A methyl jasmonate-dependent MYB-ZML regulatory mechanism links wounding stress to the derepression of lignin genes in maize.
Abstract
Lignin is an essential polymer in vascular plants that plays key structural roles in vessels and fibers. Lignification is induced by external inputs such as wounding, but the molecular mechanisms that link this stress to lignification remain largely unknown. In this work, we provide evidence that three maize (Zea mays) lignin repressors, MYB11, MYB31, and MYB42, participate in wound-induced lignification by interacting with ZML2, a protein belonging to the TIFY family. We determined that the three R2R3-MYB factors and ZML2 bind in vivo to AC-rich and GAT(A/C) cis-elements, respectively, present in a set of lignin genes. In particular, we show that MYB11 and ZML2 bind simultaneously to the AC-rich and GAT(A/C) cis-elements present in the promoter of the caffeic acid O-methyl transferase (comt) gene. We show that, like the R2R3-MYB factors, ZML2 also acts as a transcriptional repressor. We found that upon wounding and methyl jasmonate treatments, MYB11 and ZML2 proteins are degraded and comt transcription is induced. Based on these results, we propose a molecular regulatory mechanism involving a MYB/ZML complex in which wound-induced lignification can be achieved by the derepression of a set of lignin genes.
INTRODUCTION
Lignin is essential in vascular plants and its deposition in the secondary cell walls improves the hydrophobicity of vessels, reinforces the fibers, and acts as a physical barrier against pathogen attacks (Sarkanen and Ludwig, 1971; Carpita and Gibeaut, 1993; Jouanin et al., 2000; Boerjan et al., 2003). Several studies have identified different regulators of lignin biosynthesis in grasses (reviewed in Gray et al., 2012), such as maize (Zea mays) MYB31 and MYB42, two subgroup 4 R2R3-MYB transcription factors that act as repressors of the lignin pathway (Fornalé et al., 2006, 2010; Sonbol et al., 2009). Despite their different roles in lignin regulation, both factors repress the maize caffeic acid O-methyl transferase (comt) gene (AC196475). Mutations in this gene have been shown to produce the brown midrib3 phenotype (Vignols et al., 1995; Fornalé et al., 2006, 2010). The expression of maize comt is induced by wounding (Capellades et al., 1996) through a mechanism that remains unknown, while it has been demonstrated that the wound induction of the Arabidopsis thaliana COMT is CORONATINE-INSENSITIVE PROTEIN1 COI1 dependent (Reymond et al., 2000).
Plants respond to wounding by triggering the induction of lignification in tissues that normally do not accumulate this polymer (Vance et al., 1980; Lawton and Lamb, 1987; Rogers and Campbell, 2004). Consequently, several genes of the lignin pathway are induced by wounding and/or methyl jasmonate (MeJA) (Ellard-Ivey and Douglas, 1996; Bell-Lelong et al., 1997; Mizutani et al., 1997; Ehlting et al., 1999; Reymond et al., 2000; Devoto et al., 2005; Pauwels et al., 2008). In addition, the induction of MeJA cascade has been positively correlated with an increase in lignin content in several species, such as Bryonia dioica (Kaiser et al., 1994), Cassia tora (Xue et al., 2008), and rice (Oryza sativa; Tianpei et al., 2015).
One of the most important groups of proteins involved in wounding/MeJA responses is the TIFY family (Vanholme et al., 2007). TIFYs comprise four subgroups of proteins: ZML, TIFY, PPD, and JASMONATE ZIM domain (JAZ) proteins. JAZ proteins are coreceptors of the hormone and targets of the SCF(COI1) complex and function as negative regulators of the jasmonate signaling pathway (Chini et al., 2007; Thines et al., 2007). Since their discovery in Arabidopsis, JAZ proteins have been implicated in protein-protein interactions with multiple transcription factors, such as the basic helix-loop-helix factor MYC2 (Lorenzo et al., 2004; Chini et al., 2007). More recent studies in Arabidopsis have shown that the activity of some R2R3-MYB transcription factors is regulated through their interaction with JAZ proteins. Upon MeJA treatment, R2R3-MYBs are released by the MeJA-induced degradation of the JAZ proteins, and the target genes are subsequently activated (Qi et al., 2011; Song et al., 2011). Within the ZML subfamily, Arabidopsis ZML1 and ZML2 can bind DNA, suggesting that these proteins can act as transcriptional regulators (Shaikhali et al., 2012). How ZML factors cooperate with other regulators to activate or repress gene expression and to what extent protein degradation is part of the regulatory function of ZML factors remain unknown.
Here, we present a regulatory mechanism by which the expression of lignin genes can be induced upon wounding/MeJA by the action of MYB/ZML transcriptional repressors in maize. In particular, we showed that the jasmonate signaling cascade in maize triggers the degradation of MYB11 and ZML2, leading to the derepression of the lignin comt gene. The regulatory mechanism described for MYB11 can be extended to the lignin repressors MYB31 and MYB42. Our data further show that these MYB/ZML proteins also regulate other genes of the lignin biosynthetic pathway.
RESULTS
Maize MYB11 Binds to the comt Promoter in Vivo and Represses Its Expression
Based on sequence similarity with MYB31 and MYB42, two transcriptional repressors of comt (Fornalé et al., 2006, 2010), we identified myb11 (GRMZM2G000818) as a gene encoding a subgroup 4 R2R3-MYB factor (Supplemental Figure 1). Gene expression analyses revealed that myb11 is expressed at various stages of maize development, both in roots and in the aerial parts of the plant. In addition, myb11 is induced 1 h after wounding (Supplemental Figure 1).
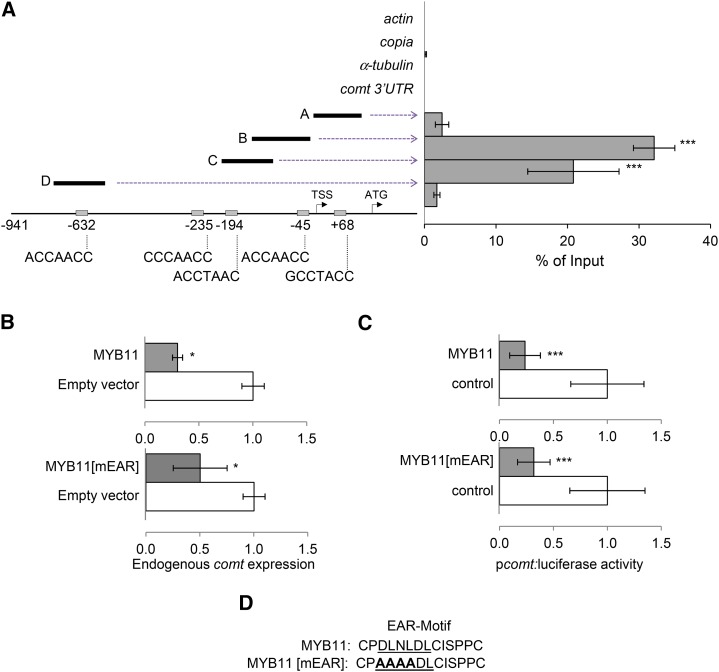
MYB31 and MYB42 repress comt expression in vivo (Fornalé et al., 2006, 2010). In the case of MYB31, this repression occurs through its binding to a typical AC-rich MYB motif present in the comt promoter (Fornalé et al., 2006). Therefore, we raised specific antibodies against MYB11 (Supplemental Figure 2) to investigate whether this factor is also able to bind to comt by chromatin immunoprecipitation (ChIP) experiments using actin, copia, and α-tubulin as negative controls. Our results demonstrate that MYB11 binds in vivo to the comt gene promoter, preferentially to sequences within the first 250 bp from the transcription start site containing the AC-rich MYB binding motifs (Figure 1A).
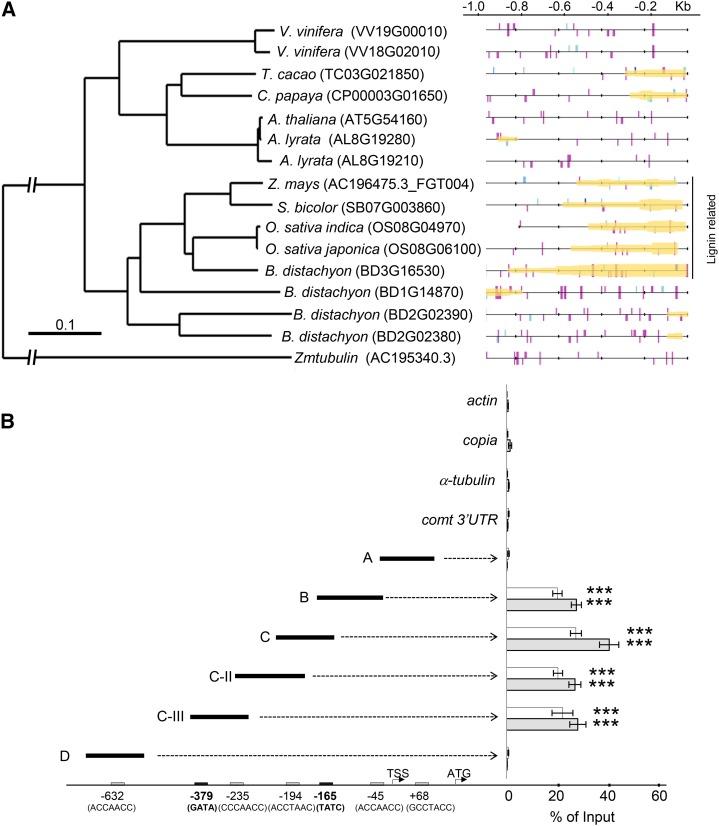
Figure 1.
Maize MYB11 Binds to the comt Promoter to Repress Its Expression.
(A) ChIP-qPCR analyses of MYB11 binding to the comt promoter. Fragments A, B, C, and D represent the different comt promoter regions analyzed. The negative controls were actin, copia, α-tubulin, and comt 3′UTR. The position and sequence of the five AC-rich elements present in the comt promoter or 5′UTR are indicated on the bottom of the scheme. Results are represented as percentage of input, and the error bars indicate the se of the data obtained from three independent biological replicates. Statistical analysis of differences between fragments of comt promoter and actin, copia, α-tubulin, and comt 3′UTR was performed using Student’s t test (***P < 0.005).
(B) Effect of MYB11 and MYB11[mEAR] on comt mRNA accumulation. Maize protoplasts were transiently transformed with p35S:MYB11:C-GFP (top panel) or p35S:MYB11[mEAR]:C-GFP (bottom panel) and the empty vector (p35S:C-GFP) as control. comt gene expression was measured by qPCR using actin as internal control. Data are the mean of three independent transformations. Error bars indicate the se. Statistical analysis of differences between samples was performed using the Student’s t test (*P < 0.05).
(C) Effect of MYB11 and MYB11[mEAR] on comt promoter-driven luciferase expression. Maize protoplasts were transiently cotransformed with p35S:MYB11:C-GFP (top panel), p35S:MYB11[mEAR]:C-GFP (bottom panel), or 35S:C-GFP and Luciferase fused to the comt promoter (pcomt:luciferase). Transactivation assays were done in biological triplicates, and data were normalized for Renilla activity. Error bars indicate the se. Statistical analysis of differences between samples was performed using the Student’s t test (***P < 0.005).
(D) The mutated EAR motif of MYB11.
To elucidate whether MYB11 regulates the expression of comt, maize protoplasts were transformed to transiently express the MYB11 protein fused to GFP. The subsequent qRT-PCR analyses showed that comt mRNA accumulation was reduced (Figure 1B). Similar repression was observed when maize protoplasts were cotransformed with MYB11-GFP and the luciferase gene under the control of the comt promoter (pcomt:luciferase) (Figure 1C).
MYB11 belongs to subgroup 4 of the R2R3-MYB family, whose characteristic feature is the presence of an ethylene-responsive element binding factor-associated amphiphilic repression (EAR) motif in the C-terminal portion of the protein (Kagale and Rozwadowski, 2011). To establish whether the EAR motif is essential for MYB11 repression activity, we mutated this motif (MYB11-mEAR, Figure 1D) and investigated the capacity of the mutated protein to repress the expression of comt in maize protoplasts. Analyses of endogenous comt and pcomt:luciferase expression indicate that MYB11-mEAR maintains its capacity to repress comt expression (Figures 1B and 1C), suggesting that this mutated EAR motif does not significantly affect the transcriptional repression activity of MYB11.
MYB11 and ZML2 Are Degraded upon Wounding
As MYB11 represses comt gene expression by directly binding its promoter and comt is also induced by wounding (Capellades et al., 1996; Fornalé et al., 2006), we investigated whether the main function of MYB11 could be associated with the regulation of lignin biosynthesis in response to this stress.
Some proteins belonging to the TIFY family can interact with R2R3-MYB factors to modulate their function (Qi et al., 2011; Song et al., 2011). As members of the TIFY family regulate wounding/MeJA responses (Vanholme et al., 2007), we investigated whether maize TIFY proteins can regulate the function of MYB11 upon wounding. Within the maize TIFY family, we found three proteins that belong to the ZML subfamily, ZML1, ZML2, and ML3 (Bai et al., 2011) (Supplemental Figure 3A). We surveyed the expression profile of each of the zml genes using the qTeller bioinformatics tool and focused on zml2 (GRMZM2G058479), as it was the one with the most similar gene expression pattern to myb11 (Supplemental Figure 3C) (Sekhon et al., 2013). In addition, as ChIP-qPCR with MYB11 was done in 9-d-old maize samples, we also tested the expression of these three genes in this developmental stage. Our results indicate that zml2 is the main (if not the only) zml gene expressed at this stage (Supplemental Figure 3D).
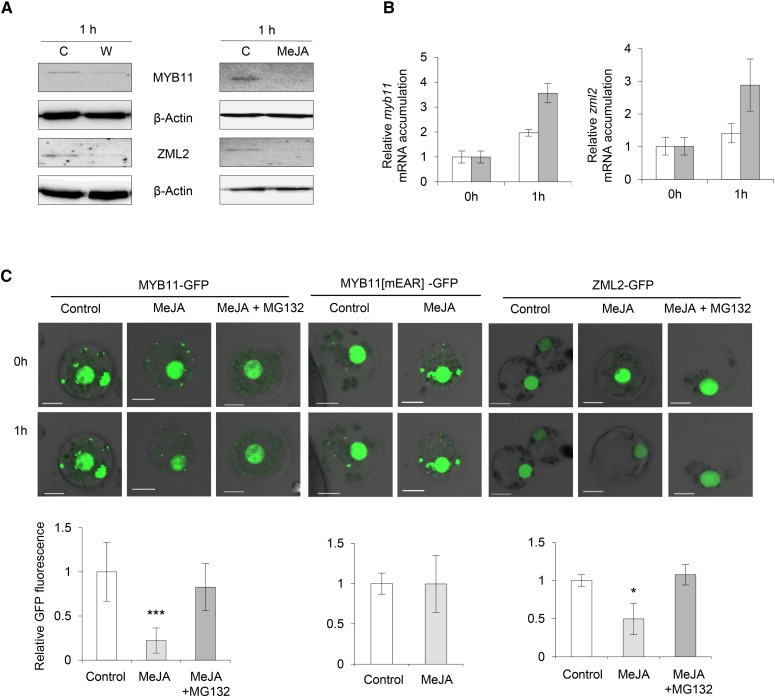
We raised specific antibodies against ZML2 (Supplemental Figure 4) and investigated whether MYB11 and ZML2 protein levels are affected by wounding or MeJA treatment in 9-d-old maize leaves. Immunoblot analysis showed that MYB11 and ZML2 levels decreased 1 h after treatments (Figure 2A). However, the decrease observed after MeJA treatment was not due to a reduced mRNA accumulation of these two genes (Figure 2B), indicating that MeJA may trigger the degradation of MYB11 and ZML2. Therefore, we transiently expressed MYB11 and ZML2 fused to GFP in maize protoplasts (MYB11-GFP and ZML2-GFP, respectively) and GFP alone as a control (Figure 2C; Supplemental Figure 5B). The fluorescence from these two fusion proteins was severely decreased after MeJA treatment, and this decrease did not occur in the presence of the proteasome inhibitor MG132 (Figure 2C). These results suggest that the decrease in the accumulation of MYB11 and ZML2 is due to degradation, likely mediated by the proteasome. Interestingly, when we assayed MYB11 with the mutated EAR domain fused to GFP (MYB11-mEAR-GFP) in maize protoplasts, we found that the MeJA-induced degradation was abolished, suggesting that the EAR motif plays an important role in the proteasomal degradation of MYB11 (Figure 2C).
Figure 2.
MYB11 and ZML2 Proteins Are Degraded by Wounding and MeJA.
(A) Immunodetection of MYB11 and ZML2 in protein extracts from 9-d-old maize leaves using anti-MYB11 and anti-ZML2 antibodies. Protein levels were determined 1 h after wounding (W, wounded samples; C, control samples) and after 1 h of 0.01% MeJA treatments using 0.01% DMSO as a control (C). β-Actin was used as protein loading control.
(B) qRT-PCR analysis of myb11 and zml2 expression in 9-d-old maize leaves from plants treated for 1 h with 25 µM MeJA or 0.01% DMSO. actin was used as internal control gene. White and gray bars refer to control and MeJA-treated samples, respectively. Error bars indicate the se of the data obtained from three independent biological replicates.
(C) Effect of MeJA on MYB11 and ZML2 stability in maize protoplasts transiently transformed with p35S:MYB11:C-GFP, p35S:MYB11[mEAR]:C-GFP, and p35:ZML2:C-GFP. Samples were treated 1 h with 25 µM MeJA or 25 µM MeJA plus 5 µM MG132. GFP quantification is shown at the bottom of each panel. Assays were done in biological triplicates and data were normalized to T0. Error bars indicate the sd (n = 3). Statistical analysis of differences between samples was performed using the Student’s t test (***P < 0.005 and *P < 0.05). Bars = 10 μm.
MYB11 Interacts with ZML2
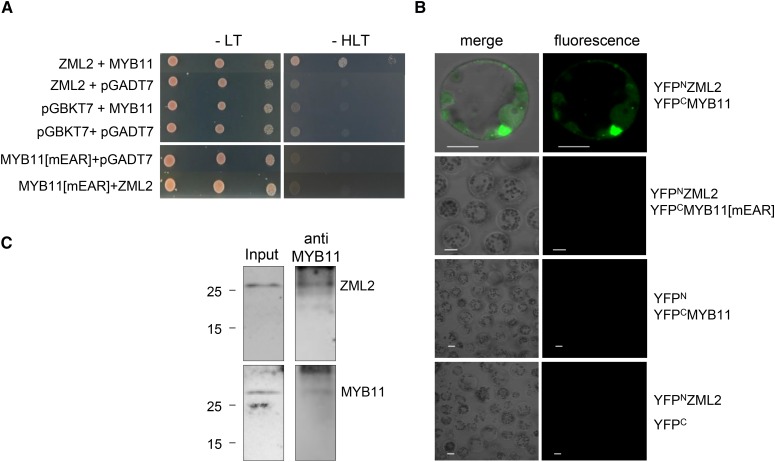
Since myb11 and zml2 have similar expression patterns, and MeJA promotes the degradation of both proteins, we investigated whether they could physically interact in yeast two-hybrid mating assays. MYB11 and ZML2 proteins interacted to allow yeast growth on selective plates and thus could be part of a protein complex (Figure 3A). However, this interaction was disrupted when the EAR motif of MYB11 was mutated, suggesting that this motif is essential for protein-protein interaction (Figure 3A). The specific interaction of these two proteins was also analyzed by bimolecular fluorescence complementation (BiFC) assays. When maize protoplasts were transfected to express MYB11 and ZML2 fused to the C-terminal and N-terminal part of YFP, respectively, the YFP fluorescence was reconstituted, indicating the occurrence of a physical interaction between these two factors (Figure 3B; Supplemental Figure 5C). However, YFP fluorescence was not reconstituted when the EAR motif of MYB11 was mutated (Figure 3B). Additionally, in vivo coimmunoprecipitation (CoIP) assays were performed using maize protein extracts and MYB11 antibodies. The immunoprecipitated samples were analyzed by immunoblot, revealing the presence of ZML2 (Figure 3C; Supplemental Figure 5A). These results indicate that MYB11 and ZML2 can interact in vivo.
Figure 3.
MYB11 Interacts with ZML2 through Its EAR Motif.
(A) Yeast two-hybrid mating assays were used to detect interaction between ZML2 and MYB11 or MYB11[mEAR] proteins. pGBKT7 vector and pGADT7 empty vectors were used as controls.
(B) Determination of the interactions between MYB11 or MYB11[mEAR] and ZML2 by BiFC assay. MYB11 or MYB11[mEAR] was fused to the C-terminal fragment of YFP (YFPC), and ZML2 was fused to the N-terminal fragment of YFP (YFPN). Both constructs were coinfiltrated into maize protoplasts. Empty vectors (YFPN and YFPC) were used as controls. Bars = 10 μm.
(C) CoIP of ZML2 and MYB11. The presence of ZML2 and MYB11 was detected by immunoblot analyses using ZML2 and MYB11 antibodies. Input extracts from 9-d-old maize leaves were immunoprecipitated with MYB11 antibodies, and ZML2 was detected by immunoblot. The presence of MYB11 in the immunoprecipitated samples was also detected as a control.
ZML2 Binds to the GAT(A/C) cis-Regulatory Elements in comt
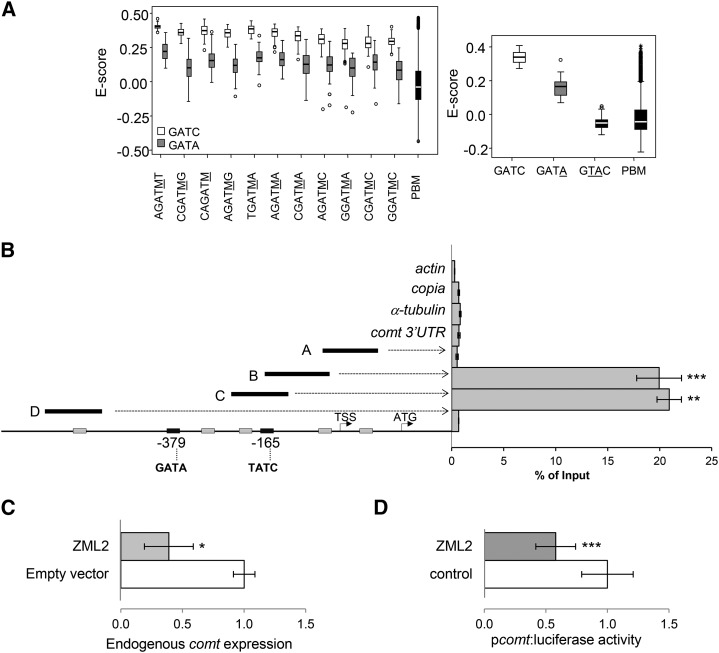
Since ZML2 has a putative GATA-zinc finger domain (Supplemental Figure 3B), we investigated whether this protein is able to bind DNA. Using an in vitro protein binding microarray (PBM) assay containing all possible double-stranded 6-mers (Godoy et al., 2011), we determined the DNA binding specificity of ZML2. We obtained significant differences for the sequences containing the consensus sequence GAT(A/C). The highest signal intensities and E-scores were obtained for the core 5′-GATC-3′, a medium to low affinity was observed for the 5′-GATA-3′ element, and no binding affinity was observed for an irrelevant element (5′-GTAC-3′) (Figure 4A).
Figure 4.
ZML2 Binds the GATA cis-Elements of the Maize comt Promoter in Vivo.
(A) Determination of the DNA binding specificity of ZML2 in vitro using protein binding microarrays. Left: box plot of enrichment scores (E-scores) of all the possible 6-mers containing the 4-mer core GATA (gray) or GATC (white). Underlined M corresponds to C or A. Right: box plot of E-scores of all the possible 6-mers containing the 4-mer core indicated. Boxes represent quartiles 25 to 75%. Horizontal line represents the median of the distribution (quartile 50%). Bars indicate quartiles 1 to 25% (above) and 75 to 100% (below), and dots denote outliers of the distribution. PBM indicates the distribution of E-scores of all the possible 6-mers represented in the microarray.
(B) ChIP-qPCR analyses of ZML2 binding to the comt gene promoter. Fragments A, B, C, and D represent the comt promoter regions analyzed. actin, copia, α-tubulin, and comt 3′UTR were used as a controls. The position of the two GATA-rich elements is indicated at the bottom. Results are represented as percentage of input. Error bars indicate the se of the data obtained from three biological replicates. Statistical analysis of differences between fragments of comt promoter and internal negative controls was performed using Student’s t test (**P < 0.01 and ***P < 0.005).
(C) Effect of ZML2 on the expression of the endogenous comt gene. Maize protoplasts were transformed with p35S:ZML2:C-GFP and the empty vector (p35S:C-GFP) as control. comt expression was measured by qPCR using actin as control. Data are the means of three independent transformations. The error bars indicate the se. Statistical analysis of differences between samples was performed using the Student’s t test (*P < 0.05).
(D) Effect of ZML2 on the comt gene promoter-driven luciferase expression (pcomt:luciferase). Maize protoplasts were cotransformed with p35S:ZML2:C-GFP or 35S:C-GFP and pcomt:luciferase. Transactivation assays were done in biological triplicates, and data were normalized for Renilla activity. Error bars indicate the se. Statistical analysis of differences between samples was performed using the Student’s t test (*P < 0.05 and ***P < 0.005).
The maize comt promoter contains two GATA cis-elements (Figure 4B). Thus, we investigated whether ZML2 can bind directly to one or both of these elements in this promoter in vivo. ChIP assays showed that ZML2 binds to the comt gene promoter in a region containing a GATA cis-element (fragments B and C), while no enrichment was observed in other fragments of comt lacking this cis-element or in the negative controls (Figure 4B).
To elucidate whether ZML2 regulates the expression of comt, maize protoplasts were transiently transformed to express GFP alone or ZML2 fused to GFP and the expression of comt was measured by qRT-PCR. The results indicated that the expression of the endogenous comt gene is reduced in presence of high levels of ZML2 (Figure 4C). Similar results were obtained when maize protoplasts were cotransformed with ZML2-GFP and pcomt:luciferase as a reporter gene (Figure 4D), indicating that ZML2, like MYB11, acts as a transcriptional repressor of the maize comt gene.
MYB11 and ZML2 Bind Simultaneously to the comt Promoter
Matrix-Scan software (http://rsat.ulb.ac.be/) was used to predict putative cis-regulatory modules (CRMs) to establish whether the 5′ region of the comt genes of different plant species were enriched in the AC-rich (Supplemental Figure 6A) and GAT(A/C) motifs recognized by MYB11 and ZML2, respectively. Our results revealed that the comt promoters of grasses are enriched in AC-GAT(A/C) cis-regulatory elements, suggesting the existence of a conserved regulatory module (Figure 5A).
Figure 5.
MYB11 and ZML2 Bind Simultaneously to a AC-GAT(A/C) CRM That Is Present in the comt Promoter of Maize and Other Plant Species.
(A) Phylogenetic tree of COMT from several species. On the right, predictions of AC and GAT(A/C) elements and CRERs in the comt promoter of different species. AC elements are represented by lines in different shades of blue, and GAT(A/C) boxes are shown as purple lines. Predicted cis-regulatory modules are shaded in yellow.
(B) Sequential chromatin immunoprecipitation (ChIP-reChIP) using antibodies against MYB11 and ZML2. qPCR assays were performed for fragments A, B, C, C-II, C-III, and D of the comt promoter and for actin, copia, α-tubulin, and comt 3′UTR as a control. AC (gray boxes) and GATA (black boxes) elements in the maize comt promoter or 5′UTR are indicated at the bottom. Gray bars refer to ChIP-reChIP performed using MYB11 (ChIP) and ZML2 antibodies (reChIP). White bars refer to control ChIP-reChIP using ZML2 antibodies only. Results are represented as percentage of input. Error bars indicate the se obtained from three independent biological replicates. Statistical analysis was performed using Student’s t test (***P < 0.005).
Since our protein-protein interaction results indicate that MYB11 and ZML2 can be part of the same protein complex and both bind the comt promoter in vivo, we tested whether MYB11 and ZML2 bind to these promoter regions in the same cells simultaneously using serial ChIP analyses. In a first step, we performed two control assays by sequentially immunoprecipitating chromatin from maize leaves using (1) anti-MYB11 antibodies (ChIP) and then the preimmune serum (reChIP) and (2) anti-ZML2 antibodies to perform both ChIP and reChiP (Supplemental Figure 6B). These results were compared with those from sequential immunoprecipitation performed using anti-MYB11 (ChIP) and anti-ZML2 (reChIP) antibodies by analyzing the relative enrichment of fragment C of the maize comt promoter by qPCR, using actin as a control (Xie and Grotewold, 2008). No enrichment was observed when reChIP was performed using preimmune serum, indicating that the C fragment ChIPed by anti-MYB11 antibodies was not retained when the sample was unspecifically reChIPed. By contrast, the significant enrichment observed when anti-ZML2 antibodies were used for ChIP and reChIP suggests that these antibodies are suitable to perform the reChIP step of this assay. Finally, the significant enrichment obtained using anti-MYB11 and anti-ZML2 antibodies gave a first indication that these two factors bind simultaneously to the maize comt promoter (Supplemental Figure 6B).
Thus, we investigated the simultaneous binding of MYB11 and ZML2 to all the AC-rich and GATA cis-elements present within the first kilobase of the maize comt promoter by nano-fluidic qPCR (Fluidigm) using actin, α-tubulin, copia, and the comt-3′UTR as controls. While no enrichment was observed when using the control genes, a high enrichment of the comt promoter fragments containing both the AC-rich and GATA cis-elements was observed (Figure 5B), indicating that MYB11 and ZML2 can co-occupy these promoter regions in vivo. In particular, our results suggest that MYB11 and ZML2 bind cooperatively to DNA, given the higher enrichment in ChIP assays of the fragment C, that contains both AC (coordinate −195) and GATA/C boxes (coordinate −165) (Figure 5B).
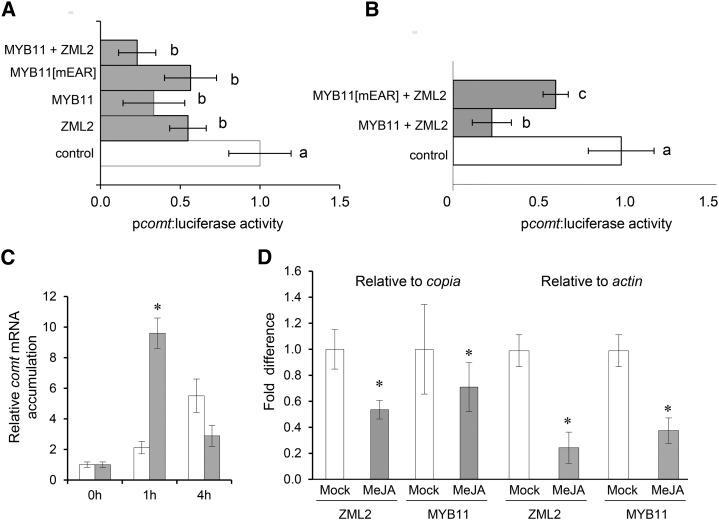
Additionally, we investigated whether the interaction between the two factors played a role in the repression of the comt gene. Thus, maize protoplasts were cotransformed with the comt promoter fused to luciferase and zml2 or myb11 fused to GFP. The resulting luciferase activity confirmed that both transcription factors could individually repress comt expression. However, the extent of comt repression was higher when ZML2 and MYB11 were simultaneously present (Figure 6A). However, when these transient expression assays were performed with ZML2 and MYB11-mEAR, the repression of comt was similar to the observed when both proteins were assayed separately (Figures 6A and 6B). These results are in line with previous results showing that the mutated MYB11 protein cannot interact with ZML2 (Figure 3).
Figure 6.
comt Repression Is Enhanced by the Simultaneous Binding of MYB11 and ZML2 and Is Relieved by MeJA.
(A) Effect of ZML2 and MYB11 on the comt gene promoter-driven luciferase expression. Maize protoplasts were transiently cotransformed with Luciferase fused to the comt promoter (pcomt:luciferase) with p35S:C-GFP (control) or with p35S:ZML2:C-GFP + p35S:MYB11:C-GFP, with p35S:MYB11[mEAR]:C-GFP, with p35S:ZML2:C-GFP, or with p35S:MYB11:C-GFP.
(B) Transient expression assays using maize protoplasts transformed with pcomt:Luc and p35S:C-GFP (control) or p35S:MYB11:C-GFP + p35S:ZML2:C-GFP or p35S:MYB11[mEAR]:C-GFP + p35S:ZML2:C-GFP. The transient transactivation assays of (A) and (B) were done in biological triplicates, and data were normalized for Renilla activity. Letters indicate significant differences in comt-driven luciferase intensity using a one-way ANOVA followed by a Tukey’s test (P < 0.05; Tukey’s LSD).
(C) qRT-PCR analysis of comt expression in 9-d-old maize leaves after 1 or 4 h of MeJA (gray bars) treatments or after 1 or 4 h of 0.01% DMSO (white bars) treatments. The fold change in comt expression was normalized using actin. The error bars indicate the se of the data obtained from three independent biological replicates.
(D) qPCR analyses comparing the enrichment of fragment C of the comt promoter from MeJA-treated (gray bars) and untreated (white bars) samples. copia and actin were used as reference genes. The difference in fold enrichment between treated and untreated samples was determined by dividing the fold enrichment of MeJA-treated samples by the fold enrichment of untreated samples. The error bars indicate the se of the data obtained from two independent biological replicates. Statistical analysis of differences between samples was performed using the Student’s t test (*P < 0.05).
Finally, when both the AC-rich and GATA binding motifs were mutated in the comt gene promoter, MYB11 and ZML2 could not repress the expression of the reporter gene (Supplemental Figure 7).
MeJA Derepresses comt through MYB11 and ZML2 Degradation
As already shown, both MYB11 and ZML2 repress comt and are degraded in a MeJA-dependent manner. Therefore, to investigate whether the degradation of these proteins affects the expression of comt, we treated maize leaves with MeJA and determined comt mRNA accumulation at different time points (1 and 4 h). The expression of comt was induced 1 h after the addition of the elicitor and the effect of MeJA on comt expression reverted after 4 h of treatment (Figure 6C).
To investigate whether MeJA affects the binding capacity of MYB11 and ZML2 to comt gene promoter, we performed ChIP assays using MYB11 and ZML2 antibodies to immunoprecipitate chromatin from control and MeJA-treated maize leaves. By qPCR analyses, we determined and compared the enrichment of fragment C of the comt promoter from treated and untreated samples. By fixing the fragment C enrichment of untreated samples as 1, and using copia and actin as control genes, we observed that the enrichment of fragment C in the MeJA-treated samples using MYB11 or ZML2 antibodies was reduced (Figure 6D). This result is in line with the degradation of these two transcription factors upon MeJA treatment (Figure 2) suggesting that the induced expression of comt gene upon MeJA exposure is, at least partially, caused by the derepression achieved by the release of MYB11 and ZML2 from the comt promoter.
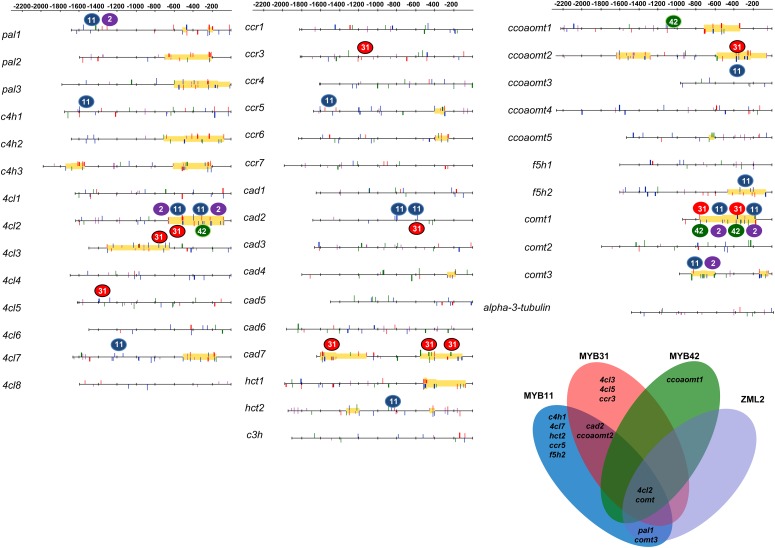
pal1, 4cl2, and comt3 Lignin Genes Are Targets of MYB11 and ZML2
To determine whether MYB11 and ZML2 act on other genes involved in lignin biosynthesis, we developed a catalog of putative genes involved in this process. Based on phylogenetic and expression analyses of maize databases, 40 putative lignin genes were selected (Supplemental Table 1) to perform ChIP-qPCR experiments using antibodies against ZML2 and MYB11 and actin, copia, α-tubulin, and the 3′UTR region of the comt gene as negative controls. In addition to comt, 10 gene promoters were in vivo targets of MYB11. Among them, pal1, 4cl2, and comt3 genes were also recognized by ZML2 (Figure 7; Supplemental Figure 8) and their expression was induced (or derepressed) by MeJA (Supplemental Figure 9), suggesting that these three genes can be regulated in a way similar to that described for comt.
Figure 7.
Schematic Representation of the DNA Binding Sites of MYB11, MYB31, MYB42, and ZML2 and Prediction of an AC-GAT(A/C) CRM in All the Lignin Genes Analyzed.
Blue, red, and green filled circles refer to MYB11, MYB31, and MYB42 binding sites identified by ChIP-qPCR assays, respectively. Predictions of AC and GAT(A/C) elements and CRERs in the promoter of different lignin genes were performed using the online Matrix-Scan software (see Methods) by scanning the upstream sequences with matrices representing binding motifs for the MYB transcription factors and ZML2. The AC elements are represented by blue (for MYB11), red (MYB31), and green (MYB42) lines, and GAT(A/C) boxes are shown as purple lines. Predicted cis-regulatory modules are shaded in yellow.
The Lignin Repressors MYB31 and MYB42 Interact with ZML2 and Share Common Targets among the Lignin Genes
As previously mentioned, MYB11 is evolutionarily closely related to MYB31 and MYB42, which are already described as lignin repressors (Fornalé et al., 2006, 2010; Sonbol et al., 2009). We performed ChIP-qPCR using specific antibodies against MYB31 and MYB42 and determined that they bind in vivo to 7 and 5 lignin genes, respectively (Figure 7; Supplemental Figure 8). Among them, only 4cl2 and comt were recognized by both these two MYB factors and ZML2. However, we did not detect lignin genes that were recognized solely by ZML2 (Figure 7).
We also analyzed the capacity of these transcription factors to bind to several cellulose synthase (cesa) genes. MYB42 was able to interact in vivo with the cesa7 promoter (Supplemental Figure 8). This is in line with previous results showing that MYB42 alters cell wall polysaccharides biosynthesis when expressed in Arabidopsis (Sonbol et al., 2009).
In addition, yeast two-hybrid and BiFC assays indicate that both MYB31 and MYB42 interact with ZML2 (Supplemental Figure 10), suggesting for MYB31 and MYB42 the existence of a mechanism similar to the one described for comt regulation by the MYB11/ZML2 complex. Thus, using the consensus sequence of the binding site of the three MYB factors derived from ChIP-qPCR data assays, the binding site of ZML2 obtained from PBM assay and a pattern-matching procedure, we predicted putative cis-regulatory modules containing the AC-rich and GAT(A/C) motifs in 15 lignin gene promoters (Figure 7). Interestingly, the region of 4cl2 and comt3 promoters in which MYBs and ZML2 bound corresponded to the region in which the AC-rich and GAT(A/C) module was predicted.
DISCUSSION
Lignin biosynthesis is affected by biotic and abiotic stresses. However, the molecular mechanisms governing these processes, from the stress perception to the alteration of the lignin gene expression, are largely unknown. In this study, we identified and characterized two new regulators that act as repressors of the maize comt gene. One of them, MYB11, belongs to the subgroup 4 of the R2R3-MYB family, and the second, ZML2, belongs to the TIFY family. MYB11 and ZML2 bind to the AC-rich and GATA cis-elements of the comt promoter in vivo, respectively. MYB11 constitutes the third R2R3-MYB factor, after MYB31 and MYB42 (Fornalé et al., 2006), that represses maize comt gene, while ZML2 is a member of the ZML subfamily of TIFY factors that we propose as a newly described regulator of a lignin gene.
Members of the TIFY family regulate wounding/MeJA responses (Vanholme et al., 2007). In line with this, we showed that both treatments trigger upregulation of the maize comt gene through the simultaneous degradation of ZML2 and MYB11, indicating that both regulators can participate in the wound-induced activation of lignin biosynthesis. The wound-induced expression of MYB11 and ZML2 can be considered as a part of the already described mechanism aimed to adjust the abundance of these two transcription factors after their protein degradation (Vom Endt et al., 2002).
We show that ZML2 interacts with MYB11, MYB31, and MYB42. Similar interactions have been described for R2R3-MYB factors and TIFY proteins belonging to the JAZ-domain subfamily (Qi et al., 2011; Song et al., 2011). In these cases, when the JAZ protein is degraded through the jasmonate signaling cascade, the R2R3-MYB partner is released to trigger the activation of the target genes (Qi et al., 2011). In the case of MYB11 and ZML2, both factors are degraded through the jasmonate signaling cascade, and this could lead to the derepression of comt. Such a mechanism of gene regulation by transcription factor degradation has been described previously (Catic et al., 2013; McShane and Selbach, 2014).
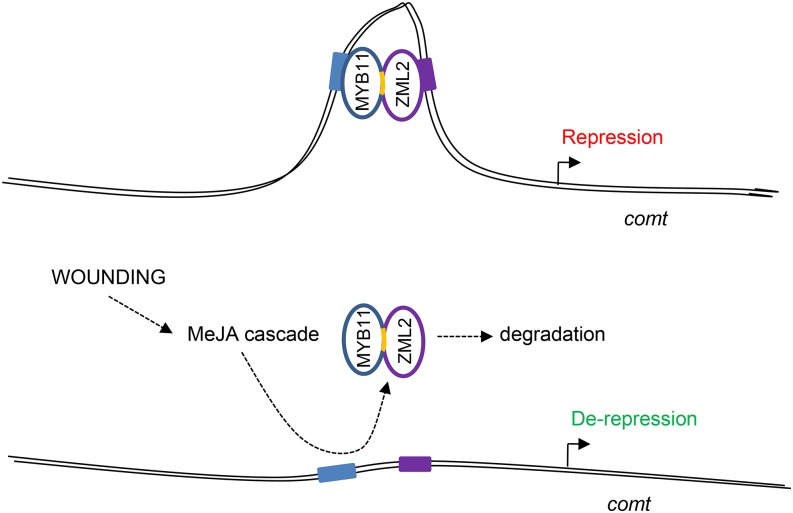
Regarding the mechanism by which the EAR motif inhibits transcription, the JAZ proteins in Arabidopsis recruit the Groucho/Tup1-type corepressor TOPLESS8 (TPL8) and TPL-related proteins (TPRs) through NOVEL INTERACTOR OF JAZ (NINJA), whose transcriptional repressor activity is mediated by a functional TPL binding EAR repression motif. Both NINJA and TPL proteins function as negative regulators of jasmonate responses (Pauwels et al., 2010). Although the mutation of the EAR motif of MYB11 does not seem to affect its repression activity significantly, it is critical for its interaction with ZML2, as these two factors cannot interact when the EAR motif of MYB11 is mutated. The importance of the EAR motif for protein-protein interactions has been reported for EAR-containing proteins, such as a MADS-domain protein (Hill et al., 2008) and a poplar zinc finger protein (Hamel et al., 2011). We show that mutation of the EAR motif of MYB11 prevents the MeJA-mediated degradation of the protein. This result suggests that the EAR motif of MYB11 is essential to connect this protein to the MeJA signaling cascade through its interaction with ZML2. In that manner, MYB11 can be physically removed from comt gene promoter, allowing the action of transcriptional activators. The AC and GATA cis-elements present in the maize comt promoter constitute a regulatory module, suggesting that MYB11 and ZML2 can bind and act simultaneously to repress comt expression. This was demonstrated by serial ChIP assays in which we showed that both factors bind simultaneously to the comt promoter. In addition, when MYB11 and ZML2 bind simultaneously to the comt promoter, the extent of comt repression increases compared with the repression observed individually.
Our results suggest that MYB11 and ZML2 bind cooperatively to the comt promoter, given the enrichment in ChIP assays of a fragment that contains an ACII (−195 bp) and a GATA/C (−165 bp) cis-elements. As these elements are located distant from each other in the comt promoter (30 bp apart), this cooperative binding of the heterodimer would likely involve a DNA loop comprising three helical turns of the DNA (Figure 8). Such a mechanism was classically proposed in the lambda bacteriophage (Hochschild and Ptashne, 1986) and in humans (Chen et al., 2012; Sanyal et al., 2012). In the case of plants, cooperative binding via DNA-looping of heteromeric transcription factors has been demonstrated in the case of MADS box proteins. In particular, SEPALLATA1-4 are able to form quaternary complexes among themselves and with other MADS proteins for the specification of floral organ identity (Melzer and Theissen, 2009; Melzer et al., 2009; Mendes et al., 2013).
Figure 8.
Schematic Representation of the Mode of Action of MYB11 and ZML2.
The AC-II (−194 bp) and GATC (−165 bp) cis-elements of the maize comt promoter in which MYB11 and ZML2 bind are shown in blue and purple boxes, respectively. The yellow fragment represents the EAR motif of MYB11.
The AC-GAT(A/C) regulatory module is also present in the promoters of comt genes of other grasses, suggesting the existence of a conserved regulatory mechanism in this family. As an example, in Brachypodium distachyon, the promoter of four comt genes was analyzed and the AC-GATA regulatory module was detected only in the promoter of Bd-comt4, which encodes the lignin methylase in this species (Wu et al., 2013). In addition to comt, ZML2 also binds to the gene promoter of pal1, 4cl2 (early phenylpropanoid genes), and comt3. These genes are also recognized by MYB11, suggesting that the mechanism by which these transcription factors derepress the expression of comt upon wounding can be extended to these three lignin genes. Furthermore, our results suggest that the regulatory mechanism described for MYB11/ZML2 can be also extended to MYB31 and MYB42, as these two MYB factors interact with ZML2 and bind to the comt and 4cl2 gene promoters.
In summary, we show that the regulation of the maize comt expression is at least partially mediated by the interaction of subgroup 4 R2R3-MYB transcription factors and ZML2. This protein complex directly binds to comt promoter. The interaction of MYB11 with ZML2 occurs through the EAR motif, and when wounding occurs, the jasmonate signaling cascade triggers the degradation not only of ZML2 but also of MYB11, leading to the derepression of comt (Figure 8). This regulatory mechanism suggested for MYB11 and ZML2 can be extended to the lignin repressors MYB31 and MYB42, as both factors bind in vivo to the comt promoter and physically interact with ZML2.
Finally, as MYB11, MYB31, and MYB42 repressors and ZML2 share common targets within the lignin biosynthetic genes, it can be proposed that the induction of lignin biosynthesis that is required upon wounding can rely on the coordinated activation of the lignin genes through the action of a MYB/ZML protein complex. Thus, this regulatory mechanism would be part of the regulatory cascade triggering the de novo lignin biosynthesis required upon wounding.
METHODS
Plant Materials, Growth Conditions, and Treatments
Maize (Zea mays) B73 inbred plants were grown in soil during 9 d in a growth chamber (28°C, 70% relative humidity). For protoplast assays, kernels of B73xMo17 plants were grown for 13 d on soil in a growth chamber in dark conditions and at 28°C.
Maize plants were sprayed with 100 μM MeJA (Sigma-Aldrich) diluted in 0.01% DMSO, and control plants were treated with 0.01% DMSO only and kept in closed plastic containers. For mechanical wound treatments, leaves of 9-d-old maize plants were wounded several times with a razor blade and damaged leaves were harvested, immediately immersed in liquid nitrogen, and stored at −80°C until use.
DNA Constructs
The full-length cDNAs encoding MYB11, MYB31, ZmMYB42, MYB11[mEAR], ZML1, and ZML2 were cloned into pDONR207 (Invitrogen) using the specific primers detailed in Supplemental Table 2. The cDNA clones in the pDONR207 vector were recombined by LR Gateway reaction into the final pDestTH1 (bacterial MBP fusion expression vector) for MYB11 and ZML2 antibody testing, in pYFPN43 and pYFPC43 (Belda-Palazón et al., 2012) for BiFC, and in pGBKT7 and pGADT7 Gateway vectors for yeast two-hybrid mating assays.
The MYB11[mEAR] was created by primer extension mutagenesis using MYB11 pDNOR221DNA as a template and the oligonucleotides ZmMYB11_GatewayFW, ZmMYB11[mEAR]A3′RV (A), ZmMYB11[mEAR]B3′RV (B; synthetic fragment), ZmMYB11[mEAR]A+B3′RV (A+B), and ZmMYB11[mEAR]C5′FW (C).
First, PCR was performed using ZmMYB11_GatewayFW and ZmMYB11[mEAR]A3′RV (A) to produce fragment A; then, ZmMYB11[mEAR]B3′RV (B) was annealed with fragment A over seven cycles and ZmMYB11_GatewayFW and ZmMYB11[mEAR]A+B3′RV (A+B) were added to the mix containing fragments A and B for PCR using 25 cycles. After that, PCR with ZmMYB11[mEAR]C5′FW (C) and ZmMYB11_GatewayRV was performed to obtain the fragment C. Finally, fragments A+B and C were annealed and amplified as above and the MYB11[mEAR] full-length cDNA clone was generated. The primer sequences are available in the Supplemental Table 2.
Production of MYB11, MYB42, and ZML2 Antibodies
Antibodies against MYB31 were obtained as already described (Fornalé et al., 2010) and anti-MYB11 and anti-MYB42 antibodies were raised against specific fragments spanning from amino acids 128 to 201 and 128 to 205, respectively (Supplemental Figure 2A; see Supplemental Table 2 for primer sequences). In the case of ZML2, the antibodies were obtained against the full-length protein. In all cases, purified proteins (or fragments) were injected in rabbits to produce polyclonal antibodies that were subsequently purified according to (Bar-Peled and Raikhel, 1996; Fornalé et al., 2010).
ChIP, Serial ChIP, and Gene Expression Analyses
ChIP experiments were performed as described previously (Morohashi et al., 2007; Morohashi and Grotewold, 2009) with minor modifications (Supplemental Methods).
Serial ChIP for sequential chromatin immunoprecipitations was performed as previously described (Xie and Grotewold, 2008) with minor modifications. Cross-linking between Dynabeads/Protein A (Invitrogen) and the MYB11 and ZML2 antibodies was performed to avoid nonspecific binding to the beads. The first elution was performed according to the previous protocol.
Nano-quantitative (real-time) PCR analyses of ChIP and serial ChIP samples were performed using the BioMark System based on Fluidigm microfluidic chips at the Genomics and NGS Core Facility of the Centre for Research in Agricultural Genomics (CRAG), according to the BioMark System protocol (Supplemental Methods).
RNA extraction of maize leaves or protoplasts was performed with Trizol reagent (Invitrogen) following the manufacturer's protocol. The cDNA was synthesized from 1 μg total RNA using QuantiTect reverse transcription kit (Qiagen).
Quantitative PCR of ChIP samples and gene expression analyses were performed using LightCycler 480 and DNA SYBR Green I Master Mix (Roche Diagnostics) (Supplemental Methods).
GFP Visualization and BiFC Assays in Maize Protoplasts
Maize protoplasts were isolated from 13-d-old etiolated plants as previously described (Vélez-Bermúdez et al., 2015). For BiFC experiments, all constructs were transformed by electroporation in maize protoplasts as described (Vélez-Bermúdez et al., 2015). Visualization of the YFP was performed 22 h after electroporation, and analysis of single fluorescence images was done as described below. Quantification of fluorescence images acquired with high magnification objective lens of 60× (BiFC signals) was performed as described previously (Walter et al., 2004) using ImageJ software (Schneider et al., 2012). The filter sets used for excitation and emission were the following: yellow fluorescent protein (EYFP), 514nm (excitation)/ 490 to 500 nm (emission); bright-field, 633 nm. Images were analyzed and processed with an FV10-ASW 2.0 viewer.
Maize protoplasts were treated with 25 μM MeJA (Sigma-Aldrich) dissolved in DMSO 18 h after electroporation. The elicitor was added to 100 μL protoplast suspension and incubated for different time periods at room temperature. For the treatments with MeJA+MG132, the protoplasts were treated with the same concentration of MeJA (25 μM) and 5 μM MG132. In both cases, DMSO-treated protoplasts were used as control. The GFP was visualized at 0 h and after 1 h in each sample with a 488-nm argon-ion laser (500- to 545-nm emission filter). The different captured images of maize protoplasts harboring p35S:MYB11:C-GFP, p35S:ZML2:C-GFP, p35S:MYB11[mEAR]:C-GFP, and p35S:C-GFP treated with MeJA (25 μM), 5 μM MG132, and DMSO are presented as 3D projected stacks made of 14 layers of 1 mm. The images were processed with the Olympus FV software and analyzed and quantified using the image processing software Image J (Schneider et al., 2012). The images of proteins labeling were obtained using a FV 1000 confocal microscope (Olympus) with a ×60 oil immersion lens.
Protein Purification and Determination of ZML2 DNA Binding Motifs
The DNA binding specificity of ZML2 was determined using protein binding microarrays (PBM11) as previously described (Godoy et al., 2011). The cDNA of zml2 cloned in fusion to MBP in the pDESTH1 vector using Gateway technology was used for this experiment. Donor template was obtained through PCR amplification of zml2 with gene-specific oligonucleotides (Supplemental Table 1). Recombinant insert was verified by sequencing and plasmid introduced into BL-21 strain. Expression of recombinant protein was performed as described for the pMAL purification system (New England Biolabs).
CoIP Assays
Maize leaves extracts resuspended in immunoprecipitation buffer supplemented with 3× plant inhibitor cocktail (Sigma-Aldrich), 50 μM MG132, 1 mM phenylmethylsulfonyl fluoride, 100 mM NaCl, and 2 mM DTT were used for CoIP experiments using the Dynabeads coimmunoprecipitation kit (Invitrogen) following the supplier’s instructions with modifications and added to 50 μL Dynabeads previously incubated with MYB11 antibodies. The Dynabeads were washed and eluted with 30 μL SDS-PAGE 1.5× sample buffer (62.5 mM Tris-HCl, pH 6.8, 2 mM β-mercaptoethanol, 10% glycerol, 2% SDS, and 0.01% bromophenol blue) for the immunoblotting analysis. The ZML2 and MYB11 antibodies were used at a 1:100 dilution. The secondary antibody (GE Healthcare; NA934-100UL) was used at a 1:5000 dilution.
Luciferase Assays
Maize protoplast transformation efficiency for the luciferase assays was estimated based on GFP expression. For transient expression assays, the Dual-Luciferase Reporter Assay System (Promega) was used (Supplemental Methods).
Phylogenetic Analysis
Dendrograms were assembled from protein-coding sequences by the neighbor-joining method using ClustalW software, version 1.83 (http://www.genome.jp/tools/clustalw/) (Saitou and Nei, 1987; Chenna et al., 2003) (Supplemental Data Sets 1 to 3). The parameters used were as already described for a slow, accurate tree with gap open penalty of 10, gap extension penalty of 0.05, and a Gonnet weight matrix for proteins for multiple alignments; a gap open penalty of 10, gap extension penalty of 0.1, and a Gonnet weight matrix for proteins for pairwise alignments; and bootstrapped 1000 times (Penning et al., 2009). At-MYB6 was used as the outgroup for phylogenetic tree of MYB proteins. Dendrograms were drawn using TreeDyn available at http://www.phylogeny.fr. For the case of MYBs, we used the online tool http://www.trex.uqam.ca for the tree representation. The protein sequences were obtained from maize sequencing, version 5b.60 (Schnable et al., 2009; http://www.maizesequence.org), and GRASSIUS database (Yilmaz et al., 2009).
Analysis of Promoter Motif Enrichment within Modules
Matrix-Scan software (http://rsat.ulb.ac.be/) was used to perform computer-based predictions of CRMs to establish whether the 5′ region of the comt gene of different plant species was enriched in the AC-GAT(A/C) CRM. This software detects cis-regulatory element enriched regions (CRERs; short sequence regions with a significant high density of predicted sites). A P value is associated to each CRER, using the binomial distribution of probability (Turatsinze et al., 2008). The parameters used were as follows: CRERs, background model: Markov Chain Order 0 (the model was trained from the input sequences), CRER size: 10 to 500, site P value: 1e-2 and CRER sig:2 (Turatsinze et al., 2008). We used TRANSFAC format matrices that represent the binding site affinity of MYB11 and ZML2. MYB11 ChIP experiment data and ZML2 PMB11 data were used to construct the matrices. We used MEME software available with the MEME suite (Bailey, 2011) to identify the motif within the genomic regions that the transcription factors can bind according to ChIP-qPCR data. The parameters used were the same as described above (Turatsinze et al., 2008). In addition, we used DNA pattern software for identification the AC and GAT(A/C) box in the promoter regions (Thomas-Chollier et al., 2008).
Matrix-Scan software was used to establish whether the 5′ region of the lignin genes was enriched in the regulatory module AC-GATA(C). TRANSFAC matrices for the four transcription factors were employed to identify CRMs in the 5′ region of the genes. The TRANSFAC matrices for MYB31 and MYB42 were generated using the same protocol as for MYB11.
The parameters used in Matrix-Scan were as follows: CRERs, background model: Markov Chain Order 3 (constructed with the sequence region corresponding to 1500 bp upstream of all genes identified in the maize genome), CRER size: 30 to 500, site P value: 1e-3, CRER sites: 1e-3, CRER P-value: 1e-3 y CRER sig: 1. In addition, we used the DNA pattern software for identification the AC and GATA(C) box in the promoter regions (Thomas-Chollier et al., 2008).
Accession Numbers
Sequence data from this article can be found in the GenBank/EMBL databases under the following accession numbers: myb11 (GRMZM2G000818), myb31 (GRMZM2G050305), myb42 (GRMZM2G419239), zml2 (GRMZM2G058479), and comt (AC196475.3_FGT004).
Supplemental Data
Supplemental Figure 1. MYB11 belongs to the R2R3MYB subgroup 4 family and is induce by wounding.
Supplemental Figure 2. Production of specific antibodies against MYB11.
Supplemental Figure 3. Structure and phylogenetic analysis of ZML2 and gene expression analyses.
Supplemental Figure 4. Production of specific antibodies against ZML2.
Supplemental Figure 5. MYB11 interacts with ZML2.
Supplemental Figure 6. Consensus binding sequences for MYB11, MYB31, and MYB42 and ChIP-reChIP of MYB11 and ZML2.
Supplemental Figure 7. Effect of ZML2 and MYB11 on the luciferase expression driven by comt promoter mutated in AC and GAT(A/C) boxes.
Supplemental Figure 8. ChIP-qPCR analyses of MYB11, MYB31, Zm-MYB42, and ZML2 binding to the comt promoter.
Supplemental Figure 9. pal1, 4cl2, and comt3 expression is induced by MeJA.
Supplemental Figure 10. MYB31 and MYB42 interact with ZML2.
Supplemental Table 1. List of genes used for ChIP-qPCR analyses.
Supplemental Table 2. Primers used in this work.
Supplemental Methods. Biochemical (immunoblot and yeast two hybrid) and molecular (ChIP and serial ChIP) techniques.
Supplemental Data Set 1. Text file of the alignment used for the phylogenetic analysis shown in Supplemental Figure 1A.
Supplemental Data Set 2. Text file of the alignment used for the phylogenetic analysis shown in Supplemental Figure 3A.
Supplemental Data Set 3. Text file of the alignment used for the phylogenetic analysis shown in Figure 5A.
Supplementary Material
Acknowledgments
We thank Kengo Morohashi, Maria Katherine Mejía-Guerra, and Maria Isabel Casas (Center for Applied Plant Sciences, Ohio State University) for assistance with ChIP assays, for suggestions, and for providing B73 and Mo17xB73 seeds. We also thank Maria José Molina (CRAG, Consortium CSIC-IRTA-UAB-UB) for her assistance with yeast two-hybrid assays. We thank Tsuyoshi Nakagawa (Shimane University) for providing Gateway binary vectors that contain the bar gene, which was identified by Meiji Seika Kaisha. We thank Chung-Ju Rachel Wang (Academia Sinica) for providing the B73 and MO17xB73 maize seeds. Research in D.C.-R.’s laboratory was supported by a grant from the Spanish Ministry of Science and Education (AGL2011-30545-C02-01), the “Xarxa de Referència de Biotecnologia” (XarBa) from the Autonomous Government of Catalonia, the CONSOLIDER-INGENIO program (CSD2007-00036) from the Spanish Ministry of Science and Innovation, and the SGR programs (SGR2009-GRC703). Research in M.P.’s laboratory was supported by two grants from the Spanish Ministry of Science and Education (BIO2009-13044-C02-01 and BIO2012-31860), the framework of the XarBa, and the SGR programs (SGR2009-GRC626) from the Autonomous Government of Catalonia. Research in R.S.’s laboratory was supported by grants from the Ministry of Science and Innovation to R.S. (BIO2013-44407). M.P. and R.S. received financial support from the CONSOLIDER-INGENIO program (CSD2007-00057-B) from the Spanish Ministerio de Ciencia e Innovación. Research in the W.S. laboratory is supported by grants from the Ministry of Science and Technology and Academia Sinica. Research in phenylpropanoid gene regulation in the laboratories of E.G. and J.G. was supported by a grant from the National Science Foundation (IOS-1125620). I.-C.V.-B. was supported by a Spanish FPI Fellowship (BES-2007-17316). J.E.S.-H. was supported by the Department of Innovation, Universities and Enterprise of the Generalitatde Catalunya, the European Social Fund FI Fellowship (AGAUR: FI-2006, Resolució EDU/3600/2006; FI-2008, Resolució IUE/2658/2007 and BE-DGR2010), and CRAG.
AUTHOR CONTRIBUTIONS
D.C.-R., M.P., E.G., R.S., J.G., M.R., S.F., I.-C.V.-B., and J.E.S.-H. designed the research. I.-C.V.-B., J.E.S.-H., S.F., I.L.-V., J.-M.F.-Z., and M.R. performed research. D.C.-R., M.P., E.G., R.S., M.R., S.F., I.-C.V.-B., J.E.S.-H., I.L.-V., W.S., and J.-M.F.-Z. analyzed data. D.C.-R., S.F., M.R., and M.P. wrote the article. All authors read and approved the article.
Glossary
- MeJA
methyl jasmonate
- ChIP
chromatin immunoprecipitation
- BiFC
bimolecular fluorescence complementation
- CoIP
coimmunoprecipitation
- PBM
protein binding microarray
- CRM
cis-regulatory module
- CRER
cis-regulatory element enriched region
- ChIP-reChIP
sequential chromatin immunoprecipitation
References
- Bai Y., Meng Y., Huang D., Qi Y., Chen M. (2011). Origin and evolutionary analysis of the plant-specific TIFY transcription factor family. Genomics 98: 128–136. [DOI] [PubMed] [Google Scholar]
- Bailey T.L. (2011). DREME: motif discovery in transcription factor ChIP-seq data. Bioinformatics 27: 1653–1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Peled M., Raikhel N.V. (1996). A method for isolation and purification of specific antibodies to a protein fused to the GST. Anal. Biochem. 241: 140–142. [DOI] [PubMed] [Google Scholar]
- Belda-Palazón B., Ruiz L., Martí E., Tárraga S., Tiburcio A.F., Culiáñez F., Farràs R., Carrasco P., Ferrando A. (2012). Aminopropyltransferases involved in polyamine biosynthesis localize preferentially in the nucleus of plant cells. PLoS One 7: e46907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell-Lelong D.A., Cusumano J.C., Meyer K., Chapple C. (1997). Cinnamate-4-hydroxylase expression in Arabidopsis. Regulation in response to development and the environment. Plant Physiol. 113: 729–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boerjan W., Ralph J., Baucher M. (2003). Lignin biosynthesis. Annu. Rev. Plant Biol. 54: 519–546. [DOI] [PubMed] [Google Scholar]
- Capellades M., Torres M.A., Bastisch I., Stiefel V., Vignols F., Bruce W.B., Peterson D., Puigdomènech P., Rigau J. (1996). The maize caffeic acid O-methyltransferase gene promoter is active in transgenic tobacco and maize plant tissues. Plant Mol. Biol. 31: 307–322. [DOI] [PubMed] [Google Scholar]
- Carpita N.C., Gibeaut D.M. (1993). Structural models of primary cell walls in flowering plants: consistency of molecular structure with the physical properties of the walls during growth. Plant J. 3: 1–30. [DOI] [PubMed] [Google Scholar]
- Catic A., Suh C.Y., Hill C.T., Daheron L., Henkel T., Orford K.W., Dombkowski D.M., Liu T., Liu X.S., Scadden D.T. (2013). Genome-wide map of nuclear protein degradation shows NCoR1 turnover as a key to mitochondrial gene regulation. Cell 155: 1380–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Bates D.L., Dey R., Chen P.-H., Machado A.C., Laird-Offringa I.A., Rohs R., Chen L. (2012). DNA binding by GATA transcription factor suggests mechanisms of DNA looping and long-range gene regulation. Cell Reports 2: 1197–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chenna R., Sugawara H., Koike T., Lopez R., Gibson T.J., Higgins D.G., Thompson J.D. (2003). Multiple sequence alignment with the Clustal series of programs. Nucleic Acids Res. 31: 3497–3500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chini A., Fonseca S., Fernández G., Adie B., Chico J.M., Lorenzo O., García-Casado G., López-Vidriero I., Lozano F.M., Ponce M.R., Micol J.L., Solano R. (2007). The JAZ family of repressors is the missing link in jasmonate signalling. Nature 448: 666–671. [DOI] [PubMed] [Google Scholar]
- Devoto A., Ellis C., Magusin A., Chang H.S., Chilcott C., Zhu T., Turner J.G. (2005). Expression profiling reveals COI1 to be a key regulator of genes involved in wound- and methyl jasmonate-induced secondary metabolism, defence, and hormone interactions. Plant Mol. Biol. 58: 497–513. [DOI] [PubMed] [Google Scholar]
- Ehlting J., Büttner D., Wang Q., Douglas C.J., Somssich I.E., Kombrink E. (1999). Three 4-coumarate:coenzyme A ligases in Arabidopsis thaliana represent two evolutionarily divergent classes in angiosperms. Plant J. 19: 9–20. [DOI] [PubMed] [Google Scholar]
- Ellard-Ivey M., Douglas C.J. (1996). Role of jasmonates in the elicitor- and wound-inducible expression of defense genes in parsley and transgenic tobacco. Plant Physiol. 112: 183–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornalé S., Sonbol F.M., Maes T., Capellades M., Puigdomènech P., Rigau J., Caparrós-Ruiz D. (2006). Down-regulation of the maize and Arabidopsis thaliana caffeic acid O-methyl-transferase genes by two new maize R2R3-MYB transcription factors. Plant Mol. Biol. 62: 809–823. [DOI] [PubMed] [Google Scholar]
- Fornalé S., et al. (2010). ZmMYB31 directly represses maize lignin genes and redirects the phenylpropanoid metabolic flux. Plant J. 64: 633–644. [DOI] [PubMed] [Google Scholar]
- Godoy M., Franco-Zorrilla J.M., Pérez-Pérez J., Oliveros J.C., Lorenzo O., Solano R. (2011). Improved protein-binding microarrays for the identification of DNA-binding specificities of transcription factors. Plant J. 66: 700–711. [DOI] [PubMed] [Google Scholar]
- Gray J., Caparrós-Ruiz D., Grotewold E. (2012). Grass phenylpropanoids: regulate before using! Plant Sci. 184: 112–120. [DOI] [PubMed] [Google Scholar]
- Hamel L.P., Benchabane M., Nicole M.C., Major I.T., Morency M.J., Pelletier G., Beaudoin N., Sheen J., Séguin A. (2011). Stress-responsive mitogen-activated protein kinases interact with the EAR motif of a poplar zinc finger protein and mediate its degradation through the 26S proteasome. Plant Physiol. 157: 1379–1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill K., Wang H., Perry S.E. (2008). A transcriptional repression motif in the MADS factor AGL15 is involved in recruitment of histone deacetylase complex components. Plant J. 53: 172–185. [DOI] [PubMed] [Google Scholar]
- Hochschild A., Ptashne M. (1986). Cooperative binding of λ repressors to sites separated by integral turns of the DNA helix. Cell 44: 681–687. [DOI] [PubMed] [Google Scholar]
- Jouanin L., Goujon T., de Nadaï V., Martin M.T., Mila I., Vallet C., Pollet B., Yoshinaga A., Chabbert B., Petit-Conil M., Lapierre C. (2000). Lignification in transgenic poplars with extremely reduced caffeic acid O-methyltransferase activity. Plant Physiol. 123: 1363–1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagale S., Rozwadowski K. (2011). EAR motif-mediated transcriptional repression in plants: an underlying mechanism for epigenetic regulation of gene expression. Epigenetics 6: 141–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser I., Engelberth J., Groth B., Weiler E.W. (1994). Touch-induced and methyl jasmonate-induced lignification in tendrils of Bryonia dioica Jacq. Bot. Acta 107: 24–29. [Google Scholar]
- Lawton M.A., Lamb C.J. (1987). Transcriptional activation of plant defense genes by fungal elicitor, wounding, and infection. Mol. Cell. Biol. 7: 335–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzo O., Chico J.M., Sánchez-Serrano J.J., Solano R. (2004). JASMONATE-INSENSITIVE1 encodes a MYC transcription factor essential to discriminate between different jasmonate-regulated defense responses in Arabidopsis. Plant Cell 16: 1938–1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McShane E., Selbach M. (2014). Gene expression: degrade to derepress. EMBO J. 33: 407–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melzer R., Theissen G. (2009). Reconstitution of ‘floral quartets’ in vitro involving class B and class E floral homeotic proteins. Nucleic Acids Res. 37: 2723–2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melzer R., Verelst W., Theissen G. (2009). The class E floral homeotic protein SEPALLATA3 is sufficient to loop DNA in ‘floral quartet’-like complexes in vitro. Nucleic Acids Res. 37: 144–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendes M.A., Guerra R.F., Berns M.C., Manzo C., Masiero S., Finzi L., Kater M.M., Colombo L. (2013). MADS domain transcription factors mediate short-range DNA looping that is essential for target gene expression in Arabidopsis. Plant Cell 25: 2560–2572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizutani M., Ohta D., Sato R. (1997). Isolation of a cDNA and a genomic clone encoding cinnamate 4-hydroxylase from Arabidopsis and its expression manner in planta. Plant Physiol. 113: 755–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morohashi K., Grotewold E. (2009). A systems approach reveals regulatory circuitry for Arabidopsis trichome initiation by the GL3 and GL1 selectors. PLoS Genet. 5: e1000396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morohashi K., Zhao M., Yang M., Read B., Lloyd A., Lamb R., Grotewold E. (2007). Participation of the Arabidopsis bHLH factor GL3 in trichome initiation regulatory events. Plant Physiol. 145: 736–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauwels L., Morreel K., De Witte E., Lammertyn F., Van Montagu M., Boerjan W., Inzé D., Goossens A. (2008). Mapping methyl jasmonate-mediated transcriptional reprogramming of metabolism and cell cycle progression in cultured Arabidopsis cells. Proc. Natl. Acad. Sci. USA 105: 1380–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauwels L., et al. (2010). NINJA connects the co-repressor TOPLESS to jasmonate signalling. Nature 464: 788–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penning B.W., et al. (2009). Genetic resources for maize cell wall biology. Plant Physiol. 151: 1703–1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi T., Song S., Ren Q., Wu D., Huang H., Chen Y., Fan M., Peng W., Ren C., Xie D. (2011). The Jasmonate-ZIM-domain proteins interact with the WD-Repeat/bHLH/MYB complexes to regulate Jasmonate-mediated anthocyanin accumulation and trichome initiation in Arabidopsis thaliana. Plant Cell 23: 1795–1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reymond P., Weber H., Damond M., Farmer E.E. (2000). Differential gene expression in response to mechanical wounding and insect feeding in Arabidopsis. Plant Cell 12: 707–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers L.A., Campbell M.M. (2004). The genetic control of lignin deposition during plant growth and development. New Phytol. 164: 17–30. [DOI] [PubMed] [Google Scholar]
- Saitou N., Nei M. (1987). The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 4: 406–425. [DOI] [PubMed] [Google Scholar]
- Sanyal A., Lajoie B.R., Jain G., Dekker J. (2012). The long-range interaction landscape of gene promoters. Nature 489: 109–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkanen K.V., Ludwig C.H. (1971). Lignins: Occurrence, Formation, Structure, and Reactions. (New York: Wiley-Interscience; ). [Google Scholar]
- Schnable P.S., et al. (2009). The B73 maize genome: complexity, diversity, and dynamics. Science 326: 1112–1115. [DOI] [PubMed] [Google Scholar]
- Schneider C.A., Rasband W.S., Eliceiri K.W. (2012). NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 9: 671–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekhon R.S., Briskine R., Hirsch C.N., Myers C.L., Springer N.M., Buell C.R., de Leon N., Kaeppler S.M. (2013). Maize gene atlas developed by RNA sequencing and comparative evaluation of transcriptomes based on RNA sequencing and microarrays. PLoS One 8: e61005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaikhali J., de Dios Barajas-Lopéz J., Ötvös K., Kremnev D., Garcia A.S., Srivastava V., Wingsle G., Bako L., Strand Å. (2012). The CRYPTOCHROME1-dependent response to excess light is mediated through the transcriptional activators ZINC FINGER PROTEIN EXPRESSED IN INFLORESCENCE MERISTEM LIKE1 and ZML2 in Arabidopsis. Plant Cell 24: 3009–3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonbol F.M., Fornalé S., Capellades M., Encina A., Touriño S., Torres J.L., Rovira P., Ruel K., Puigdomènech P., Rigau J., Caparrós-Ruiz D. (2009). The maize ZmMYB42 represses the phenylpropanoid pathway and affects the cell wall structure, composition and degradability in Arabidopsis thaliana. Plant Mol. Biol. 70: 283–296. [DOI] [PubMed] [Google Scholar]
- Song S., Qi T., Huang H., Ren Q., Wu D., Chang C., Peng W., Liu Y., Peng J., Xie D. (2011). The Jasmonate-ZIM domain proteins interact with the R2R3-MYB transcription factors MYB21 and MYB24 to affect jasmonate-regulated stamen development in Arabidopsis. Plant Cell 23: 1000–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thines B., Katsir L., Melotto M., Niu Y., Mandaokar A., Liu G., Nomura K., He S.Y., Howe G.A., Browse J. (2007). JAZ repressor proteins are targets of the SCF(COI1) complex during jasmonate signalling. Nature 448: 661–665. [DOI] [PubMed] [Google Scholar]
- Thomas-Chollier M., Sand O., Turatsinze J.V., Janky R., Defrance M., Vervisch E., Brohée S., van Helden J. (2008). RSAT: regulatory sequence analysis tools. Nucleic Acids Res. 36: W119–W127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tianpei X., Li D., Qiu P., Luo J., Zhu Y., Li S. (2015). Scorpion peptide LqhIT2 activates phenylpropanoid pathways via jasmonate to increase rice resistance to rice leafrollers. Plant Sci. 230: 1–11. [DOI] [PubMed] [Google Scholar]
- Turatsinze J.V., Thomas-Chollier M., Defrance M., van Helden J. (2008). Using RSAT to scan genome sequences for transcription factor binding sites and cis-regulatory modules. Nat. Protoc. 3: 1578–1588. [DOI] [PubMed] [Google Scholar]
- Vance, C.P., Kirk, T.K., and Sherwood, R.T. (1980). Lignification as a mechanism of disease resistance. Annu. Rev. Phytopothol. 18: 259–288.
- Vanholme B., Grunewald W., Bateman A., Kohchi T., Gheysen G. (2007). The tify family previously known as ZIM. Trends Plant Sci. 12: 239–244. [DOI] [PubMed] [Google Scholar]
- Vélez-Bermúdez I.C., Carretero-Paulet L., Legnaioli T., Ludevid D., Pagès M., Riera M. (2015). Novel CK2α and CK2β subunits in maize reveal functional diversification in subcellular localization and interaction capacity. Plant Sci. 235: 58–69. [DOI] [PubMed] [Google Scholar]
- Vignols F., Rigau J., Torres M.A., Capellades M., Puigdomènech P. (1995). The brown midrib3 (bm3) mutation in maize occurs in the gene encoding caffeic acid O-methyltransferase. Plant Cell 7: 407–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vom Endt D., Kijne J.W., Memelink J. (2002). Transcription factors controlling plant secondary metabolism: what regulates the regulators? Phytochemistry 61: 107–114. [DOI] [PubMed] [Google Scholar]
- Walter M., Chaban C., Schütze K., Batistic O., Weckermann K., Näke C., Blazevic D., Grefen C., Schumacher K., Oecking C., Harter K., Kudla J. (2004). Visualization of protein interactions in living plant cells using bimolecular fluorescence complementation. Plant J. 40: 428–438. [DOI] [PubMed] [Google Scholar]
- Wu X., Wu J., Luo Y., Bragg J., Anderson O., Vogel J., Gu Y.Q. (2013). Phylogenetic, molecular, and biochemical characterization of caffeic acid o-methyltransferase gene family in Brachypodium distachyon. Int. J. Plant Genomics 2013: 423189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Z., Grotewold E. (2008). Serial ChIP as a tool to investigate the co-localization or exclusion of proteins on plant genes. Plant Methods 4: 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue Y.J., Tao L., Yang Z.M. (2008). Aluminum-induced cell wall peroxidase activity and lignin synthesis are differentially regulated by jasmonate and nitric oxide. J. Agric. Food Chem. 56: 9676–9684. [DOI] [PubMed] [Google Scholar]
- Yilmaz A., Nishiyama M.Y. Jr., Fuentes B.G., Souza G.M., Janies D., Gray J., Grotewold E. (2009). GRASSIUS: a platform for comparative regulatory genomics across the grasses. Plant Physiol. 149: 171–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.