenance based on deletion of the Notch2 gene in the embryonic RP. However, despite a progressive decrease in the number of pituitary progenitors, these cells remain in the postnatal gland in this animal model, particularly in the anterior lobe (Nantie et al., 2014). An animal model with specific and complete depletion of Notch signaling is required to provide an unambiguous answer.
At birth, all of the endocrine cell lineages are present in the mouse pituitary gland, but the gland continues to grow and mature substantially after birth, particularly during the first few postnatal weeks. It has been documented that this postnatal pituitary gland expansion in the rat is only partially brought about via proliferation of preexisting differentiated hormone-producing cells (Carbajo-Pérez and Watanabe, 1990, Taniguchi et al., 2000, Taniguchi et al., 2001a, Taniguchi et al., 2001b, Taniguchi et al., 2002). Double immunolabeling of hormone and proliferation markers reveals that 10%–30% of the proliferating cells are differentiated endocrine cells, implying that some of the postnatal proliferation might take place in undifferentiated cells. On the other hand, the mature pituitary gland has a low turnover rate under basal conditions (Florio, 2011). However, one important feature of the pituitary gland is its plasticity. The cellular composition of the mature gland can change flexibly to adapt to the physiological or pathological demands of the organism (Levy, 2002). Recently, postnatal pituitary stem cells have been identified based on expression of a variety of stem cell-specific markers, including SOX2, SOX9, E-Cadherin, NES, and the pituitary-specific transcription factor LHX3 (Chen et al., 2009, Fauquier et al., 2008, Garcia-Lavandeira et al., 2009, Gleiberman et al., 2008, Rizzoti, 2010, Vankelecom and Chen, 2014). These cells are localized in the marginal region between the intermediate lobe and the anterior lobe, and, when cultured in vitro, they are capable of self-renewal and differentiation into diverse hormone-producing pituitary cell types, implying their “stemness.” In vivo characterizations of these SOX2+ cells have shown that they are most abundant in the neonatal pituitary gland (Gremeaux et al., 2012). Recent studies of cell ablation of terminally differentiated cells have suggested that these SOX2+ cells may contribute to pituitary regeneration (Fu et al., 2012, Fu and Vankelecom, 2012). In addition, lineage tracing of SOX2+, SOX9+ cells have provided genetic evidence that these cells can contribute to organ homeostasis and tissue adaptation (Andoniadou et al., 2013, Rizzoti et al., 2013). Expression of the Notch signaling component in postnatal stem cells has been described previously (Chen et al., 2006, Chen et al., 2009, Nantie et al., 2014, Tando et al., 2013, Vankelecom and Gremeaux, 2010). Interfering with Notch activation affects stem cell number in pituitary primary culture, implicating a critical role of Notch signaling in stem cell proliferation (Chen et al., 2006, Nantie et al., 2014, Tando et al., 2013). However, whether Notch signaling is essential for pituitary stem cell proliferation in vivo and whether these cells are necessary for any normal physiological pituitary functions remains unknown.
Here we demonstrate that postnatal pituitary SOX2+ stem cells are derived from the embryonic RP and that the Notch signaling pathway is essential for their proliferation, maintenance, and postnatal pituitary expansion. Furthermore, we present evidence that SOX2+ stem cells make a significant contribution to neonatal pituitary expansion but that they gradually switch to an essentially quiescent state so that they are no longer required for homeostasis and tissue plasticity in the mature gland. Our results suggest that committed cells are capable of adapting to physiological demands and contribute to pituitary gland plasticity.
Results
Notch Signaling Is Active in Pituitary Postnatal Stem Cells
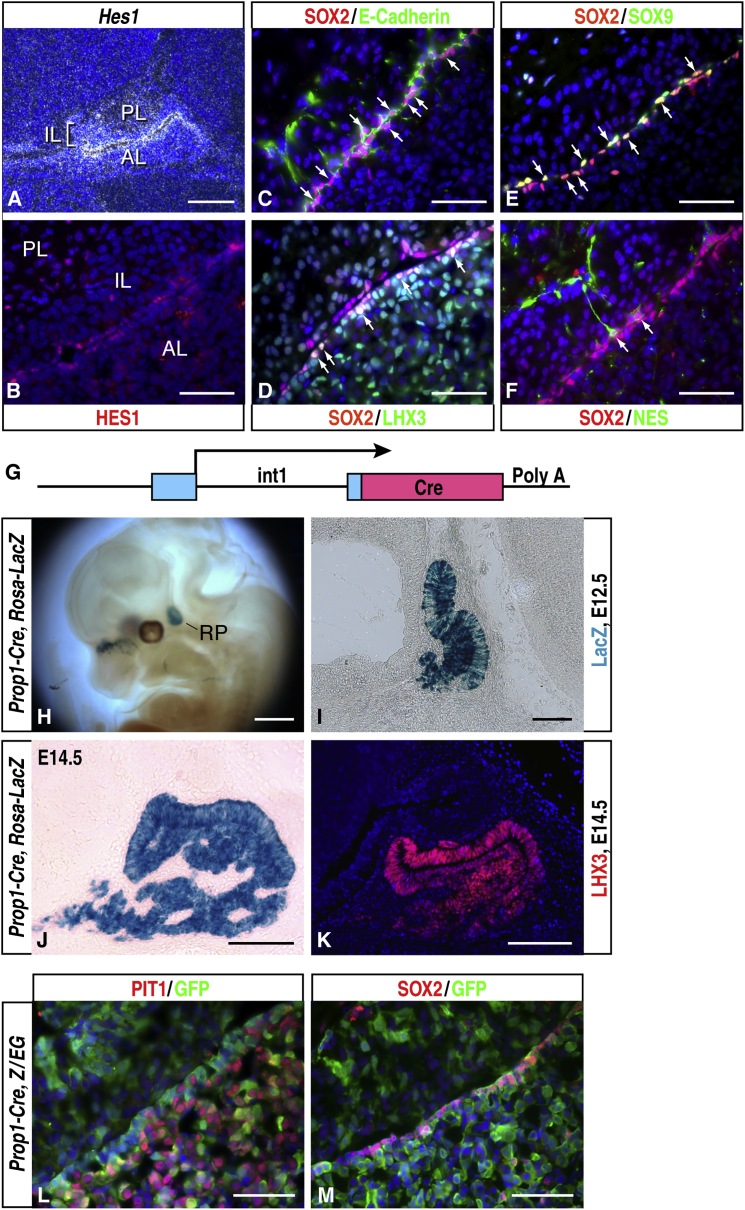
Components of the Notch signaling pathway exhibit dynamic expression patterns during pituitary organogenesis. Their expression is high in the RP but becomes attenuated at E13.5 in the perspective anterior lobe as cells begin to undergo lineage commitment (Raetzman et al., 2004, Zhu et al., 2006). However, we observed that the expression of Notch2, Dll1, Dll3, and the downstream target gene Hes1 persists in the periluminal region at E17.5, where the prospective postnatal pituitary stem cells reside (Figure 1A; Figures S1A–S1D). To further elucidate the status of Notch signaling in postnatal stem cells, we performed immunofluorescence staining using a specific anti-Hes1 antibody (Figure 1B). All periluminal cells express SOX2 and E-Cadherin, and most of them express Hes1, suggesting that Notch signaling remains active in these cells. Double immunolabeling revealed that some of the SOX2+ stem cells co-expressed SOX9, LHX3, NES, and PROP1 (Figures 1C–1F; Figures S1E–S1H). Interestingly, SOX9 appears to be more highly expressed in the periluminal layer next to the intermediate lobe, whereas LHX3 and PROP1 are more expressed toward the anterior lobe. These results, collectively, imply heterogeneity within the stem cell population.
Figure 1.
Characterizations of Pituitary Stem Cells and Prop1-Cre Transgenic Mice
(A) In situ hybridization of the Notch target gene Hes1 at E17.5 revealed that it is highly expressed in the periluminal region.
(B–F) Immunofluorescence staining of Hes1 (B), SOX2/E-Cadherin (C), SOX2/LHX3 (D), SOX2/SOX9 (E), and SOX2/NES (F) in a 2-month-old pituitary gland. Arrows indicate representative double-positive cells.
(G) The Prop1-Cre transgenic line is driven by the 2.2-kb promoter/enhancer region of the Prop1 gene. Blue boxes, exons 1 and 2; int1, intron 1.
(H–J) LacZ reporter analysis of Prop1-Cre, Rosa-LacZ embryos in whole-mount (H) and middle sagittal frozen sections at E12.5 (I) and E14.5 (J). LacZ activity at E14.5 is detected in all cells in the anterior and intermediate lobes of the embryonic pituitary gland. It is noted that ectopic Cre activity is detected in the olfactory epithelium (H).
(K) Immunofluorescence staining of LHX3 at E14.5.
(L and M) Dual immunofluorescence staining of GFP/PIT1 and GFP/SOX2 in a Prop1-Cre, Z/EG postnatal day 22 pituitary gland.
AL, anterior lobe; IL, intermediate lobe; PL, posterior lobe. Scale bars, 200 μm (A); 50 μm (B–F, L, and M), 500 μm (H), 100 μm (I–K).
Generation of the Prop1-Cre Transgenic Mice
To circumvent the neonatal lethality and early developmental defects in the Rbp-Jf/f, Pitx1-Cre mice as described before (Zhu et al., 2006), we generated a Cre line using the genetic information of the Prop1 gene to drive expression exclusively in the RP during pituitary organogenesis. We learned that the first intron of the Prop1 gene is essential for its expression based on the following observations. First, in the previously generated Prop1 knockout mice, where exons 2 and 3 and all introns were replaced with in-frame fusion of the LacZ cassette, LacZ failed to be expressed (Olson et al., 2006). Second, the first intron of the Prop1 gene is evolutionally conserved and is required to maintain Prop1 gene expression in response to early Notch signaling (Zhu et al., 2006). Consistent with these findings, it was shown later that the first intron of the Prop1 gene can function as a pituitary-specific enhancer when placed together with a heterogeneous promoter (Ward et al., 2007). We mapped the critical region to a 2.2-kb promoter/enhancer region of the Prop1 gene, comprised of approximately 0.7 kb of promoter sequence, the first exon, and the first intron region (unpublished data). We subsequently generated Prop1-Cre transgenic mice using the same genomic information (Figure 1G). This Cre line expresses CRE in many cells in the RP at E12.5, similar to endogenous PROP1 expression (Figures S1I–S1J) and, consistently, when crossed with Rosa26-LacZ reporter mice, can mediate LoxP recombination in many RP progenitors at E12.5, as evidenced by LacZ staining on whole mounts and sections (Figures 1H and 1I) and in almost all cells in the anterior and intermediate lobes of the pituitary gland at E14.5 and postnatal day (P) 0 (Figure 1J; Figure S1K). The LacZ activity at E14.5 is almost identical to the expression pattern of LHX3 (Figure 1K), a critical transcription factor required for early pituitary development and expressed in every embryonic endocrine cell type and their precursors. Therefore, the Prop1-Cre line can effectively mediate recombination in the RP and label almost all cells in the anterior and intermediate lobes of the pituitary gland. Moreover, to probe the origin of pituitary postnatal SOX2+ cells, we crossed Prop1-Cre with Z/EG reporter mice, with double immunofluorescence labeling in the postnatal pituitary gland from Prop1-Cre, Z/EG mice, revealing that EGFP is co-labeled with SOX2+ as well as PIT1+ cells, suggesting that postnatal SOX2+ stem cells are derived from the embryonic RP progenitors (Figures 1L and 1M).
Notch Signaling Is Required to Maintain Pituitary Stem Cells
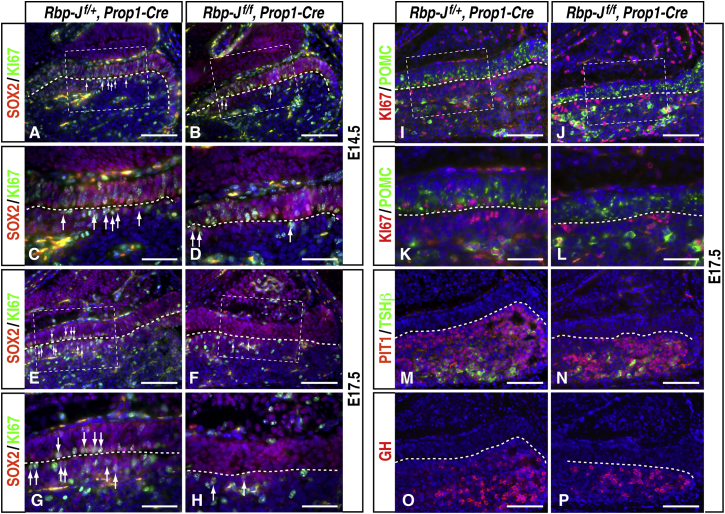
To explore the potential functions of Notch signaling in late pituitary organogenesis and postnatal stem cells, we generated Rbp-Jf/f, Prop1-Cre mice. At E12.5 in pituitary development, all RP progenitor cells express SOX2, and most of the luminal SOX2+ cells are proliferating, as revealed by double-labeling of SOX2 and KI67 (Figure S1L). By E14.5, expression of SOX2 is confined to the intermediate lobe and periluminal cells in the anterior lobe (Figures 2A and 2C). In the mutant embryos, the number of proliferating SOX2+KI67+ progenitor cells was decreased at E14.5 (Figures 2B and 2D). By E17.5, there were no KI67+ cells detectable in the intermediate lobe, and they were decreased markedly in the periluminal region of the anterior lobe (Figures 2E–2H). Expression of LHX3 at E17.5 is similar to that of SOX2, high in the intermediate lobe and periluminal cells of the anterior lobe. Consistently, in the mutant embryos, there was a reduction in the number of LHX3+ KI67+ cells in the periluminal region (Figures S2A–S2D). No increased apoptosis was observed in the mutants by either terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL) assay or immunofluorescence labeling with activated caspase-3 (Figures S2E–S2H). In addition, the intermediate lobe at E17.5 in the mutant was populated with POMC+ melanotropes, consistent with the idea that SOX2+ KI67+ cells may differentiate prematurely into melanotropes (Figures 2I–2L). On the other hand, the ontogeny of PIT1+ lineages in the anterior lobe, which is mutually exclusive with SOX2+ cells (Figures S2I–S2L), occurred at the appropriate times. There was, however, a decrease in the number of PIT1+ cells, including GH+ somatotropes and TSHβ+ thyrotropes (Figures 2M–2P; Figures S2M and S2N), perhaps because of progressive depletion of the SOX2+ KI67+ progenitors.
Figure 2.
Proliferating SOX2+ Progenitor Cells in the Embryonic Pituitary Gland Are Reduced in Rbp-Jf/f, Prop1-Cre Mutants
(A–H) Double immunofluorescence labeling of SOX2 and KI67 of wild-type controls (A, C, E, and G) and Rbp-Jf/f, Prop1-Cre mutants (B, D, F, and H) at E14.5 (A–D) and E17.5 (E–H) showed reduced numbers of SOX2+ KI67+ cells in the Rbp-Jf/f, Prop1-Cre mutants, initially in the anterior lobe at E14.5 and later in the intermediate lobe at E17.5. Arrows indicate representative double-positive cells.
(I–L) Double immunofluorescence labeling of POMC and KI67 at E17.5 revealed that periluminal KI67+ cells in the intermediate lobe (I and K) are absent in the Rbp-Jf/f, Prop1-Cre mutants (J and L).
(M and N) Double immunofluorescence labeling of PIT1 and TSHβ at E17.5. The anterior lobe in the mutant (N) is smaller than in the wild-type control (M), with reduced numbers of PIT1+ and TSHβ+ cells.
(O and P) Immunofluorescence labeling of GH in the wild-type control (O) and Rbp-Jf/f, Prop1-Cre mutants (P) at E17.5.
The dashed lines indicate the lumen between the intermediate and anterior lobes. The dashed areas in (A), (B), (E), (F), (I), and (J) are also presented in (C), (D), (G), (H), (K), and (L), respectively. Scale bars, 100 μm (A, B, E, F, I, J, and M–P) and 50 μm (C, D, G, H, K, and L).
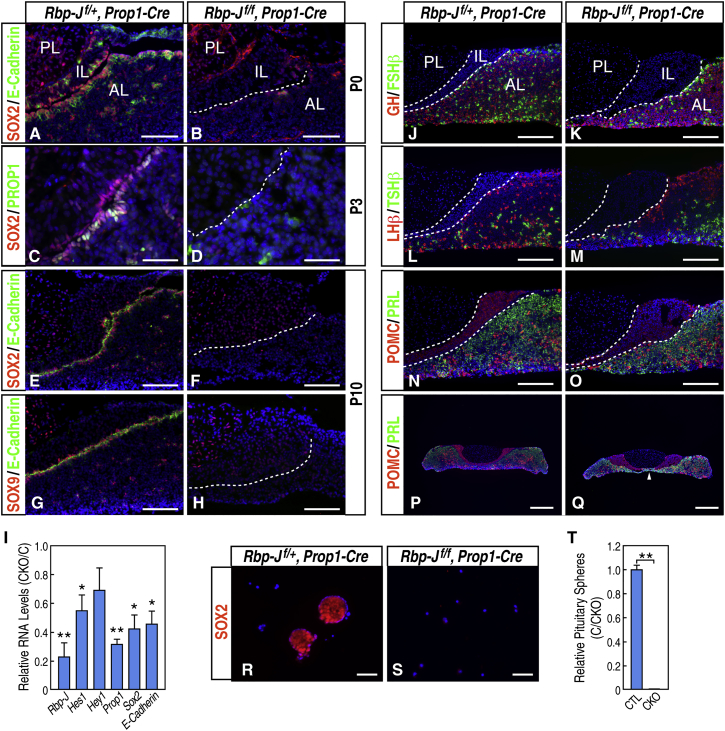
The SOX2+ cells were examined further in postnatal pituitary glands (Figure 3). At P0, SOX2+ E-Cadherin+ stem cells were present in the periluminal region and as small clusters in the anterior lobe in the wild-type pituitaries. By contrast, in the mutant gland, these SOX2+ cells were largely absent, except for a few remaining cells with reduced levels of SOX2 and E-Cadherin (Figures 3A and 3B). Expression of PROP1, which was present in some SOX2+ stem cells in the wild-type control, was largely undetectable in the mutant gland (Figures 3C and 3D). By P10, the periluminal pituitary stem cells as well as stem cell clusters in the anterior lobe, characterized by SOX2, SOX9, and E-Cadherin labeling, were almost completely absent in the mutant glands (Figures 3E–3H). Consistent with these observations, RNA levels of the Sox2, E-Cadherin, and Prop1 genes as well as the Notch downstream target Hes1 were downregulated in the mutant pituitary glands, detected by qRT-PCR (Figure 3I). Therefore, in the absence of Notch signaling after the onset of Prop1 expression in the RP, the SOX2+ progenitor cells in the RP fail to maintain self-renewal, leading to a reduced population of proliferative progenitors and, consequently, a complete depletion of pituitary stem cells in the postnatal pituitary gland.
Figure 3.
SOX2+ E-Cadherin+ Pituitary Stem Cells Are Depleted Gradually in Hypoplastic and Dysmorphic Pituitary Glands in Postnatal Rbp-Jf/f, Prop1-Cre Mutants
(A–H) Double immunofluorescence labeling of SOX2/E-Cadherin (A, B, E, and F; magnification, 200×), SOX2/PROP1 (C and D; magnification, 400×), and SOX9/E-Cadherin (G and H; magnification, 200×) at P0 (A and B), P3 (C and D), and P10 (E–H) in wild-type controls (A, C, E, and G) and Rbp-Jf/f, Prop1-Cre mutants (B, D, F, and H).
(I) qRT-PCR of a 3-month-old wild-type control and Rbp-Jf/f, Prop1-Cre mutant pituitary revealed reduced expression of Rbp-J, Hes1, Hey1, Prop1, Sox2, and E-Cadherin. Data are represented as mean ± SEM (n = 3 mice). ∗p < 0.05, ∗∗p < 0.01. CKO/C, conditional knockout/control.
(J–Q) Immunofluorescence labeling of GH/FSHβ (J and Κ), LHβ/TSHβ, and POMC/PRL (N–Q) in 1-month-old wild-type controls (J, L, N, and P) and Rbp-Jf/f, Prop1-Cre mutants (K, M, O, and Q) showed that all cell types are present in the mutant glands. Lower magnification (25×) of the gland (P and Q) revealed that, in the Rbp-Jf/f, Prop1-Cre mutants, the intermediate lobe is expanded laterally and discontinued in the ventral medial region, leading to a direct interaction of the anterior lobe with the posterior lobe (arrowhead).
(R–T) Immunofluorescence labeling of SOX2 (R and S) and quantification (T) of pituitary spheres cultured from a 3-month-old wild-type control (CTL) and Rbp-Jf/f, Prop1-Cre mutant. Data are represented as mean ± SEM (n = 3 mice). ∗p < 0.05, ∗∗p < 0.01.
Scale bars, 100 μm (A, B, and E–H), 50 μm (C and D), 200 μm (J and O), 500 μm (P and Q), and 75 μm (R and S).
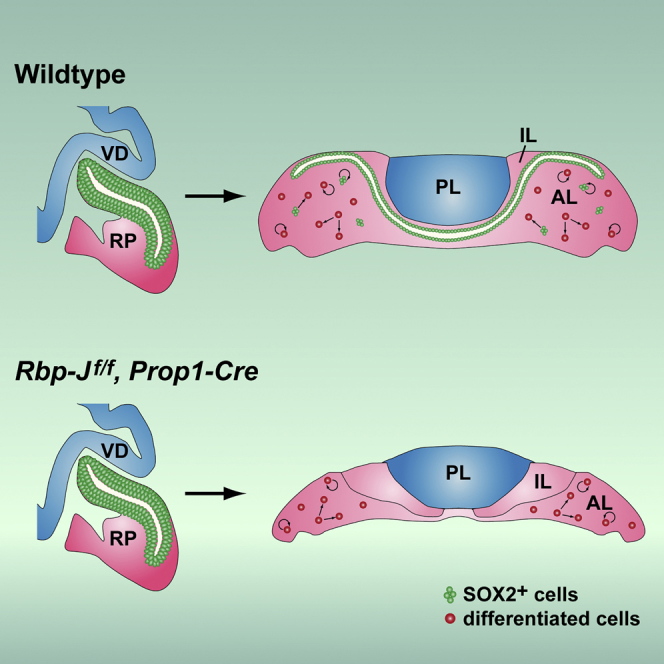
As expected, the mutant mice were able to survive until adulthood. They were smaller than their age-matched wild-type controls by body weight (Figures S3A and S3B), and the pituitary glands of mutant animals were hypoplastic. Immunofluorescence staining revealed that all cell types in the anterior lobe were present but with a reduced number (Figures 3J–3Q). The intermediate lobe, labeled by POMC staining, which encloses the posterior lobe and separates the posterior lobe from the anterior lobe in wild-type glands, expanded laterally in the mutants, whereas the medial regions of the gland lacked intermediate lobe cells and, as a result, allowed direct contact between the anterior and posterior lobes (Figures 3P and 3Q). Moreover, to further verify that SOX2+ stem cells were absent in mutant glands, in vitro pituitary cultures were established. Pituitary spheres, labeled by SOX2 staining, were readily detectable in wild-type culture, whereas there were barely any in the mutant culture (Figures 3R–3T). Collectively, these data demonstrate that Notch signaling is essential for the proliferation and maintenance of postnatal pituitary stem cells as well as the size and morphology of the pituitary gland.
To examine whether Notch signaling is required cell-autonomously in SOX2+ cells for their maintenance, we generated Rbp-Jf/f, Sox2-CreERT2 mice. Tamoxifen was injected for two consecutive days, and pituitary glands were characterized 21 days later. There was a clearly decrease in the number of SOX2+ cells in the mutant, particularly in the very lateral periluminal region (Figures S3C–S3E). However, large numbers of SOX2+ cells still remained in the periluminal region as well as in the anterior lobe, probably because Sox2-CreERT2 cannot effectively label all SOX2+ cells after tamoxifen induction (Rizzoti et al., 2013; unpublished data). Additionally, chemical inhibition of Notch signaling by N-[N-(3,5-difluorophenacetyl)-L-alanyl]-S-phenylglycine t-butyl ester (DAPT) in the pituitary sphere culture resulted in a significant reduction in the number of pituitary spheres (Figure S3F). Therefore, Notch activation in SOX2+ cells is necessary for their proliferation and maintenance.
Prop1 Functions Downstream of Sox2
It has been shown previously that expression of Prop1 in RP is directly regulated by Notch signaling (Nantie et al., 2014, Zhu et al., 2006). Here we demonstrated that Notch signaling is required for maintaining SOX2+ cells in the postnatal gland and that expression of PROP1 in SOX2+ stem cells is absent when Notch signaling is ablated (Figures 3C and 3D), suggesting that PROP1 may function downstream of SOX2. Consistent with this notion, it has been reported that Prop1 is downregulated in pituitary-specific Sox2 conditional knockout embryos (Jayakody et al., 2012). To understand whether PROP1 might reciprocally regulate SOX2 expression, we examined SOX2+ cells in Prop1−/− mice. Immunofluorescence of SOX2 at P10 revealed that SOX2+ stem cells still persist in the periluminal region, as well as in the anterior lobe, in the absence of Prop1 (Figures S3G and S3H). Together, these results suggest that SOX2 functions upstream of PROP1.
Functions of SOX2+ Stem Cells and Notch Signaling in the Postnatal Expansion of the Pituitary Gland
Through deletion of Rbp-J using Prop1-Cre, we generated animals that survive after birth but lack SOX2+ stem cells and are defective in Notch activation in the pituitary gland. This provided us with an animal model in which to investigate the roles of SOX2+ cells and Notch signaling in postnatal pituitary gland function and homeostasis.
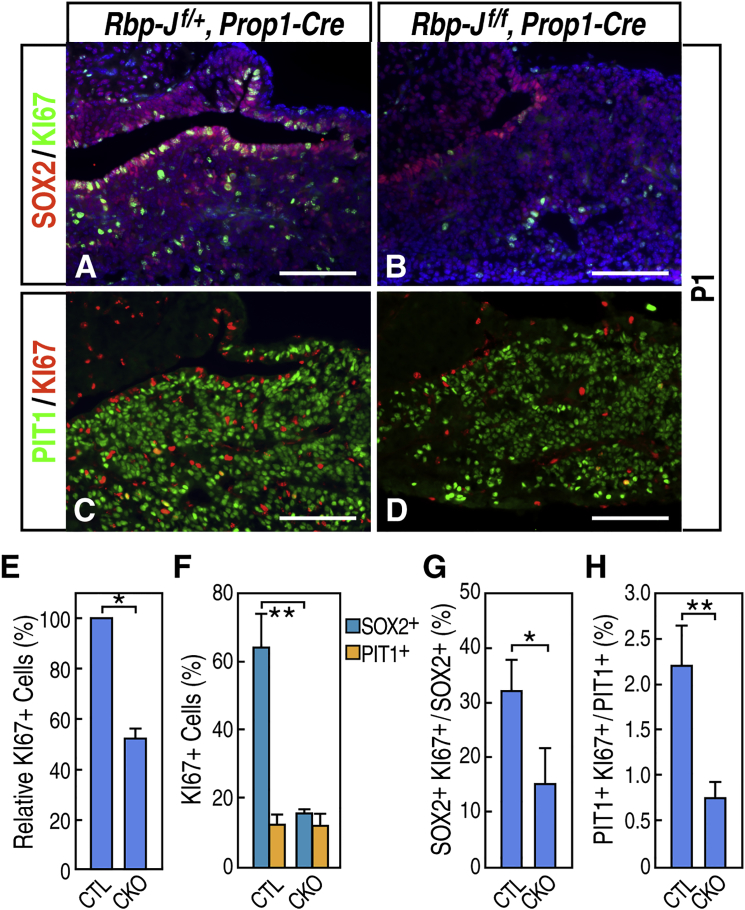
Because pituitary glands undergo expansion after birth, we first examined whether postnatal pituitary stem cells are required for this initial postnatal growth. At P1 in the wild-type pituitary gland, both periluminal stem cells and stem cell clusters in the anterior lobe continued to proliferate, and they represented approximately 64% of all KI67+ proliferating cells in the anterior lobe (Figures 4A and 4F). Meanwhile, after an initial cell-cycle arrest accompanying lineage commitment and terminal differentiation during embryonic development, PIT1+ cells resumed proliferation, and they represented approximately 12% of all KI67+ cells in the anterior lobe (Figures 4C and 4F). By contrast, in the mutant glands, where most of the SOX2+ stem cells were absent, the total number of KI67+ proliferating cells was reduced significantly to 51% of the controls (Figure 4E), of which about 16% represented the remaining SOX2+ cells (Figure 4F). Furthermore, the proportion of SOX2+ cells that were proliferating (SOX2+KI67+/SOX2+) was also decreased greatly (Figure 4G), consistent with the idea that the remaining SOX2+ stem cells failed to maintain self-renewal in the absence of Notch signaling. Interestingly, the proportion of PIT1+ cells that were proliferating (PIT1+KI67+/PIT1+) in the mutants was also decreased significantly (Figure 4H), suggesting a delay of PIT1+ cells in their re-entry into the cell cycle in the mutants. Taken together, these data suggest that SOX2+ stem cells make a significant contribution to neonatal pituitary expansion and that SOX2+ stem cells and Notch signaling are also required to ensure proper timing of re-entry of PIT1+ cells into the cell cycle.
Figure 4.
Both SOX2+ Stem Cells and PIT1+ Cells Exhibit Reduced Proliferation in the Anterior Lobe of Rbp-Jf/f, Prop1-Cre Mutants at P1
(A–D) Double immunofluorescence labeling of SOX2/KI67 (A and B) and PIT1/KI67 (C and D) in wild-type control (A and C) and Rbp-Jf/f, Prop1-Cre mutants (B and D) at P1.
(E) Quantification of KI67+ proliferating cells in the anterior lobe in the control and mutant (CKO) at P1. Data are represented as mean ± SEM (n = 3 mice, ∗p < 0.05).
(F) Quantification of SOX2+ and PIT1+ cells among KI67+ proliferating cells in the control and mutant. Data are represented as mean ± SEM (n = 3 mice, ∗∗p < 0.01).
(G) Quantification of SOX2+ KI67+ cells among SOX2+ cells. Data are represented as mean ± SEM (n = 3 mice, ∗p < 0.05).
(H) Quantification of PIT1+ KI67+ cells among PIT1+ cells. Data are represented as mean ± SEM (n = 3 mice, ∗∗p < 0.01).
Scale bars, 100 μm.
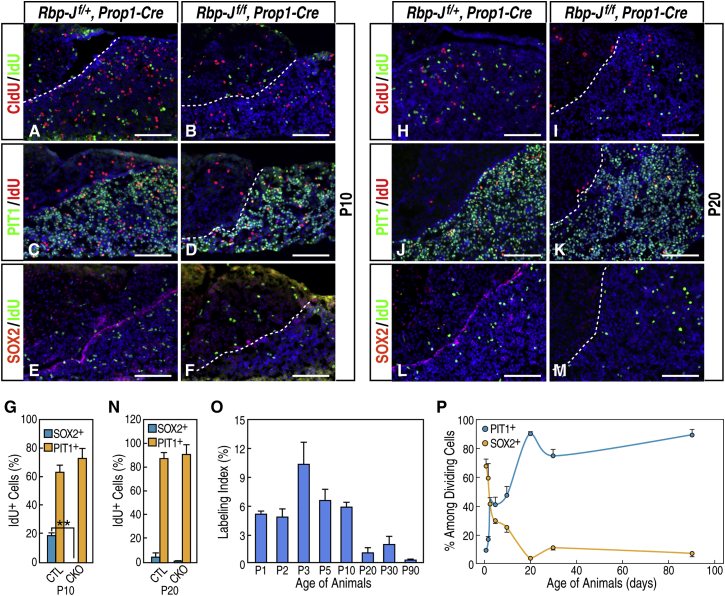
However, despite a progressively diminished postnatal stem cell population and decreased cell proliferation at P1, the mutant pituitary glands continued to proliferate and expand postnatally. At P10, the labeling index in the anterior lobe of the mutant gland is similar to that of the wild-type control, in contrast to P1, when a significant difference was observed (Figure S4). To further examine the proliferation dynamics, we performed a double-thymidine analog incorporation assay by administering 5-chloro-2′-deoxyuridine (CldU) and 5-Iodo-2′-deoxyuridine (IdU) on consecutive days. CldU/IdU double labeling starting at P10 revealed that, in wild-type animals, dividing cells were present in all three lobes of the pituitary gland (Figures 5A–5F; and data not shown). At this stage, 63% of the dividing cells in the anterior lobe were PIT1+ cells (PIT1+ IdU+/IdU+), and a relatively smaller proportion of dividing cells (19%) were SOX2+ stem cells. Similarly, in the mutants, PIT1+ cells continued to proliferate in the absence of postnatal SOX2+ stem cells and represented the majority of dividing cells (72%) (Figure 5G). Therefore, expansion of the postnatal gland at this stage was largely contributed to by proliferation of existing, committed cells rather than pituitary SOX2+ stem cells. Furthermore, double labeling of CldU and IdU revealed that there were barely any detectable CldU+ IdU+ cells in either the anterior lobe or in the periluminal region (Figures 5A and 5B), suggesting that, in contrast to the intestine or other organs with fast cell turnover, there were no transiently amplifying progenitor cells that may contribute to the postnatal expansion of the pituitary gland at this stage. Instead, dividing cells in the pituitary gland appear to enter a resting period and do not immediately reenter the cell cycle. Similar CldU/IdU labeling performed at P20 showed that postnatal SOX2+ stem cells were mostly quiescent and not dividing actively at this stage, whereas PIT1+ cells made up about 87% of the dividing cells in the anterior lobe in both the wild-type and mutants, and no transiently amplifying progenitor cells were detected (Figures 5H–5N).
Figure 5.
Proliferation of SOX2+ Cells Is Diminished Gradually, and PIT1+ Cells Make up the Majority of Proliferating Cells in the Postnatal Pituitary Gland
(A–F) Double immunofluorescence labeling of CldU/IdU (A and B), PIT1/IdU (C and D), and SOX2/IdU (E and F) in the wild-type control (A, C, and E) and Rbp-Jf/f, Prop1-Cre mutants (B, D, and F) at P10.
(G) Quantification of PIT1+ or SOX2+ cells among dividing cells at P10. Data are represented as mean ± SEM (n = 3 mice, ∗∗p < 0.01).
(H–M) Double immunofluorescence labeling of CldU/IdU (H and I), PIT1/IdU (J and K), and SOX2/IdU (L and M) in the wild-type control (H, J, and L) and Rbp-Jf/f, Prop1-Cre mutants (I, K, and M) at P20.
(N) Quantification of PIT1+ or SOX2+ cells among dividing cells at P20. Data are represented as mean ± SEM (n = 3 mice).
(O) Labeling index of the wild-type pituitary glands at different postnatal stages. Data are represented as mean ± SEM (n = 3 mice).
(P) Respective quantification of PIT1+ and SOX2+ cells among IdU+ dividing cells in the anterior lobe of wild-type pituitaries at different postnatal stages. Data are represented as mean ± SEM (n = 3 mice).
Scale bars, 100 μm.
To further characterize cell divisions in postnatal pituitary gland expansion, single IdU labeling at different postnatal time points was performed in wild-type animals. Immunofluorescence assays and further analyses revealed that the overall labeling index in the anterior lobe peaked at P3 and then decreased gradually (Figure 5O). Quantifications of dividing cells among SOX2+ and PIT1+ cells, respectively, revealed that the actively dividing SOX2+ stem cells declined significantly, transitioning from a highly proliferative state in the neonatal stages to near quiescence a few weeks after birth, whereas PIT1+ cells exhibited a temporally delayed wave of proliferation and became mostly quiescent as well (Figures S5A and S5B). In the process of postnatal expansion, PIT1+ cells gradually increased in cell number and, ultimately, represented large proportions of dividing cells in the mature pituitary gland (Figure 5P; Figure S5C).
To examine the contribution of SOX2+ cells in the postnatal pituitary gland, we carried out lineage tracing experiments using Sox2-CreERT2, ROSA26-EYFP mice (Arnold et al., 2011). Tamoxifen was injected for two consecutive days at P1 and P2, and animals were analyzed at P15. Consistent with observations from others (Andoniadou et al., 2013, Rizzoti et al., 2013), we observed that the majority of YFP+ cells remained as SOX2+ stem cells and that only a small percentage of YFP+ stem cells eventually differentiated into distinct pituitary endocrine cells (Figure S5D; unpublished data).
In conclusion, these results, collectively, suggest that SOX2+ stem cells are critical for neonatal pituitary expansion by self-renewal, differentiating into distinct pituitary cell lineages, and that Notch signaling is essential for postnatal expansion largely by maintaining SOX2+ cells and regulating the proper timing of PIT1+ cell re-entry into the cell cycle.
Roles of Pituitary Postnatal Stem Cells in Pituitary Plasticity
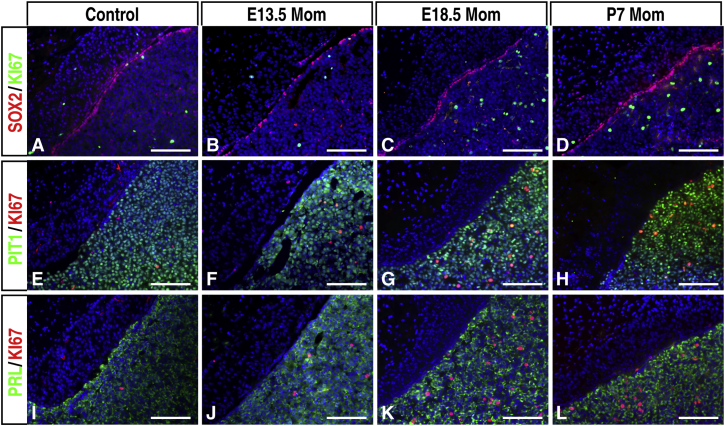
An interesting feature of the pituitary gland is its plasticity. The composition and cell content of the gland changes to meet the physiological demands. One example is that the number of lactotropes in the rat pituitary gland increases 3-fold during pregnancy and lactation (Levy, 2002). It is generally believed that transdifferentiation and increased lactotrope proliferation account for this change, although, in the mouse, no lactotroph transdifferentiation has been observed during pregnancy and lactation (Castrique et al., 2010). However, it is not known whether pituitary stem cells play a role in these processes. We therefore probed the possibility of whether pituitary stem cells, although mostly quiescent during their adult life, might reenter the cell cycle and function in these processes. We examined cell proliferation in the pituitary glands of wild-type pregnant females on days 13 and 18 of pregnancy and day 7 of lactation as well as in age-matched virgin females (Figure 6). Overall, cell proliferation in the pituitary gland increased toward the end of pregnancy at E18.5 and while lactating at P7. Double labeling of SOX2/KI67 and PIT1/KI67 revealed that SOX2+ stem cells did not undergo enhanced proliferation under these conditions. In contrast, PIT1+ cells were still the major proliferating cells in the anterior lobe. Among them, only a very small number of PRL+ KI67+ cells could be identified (Figure 6). Similar proliferation dynamics were observed in mutant pituitary glands (Figure S6). These data suggest that SOX2+ stem cells remain mostly quiescent in these processes and are unlikely to play a significant role during pregnancy and lactation. This is consistent with our observation that the number and size of litters generated from mutant females were not significantly different from those of control wild-type females, and the mutant females did not exhibit any detectable defects in lactation (8.4 ± 0.9/litter for wild-type and 7.7 ± 0.9/litter for mutant animals, mean ± SEM, n = 3 litters, p = 0.12; data not shown). Therefore, we conclude that Notch signaling and Notch-dependent SOX2+ stem cells are not absolutely required for pituitary functions in the process of pregnancy and lactation.
Figure 6.
Characterization of Proliferating Cells in 3-Month-Old Wild-Type Control Mice during Pregnancy and Lactation
(A–L) Double immunofluorescence labeling of SOX2/KI67 (A–D), PIT1/KI67 (E–H), and PRL/KI67 (I–L) at different stages of pregnancy and lactation. Scale bars, 100 μm.
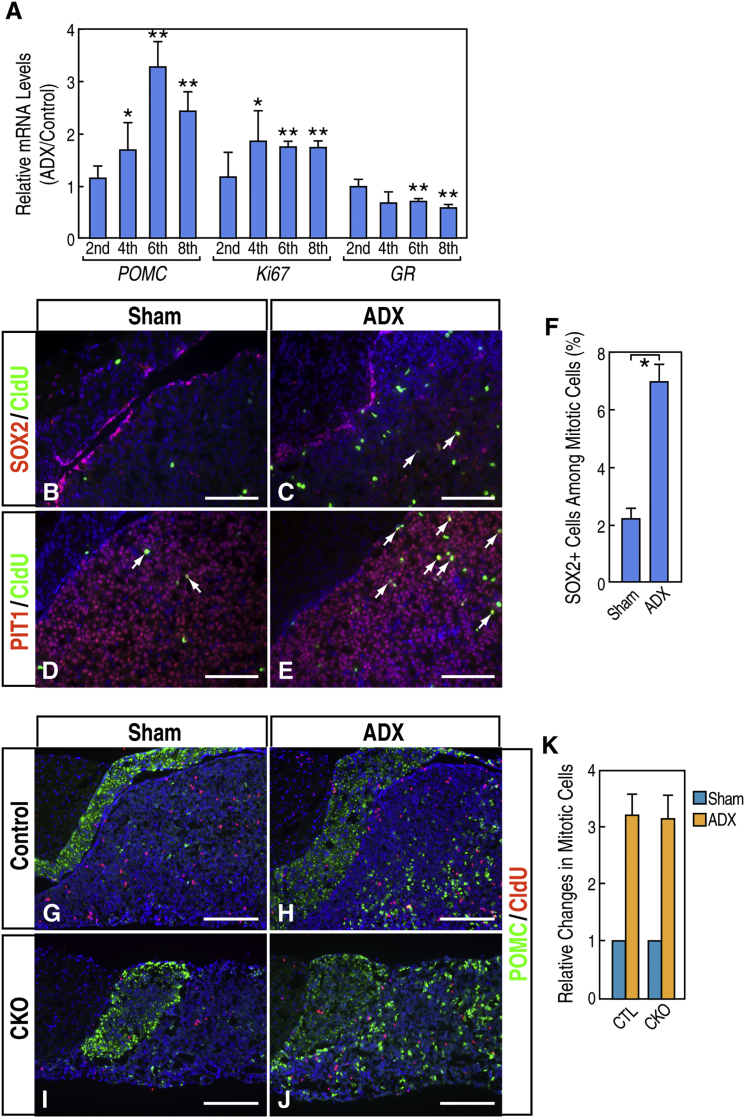
A second example of pituitary plasticity is characterized by the fact that there are increases in the number of proliferating cells and in the number of corticotropes in response to bilateral adrenalectomy (ADX) because of disruption of the negative feedback imposed by glucocorticoid from the target organ, the adrenal gland (Levy, 2002, Nolan and Levy, 2006). qRT-PCR from wild-type pituitary glands after ADX showed increases in the RNA levels of Pomc and the proliferating marker Ki67 (Figure 7A). Interestingly, RNA levels of glucocorticoid receptor (GR) declined gradually, compatible with the idea that there is a disruption of negative feedback upon ADX. We questioned whether postnatal pituitary stem cells make up those proliferating cells after ADX in the pituitary gland. We performed surgery in wild-type animals. Double immunofluorescence labeling of SOX2/CldU in wild-type animals revealed that SOX2+ cells contributed to 2.2% of cells labeled with CldU in sham surgery, and the respective contribution changed to 6.9% upon ADX (Figures 7B–7F). These data suggest that SOX2+ stem cells could be reactivated to proliferate by ADX. However, SOX2+ stem cells were not the only cell types that were stimulated to proliferate upon ADX. PIT1+ cells as well as ACTH+ corticotropes also exhibited increased proliferation, with PIT1+ cells representing the majority of proliferating cells and ACTH+ corticotropes representing a much lower proportion than SOX2+ cells (Figures 7D, 7E, 7G, and 7H; data not shown). Consistently, GR expression was detected in PIT1+ cells (Figure S7). These results suggest that ADX-induced cell proliferation in the pituitary gland is not cell type specific. We also performed similar experiments in both control littermates and the mutant mice. ADX induced an ∼3-fold increase in the number of cells incorporating CldU in the anterior lobe of wild-type pituitary glands. We did not detect a significant deficit in the responses to ADX in the mutant animals with respect to both the increase in the number of proliferating cells and the increase of ACTH+ cells (Figures 7G–7K). These results reveal that ADX can elicit general reactivation of multiple cell types in the pituitary gland and that SOX2+ stem cells make a limited contribution to the overall responses in the pituitary gland.
Figure 7.
SOX2+ Cells Are Mobilized upon Target Organ Ablation but Contribute a Small Percentage of Overall Pituitary Gland Activation
(A) qRT-PCR of Pomc, Ki67, and glucocorticoid receptor (GR) in the pituitary glands after bilateral ADX in wild-type control mice. Data are represented as mean ± SEM (n = 3 mice, ∗p < 0.05, ∗∗p < 0.01).
(B–E) Double immunofluorescence labeling of SOX2/CldU (B and C) and PIT1/CldU (D and E) in sham- (B and D) and ADX-operated (C and E) wild-type pituitary glands. The arrows indicate co-labeling.
(F) Quantification of SOX2+ cells among mitotic cells in sham- and ADX-operated wild-type pituitary glands. Data are represented as mean ± SEM (n = 3 mice, ∗p < 0.05).
(G–J) Double immunofluorescence labeling of POMC/CldU in sham- (G) and ADX-operated (H) control animals and Rbp-Jf/f, Prop1-Cre mutants (CKO, I and J).
(K) Quantification of relative changes in mitotic cells in response to ADX in wild-type control and Rbp-Jf/f, Prop1-Cre mutants. Data are represented as mean ± SEM (n = 3 mice).
Scale bars, 100 μm (B–E) and 200 μm (G–J).
Discussion
Adult stem cells have been identified in the postnatal pituitary gland. These are groups of heterogeneous cells expressing SOX2, SOX9, LHX3, PROP1, E-Cadherin, and NES located either in the marginal zone between the intermediate and anterior lobes or scattered in the anterior lobe (Chen et al., 2009, Fauquier et al., 2008, Garcia-Lavandeira et al., 2009, Gleiberman et al., 2008, Rizzoti, 2010, Vankelecom and Chen, 2014). It has been suggested previously that mouse pituitary adult stem cells are uniquely set aside during embryonic pituitary development and only contribute prominently to postnatal pituitary growth (Gleiberman et al., 2008). In this study, we showed that these stem cells were labeled by the LacZ or GFP reporters in the lineage-tracing experiment using the RP-specific Prop1-Cre line, suggesting that pituitary stem cells are derived from the embryonic RP progenitors and that they continue to proliferate and differentiate to contribute to postnatal pituitary expansion. One common feature of RP progenitors and postnatal stem cells is their expression of the transcription factor SOX2, which has been determined to be expressed in many adult stem cells (Andoniadou et al., 2013, Arnold et al., 2011, Rizzoti et al., 2013).
In this study, we demonstrate that postnatal pituitary SOX2+ stem cells are maintained by the canonical Notch signaling pathway. Our result is consistent with the finding that Notch signaling is essential for the long-term maintenance of neural stem cells in the adult brain hippocampus, where it has been shown that RBP-J is recruited to the Sox2 promoter and activates its expression (Ehm et al., 2010). In the absence of Notch signaling, the pituitary stem cells were depleted gradually in the postnatal pituitary gland. This was accomplished by employing Prop1-Cre, which is expressed in all RP progenitors, but not so early to interfere with the lineage commitment program, in which Notch signaling plays a pivotal role (Zhu et al., 2006). In addition, there was a reduction in the number of SOX2+ cells in mutant mice in which Rbp-J was specifically deleted in SOX2+ cells. Consistent with these data, chemical inhibition of Notch activation in primary culture resulted in a decreased number of pituitary spheres. Taken together, these results reveal that Notch signaling controls the fate choices of RP progenitors in a narrow developmental window, whereas it is required continuously for the maintenance of SOX2+ pituitary stem cells.
During embryonic pituitary organogenesis, SOX2+ cells exhibit a remarkable capacity for proliferation (Figure S1L; Davis et al., 2011), whereas, after birth, these cells remain mitotically active for the initial 3 weeks and then become mostly quiescent after this period. For the first 3 days after birth, they are the dominant cell type undergoing cell division, and their postnatal proliferation makes a significant contribution to pituitary gland expansion. We show that postnatal pituitary expansion can be attributed to successive and overlapping waves of proliferation of SOX2+ stem cells and committed/differentiated cells, as exemplified by PIT1+ cells. How the mitotic properties of SOX2+ stem cells are regulated dynamically during embryonic development, postnatal growth, and subsequent homeostasis remains to be fully elucidated.
In the absence of SOX2+ stem cells and Notch signaling in Rbp-Jf/f, Prop1-Cre mutant mice, the pituitary glands are hypoplastic and dysmorphic. Interestingly, re-entry of PIT1+ lineages into the cell cycle after birth is delayed in the mutant. Consistent with our finding, reduced postnatal pituitary proliferation has been observed in Notch2f/f, Foxg1-Cre mice with limited loss of SOX2+ stem cells and partial loss of Notch signaling (Nantie et al., 2014). We have shown previously that ablating Notch signaling in PIT1+ cells by expressing a dominant-negative form of RBP-J did not result in any detectable phenotype, suggesting that Notch signaling is not required in lineage-committed cells (Zhu et al., 2006). Taken together, these results imply an additional role of Notch signaling during late pituitary development in regulating cell maturation and setting up the gland for postnatal expansion. Interestingly, it has been demonstrated recently that ectopic expression of the activated form of β-catenin in SOX2+ stem cells gives rise to pituitary tumors in a non-cell-autonomous manner (Andoniadou et al., 2013), consistent with the idea that SOX2+ cells can function as a signaling center under specific conditions to have a profound effect in the pituitary gland. It is tempting to speculate that SOX2+ stem cells may additionally contribute to postnatal pituitary expansion by providing a paracrine signal for regulating the proper timing of PIT1+ cell re-entry into the cell cycle.
Tissue homeostasis in the adult stage is maintained by different mechanisms. In fast turnover tissues, e.g., the intestine and epidermis, tissue maintenance is coordinated by tissue-specific stem cells through self-renewal and differentiation processes, whereas initial analysis in slow turnover tissue, e.g., the pancreas, tissue homeostasis can be largely mediated by replication of differentiated cells (Brennand and Melton, 2009, Simons and Clevers, 2011). Our data reveal that SOX2+ stem cells and PIT1+ differentiated cells become essentially quiescent a few weeks after birth and that they divide slowly in the adult pituitary gland. Our findings suggest that, after an initial postnatal expansion period, the adult pituitary gland is maintained by stochastic cellular self-replication rather than stem cell replenishment. Consistent with this notion, genetic lineage tracing of SOX2+ cells in the young adult pituitary gland has shown that SOX2+ labeled cells rarely differentiate into different pituitary lineages and mostly remain as SOX2+ stem cells (Andoniadou et al., 2013, Rizzoti et al., 2013). In addition, we showed that, during pregnancy and lactation, SOX2+ cells are not mobilized readily and not essential for these processes. However, we found that SOX2+ cell can be re-activated following bilateral adrenalectomy when the negative feedback inhibition is eliminated, although they represent only a minor proportion of cells that were mobilized for proliferation, consistent with recent findings (Langlais et al., 2013, Rizzoti et al., 2013). Indeed, ADX responses were not affected significantly in the absence of SOX2+ cells, suggesting that, although SOX2+ cells can be mobilized and make a limited contribution to corticotrope expansion (Rizzoti et al., 2013), they are not an essential component for the functions of a mature gland under the conditions we examined. Interestingly, we observed that PIT1+ cells are also mobilized to proliferate under pregnancy/lactation, target organ ablation, and partial hypophysectomy (unpublished data) and that they constitute a rather large proportion of proliferating cells. These findings raise the intriguing possibility that PIT1+ cells, and potentially other cell types, may function as reserve cells and contribute to pituitary plasticity in response to physiological demands. Indeed, it has been demonstrated recently that differentiated cells of the stomach and lung can function as stem cells and give rise to various cell types of the tissues, revealing enormous plasticity of cells (Stange et al., 2013, Tata et al., 2013). Whether and how PIT1+ cells and other cells in the pituitary gland respond to physiological demands and whether transdifferentiation is involved are interesting questions to be investigated.
Experimental Procedures
Mice
Prop1-Cre transgenic mice were generated by using the 2.2-kb promoter and enhancer region of the Prop1 gene to drive CRE recombinase expression. Rbp-Jf/f mice were provided by Dr. T. Honjo (Tanigaki et al., 2002). Sox2-CreERT2 (Arnold et al., 2011), Rosa26-LacZ reporter Gt(ROSA)26Sortm1Sor (Soriano, 1999), Z/EG (Tg(CAG-Bgeo/GFP) (Novak et al., 2000), and Rosa26-EYFP (Srinivas et al., 2001) mice were obtained from Jackson Laboratory. Noon of the day of the vaginal plug was considered E0.5. For lineage-tracing experiments, tamoxifen was injected at P1 and P2 at 0.075 μg/g. Care and experimentation were carried out in accordance with the Institutional Animal Care and Use Committee of University of California at San Diego.
CldU/IdU Labeling
For CldU and IdU labeling, the animals received one intraperitoneal injection of CldU for 1 day at noon, followed by a similar injection of IdU for 1 day (at 100 μg/g body weight), and were sacrificed 1 day after the last injection. For the postnatal labeling index experiment, the animals were injected with IdU at noon and sacrificed 2 hr later. CldU and IdU were obtained from Sigma.
Cell Counting and Statistical Analysis
At least 2,000 DAPI+ cells from the anterior lobe and periluminal region from at least four comparable fields of different sections were counted manually per sample. Results are presented as mean ± SEM. Two-tailed Student’s t test was used to compare the difference between two sets of values. p ≤ 0.05 was considered to indicate statistical significance (∗p ≤ 0.05, ∗∗p ≤ 0.01).
Acknowledgments
We would like to thank Drs. K. Scully, D. Skowronska-Krawczyk, and C. Lin in the M.G.R. laboratory and Dr. Hoi U for discussions and suggestions and J. Hightower for figure preparation. We thank Dr. N. Justice for ADX surgery training. X.Z. was partially supported by NICHD R03HD060779. M.G.R. is an investigator with the Howard Hughes Medical Institute. This research was supported by a grant from the NIDDK (to M.G.R.).
Published: December 8, 2015
Footnotes
This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
Supplemental Information includes Supplemental Experimental Procedures, seven figures, and one table and can be found with this article online at http://dx.doi.org/10.1016/j.stemcr.2015.11.001.
Contributor Information
Xiaoyan Zhu, Email: xzhu@salk.edu.
Michael G. Rosenfeld, Email: mrosenfeld@ucsd.edu.
Supplemental Information
References
- Andoniadou C.L., Matsushima D., Mousavy Gharavy S.N., Signore M., Mackintosh A.I., Schaeffer M., Gaston-Massuet C., Mollard P., Jacques T.S., Le Tissier P. Sox2(+) stem/progenitor cells in the adult mouse pituitary support organ homeostasis and have tumor-inducing potential. Cell Stem Cell. 2013;13:433–445. doi: 10.1016/j.stem.2013.07.004. [DOI] [PubMed] [Google Scholar]
- Arnold K., Sarkar A., Yram M.A., Polo J.M., Bronson R., Sengupta S., Seandel M., Geijsen N., Hochedlinger K. Sox2(+) adult stem and progenitor cells are important for tissue regeneration and survival of mice. Cell Stem Cell. 2011;9:317–329. doi: 10.1016/j.stem.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennand K., Melton D. Slow and steady is the key to beta-cell replication. J. Cell. Mol. Med. 2009;13:472–487. doi: 10.1111/j.1582-4934.2008.00635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbajo-Pérez E., Watanabe Y.G. Cellular proliferation in the anterior pituitary of the rat during the postnatal period. Cell Tissue Res. 1990;261:333–338. doi: 10.1007/BF00318674. [DOI] [PubMed] [Google Scholar]
- Castrique E., Fernandez-Fuente M., Le Tissier P., Herman A., Levy A. Use of a prolactin-Cre/ROSA-YFP transgenic mouse provides no evidence for lactotroph transdifferentiation after weaning, or increase in lactotroph/somatotroph proportion in lactation. J. Endocrinol. 2010;205:49–60. doi: 10.1677/JOE-09-0414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Crabbe A., Van Duppen V., Vankelecom H. The notch signaling system is present in the postnatal pituitary: marked expression and regulatory activity in the newly discovered side population. Mol. Endocrinol. 2006;20:3293–3307. doi: 10.1210/me.2006-0293. [DOI] [PubMed] [Google Scholar]
- Chen J., Gremeaux L., Fu Q., Liekens D., Van Laere S., Vankelecom H. Pituitary progenitor cells tracked down by side population dissection. Stem Cells. 2009;27:1182–1195. doi: 10.1002/stem.51. [DOI] [PubMed] [Google Scholar]
- Davis S.W., Mortensen A.H., Camper S.A. Birthdating studies reshape models for pituitary gland cell specification. Dev. Biol. 2011;352:215–227. doi: 10.1016/j.ydbio.2011.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehm O., Göritz C., Covic M., Schäffner I., Schwarz T.J., Karaca E., Kempkes B., Kremmer E., Pfrieger F.W., Espinosa L. RBPJkappa-dependent signaling is essential for long-term maintenance of neural stem cells in the adult hippocampus. J. Neurosci. 2010;30:13794–13807. doi: 10.1523/JNEUROSCI.1567-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauquier T., Rizzoti K., Dattani M., Lovell-Badge R., Robinson I.C. SOX2-expressing progenitor cells generate all of the major cell types in the adult mouse pituitary gland. Proc. Natl. Acad. Sci. USA. 2008;105:2907–2912. doi: 10.1073/pnas.0707886105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florio T. Adult pituitary stem cells: from pituitary plasticity to adenoma development. Neuroendocrinology. 2011;94:265–277. doi: 10.1159/000330857. [DOI] [PubMed] [Google Scholar]
- Fu Q., Vankelecom H. Regenerative capacity of the adult pituitary: multiple mechanisms of lactotrope restoration after transgenic ablation. Stem Cells Dev. 2012;21:3245–3257. doi: 10.1089/scd.2012.0290. [DOI] [PubMed] [Google Scholar]
- Fu Q., Gremeaux L., Luque R.M., Liekens D., Chen J., Buch T., Waisman A., Kineman R., Vankelecom H. The adult pituitary shows stem/progenitor cell activation in response to injury and is capable of regeneration. Endocrinology. 2012;153:3224–3235. doi: 10.1210/en.2012-1152. [DOI] [PubMed] [Google Scholar]
- Garcia-Lavandeira M., Quereda V., Flores I., Saez C., Diaz-Rodriguez E., Japon M.A., Ryan A.K., Blasco M.A., Dieguez C., Malumbres M., Alvarez C.V. A GRFa2/Prop1/stem (GPS) cell niche in the pituitary. PLoS ONE. 2009;4:e4815. doi: 10.1371/journal.pone.0004815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleiberman A.S., Michurina T., Encinas J.M., Roig J.L., Krasnov P., Balordi F., Fishell G., Rosenfeld M.G., Enikolopov G. Genetic approaches identify adult pituitary stem cells. Proc. Natl. Acad. Sci. USA. 2008;105:6332–6337. doi: 10.1073/pnas.0801644105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gremeaux L., Fu Q., Chen J., Vankelecom H. Activated phenotype of the pituitary stem/progenitor cell compartment during the early-postnatal maturation phase of the gland. Stem Cells Dev. 2012;21:801–813. doi: 10.1089/scd.2011.0496. [DOI] [PubMed] [Google Scholar]
- Jayakody S.A., Andoniadou C.L., Gaston-Massuet C., Signore M., Cariboni A., Bouloux P.M., Le Tissier P., Pevny L.H., Dattani M.T., Martinez-Barbera J.P. SOX2 regulates the hypothalamic-pituitary axis at multiple levels. J. Clin. Invest. 2012;122:3635–3646. doi: 10.1172/JCI64311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelberman D., Rizzoti K., Lovell-Badge R., Robinson I.C., Dattani M.T. Genetic regulation of pituitary gland development in human and mouse. Endocr. Rev. 2009;30:790–829. doi: 10.1210/er.2009-0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kita A., Imayoshi I., Hojo M., Kitagawa M., Kokubu H., Ohsawa R., Ohtsuka T., Kageyama R., Hashimoto N. Hes1 and Hes5 control the progenitor pool, intermediate lobe specification, and posterior lobe formation in the pituitary development. Mol. Endocrinol. 2007;21:1458–1466. doi: 10.1210/me.2007-0039. [DOI] [PubMed] [Google Scholar]
- Langlais D., Couture C., Kmita M., Drouin J. Adult pituitary cell maintenance: lineage-specific contribution of self-duplication. Mol. Endocrinol. 2013;27:1103–1112. doi: 10.1210/me.2012-1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy A. Physiological implications of pituitary trophic activity. J. Endocrinol. 2002;174:147–155. doi: 10.1677/joe.0.1740147. [DOI] [PubMed] [Google Scholar]
- Nantie L.B., Himes A.D., Getz D.R., Raetzman L.T. Notch signaling in postnatal pituitary expansion: proliferation, progenitors, and cell specification. Mol. Endocrinol. 2014;28:731–744. doi: 10.1210/me.2013-1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan L.A., Levy A. A population of non-luteinising hormone/non-adrenocorticotrophic hormone-positive cells in the male rat anterior pituitary responds mitotically to both gonadectomy and adrenalectomy. J. Neuroendocrinol. 2006;18:655–661. doi: 10.1111/j.1365-2826.2006.01459.x. [DOI] [PubMed] [Google Scholar]
- Novak A., Guo C., Yang W., Nagy A., Lobe C.G. Z/EG, a double reporter mouse line that expresses enhanced green fluorescent protein upon Cre-mediated excision. Genesis. 2000;28:147–155. [PubMed] [Google Scholar]
- Olson L.E., Tollkuhn J., Scafoglio C., Krones A., Zhang J., Ohgi K.A., Wu W., Taketo M.M., Kemler R., Grosschedl R. Homeodomain-mediated beta-catenin-dependent switching events dictate cell-lineage determination. Cell. 2006;125:593–605. doi: 10.1016/j.cell.2006.02.046. [DOI] [PubMed] [Google Scholar]
- Raetzman L.T., Ross S.A., Cook S., Dunwoodie S.L., Camper S.A., Thomas P.Q. Developmental regulation of Notch signaling genes in the embryonic pituitary: Prop1 deficiency affects Notch2 expression. Dev. Biol. 2004;265:329–340. doi: 10.1016/j.ydbio.2003.09.033. [DOI] [PubMed] [Google Scholar]
- Raetzman L.T., Cai J.X., Camper S.A. Hes1 is required for pituitary growth and melanotrope specification. Dev. Biol. 2007;304:455–466. doi: 10.1016/j.ydbio.2006.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzoti K. Adult pituitary progenitors/stem cells: from in vitro characterization to in vivo function. Eur. J. Neurosci. 2010;32:2053–2062. doi: 10.1111/j.1460-9568.2010.07524.x. [DOI] [PubMed] [Google Scholar]
- Rizzoti K., Akiyama H., Lovell-Badge R. Mobilized adult pituitary stem cells contribute to endocrine regeneration in response to physiological demand. Cell Stem Cell. 2013;13:419–432. doi: 10.1016/j.stem.2013.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons B.D., Clevers H. Strategies for homeostatic stem cell self-renewal in adult tissues. Cell. 2011;145:851–862. doi: 10.1016/j.cell.2011.05.033. [DOI] [PubMed] [Google Scholar]
- Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat. Genet. 1999;21:70–71. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- Srinivas S., Watanabe T., Lin C.S., William C.M., Tanabe Y., Jessell T.M., Costantini F. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev. Biol. 2001;1:4. doi: 10.1186/1471-213X-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stange D.E., Koo B.K., Huch M., Sibbel G., Basak O., Lyubimova A., Kujala P., Bartfeld S., Koster J., Geahlen J.H. Differentiated Troy+ chief cells act as reserve stem cells to generate all lineages of the stomach epithelium. Cell. 2013;155:357–368. doi: 10.1016/j.cell.2013.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tando Y., Fujiwara K., Yashiro T., Kikuchi M. Localization of Notch signaling molecules and their effect on cellular proliferation in adult rat pituitary. Cell Tissue Res. 2013;351:511–519. doi: 10.1007/s00441-012-1532-3. [DOI] [PubMed] [Google Scholar]
- Tanigaki K., Han H., Yamamoto N., Tashiro K., Ikegawa M., Kuroda K., Suzuki A., Nakano T., Honjo T. Notch-RBP-J signaling is involved in cell fate determination of marginal zone B cells. Nat. Immunol. 2002;3:443–450. doi: 10.1038/ni793. [DOI] [PubMed] [Google Scholar]
- Taniguchi Y., Kominami R., Yasutaka S., Kawarai Y. Proliferation and differentiation of pituitary corticotrophs during the fetal and postnatal period: a quantitative immunocytochemical study. Anat. Embryol. (Berl.) 2000;201:229–234. doi: 10.1007/s004290050313. [DOI] [PubMed] [Google Scholar]
- Taniguchi Y., Yasutaka S., Kominami R., Shinohara H. Proliferation and differentiation of pituitary somatotrophs and mammotrophs during late fetal and postnatal periods. Anat. Embryol. (Berl.) 2001;204:469–475. doi: 10.1007/s429-001-8003-x. [DOI] [PubMed] [Google Scholar]
- Taniguchi Y., Yasutaka S., Kominami R., Shinohara H. Proliferation and differentiation of thyrotrophs in the pars distalis of the rat pituitary gland during the fetal and postnatal period. Anat. Embryol. (Berl.) 2001;203:249–253. doi: 10.1007/s004290100161. [DOI] [PubMed] [Google Scholar]
- Taniguchi Y., Yasutaka S., Kominami R., Shinohara H. Proliferation and differentiation of rat anterior pituitary cells. Anat. Embryol. (Berl.) 2002;206:1–11. doi: 10.1007/s00429-002-0271-8. [DOI] [PubMed] [Google Scholar]
- Tata P.R., Mou H., Pardo-Saganta A., Zhao R., Prabhu M., Law B.M., Vinarsky V., Cho J.L., Breton S., Sahay A. Dedifferentiation of committed epithelial cells into stem cells in vivo. Nature. 2013;503:218–223. doi: 10.1038/nature12777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vankelecom H., Chen J. Pituitary stem cells: where do we stand? Mol. Cell. Endocrinol. 2014;385:2–17. doi: 10.1016/j.mce.2013.08.018. [DOI] [PubMed] [Google Scholar]
- Vankelecom H., Gremeaux L. Stem cells in the pituitary gland: A burgeoning field. Gen. Comp. Endocrinol. 2010;166:478–488. doi: 10.1016/j.ygcen.2009.11.007. [DOI] [PubMed] [Google Scholar]
- Ward R.D., Davis S.W., Cho M., Esposito C., Lyons R.H., Cheng J.F., Rubin E.M., Rhodes S.J., Raetzman L.T., Smith T.P., Camper S.A. Comparative genomics reveals functional transcriptional control sequences in the Prop1 gene. Mamm. Genome. 2007;18:521–537. doi: 10.1007/s00335-007-9008-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X., Zhang J., Tollkuhn J., Ohsawa R., Bresnick E.H., Guillemot F., Kageyama R., Rosenfeld M.G. Sustained Notch signaling in progenitors is required for sequential emergence of distinct cell lineages during organogenesis. Genes Dev. 2006;20:2739–2753. doi: 10.1101/gad.1444706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X., Gleiberman A.S., Rosenfeld M.G. Molecular physiology of pituitary development: signaling and transcriptional networks. Physiol. Rev. 2007;87:933–963. doi: 10.1152/physrev.00006.2006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.