Microtubule interacting proteins, which connect various cellular compartments with microtubules, regulate specific aspects of tracheary element differentiation and secondary cell wall formation.
Abstract
Plant vascular cells, or tracheary elements (TEs), rely on circumferential secondary cell wall thickenings to maintain sap flow. The patterns in which TE thickenings are organized vary according to the underlying microtubule bundles that guide wall deposition. To identify microtubule interacting proteins present at defined stages of TE differentiation, we exploited the synchronous differentiation of TEs in Arabidopsis thaliana suspension cultures. Quantitative proteomic analysis of microtubule pull-downs, using ratiometric 14N/15N labeling, revealed 605 proteins exhibiting differential accumulation during TE differentiation. Microtubule interacting proteins associated with membrane trafficking, protein synthesis, DNA/RNA binding, and signal transduction peaked during secondary cell wall formation, while proteins associated with stress peaked when approaching TE cell death. In particular, CELLULOSE SYNTHASE-INTERACTING PROTEIN1, already associated with primary wall synthesis, was enriched during secondary cell wall formation. RNAi knockdown of genes encoding several of the identified proteins showed that secondary wall formation depends on the coordinated presence of microtubule interacting proteins with nonoverlapping functions: cell wall thickness, cell wall homogeneity, and the pattern and cortical location of the wall are dependent on different proteins. Altogether, proteins linking microtubules to a range of metabolic compartments vary specifically during TE differentiation and regulate different aspects of wall patterning.
INTRODUCTION
The early microscopist Malpighi (1675) named the hollow, conducting wood cells of plants after vertebrate trachea since both conducting structures display characteristic transverse thickenings. To operate as sap conduits, tracheary elements (TEs) undergo programmed cell death. This hollows the cell lumen, while cell wall modifications reinforce and alter the sidewalls of the emptied tube. Circumferential deposits of secondary cell wall prevent the tube from collapsing and are organized to form annular, spiral, reticulate, or pitted motifs (Pesquet and Lloyd, 2011). These uniformly thick secondary walls maintain an open lumen for the hydrodynamic sap flow (Ménard and Pesquet, 2015), while the intervening areas of thinned and modified primary cell walls allow lateral distribution of the sap content (Benayoun, 1983; Ryser et al., 1997).
Different patterns of wall thickening are known to be associated with different phases of plant growth. Annular and spiral patterns (i.e., protoxylem) appear during early primary growth, while reticulate and pitted patterns (i.e., metaxylem) form later to further strengthen the plant organs (Esau, 1977). All of these specific patterns of secondary cell wall are based upon underlying templates of bundled microtubules (Hepler and Newcomb, 1964; Pesquet and Lloyd, 2011; Oda and Fukuda, 2012). Studies in Arabidopsis thaliana show that the overall pattern of microtubules is regulated by specific microtubule-associated proteins (MAPs). Some MAP complexes that stabilize microtubules delimit the sites of secondary cell wall deposition, while other MAP complexes exclude the possibility of thickening by destabilizing microtubules. For example, MAP70-5, which stabilizes microtubules in vitro (Korolev et al., 2007), directly influences TE cell wall patterning; its overexpression leads to an increase in spiral patterning, whereas RNAi knockdown leads to more pitted TEs (Pesquet et al., 2010). In contrast, MIDD1 (MICROTUBULE DEPLETION DOMAIN1), shown to associate with the destabilizing MAP, KINESIN13A (Oda et al., 2010; Oda and Fukuda, 2013), appears to regulate pit formation in metaxylem TEs. Its silencing causes abolition of pits in TEs, leading to the formation of vessels completely covered with unpatterned secondary cell walls (Oda et al., 2010).
Such studies illustrate the roles of different MAPs in fine-tuning the patterns of microtubules, thereby sculpturing the overlying secondary cell wall. However, other classes of protein can be anticipated to interact with microtubules during secondary cell wall assembly. The secondary cell wall of TEs is 10 to 15 times thicker than the primary cell wall of expanding cells and, remarkably, is deposited within a 12- to 16-h time frame (Pesquet et al., 2010, 2011). This presents a major logistical task of delivering secretory vesicles along the microtubules to the overlying secondary wall thickening. During primary wall synthesis, microtubules directly guide cellulose synthesizing complexes via two microtubule interacting proteins, CELLULOSE SYNTHASE-INTERACTING PROTEIN1 (CSI1) and CSI3 (Li et al., 2012; Lei et al., 2013). CSI1 associates with microtubules (Li et al., 2012; Mei et al., 2012), with plasma membrane cellulose synthase complexes, as well as with the microtubule-associated cellulose synthase compartment (MASC) (Crowell et al., 2009; Bringmann et al., 2012), which may form part of the post-Golgi delivery system. Experimental modulation of CSI1 and CSI3 causes changes in both the tracking of cellulose synthase complexes along microtubules and their processivity (Bringmann et al., 2012; Lei et al., 2013), highlighting the tight link between microtubules and cell wall synthesis in primary walls. While similar principles may apply to secondary walls, our understanding of this process is less advanced.
Earlier proteomic analysis of microtubule pull-downs in Arabidopsis identified novel MAPs in bands cut from gels (Korolev et al., 2005) and from whole extracts (Hamada et al., 2013), but these studies were performed on unsynchronized, undifferentiating cells. In this study, we employed quantitative proteomics on paired 14N/15N samples of differentiating Arabidopsis TEs in vitro. This enabled us to compare the entire microtubule pull-down of noninduced cells with the coanalyzed microtubule pull-down of induced cells at morphologically defined stages of TE formation. We also performed in silico interactomics to support the interaction of the identified proteins with each other and with microtubules. Selected candidate genes were then subjected to functional analysis using RNAi knockdown, producing various defects in secondary wall deposition and patterning.
This extensive analysis of an entire functional microtubule interactome and its changes during TE formation widens our understanding of the complexity of microtubule function and defines the level at which microtubules are regulated at key stages of a plant developmental process.
RESULTS
TE Differentiation
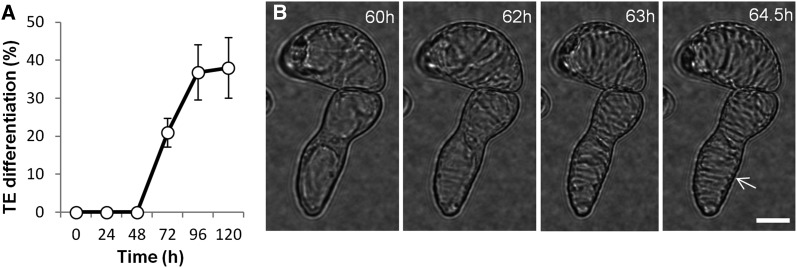
In differentiating Arabidopsis cell cultures, TEs start to develop a visibly patterned secondary cell wall at 60 h after induction and the full differentiation process is completed after 96 h (Figure 1A). Live-cell imaging enabled us to follow the chronology of morphological events marking TE formation: cell elongation occurs between 0 and 60 h and then the first TEs gradually begin to deposit their polysaccharide secondary cell wall, starting at 60 h (Figure 1B) and continuing over the next 12 to 24 h. This is followed by programmed cell death, which is executed in <15 min between 96 and 102 h, concluded by postmortem lignification of the formed secondary cell walls (Supplemental Figure 1 and Supplemental Movies 1 and 2) (Pesquet et al., 2010, 2011, 2013).
Figure 1.
Synchronous TE Differentiation of Arabidopsis Cell Suspension Cultures.
(A) Appearance of secondary walls in populations of TEs visualized by cell wall birefringence during the differentiation time course (average values of 10 × 400 to 500 cells ± sd).
(B) Live-cell imaging of induced single cells between 60 and 65 h beginning their secondary cell wall deposition (Supplemental Figure 1 presents the full chronology of TE development in this study). Birefringence of secondary cell walls of TEs at 64.5 h; note that these were counted as differentiated TEs in (A). Note that TE secondary thickenings (arrow) are gradually deposited and orientated transversely. Bar = 8 µm. See Supplemental Movies 1 and 2.
Transcriptomic Analysis of Marker Genes during TE Differentiation
To confirm that in vitro TE differentiation reliably reflects the TE formation processes in planta, we monitored classical gene markers associated with distinct phases of TE development identified in studies of whole plants. This was performed using Affymetrix microarrays at 12-h intervals from initial induction to 120 h of culture. These included gene markers for the loss of vascular meristem activity (TRACHEARY ELEMENT DIFFERENTIATION INHIBITION FACTOR [TDIF/CLE41]) (Hirakawa et al., 2008), the initiation of xylem mother cells (ATHB8, a member of the homeobox-leucine zipper family) (Baima et al., 2001), the induction of TEs (VASCULAR-RELATED NAC-DOMAIN6 [VND6]) (Kubo et al., 2005), the formation of TE secondary cellulose (cellulose synthase gene CesA7/IRX3) (Brown et al., 2005), and TE autolysis (Xylem cysteine protease gene 1 [XCP1]) (Funk et al., 2002) (Supplemental Figures 2A to 2E). Expression profiles of all marker genes confirmed that the chronological succession of gene expression in this in vitro system corresponded to the different phases of TE development defined for whole plants. We also looked at MAPs already shown to be upregulated during secondary wall formation in TEs between 60 and 84 h: MAP70-5 (Pesquet et al., 2010), MIDD1 (Oda et al., 2010), and MAP65-8 (Soyano et al., 2008) (Supplemental Figures 2H to 2J). Genes previously predicted in whole plants to be associated with cellulose and xylan synthesis in xylem secondary cell walls (IRX6/COBL4 and IRX8/GAUT12) (Brown et al., 2005) were clearly upregulated when TEs were actively depositing secondary cell walls between 60 and 84 h (Supplemental Figures 2F and 2G). Tubulins and KINESIN13A genes exhibited high expression levels irrespective of the particular phase of TE formation, while MAP70-1 and the GTPase gene ROP11 presented relatively low expression levels and expression maximum associated to active secondary cell wall formation (Supplemental Figures 2K to 2O and Supplemental Data Set 1).
Microtubule Pull-Downs and Quantitative Proteomics
Microtubule pull-downs were performed at key stages of TE formation every 24 h from initial induction to 96 h of culture (Supplemental Figure 3A). In pilot studies, mass spectrometry was used to evaluate the release of microtubule interacting proteins from microtubule pull-downs over a range of salt concentrations (Supplemental Figure 4B). Proteins such as dynamin DRP3A (Hamada et al., 2006), ABNORMAL INFLORESCENCE MERISTEM1 (AIM1) (Chuong et al., 2002), and PHOSPHOLIPASE D (PLD) (Gardiner et al., 2001) eluted at 150 mM (Supplemental Figure 4D). Others, such as MAP65 (Mao et al., 2005) and family 7 kinesins (AT5G06670 and AT3G12020) (Zhu and Dixit, 2012), showed medium avidity binding, eluting at 600 mM (Supplemental Figure 4E), while other avidly binding proteins, such as a family 7 kinesins (AT2G21380 and AT1G21730) (Zhu and Dixit, 2012) and CCT chaperone (Leroux and Hartl, 2000), were firmly bound, only eluting at 1200 mM (Supplemental Figure 4F).
Analyses were then placed on a quantitative footing using nitrogen isotope enrichment. Proteins were evenly labeled by replacing all nitrogen-containing macroelements from the Murashige and Skoog (MS) medium with 15N compared with the 14N (standard nitrogen) control. After three weekly subcultures, the heavy nitrogen labeling of proteins rose to 99.5% (Supplemental Figure 4A). No changes in cell growth, cell elongation, or TE differentiation were observed in cells grown in 15N salts. Extracts from the 0 h control (either 15N or 14N) and 24, 48, 72, and 96 h differentiating (either 14N or 15N) cultures were then mixed. Proteins isolated by microtubule pull-down on exogenous microtubules made from porcine tubulin (Supplemental Figure 3A) were separated on gels (Supplemental Figure 4C), and the relative amount of any particular protein following TE induction was quantitatively determined by ratiometric mass spectrometry. Supplemental Figures 3B and 3C show that swapping labels between control and differentiating cultures had no effect on the detection outcome. Scatterplot analysis of variation in the amount of protein observed over the three independent experiments showed that 91% ± 5% of proteins varied within a 2-fold ratio at any particular stage of the TE differentiation process (Supplemental Figure 3D). Significant differences in specific proteins at different stages of the TE differentiation pathway were therefore defined as above 2-fold (Supplemental Figure 3E). The reproducibility of the protein accumulation profiles over the time course was followed in three independent biological experiments, each comprising four sequential time points compared with 0 h, which all showed similar accumulation trends (Supplemental Figure 5).
Immunoblot analysis was then used to check whether changes in the amounts of protein isolated on microtubules across the differentiation process were due to corresponding changes in the total amount of protein (Supplemental Figures 6A to 6F). Candidates included histone deacetylase HDT1 and HDT3, heat shock proteins 70-1 and 90, clathrin heavy chain, and γ-coatomer protein, which were chosen because they showed different accumulation profiles during TE differentiation. The accumulation of each tested protein reflected the same trend as measured during the quantitative proteomic study. Clathrin heavy chain and γ-coatomer exhibited distinct protein accumulation at 72 h (Supplemental Figures 6D and 6F), while HDT1 and HDT3 presented a clear reduction after 24 h (Supplemental Figures 6A and 6C). However, both heat shock proteins showed no significant changes in accumulation during the time course (Supplemental Figures 6B and 6E). The observed changes in protein accumulation measured by isotope enrichment can therefore be related to differences in the amount of proteins present.
Proteomic Analysis of Microtubule Interacting Proteins during TE Differentiation
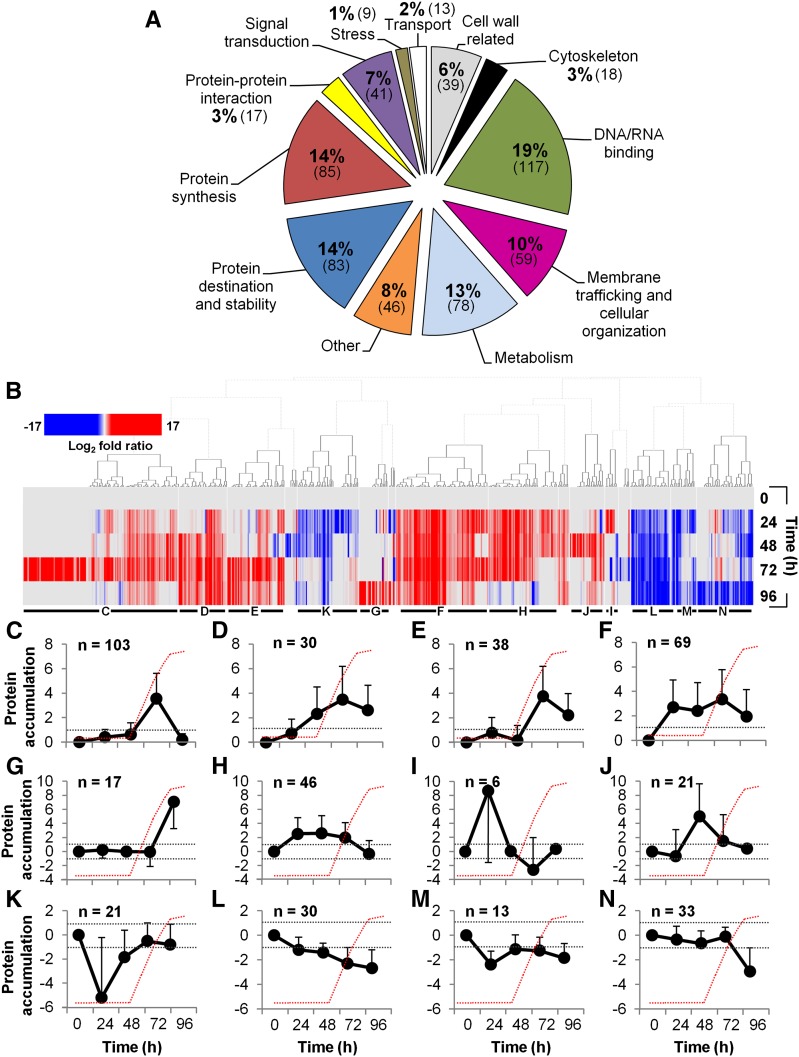
We performed three independent series of experiments, quantitatively analyzing microtubule interacting proteins at the defined stages of TE development; 605 proteins were identified. In these experiments, we measured how the amount of each candidate microtubule interacting protein changed over the course of the differentiation process. Therefore, each experiment consisted of pairwise comparisons between the zero time sample and a TE-induced sample taken at 24, 48, 72, and 96 h. This was done in three separate experiments performed on different days. A core group of 27.6% of proteins was common to all three experiments; 22.6% overlapped between two experiments, whereas 49.8% were specific to individual experiments (Supplemental Figure 7A). Venn diagrams for the three experiments show a similar degree of overlap at each time point (Supplemental Figures 7B, 7D, 7F, and 7H). Twelve main functional groups based on TAIR annotation and known or predicted function, together with known or predicted domains based on EMBL-EBI annotations, could be identified (Figure 2A; Supplemental Data Set 2). The largest groups were represented by DNA/RNA binding (19%), protein synthesis (14%), protein destination and stability (14%), metabolism (13%), and membrane trafficking/cellular organization (10%). Smaller groups were represented by other unclassified proteins (8%), which were mostly unknowns, signal transduction (7%), cell wall related (6%) (over half were related to primary cell walls), protein-protein interaction (3%), transport (2%), and stress (1%).
Figure 2.
Functional Analysis of the Microtubule Interactome and the Different Accumulation Profiles of Microtubule Interacting Proteins during TE Differentiation.
(A) Functional classification of the 605 microtubule interacting proteins isolated in differentiating TE cells according to TAIR classification (Supplemental Data Set 2 provides the identity of each protein of the functional groups).
(B) Color-coded heat map shows the hierarchical clustering of subsets of proteins with distinct accumulation profiles during TE differentiation, expressed as log2-fold ratio (relative to t0 h). Distinct clusters, presented below, are indicated by horizontal black bars and letters. Supplemental Data Set 2 provides the protein accumulation profiles during TE formation of the isolated microtubule interacting proteins.
(C) to (N) Average log2-fold ratio ± sd of specific protein accumulation clusters peaking/dipping at specific times during TE differentiation (n = number of proteins in each cluster). Dotted gray lines indicate the log2-fold thresholds of significant protein accumulation and the red dotted lines represent the appearance of visible TEs as observed in Figure 1A.
Eighteen proteins (3%) were annotated as being part of the cytoskeleton (Supplemental Data Set 2). These include well-established MAPs such as MAP65-1 and MAP65-2 (Smertenko et al., 2004; Mao et al., 2005), seven kinesins (two family 14) (Reddy and Day, 2001; Lee and Liu, 2004; Zhu and Dixit, 2012), AIR9 (Buschmann et al., 2006), and AIM1 (Chuong et al., 2002). Other candidate MAPs based on bibliographical cross-referencing (Supplemental Data Set 3) included MAP190/EMB1579 (Igarashi et al., 2000), seven dynamin-related proteins (Maeda et al., 1992; Hong et al., 2003; Hamada et al., 2006), PLD (Gardiner et al., 2001), CSI1 (Bringmann et al., 2012; Li et al., 2012; Mei et al., 2012), TCTP (Kim et al., 2012), and THO2 (Hamada et al., 2009). Proteins associated with the posttranslational modification of microtubules were also isolated, such as TUBULIN-TYROSINE LIGASE (Szyk et al., 2011), casein kinases (Ben-Nissan et al., 2008), and histone deacetylases (Hubbert et al., 2002; Cho and Cavalli, 2012). Other proteins generally recovered in microtubule pull-down experiments, and which decorate microtubule structures (e.g., the spindle), were also found, such as GLYCERALDEHYDE-3-PHOSPHATE DEHYDROGENASE (Kumagai and Sakai, 1983), heat shock protein 70 (Parrotta et al., 2013), heat shock protein 90 (Petrášek et al., 1998; Krtková et al., 2012), clathrin heavy chains (Royle, 2012), and elongation factor 1α (Hugdahl et al., 1995). Comparison with other Arabidopsis proteomic data sets focusing on identification of proteins binding to tubulin or microtubules (Chuong et al., 2004; Korolev et al., 2005; Hamada et al., 2013) revealed 64 proteins common to our data set (Supplemental Data Set 3). These include candidate MAPs such as SC35-like splicing factor (AT5G64200), several transducins, and SNARE-like protein (AT1G15370) (Supplemental Data Set 3). The isolated proteins also included 19 proteins known to bind MAPs (i.e., interactors of interactors). These included GRF5, which interacts with the MAP EDE1 (Pignocchi and Doonan, 2011), the chaperonin AT1G55490, which interacts with γ-tubulin complex proteins GCP2 and GCP3 (Nakamura et al., 2010), and two interacting partners of MAP70-5, AT1G15200 and IMPA-2 (Arabidopsis Interactome Mapping Consortium, 2011).
Further bibliographic analysis was performed to confirm the biological relationship of the identified proteins with cell wall synthesis and/or xylem/vascular formation (i.e., defects observed in loss-of-function mutation or antisense/RNAi) and cytoskeleton-related TAIR Gene Ontology terms (Supplemental Data Set 3). This comparison yielded 16 genes required for correct vascular development or normal wall deposition. For example, loss-of-function mutations in LOW EXPRESSION OF OSMOTICALLY RESPONSIVE GENES2 (LOS2) affect vascular development (Eremina et al., 2015), while those in CULLIN-ASSOCIATED AND NEDDYLATION-DISSOCIATED1 (CAND1) or the multicopper oxidase-like gene SKS6 alter the venation pattern in leaves (Jacobs and Roe, 2005; Alonso-Peral et al., 2006), and mutations of histone deacetylase genes HDT1 and HDT2 result in filamentous leaves with no xylem when in the background of ASYMMETRIC LEAVES1 (AS1) or AS2 mutations (Ueno et al., 2007). Proteins were also cross-referenced for homology to those with known microtubule association in mammalian and other systems. This included searching for protein domains such as tetratricopeptide repeats (Gindhart and Goldstein, 1996) and ankyrin repeats (Bennett and Davis, 1981; Cheng et al., 2010). In total, 238 (39.3%) of the 605 identified proteins could be associated with MAPs or microtubules/tubulin in both plant and animal systems, as well as cell wall and vascular development in plants (Supplemental Data Set 3).
Differential Accumulation of Microtubule Interacting Proteins during TE Differentiation
Ratiometric analysis revealed proteins that exhibited distinct accumulation profiles at defined time points during TE formation (Figure 2B). Twenty-five percent of the 605 identified proteins showed no significant change in accumulation during TE formation, while 57% overaccumulated and 21% underaccumulated in at least one time point during the differentiation time course (Supplemental Figures 7C, 7E, 7G, and 7I and Supplemental Data Set 2). For example, compared with controls, 161 proteins were overaccumulated at 24 h, 185 at 48 h, 285 at 72 h, and 136 at 96 h (Supplemental Figures 7C, 7E, 7G, and 7I). Conversely, some proteins were specifically underaccumulated at specific stages: 64 at 24 h, 60 at 48 h, 50 at 72 h, and 81 at 96 h (Supplemental Figures 7C, 7E, 7G, and 7I). When considering the accumulation profiles of each class, most overaccumulated proteins belong to the protein synthesis, membrane trafficking/cellular organization, and DNA/RNA binding classes (Supplemental Figures 8G, 8L, and 8M, respectively). The accumulation of all of these proteins peaked at 72 h and was reduced at 96 h. Proteins that underaccumulated during TE formation primarily included cell wall-related, metabolism, and protein destination/stability classes (Supplemental Figures 8A, 8B, and 8H, respectively). These proteins were essentially associated with cell division and elongation events occurring at 0 h prior to TE induction (Supplemental Data Set 2). A significant proportion of our identified microtubule interacting proteins were present in publicly available proteomic surveys that target other specific subcellular compartments in plants (20 independent studies; Supplemental Figure 8N). The fact that 55% of the proteins could be detected in at least two different subcellular proteomes (Supplemental Figure 8O) is consistent with the idea that microtubules bind to a wide range of metabolic compartments.
Proteins That Overaccumulated during Active Secondary Cell Wall Deposition in TEs
We found that 47% of proteins (285) overaccumulated at the key 72 h stage of active secondary cell wall deposition, which exceeded that at any other time point (Figures 2C to 2F; Supplemental Figure 7G and Supplemental Data Set 2). These proteins were dominated by signal transduction, protein synthesis, cytoskeleton, membrane trafficking/cellular organization, and DNA/RNA binding classes (Supplemental Figures 8F, 8G, 8K, 8L, and 8M, respectively). Specifically, 103 proteins accumulated only at 72 h (Figure 2C), 30 accumulated from 48 to 96 h but peaked at 72 h (Figure 2D), 38 accumulated only at 72 and 96 h but their levels were highest at 72 h (Figure 2E), and 69 overaccumulated at all four time points but their levels were highest at 72 h (Figure 2F). Proteins that exhibited the highest accumulation at 72 h included conventional MAPs (e.g., kinesins AT3G45850 and AT2G22610 increased ∼8- and ∼4-fold, respectively), proteins identified in other microtubule proteomic studies (e.g., SC35-like splicing factor AT5G64200) that increased ∼4-fold (isolated by Korolev et al., 2005), and translation initiation factor EIF4A1 that increased ∼12-fold (as also detected in Nakamura et al., 2010) (Supplemental Data Set 2). The signal transduction class included many transducins, such as AT4G04940, which accumulated over 60-fold. Several of the identified proteins have been linked to cell wall synthesis, such as CSI1 and UDP-glycosyltransferase superfamily protein (AT4G36770), which accumulated ∼11- and ∼1050-fold, respectively, at 72 h. Other proteins more specifically associated with secondary cell wall formation, such as amine oxidase 1 (Møller and McPherson, 1998) and dynamin DL3/DRP2B (Xiong et al., 2010), accumulated ∼160- and ∼19-fold, respectively. Lastly, proteins implicated in membrane trafficking, such as SEC10, coatomer β’1 protein, and clathrin heavy chains, accumulated ∼35-, ∼28-, and ∼6-fold, respectively (Supplemental Data Set 2).
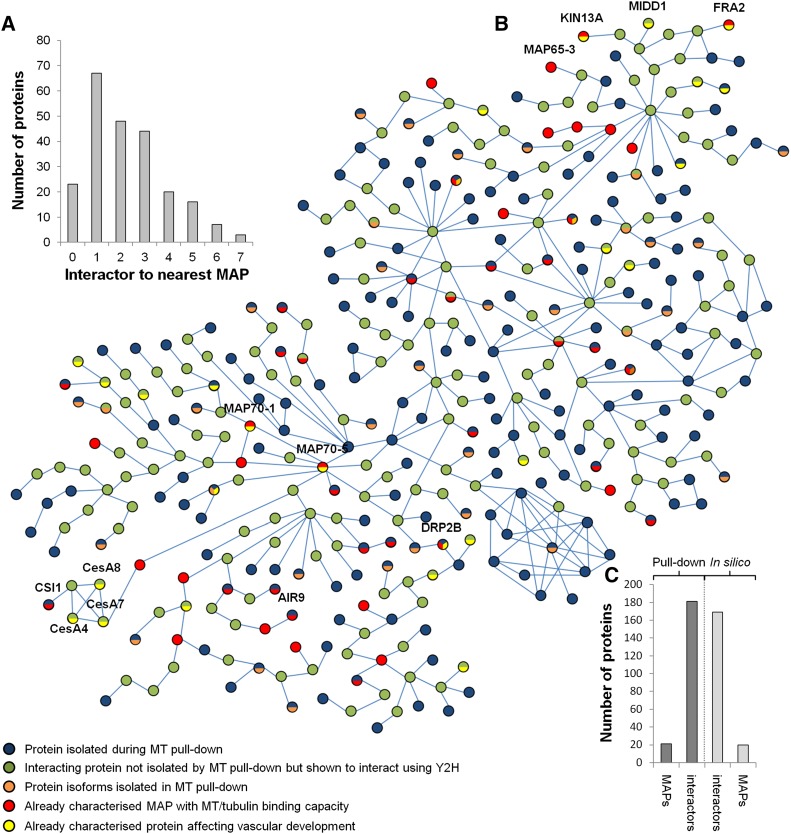
A Microtubule Interactomics Network
In silico analysis was used to assess the distance (via protein intermediates) between microtubules and each of our identified proteins for which yeast two-hybrid protein interaction(s) were publicly available in GenBank (Arabidopsis Interactome Mapping Consortium, 2011). Thirty-two percent of the identified proteins (195 proteins) were present in the Arabidopsis interactome databases. A protein-protein interaction network was constructed to include the following: identified microtubule interacting proteins, known MAPs from previous studies, and known interactors absent from our proteomic data set (Figure 3; Supplemental Figure 9). A total of 45 MAPs (24 identified in our protein data set; 10% of the proteins in the network) offered a direct link to microtubules (Figure 3A), and each line indicates direct yeast two-hybrid protein interactions between proteins identified in our pull-downs or other known interactors (Figures 3B and 3C). Distance to the closest MAPs indicated that 29% of the identified proteins (68 proteins) were direct interactors of known MAPs, 21% (48 proteins) interacted with known MAPs via one intermediate, 19% (44 proteins) interacted through two intermediates, and the remaining proteins (20% or 46 proteins) interacted with known MAPs via more than two intermediate proteins (Figure 3A). This combined analysis supports the close link between the isolated proteins and microtubules, either through direct interaction (15.4%; 93 proteins) or by indirect interaction via established MAPs (34.1%; 206 proteins).
Figure 3.
In Silico Interactomic Network of the Identified Proteins.
(A) Histogram defining the number of intermediates for each identified protein to reach the nearest MAP in the interactomic network. Note that 10% of the 229 identified proteins interact directly while 29% associate with a known MAP through a single intermediate protein.
(B) Interactomic network (n = 402 proteins in total) of the identified proteins (in blue) with known MAPs (in red) or through intermediates not identified in the pull-down experiment (in green). Protein isoforms identified in the proteomic analysis are shown in orange, while genes/proteins previously identified as affecting xylem formation are colored in yellow. Blue bars indicate protein-protein interactions defined through yeast two-hybrid interaction experiments.
(C) Composition of the interactomic network showing the number of proteins identified in the pull-down (23 known MAPs and 185 candidates) compared with the in silico identified MAPs (n = 22) and interactors (n = 172). High-resolution network image with annotation is provided in Supplemental Figure 9.
Effects of RNAi Knockdown of Microtubule Interacting Proteins on TE Formation
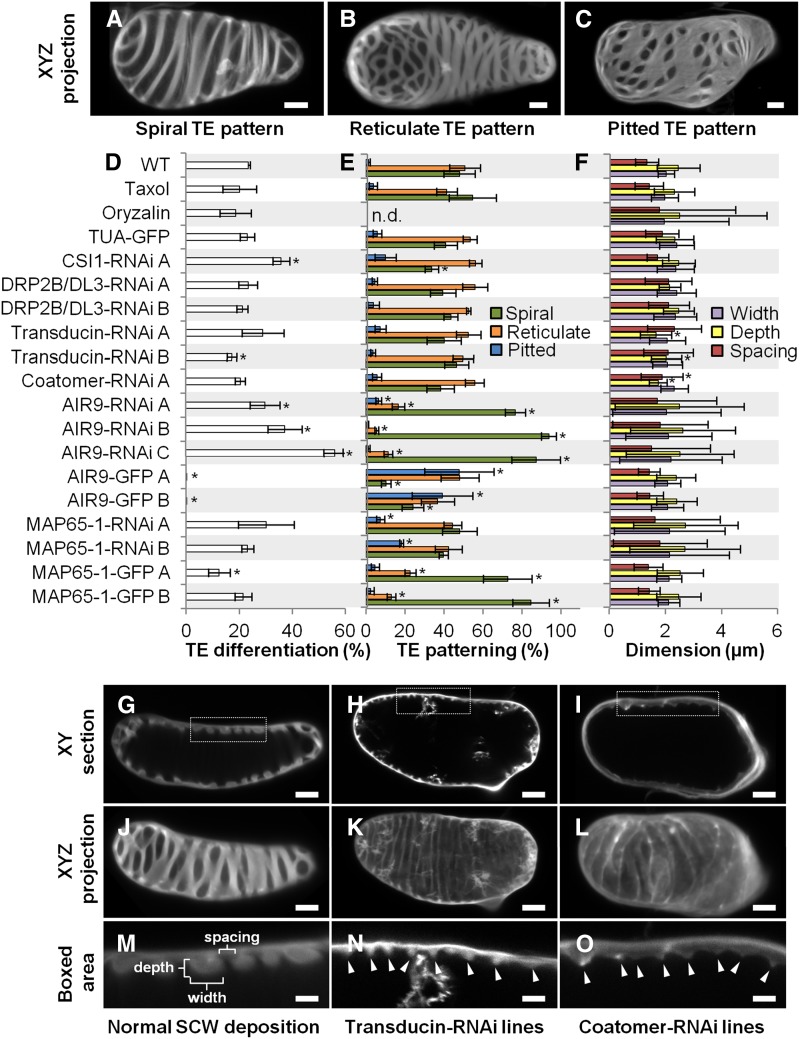
To evaluate the role(s) of the identified proteins belonging to different functional groups and interacting with microtubules, we performed functional analysis centered on microtubule-dependent TE secondary cell wall organization. Pharmacological treatment using the microtubule destabilizing drug oryzalin prior or during active secondary cell wall formation highlighted the importance of microtubules in guiding cell wall material deposition, thereby defining both cortical position and cell wall dimensions (Figures 4 and 5; Supplemental Figure 10 and Supplemental Movie 3). In contrast, microtubule stabilization using taxol did not alter TE cell wall patterning, thickening dimensions, or cortical positioning (Figures 4 and 5). Constitutive RNAi knockdown was therefore used to test whether several identified proteins displayed specific and/or overlapping functions when cells were induced to make TEs and secondary cell walls. Proteins that overaccumulated following induction were selected. Both canonical and novel candidate MAPs that might function in different aspects of secondary wall deposition include the following: (1) CSI1, a MAP that binds to the cellulose synthase machinery in cells producing primary wall (Bringmann et al., 2012; Li et al., 2012; Mei et al., 2012) but whose participation in secondary cell wall production has not been determined; (2) DRP2B/DL3, a potential MAP involved in cellulose synthesis (Li et al., 2010) and regulating cellulose synthase density (Xiong et al., 2010); (3) a putative transducin (AT4G04940) potentially associated with cell wall signaling; and (4) a putative coatomer β’1 protein potentially associated with exocytosis. Both of the latter proteins contain several characteristic WD40 domains found in other identified MAPs (such as NEDD1) (Zeng et al., 2009). These four target proteins overaccumulated more than 10-fold, with maximum levels at the key 72 h stage of secondary cell wall formation (Supplemental Figures 11A to 11D). DRP2B/DL3 and transducin were exclusive to this time point. In addition, we selected two classical MAPs: MAP65-1 (Supplemental Figure 11E), a MAP known to bundle microtubules (Smertenko et al., 2004; Mao et al., 2005); and AIR9 (Supplemental Figure 11F), another established MAP expressed in the vascular system (Buschmann et al., 2006, 2015).
Figure 4.
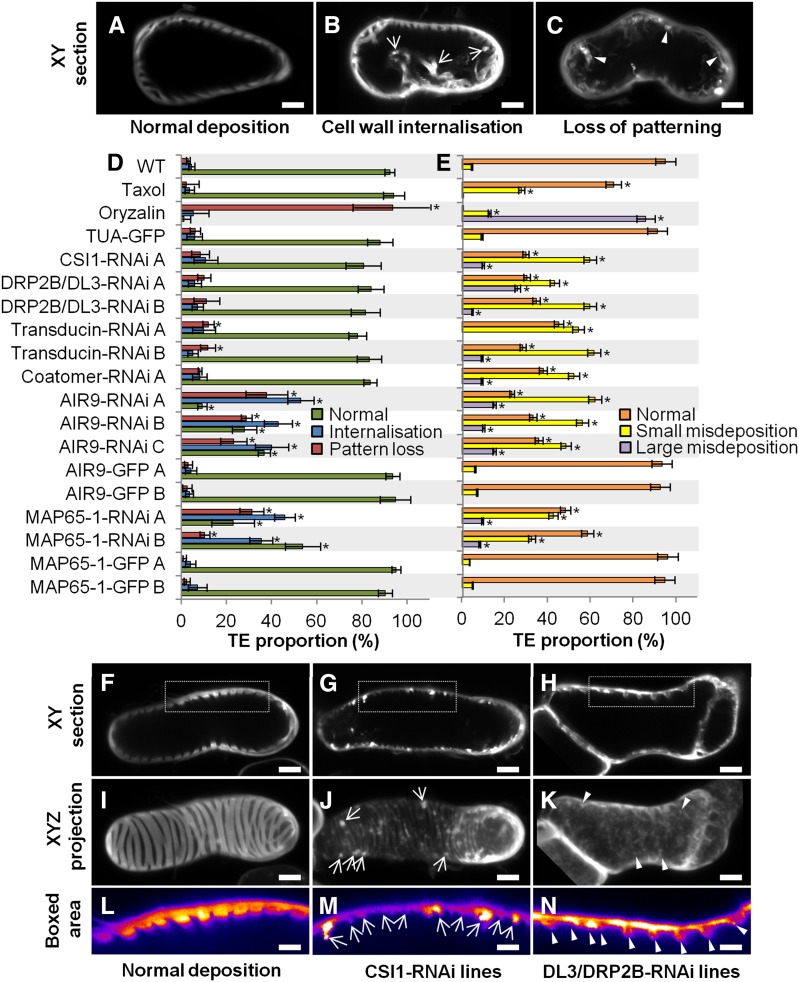
Effect of Constitutive Gene RNAi Knockdown on TE Differentiation, Secondary Cell Wall Patterning, and Dimensions.
(A) to (C) Different TE cell wall patterns shown by half-cell maximal projection of xyz confocal stacks for TEs with spiral pattern (A), reticulate pattern (B), and pitted pattern (C). Bars = 3 µm.
(D) Efficiency of TE differentiation in control, pharmacologically treated, and transgenic cell lines. Control cell lines were treated with 10 µM taxol or 1 µM oryzalin or by constitutively overexpressing (using the 35S promoter) α-tubulin, AIR9, or MAP65-1 N-terminally fused to GFP. Constitutive RNAi knockdown of transducin, coatomer β’1, CSI1, DL3/DRP2B, MAP65-1, and AIR9 (using the 35S promoter). Efficiency of TE differentiation was determined after 96 h culture (three replicates with n = 400 to 500 cells for each cell line).
(E) Distribution of the different TE patterns between control, pharmacologically treated, and transgenic cell lines. The different types of TE pattern are defined as illustrated in (A) to (C). TE pattern distribution was determined after 96 h as described in (D).
(F) TE secondary cell wall dimensions in control, pharmacologically treated, and transgenic lines. Cell wall measurements include depth, width, and spacing between thickenings within a single TE. Wall dimensions were determined after 96 h culture by measuring median sections of 5 to 20 thickenings in 20 to 25 individual spiral and reticulate TEs (n = 112 to 395 individual thickenings for each cell line). Due to cell wall internalization, the dimensions for TE secondary cell walls became highly variable in oryzalin-treated cells and during constitutive RNAi knockdown of MAP65-1 and AIR9. Note that reduction in depth and spacing of secondary thickenings is observed in the silenced transducin and coatomer β’1.
(G) to (I) Image of TEs in median section in control, silenced transducin, and silenced coatomer β’1. Bars = 3 µm.
(J) to (L) Image of half-cell maximal confocal projection for TEs in control (J), silenced transducin (K), and silenced coatomer β’1 (L). Bars = 3 µm.
(M) to (O) Close-up of the boxed area indicated in (G) to (I) showing TE secondary cell wall dimensions. Bars = 2 µm.
Arrowheads in (N) and (O) indicate the reduced but regular distribution of secondary wall thickenings in cell lines constitutively silenced for transducin ([H], [K], and [N]) and coatomer β’1 ([I], [L], and [O]). Error bars refer to sd. Asterisks in (D) to (F) indicate significant difference to control and TUA-GFP cell lines using Student’s t test with P < 0.05. Note that TE secondary cell walls are differently affected by RNAi knockdown of specific microtubule interacting proteins (Supplemental Movie 4).
Figure 5.
Effect of Constitutive Gene RNAi Knockdown on Cortical Position, Organization, and Deposition of Secondary Cell Walls.
(A) to (C) TE cell wall organization presented in median xy confocal sections for controls (A), TEs with secondary cell wall internalization (B), and TEs with a complete loss of secondary cell wall pattern (C). Arrows indicate secondary cell wall strands traversing the lumen, and arrowheads indicate large strands of wall material deposited unevenly at the cortex and protruding through the lumen. Bars = 3 µm.
(D) Defects in secondary cell wall organization in control, pharmacologically treated, and transgenic cell lines. Internalization and loss of patterning were determined after 96 h (ratio of altered TEs; three replicates, n = 400 to 500 cells per cell line).
(E) Small and large misdepositions of cell wall material in control, pharmacologically treated, and transgenic cell lines.
(F) to (N) Misdeposition seen in median confocal xy sections ([F] to [H]) and half-cell maximal projections ([I] to [K]).
(L) to (N) Eight-bit color-coded distribution (0 = black, white = 256) of Calcofluor-stained cell walls of the boxed area in (F) to (H). Bars = 3 µm.
Large (arrows in [J] and [M]) and small (arrowheads in [K] and [N]) abnormalities were determined after 96 h as the ratio of TEs with defects compared with all TEs (n = 3 replicates of 7 to 10 TEs for each cell line). Secondary wall is evenly stained by Calcofluor between TE thickenings in controls ([F], [I], and [L]), but misdepositions are observed on and between thickenings for CSI1 ([G], [J], and [M]) and DL3/DRP2B ([H], [K], and [N]) knockdown lines. Large misdepositions (arrows in [J]) cause uneven distribution of secondary wall within and between individual thickenings (arrows in [M]). Small misdepositions (arrowheads in [K]) also cause uneven distribution (arrowheads in [N]). Error bars refer to sd. Stars indicate significant difference to control and TUA-GFP cell lines using Student’s t test; P < 0.05. See Supplemental Movie 4.
Quantitative RT-PCR confirmed the downregulation of these selected genes in each independent line, ranging from 34 to 66% of residual gene expression (Supplemental Figure 12C). Cell growth and elongation were unaffected by constitutive gene RNAi knockdown for all genes studied (Supplemental Figures 12A and 12B), allowing us to focus specifically on TE differentiation and secondary cell wall organization. In all lines tested, no delay in the time of TE appearance was observed, although some varied in efficiency. CSI1-RNAi and AIR9-RNAi lines produced more TEs after 96 h (up to 2-fold more than controls), and one transducin line produced slightly fewer TEs, but the other RNAi lines were not affected (Figure 4D). Most of the resulting TEs had spiral or reticulate patterns in approximately equal proportions, with only 5 to 10% exhibiting a pitted pattern (Figures 4A to 4C). In most RNAi lines, gene knockdown did not affect the distribution of TE patterns, although CSI1-RNAi produced fewer spiral and more pitted TEs (Figure 4E). In contrast, AIR9 RNAi lines showed fewer reticulate and more spiral patterns, whereas MAP65-1 RNAi produced more pitted patterns (Figure 4E). In spiral and reticulate TEs, the patterned secondary wall normally displays ordered spacing, and the thickenings tend to have similar dimensions (width and depth), as seen in transverse section (Figures 4G, 4J, and 4M). As a population, coatomer β’1 and transducin RNAi lines had thinner cell walls than wild-type and TUA-GFP controls (Figure 4F), although within any one TE, the thickenings were uniform (Figures 4H, 4I, 4N, and 4O). Although the walls were thinner, the patterns were unaffected, except for 11 to 13% of TEs in transducin RNAi lines, which showed loss of patterning (Figures 4K and 4L). In contrast to such uniformity, the patterning and dimensions of secondary thickenings were highly variable in MAP65-1 and AIR9 RNAi lines, resembling the disorganization seen with the microtubule-depolymerizing drug oryzalin (Figure 4F; Supplemental Figures 10E to 10L). Compared with the normal cortical positioning of walls in control TEs (Figure 5A), the knockdown of some candidate genes resulted in cell wall internalization (Figure 5B) and even loss of patterning (Figure 5C). Cell wall internalization corresponded to the loss of cortical positioning of the secondary thickenings in TEs, which protrude within the cell (Figure 5B; Supplemental Figures 10E to 10L). A total of 64 to 91% of AIR9 RNAi lines contained TEs with cell wall internalization defects, while MAP65-1 RNAi showed 47 to 78% defects (Figure 5D). A small but significant increase in pattern loss was also seen in transducin RNAi lines (Figures 5C and 5D), whereas other lines were not affected. Normal secondary cell wall deposition results in alternating thickened and unthickened regions that can clearly be observed in xy confocal sections of TEs (Figures 5F, 5I, and 5L). A loss of this cell wall homogeneity, characterized by the cortical misdeposition of small patches of secondary cell wall deposited randomly between or on some of the secondary thickenings, was observed when some candidate genes were knocked down. Such perturbations could be large (Figures 5G, 5J, and 5M) or small (Figures 5H, 5K, and 5N). Small misdepositions were observed in all RNAi lines but occurred in fewer than 10% of wild-type and tubulin-GFP controls (Figure 5E). In CSI1 and DRP2B/DL3 lines, small misdepositions occurred at a higher frequency (>50%), while large misdepositions not seen in control cell lines did occur, but generally at a frequency of 7 to 27% (Figure 5E). Supplemental Movie 4 provides examples of these different modifications of TE cell walls observed by confocal through-focusing and half-cell 3D reconstructions.
Effects of MAP65 and AIR9 on Microtubule/TE Patterning
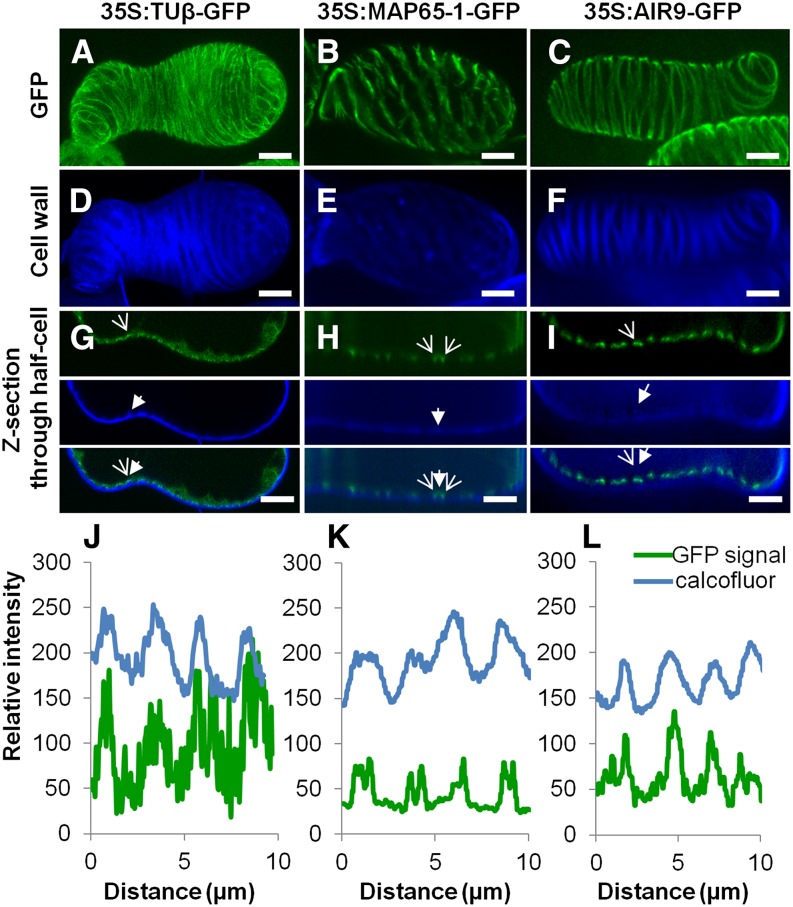
In view of their pronounced effects on TE wall patterning, we looked more closely at the localization of AIR9 and MAP65-1. Constitutive N-terminal GFP fusions of AIR9 and MAP65-1 driven by the 35S promoter were stably transformed into differentiating TEs. This dramatically reduced differentiation, by 95 to 97% for AIR9 and by 80 to 90% for MAP65-1 (Figure 4D). AIR9 overexpression induced a shift in TE patterning, significantly increasing the presence of pitted TEs, while RNAi knockdown resulted in more spiral TEs (Figure 4E). MAP65-1 overexpression also affected TE patterning by increasing the number of spiral thickenings, while RNAi knockdown resulted in more pitted TEs (Figure 4E). β-Tubulin GFP fusion was observed directly beneath the secondary thickenings of TEs and on transverse microtubules beneath the intervening unthickened areas (Figures 6A, 6D, 6G, and 6J ; Supplemental Movie 5), whereas MAP65-1 was found on microtubules flanking the secondary thickenings and beneath modified primary cell walls (Figure 6B, 6E, 6H, and 6K; Supplemental Movie 5). By contrast, GFP-AIR9 exclusively labeled the cortex directly beneath secondary thickenings but was absent from transverse microtubules in the areas between thickenings (Figures 6C, 6F, 6I, and 6L; Supplemental Movie 5). To investigate the binding of these different MAPs to microtubules, GFP fusions of MAP65-1 or AIR9 were transformed into cells already stably expressing α-tubulin RFP. For both MAPs, α-tubulin RFP microtubules were readily visible at the cortex of the interphase cells (Supplemental Figures 13A to 13F). Colocalization analysis revealed that MAP65-1 covered ∼69 to 77% of microtubules in small linear patches (Supplemental Figures 13A to 13C and 13G), while AIR9 decorated a similar number of microtubules (71 to 75%) but in a continuous manner (Supplemental Figures 13D to 13F and 13H). This analysis indicates that MAP65-1 and AIR9 label microtubules with different subcellular localizations, i.e., flanking or directly beneath TE secondary cell walls, respectively.
Figure 6.
Differential Labeling of Microtubules by MAP65-1 and AIR9.
(A) to (C) Subcellular localization in TEs of tubulinß8 (A), MAP65-1 (B), and AIR9 (C) using constitutively expressed 35S promoter N-terminal GFP fusion proteins; bars = 5µm.
(D) to (F) Corresponding TE secondary cell wall stained with Calcofluor. bars = 5µm.
(G) to (I) Confocal cross section illustrating spatial relationship between GFP fusion protein and TE secondary thickenings for tubulinß8 (G), MAP65-1 (H), and AIR9 (I). Open arrows indicate fusion protein while closed arrows indicate TE cell wall thickening. Bars = 5 µm.
(J) to (L) Analysis of fluorescence intensity (8-bit values) distribution along the cell cortex (in µm) between Calcofluor-stained wall fluorescence and GFP fusion proteins. Note that tubulin accumulates mostly underneath TE thickenings (J). MAP65-1 flanks thickenings (K), whereas AIR9 accumulates exclusively beneath TE thickenings (L). Supplemental Movie 5 illustrates the subcellular localization of tubulin β8, AIR9, and MAP65-1 in TEs.
DISCUSSION
The Diversity of Proteins Associated with Microtubules
In this study, the synchrony of TE differentiation in Arabidopsis cell suspension cultures, coupled with the quantitative analysis of proteins, allowed specific microtubule interacting proteins to be related to defined morphological stages of tracheary element formation. A major feature is the large number and variety of proteins found to interact with microtubules (Figure 2; Supplemental Figures 7 and 8 and Supplemental Data Set 2). The number and diversity of the functional groups were not entirely unexpected, since the ability of proteomics to dramatically widen the range of known microtubule interactors has been amply demonstrated by a growing variety of similar studies, such as studies of shrimp embryos (O’Connell et al., 2006), Drosophila melanogaster embryos (Hughes et al., 2008), macrophage cell lines (Patel et al., 2009), Xenopus laevis oocytes (Gache et al., 2010), mammalian brain (Kozielski et al., 2011), and Arabidopsis cells (Hamada et al., 2013). Our in silico interactomics analysis of the identified proteins further supported their association with microtubules, suggesting that many are likely to interact indirectly as parts of protein complexes (Figure 3). In addition, this proteomic survey tends to suggest that microtubules in plant cells form a hub linking together many cellular compartments, such as the actin microfilaments (Sampathkumar et al., 2011; Sambade et al., 2014), nucleus (Goto and Asada, 2007; Batzenschlager et al., 2013), peroxisomes (Chuong et al., 2005; Hamada et al., 2012), endoplasmic reticulum (Gupton et al., 2006; Hamada et al., 2012, 2014), Golgi apparatus (Wightman and Turner, 2008; Crowell et al., 2009), and cell wall, thereby coordinating the overall organization of the plant cell.
Microtubules and Secondary Wall Deposition
The primary cell wall, which can be 0.1- to 1.0-µm thick (Derbyshire et al., 2007), is synthesized by Golgi-derived vesicles containing cellulose synthase complexes or MASCs, which pause on cortical microtubules before their insertion into the plasma membrane (Crowell et al., 2009). These complexes then have an average lifetime at the plasma membrane of 7 to 8 min (Sampathkumar et al., 2013) before they are recycled. Compared with the primary wall, the secondary thickenings of TEs are much thicker (2 to 2.5µm) and are deposited in a relatively narrow 12- to 16-h window (Pesquet et al., 2010). This highlights the logistical task of maintaining such a large increase in the secretion and recycling of cellulose synthase complexes as well as the secretion of other products into the wall and plasma membrane. Microtubule-associated CSI1 has not so far been reported to be active during secondary wall formation, but our data show that it peaks at 72 h, when secondary wall cellulose deposition is active (Supplemental Figure 11A and Supplemental Data Set 2). We also show that the knocking down of CSI1 in differentiating TEs results in misguided wall deposition (Figures 5E, 5G, 5J, and 5M), confirming its functional role in secondary wall formation and more specifically in linking the cell wall synthesis machinery to sites marked by microtubules.
In addition, our analysis of microtubule pull-downs detected a variety of candidate MAP(s) and/or MAP interactor(s) that have been implicated in wall formation. Such proteins include amine oxidase 1, COBL8 (COBRA-LIKE8), a legume lectin-family protein (AT3G15356), UDP-Xylose Synthase 6, and UDP-glycosyltransferase 72C1 (AT4G36770). COBRA, a GPI-anchored protein, is distributed by a microtubule-dependent mechanism that regulates the deposition and orientation of cellulose microfibrils in the primary cell wall (Sorek et al., 2014). Its relative, COBL-8, was found in our study to overaccumulate 48,000-fold at the key stage of secondary wall formation (Supplemental Data Set 2).
Previous studies have shown a dynamic linkage between microtubules and the endoplasmic reticulum (ER) (Gupton et al., 2006; Hamada et al., 2012, 2014) and Golgi apparatus (Wightman and Turner, 2008; Crowell et al., 2009). Such a close connection between microtubules and the endomembrane system during secondary cell wall formation is supported in this study by the accumulation at 72 h of many proteins involved in secretion and recycling. These include proteins associated with (1) ER emergence sites (SEC24a/ERMO2, SPK1); (2) ER-to-Golgi vesicle transport (four Sec23/sec24-like for COPII and coatomer α1, α2, β1, β2, β’1, β’2, β’3, γ, ε, and ζ3 for COPI); (3) vesicle-mediated transport from the Golgi to the plasma membrane or vacuole (the exocyst system, SEC3A, SEC6, SEC10, SEC15B, and the retromer; and ESCRT system, VPS29/MAG1, VPS35A, VSP35B, SNF7.2, and VCL1); and (4) endocytosis by clathrin-coated vesicles (seven dynamins, DL6/DRP2A, DL3/DRP2B, DL5/DRP1C, DRP1A, DRP1E, DRP3A, and DRP3B; γ-adaptin1; and two clathrin heavy chains). Notably, the levels of all exocyst components isolated in this study peaked (between 26- and 96-fold) at the key 72 h stage, strengthening the idea that the microtubule cytoskeleton marks the developing secondary thickenings as delivery sites for the exocyst. Although no colocalization between microtubules and the exocyst was observed during primary cell wall formation (Fendrych et al., 2013), a loss-of-function mutant in exocyst component EXO70A1 exhibits changes in TE patterning and misguided secondary cell wall deposition (Li et al., 2013). EXO70A1 interacting proteins VESICLE TETHERING1 (VETH1) and VETH2 have recently been shown to bind to the plus end of microtubules of differentiating TEs (Oda et al., 2015). ER-to-Golgi vesicle transport is also required for correct TE formation. RNAi knockdown of coatomer β’1 resulted in thinner but regularly patterned secondary walls (Figures 4F, 4I, 4L, and 4O), suggesting that other aspects of endomembrane trafficking affect microtubule-guided cell wall formation. Similarly, all endocytotic components accumulated between 4- and 20-fold during active cell wall synthesis, and knocking down of DL3/DRP2B caused misguided TE cell wall depositions (Figures 5E, 5H, 5K, and 5N), confirming the tight link between endocytosis and microtubules during secondary wall formation. These results are consistent with the phenotype observed in the rice (Oryza sativa) DRP2B null mutant (brittle culm3) (Hirano et al., 2010; Xiong et al., 2010), whose altered cell wall thickness indicates that DRP2B helps regulate trafficking of the secondary cellulose synthase complexes to and from the plasma membrane (Hirano et al., 2010; Li et al., 2010; Xiong et al., 2010). A major advance in recent years has been the discovery of cellulose synthase-containing compartments that move along microtubules for insertion into the plasma membrane (Crowell et al., 2009; Bringmann et al., 2012). These are referred to as small CesA compartments (Gutierrez et al., 2009) or MASCs (Crowell et al., 2009). The fact that our microtubule pull-downs identify a significant number of members of the secretory complex, as well as the microtubule-CesA linker CSI1, under quite stringent salt concentrations indicates that the proteomics list is likely to include constituents of the MASC/small CesA compartment isolated before and during insertion into the plasma membrane, as well as components associated with the endocytotic recycling of the synthesizing complexes.
The Multiple Roles of Microtubules during TE Secondary Cell Wall Formation
The variety of microtubule interacting proteins identified in this study emphasizes the functional range of microtubules during secondary cell wall formation in TEs. The eventual role of TEs in sap conduction relies entirely on uniformly thickened secondary walls deposited at the cortex. Our data show that experimental modification of some of the microtubule interacting proteins causes wall formation to be misdirected to internal locations, indicating that the formation of the secondary wall against the primary cell wall is not inevitable. When correctly positioned at the cortex, the secondary cells walls are organized in a limited number of specific patterns. These include protoxylem-type TEs with annular and spiral thickenings and metaxylem-type TEs with reticulated and pitted cell walls (Figures 4A to 4C). Microtubules initially function in determining the cortical patterning of TE walls through the subcellular localization of specific MAPs: MAP70s flank the thickened areas (Pesquet et al., 2010), whereas MIDD1 labels the unthickened part of the cell walls (Oda et al., 2010). In addition to these proteins, two other MAPs, MAP65-1 and AIR9, directly regulate the type of TE pattern and its location at the cortex, as shown by the knockdown and overexpression studies. MAP65-1 specifically regulates TE patterning, producing more spiral type TEs when overexpressed and more pitted TEs when knocked down (Figure 4E). Conversely, AIR9 induced more spiral TEs when knocked down and more pitted TEs when overexpressed (Figure 4E). We observed that GFP fusions of these MAPs did not destabilize the cortical microtubule network in interphase cells but labeled different microtubules surrounding the secondary thickenings of TEs. While β- or α-tubulin labeled the entire microtubule bundle that cupped the thickenings (Figures 6A, 6D, and 6G; Pesquet et al., 2010), MAP65-1 exclusively labeled microtubules flanking the edges of bundles, whereas AIR9 only labeled microtubules directly beneath secondary thickenings (Figure 6). It therefore appears that TE patterning is associated with the specific subcellular localization of different MAPs in regards to thickened and unthickened areas of the cell wall.
Different MAPs fulfill different functions: Not only do they direct precursors to the cortex, but different classes of MAPs appear to ensure a microdifferentiation in which the wall sculpturings conform to stereotypical patterns of an appropriate thickness. This is borne out by our results showing that some microtubule interacting proteins affect the delivery process, some affect the patterning of insertion into the wall, and others affect both. For example, RNAi knockdown of the endomembrane trafficking-related coatomer β’1 altered the relative thickness of the wall without affecting the overall pattern of that secondary wall (Figures 4E, 4F, 4I, 4L and 4O). On the other hand, the transducin selected for functional analysis represents a protein with dual roles in patterning and delivery, as its knocking-down resulted in both a loss of patterning and reduced thickness of the wall (Figures 4E, 4F, 4H, 4K, 4N, and 5D). RNAi knockdown of both cellulose synthase-associated CSI1 and endocytosis-associated DL3/DRP2B resulted in misguided cell wall deposition and uneven secondary wall thickenings (Figures 5E, 5G, 5H, 5J, 5K, 5M, and 5N).
This study underlines the great diversity of proteins that associate with microtubules in the narrow window of time in which massive thickenings are deposited in the secondary cell wall. These proteins fulfill a variety of roles. It would therefore appear that multiple mechanisms must be coordinated in order to ensure the optimal structure of TEs for the efficient conduction of the hydro-mineral sap and, hence, the plant’s nutrition (Ménard and Pesquet, 2015).
METHODS
Arabidopsis Cell Culture and Induction of Tracheary Elements
The habituated stem cell-like suspension culture used in this study was derived from roots of Arabidopsis thaliana Col-0 (Pesquet et al., 2010). Subculturing of cells was performed weekly by transferring 10% of 7-d-old cells into 1× MS medium + 3% (w/v) sucrose at pH 5.7. Cell growth was maintained in the dark at 24 to 25°C with orbital agitation at 120 rpm (3-cm agitation radius). TE induction was performed as described previously (Pesquet et al., 2010) with 6 µg/mL of 1-naphthalene acetic acid (N0640; Sigma-Aldrich), 1 µg/mL of 6-benzylaminopurine (B3408; Sigma-Aldrich), and 4 µM epibrassinolide (E1641; Sigma-Aldrich) on freshly subcultured cells. Drug treatments were performed with 10 µM taxol (T7191; Sigma-Aldrich) or 1 µM oryzalin (N12729; Sigma-Aldrich), solubilized as 1000× stock solutions in DMSO.
RNA Extraction for Marker Gene Transcriptomics
RNA extraction from cells along the TE differentiation time course was performed according to Pesquet et al. (2010). Cell suspensions were harvested using a vacuum filtration system (Sterifil; Millipore) on 100-µm nylon meshes. Filtered cells were frozen in liquid nitrogen and ground to a fine powder in liquid nitrogen using a ceramic pestle and mortar. One milliliter of TRIzol reagent solution (Sigma-Aldrich) was then added to the ground cells. Total RNA was isolated according to the manufacturer’s instructions and subjected to DNA digestion with 5 units of RQ1 RNase-free DNase I (Promega) for 1 h at 37°C. A second round of RNA extraction was then performed using TRIzol reagent solution. RNA was quantified using an RNA Biophotometer (Eppendorf) and visualized after electrophoresis on 1.5% (w/v) agarose gels. The lack of DNA contamination was confirmed by performing PCR on RNA with 18S rRNA PCR primers at 55°C annealing temperature for 40 cycles. Under these conditions, no bands could be detected. PCR primers used in this article are listed in Supplemental Table 1.
Affymetrix Microarray Hybridization and Data Analysis
Global gene expression analysis of the Arabidopsis entire transcriptome, using the Affymetrix GeneChip ATH1 genome array, was performed in three independent experiments. RNA was prepared from cell cultures every 12 h after TE induction up to 120 h. Array hybridization, signal quality and expression analysis were performed at the John Innes Centre (Norwich, UK) according to standard protocols. The resulting signal values were checked for signal quality and robust multichip average normalized using Affymetrix standard procedure using GeneSpring software. A list of the expression of selected genes is provided in Supplemental Data Set 1.
15N Labeling of Cell Cultures for Proteomics
Cells were subcultured into 1× MS medium + 3% (w/v) sucrose at pH 5.7 in which the nitrogen-containing salts NH4NO3 and KNO3 were replaced by the 15N form (CK Gas Products) at the same molar amounts as in standard (14N) 1× MS medium and maintained as above. After three weekly rounds of subculture, the incorporation of 15N into the cell proteome was calculated to be >99.5% (Supplemental Figure 4A).
Cell cultures with protein labeling saturated with 15N were induced to become tracheary elements as described above. Cells were harvested at four time points during the TE differentiation process: 24, 48, 72, and 96 h (t24-96). In parallel, cell cultures saturated in 14N were harvested as the 0 h (t0) noninduced (basal) control. Two reciprocal experiments were performed where the isotopes were switched, i.e., basal control cells grown in the presence of 15N salts and induced cells during the TE differentiation process grown in 14N salts. Cell suspensions were harvested using a vacuum filtration system as described above. Filtered cells were weighed and frozen in liquid nitrogen and stored at −80°C. Three independent induction experiments (biological replicates A, B, and C) were performed.
Total Soluble Protein Extraction from Differentiating Cell Cultures
Frozen cells were ground to a fine powder in liquid nitrogen using a mortar and pestle, resuspended in ice-cold extraction buffer (100 mM HEPES, pH 7.5, with KOH, 500 mM NaCl, 5 mM EDTA, 5 mM NaF, 1 mM DTT, 0.4 mM phenylmethylsulfonyl fluoride, 1 mg/mL polyvinylpyrrolidone, and one tablet of Protease Inhibitor Cocktail [Roche Complete Mini, EDTA-free] per 10 mL of buffer) at 1 mL/g fresh weight, mixed thoroughly by vortexing, and incubated on a rotating platform for 1 h at 4°C. Extracts were centrifuged at 95,000g for 45 min at 4°C using a Sorvall OTD Combi ultracentrifuge with the AH650 swinging bucket rotor. Supernatants were collected and concentrated using Amicon centrifugal filter units (15 mL) with a 10-kD MWCO membrane (Millipore), according to the manufacturer’s instructions, at 3900g, 4°C, on a Sorvall RC3C Plus centrifuge with the H-6000 swinging bucket rotor until most of the liquid had been removed. Protein extracts were resuspended in the filter unit for buffer exchange with 14 mL ice cold PEM buffer (50 mM PIPES, 5 mM EGTA, and 5 mM MgSO4 at pH 6.9) and centrifuged at 3900g, 4°C, until ∼12 mL buffer had passed through the filter unit. The concentrated protein extracts were resuspended in the remaining 2 mL of PEM buffer, transferred to new tubes, and frozen.
Isolation of Interacting Proteins by Microtubule Pull-Down
Taxol-stabilized microtubules were prepared by adding 25 μL PEMT (PEM buffer + 20 µM taxol) to 250 µg porcine brain tubulin (T240-A; Cytoskeleton) and incubating for 1 h at 37°C. Protein samples were thawed slowly on ice and ultracentrifuged at 95,000g for 45 min at 4°C to remove any precipitate. Total soluble protein concentrations were measured by Bradford assay. Equal amounts (6.25 mg) of proteins from t0 and one time point during the time course (t24-96) were combined on ice and supplemented with 20 µM taxol. Taxol-stabilized microtubules (250 µg) were added and gently mixed by pipette aspiration and incubated for 1 h at 4°C. The protein sample/microtubule mix was ultracentrifuged at 65,000g for 30 min at 4°C, and the supernatant containing unbound protein was removed. The pellet was washed with 1 mL ice cold PEMT and incubated on ice for 15 min, followed by further ultracentrifugation at 65,000g for 30 min at 4°C. The supernatant (wash fraction) was removed and the pellet washed in 250 μL PEMT + 1.2 M NaCl and incubated for 30 min on ice in order to elute microtubule interacting proteins from the microtubule pellet, followed by further ultracentrifugation at 65,000g for 30 min at 4°C. The procedure is summarized in Supplemental Figure 3A. In pilot experiments, sequential salt washes were performed to sequentially elute microtubule interacting proteins from the microtubule pellet with 250 μL PEMT + 0.15/0.6/1.2 M NaCl. Between each salt concentration, ultracentrifugation at 65,000g was performed as above and the supernatant collected. The supernatant containing microtubule-enriched proteins was removed and the microtubule interactome elution yield determined by Bradford protein assay. The remaining pellet was resuspended in 100 μL ice-cold PEMT and saved. The effects of increasing NaCl concentrations can be seen in Supplemental Figure 4B.
Fractionation of Microtubule Pull-Down Eluted Proteins by Gel Electrophoresis
Protein fractions isolated by microtubule pull-down (17 µg of each) were denatured in a one-quarter volume of NuPage lithium dodecyl sulfate loading buffer (Invitrogen) at 100°C for 10 min and separated on a NuPAGE 4 to 12% Bis-Tris gel (Invitrogen) in MOPs buffer in the presence of NuPAGE reducing agent (Invitrogen) according to the manufacturer’s instructions. A ColorPlus Prestained Protein Marker (P7709V; New England Biolabs) was used as a reference according to the manufacturer’s instructions. Gels were stained with Instant Blue (Expedeon) for 1 h and washed in sterile distilled water. To reduce the complexity within each time point sample, each gel lane was cut into five equal slices of ∼6 × 12 mm (Supplemental Figure 4C).
Mass Spectrometry
Each gel slice was washed, reduced, and alkylated, and treated with trypsin according to standard procedures (Shevchenko et al., 1996). Peptides were extracted with 5% (v/v) formic acid/50% acetonitrile (v/v), dried down, and redissolved in 0.1% (v/v) trifluoroacetic acid.
For liquid chromatography-tandem mass spectrometry analysis, a sample aliquot was applied via a nanoAcquity UPLC system (Waters) running at a flow rate of 250 nL min−1 to an LTQ-Orbitrap mass spectrometer (Thermo Fisher). Peptides were trapped using a precolumn (Symmetry C18, 5 µm, 180 µm × 20 mm; Waters), which was then switched in-line to an analytical column (BEH C18, 1.7 µm, 75 µm × 250 mm; Waters) for separation. Peptides were eluted with a gradient of 3 to 40% (v/v) acetonitrile in water/0.1% (v/v) formic acid at a rate of 0.67% min−1. The column was connected to a 10-µm SilicaTip nanospray emitter (New Objective) attached to a nanospray interface (Proxeon) for infusion into the mass spectrometer. The mass spectrometer was operated in positive ion mode at a capillary temperature of 200°C. The source voltage and focusing voltages were tuned for the transmission of MRFA peptide (m/z 524) (Sigma-Aldrich). Data-dependent analysis was performed in Orbitrap-IT parallel mode using collision-induced dissociation fragmentation on the six most abundant ions in each cycle. The Orbitrap was run with a resolution of 30,000 over the mass spectrometry range from m/z 350 to 1800 and a mass spectrometry target of 106 and 1-s maximum scan time. Collision energy was 35, and an isolation width of 2 was used. Only mono-isotopic 2+ and 3+ charged precursors were selected for tandem mass spectrometry. The tandem mass spectrometry was triggered by a minimal signal of 1000 with an AGC target of 3 × 104 ions and 150-ms scan time using the chromatography function for peak apex detection. Dynamic exclusion was set to 1 count and 60-s exclusion with an exclusion mass window of ±20 ppm. Mass spectrometry scans were saved in profile mode, while tandem mass spectrometry scans were saved in centroid mode.
Protein Quantitation
Data were processed and quantitated in Mascot Distiller Version 2.4.2.0 (Matrixscience). All runs from all five gel slices from the three replicates of each time point were merged in one Mascot Distiller multifile project. For peak-picking, the profile mass spectrometry scans were regridded with 500 data points per Dalton and precursors with charge 1+ to 4+ were selected. Precursor charge stage was redetermined from the parent scan. Tandem mass spectrometry scans were used in centroid mode and maximum charge stage set to 2+. Other processing options were set to default values.
The database search was performed on the TAIR10 database (https://www.arabidopsis.org/) using an in-house Mascot 2.3 server (Matrixscience) with 15 ppm precursor tolerance, 0.5 D fragment tolerance, carbamidomethylation (C) as fixed, and oxidation (M) and acetylation (N terminus) as variable modifications. For protein scoring, the MudPIT option was used, and for peptide scoring, a significance threshold of 0.05 and an expected cutoff of 0.05 were used.
The precursor protocol was used for protein quantitation based on 99.5% labeling efficiency with 15N and at least two peptides per protein. The ratio was calculated as the median of the peptide ratios, all charge stages were included, outlier removal was set to auto, and no normalization was applied. Only peptides passing the following thresholds were included in quantitation: correlation 0.8, fraction 0.45, and standard error 0.15. The resulting Mascot Distiller quantitation tables were exported into Microsoft Excel.
Data Analysis and Classification of Microtubule Interacting Proteins
For each time point, the ratios (induced/basal) from each of the three experiments were averaged. This was performed using n-weighted averaging (and n-weighted sd) to take into account the number of peptides used for quantification of each protein in each experiment (Supplemental Data Set 2). Only proteins that presented at least two peptides at one or more time points during the time course were selected. Those selected on this basis may also have single peptide ratios, which were also included in the final n-weighted averaging. Functional categorization of the proteins was performed manually through interpretation of information supplied by TAIR/EMBL-EBI. Comparison between proteomes was achieved by retrieving the protein accession numbers isolated in 20 proteomic experiments from GenBank and using Microsoft Excel database comparison tools. All assigned functions were downloaded to Excel spread sheets. The complete list of proteins with averaged ratios, functional category, and comparison with other proteomic studies is provided in Supplemental Data Set 2.
SDS-PAGE and Immunoblot Detection
Ten micrograms of total soluble protein was separated using SDS-PAGE and transferred onto nitrocellulose membrane for immunoblotting performed according to Pesquet et al. (2010). Experiments were performed in triplicate with two technical repeats for three independent biological time courses used. Antibodies used included anticlathrin heavy chain (AS10690; Agrisera), anti-SEC21p γ-coatomer (AS08327; Agrisera), antihistone deacetylase HDT1 (AS111792; Agrisera), antihistone deacetylase HDT3 (AS111753; Agrisera), anti-heat shock protein 70 (AS08371; Agrisera), and anti-heat shock protein 90 (AS08346; Agrisera), which were used at 1:2000, 1:1000, 1:500, 1:1000, 1:1000, and 1:1000 dilutions, respectively, for immunoblotting. The primary antibody was detected by enhanced chemiluminescence (Pierce) using horseradish peroxidase-conjugated secondary antibodies diluted at 1:10,000 for anti-rabbit (Agrisera).
Vectors for Transformation and Establishment of Stable Transgenic Cell Lines
RNAi knockdown constructs for genes encoding DL3/DRP2B, coatomer β’1, transducin AT4G04940, and CSI1 were made using Gateway technology (Life Technologies) as described by Pesquet et al. (2010). A BP reaction of the PCR-amplified target products (primer sequences provided in Supplemental Table 1) was produced in the pDONR207 donor vector (Invitrogen) to create the different entry clones. LR reactions using these entry clones with pGFP-N-Bin binary destination vector were performed to create the 35S:N-term-GFP fusion protein and with pFGC5941 to create the 35S:RNAi constructs. Other binary vectors used in this study included TUα6-RFP, TUβ8-GFP, MAP65-1-GFP in pGFP-N-Bin (Mao et al., 2005), AIR9-GFP in pGFP-N-Bin (Buschmann et al., 2006), MAP65-1, and AIR9 RNAi, which were made in pFGC5941 using previously published entry vectors (Mao et al., 2005; Buschmann et al., 2006). Stable transgenic cell lines were generated according to Pesquet et al. (2010) by cocultivation of cell suspensions with transformed Agrobacterium tumefaciens LBA4404 or GV3101.
Quantitative RT-PCR
Two milliliters of 72-h-old cell suspension cultures for each independent cell line was harvested by centrifugation and frozen in liquid nitrogen. Cells were ground to a powder and RNA extracted using an RNeasy Mini Kit (Qiagen) with an RNase-free DNaseI digestion step according to the manufacturer’s instructions. cDNAs were synthesized from 1 μg of RNA template using SuperScript II reverse transcriptase (Invitrogen) according to the manufacturer’s instructions. For quantitative RT-PCR, SYBR Green JumpStart Taq ReadyMix (Sigma-Aldrich) was used. Reactions were performed in 96-well plates using primers to amplify the target template and a reference template (see Supplemental Table 1 for primer sequences). Each well contained 10 μL SYBR green, 1 μL each forward and reverse primers (100 μM stocks), and 10 ng cDNA template in a final volume of 20 μL. PCR was run and fluorescence measured on a Chromo4 Real-Time PCR detector (Bio-Rad) with an initial denaturing temperature of 95°C for 2 min followed by 45 cycles of 95°C for 10 s, 55°C for 10 s, and 72°C for 20 s. Melting curve analysis was also performed at the end of the run to verify that only one product was amplified. PCR efficiencies and expression levels were calculated for target and reference gene from a serial dilution of cDNA template isolated from stably transformed TUα6-GFP cell lines. Target gene transcript levels were normalized to the transcript level of one reference gene (ubiquitin or 18sRNA from Pesquet et al., 2010). For each RNAi line, target gene transcript levels were normalized to those measured in stably transformed TUα6-GFP cell lines.
Time-Lapse Microscopy and Analysis
Live cells were imaged using a Zeiss Axioplan 2 inverted epifluorescence microscope equipped with a motorized xy and z stage, automatic shutter control, and Axiocam MRm high-sensitivity black and white CCD camera (Zeiss) controlled via Axiovision software (Zeiss). Real-time live-cell imaging of differentiating TEs was acquired at 15-min intervals in a gas-permeable biochamber as described by Pesquet et al. (2010). Lignin autofluorescence was observed using a 450- to 490-nm excitation filter/515-nm long-pass emission filter (Zeiss).
Confocal Microscopy and Image Analysis
TE cell wall staining was performed by incubating 100 μL of cells with 2 μL of 0.02% (w/v) Calcofluor (F3543; Sigma-Aldrich). Cells were mounted between glass slides and cover slips and imaged using an inverted Leica SP2 AOBS laser scanning microscope (Leica Microsystems) using a 40× or a 63× oil immersion objective. TE Calcofluor-stained cell walls were visualized using an excitation with 405-nm diode laser and collecting in the 415- to 465-nm emission window. GFP fluorescence was visualized using an excitation with 488-nm Argon laser and collecting in the 495- to 545-nm emission window, while RFP fluorescence was visualized using an excitation with 541-nm HeNe laser and collecting in the 555- to 595-nm emission window. Fluorescence intensity was range adjusted to the 8-bit scale for each independent image acquired by setting the background to 0 and maximum emission to 256. Confocal xyz stacks were acquired at 512 × 512 8-bit pixels with a 0.5- to 1-µm z step size.
Image processing and analysis was performed using ImageJ software (http://rsb.info.nih.gov/ij/) to adjust image brightness/contrast/size, compile movies, color realignment/intensity-coding/merging, pixel colocalization analysis, intensity profiling, dimension measurements, and formatting. Half-cell 3D reconstructions and animations of TEs were performed using Voxx software (Indiana University) on 8-bit xyz stacks processed using ImageJ.
Accession Numbers
Sequence data from this article can be found in the GenBank/EMBL libraries under the following accession numbers: At3g24770 (TDIF/CLE41), At4g32880 (ATHB8), At5g62380 (VND6), At5g17420 (IRX3/CesA7), At4g35350 (XCP1), At5g15630 (IRX6/COBL4), At5g54690 (IRX8/GAUT12), At4g17220 (MAP70-5), At3g53350 (MIDD1), At1g27920 (MAP65-8), At1g68060 (MAP70-1), At3g16630 (KINESIN13A), At3g51300 (ROP11), At1g04820 (TUA4), At5g23860 (TUB8), At4g33650 (DRP3A), At4g29010 (AIM1), At3g15730 (PLD), At5g55230 MAP65-1), At2g34680 (AIR9), At2g03150 (MAP190/EMB1579), At2g22125 (CSI1), At3g16640 (TCTP), At1g24706 (THO2), At5g16050 (GRF5), At4g16143 (IMPA-2), At2g36530 (LOS2), At2g02560 (CAND1), At1g41830 (SKS6), At3g44750 (HDT1), At5g22650 (HDT2), At3g13920 (EIF4A1), At4g14940 (AO1), At1g59610 (DRP2B/DL3), At5g12370 (SEC10), At1g79990 (coatomer β’1), At3g11130 and At3g08530 (clathrin heavy chains), At3g16860 (COBL8), and At2g28760 (UXS6). Microarray data have been deposited in NCBI’s Gene Expression Omnibus under accession number GSE73146.
Supplemental Data
Supplemental Figure 1. Real-time monitoring of TE differentiation in Arabidopsis cell suspension cultures.
Supplemental Figure 2. Gene expression of phase-specific markers during in vitro differentiation of TEs.
Supplemental Figure 3. Isolation of microtubule interacting proteins.
Supplemental Figure 4. Quantitative proteomic analysis of microtubule interacting proteins throughout TE differentiation using nitrogen isotope enrichment.
Supplemental Figure 5. Comparison of N-isotope-based mass spectrometry protein accumulation profile during TE formation between independent replicate experiments.
Supplemental Figure 6. Comparison between N-isotope-based mass spectrometry protein accumulation in microtubule pull-downs and immunoblot analysis in total soluble protein extracts of isolated proteins during the TE differentiation time course.
Supplemental Figure 7. Quantitative proteomic analysis of microtubule interacting proteins throughout TE differentiation using nitrogen isotope enrichment.
Supplemental Figure 8. Category and subcellular localization of identified microtubule interacting proteins along TE differentiation.
Supplemental Figure 9. High-resolution image of the in silico interactomic network of 402 proteins presented in Figure 3.
Supplemental Figure 10. Influence of microtubule disorganizing (oryzalin) drugs on tracheary element cell wall organization prior and during its synthesis.
Supplemental Figure 11. Accumulation profiles of proteins selected for RNAi analysis during TE differentiation.
Supplemental Figure 12. Constitutive genetic modulation of microtubule interacting proteins in cell cultures.
Supplemental Figure 13. Confirmation of microtubule labeling using colocalization of stably transformed cell lines with N-terminal MAP65-1 or AIR9 GFP fusions with α6tubulin-RFP.
Supplemental Table 1. List of primers used in this study for Gateway cloning and qPCR.
Supplemental Data Set 1. Transcriptomic analysis of in vitro TE differentiation time course using ATH1 Affymetrix microarray.
Supplemental Data Set 2. Quantitative proteomics, using N isotope labeling and microtubule pull-downs, of microtubule interacting proteins during TE differentiation.
Supplemental Data Set 3. Bibliographical cross-referencing of isolated microtubule interacting proteins.
Supplemental Movie 1. Visualizing the initiation of secondary cell wall deposition during the differentiation of TEs in pluripotent Arabidopsis cell suspension cultures.
Supplemental Movie 2. Visualizing the completion of secondary cell wall deposition,
Supplemental Movie 3. Impact of microtubule destabilization using oryzalin prior and during TE active secondary cell wall formation.
Supplemental Movie 4. Impact of RNA RNAi knockdown on wall dimension, deposition, cortical position, and patterning.
Supplemental Movie 5. Subcellular localization of tubulinß8, AIR9, and MAP65-1 relatively to secondary cell walls in TEs.
Supplementary Material
Acknowledgments
This work was supported by a Biotechnology and Biological Sciences Research Council Grant (BB/G008019/1) to C.W.L., by a John Innes Director’s Fund award to C.W.L., by a FP6 Marie Curie Intra-European Fellowship EIF (040433-XYLOSKELETON) to E.P., by a Vetenskapsrådet VR Swedish research council grant (2010-4620) to E.P., and by a Gunnar Öquist Fellowship from the Kempe Foundation to E.P. We thank the Swedish research council Vetenskapsrådet and the Swedish Governmental Agency for Innovation Systems Vinnova (Umeå Plant Science Centre Berzelii Centre) and Bio4Energy (a strategic research environment appointed by the Swedish government).
AUTHOR CONTRIBUTIONS
P.D., C.W.L., and E.P. conceived and designed the experiments. P.D., P.G., D.M., G.S., H.B., and E.P. performed the experiments. P.D. and E.P. analyzed the data. P.D., C.W.L., and E.P. wrote the article.
Glossary
- TE
tracheary element
- MAP
microtubule-associated protein
- MASC
microtubule-associated cellulose synthase compartment
- MS
Murashige and Skoog
- ER
endoplasmic reticulum
Footnotes
Articles can be viewed online without a subscription.
References
- Alonso-Peral M.M., Candela H., del Pozo J.C., Martínez-Laborda A., Ponce M.R., Micol J.L. (2006). The HVE/CAND1 gene is required for the early patterning of leaf venation in Arabidopsis. Development 133: 3755–3766. [DOI] [PubMed] [Google Scholar]
- Arabidopsis Interactome Mapping Consortium (2011). Evidence for network evolution in an Arabidopsis interactome map. Science 333: 601–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baima S., Possenti M., Matteucci A., Wisman E., Altamura M.M., Ruberti I., Morelli G. (2001). The Arabidopsis ATHB-8 HD-zip protein acts as a differentiation-promoting transcription factor of the vascular meristems. Plant Physiol. 126: 643–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batzenschlager M., Masoud K., Janski N., Houlné G., Herzog E., Evrard J.L., Baumberger N., Erhardt M., Nominé Y., Kieffer B., Schmit A.C., Chabouté M.E. (2013). The GIP gamma-tubulin complex-associated proteins are involved in nuclear architecture in Arabidopsis thaliana. Front. Plant Sci. 4: 480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benayoun J. (1983). A cytochemical study of cell wall hydrolysis in the secondary xylem of Poplar (Populis italica Moench). Ann. Bot. (Lond.) 52: 189–200. [Google Scholar]
- Bennett V., Davis J. (1981). Erythrocyte ankyrin: immunoreactive analogues are associated with mitotic structures in cultured cells and with microtubules in brain. Proc. Natl. Acad. Sci. USA 78: 7550–7554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Nissan G., Cui W., Kim D.J., Yang Y., Yoo B.C., Lee J.Y. (2008). Arabidopsis casein kinase 1-like 6 contains a microtubule-binding domain and affects the organization of cortical microtubules. Plant Physiol. 148: 1897–1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bringmann M., Li E., Sampathkumar A., Kocabek T., Hauser M.T., Persson S. (2012). POM-POM2/cellulose synthase interacting1 is essential for the functional association of cellulose synthase and microtubules in Arabidopsis. Plant Cell 24: 163–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D.M., Zeef L.A., Ellis J., Goodacre R., Turner S.R. (2005). Identification of novel genes in Arabidopsis involved in secondary cell wall formation using expression profiling and reverse genetics. Plant Cell 17: 2281–2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buschmann H., Chan J., Sanchez-Pulido L., Andrade-Navarro M.A., Doonan J.H., Lloyd C.W. (2006). Microtubule-associated AIR9 recognizes the cortical division site at preprophase and cell-plate insertion. Curr. Biol. 16: 1938–1943. [DOI] [PubMed] [Google Scholar]
- Buschmann H., Dols J., Kopischke S., Peña E.J., Andrade-Navarro M.A., Heinlein M., Szymanski D.B., Zachgo S., Doonan J.H., Lloyd C.W. (2015). Arabidopsis KCBP interacts with AIR9 but stays in the cortical division zone throughout mitosis via its MyTH4-FERM domain. J. Cell Sci. 128: 2033–2046. [DOI] [PubMed] [Google Scholar]
- Cheng L.E., Song W., Looger L.L., Jan L.Y., Jan Y.N. (2010). The role of the TRP channel NompC in Drosophila larval and adult locomotion. Neuron 67: 373–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuong S.D., Mullen R.T., Muench D.G. (2002). Identification of a rice RNA- and microtubule-binding protein as the multifunctional protein, a peroxisomal enzyme involved in the beta-oxidation of fatty acids. J. Biol. Chem. 277: 2419–2429. [DOI] [PubMed] [Google Scholar]
- Chuong S.D., Good A.G., Taylor G.J., Freeman M.C., Moorhead G.B., Muench D.G. (2004). Large-scale identification of tubulin-binding proteins provides insight on subcellular trafficking, metabolic channeling, and signaling in plant cells. Mol. Cell. Proteomics 3: 970–983. [DOI] [PubMed] [Google Scholar]
- Chuong S.D., Park N.I., Freeman M.C., Mullen R.T., Muench D.G. (2005). The peroxisomal multifunctional protein interacts with cortical microtubules in plant cells. BMC Cell Biol. 6: 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho Y., Cavalli V. (2012). HDAC5 is a novel injury-regulated tubulin deacetylase controlling axon regeneration. EMBO J. 31: 3063–3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowell E.F., Bischoff V., Desprez T., Rolland A., Stierhof Y.D., Schumacher K., Gonneau M., Höfte H., Vernhettes S. (2009). Pausing of Golgi bodies on microtubules regulates secretion of cellulose synthase complexes in Arabidopsis. Plant Cell 21: 1141–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derbyshire P., Findlay K., McCann M.C., Roberts K. (2007). Cell elongation in Arabidopsis hypocotyls involves dynamic changes in cell wall thickness. J. Exp. Bot. 58: 2079–2089. [DOI] [PubMed] [Google Scholar]
- Eremina M., Rozhon W., Yang S., Poppenberger B. (2015). ENO2 activity is required for the development and reproductive success of plants, and is feedback-repressed by AtMBP-1. Plant J. 81: 895–906. [DOI] [PubMed] [Google Scholar]
- Esau K. (1977). Anatomy of Seed Plants. (New York: John Wiley & Sons; ). [Google Scholar]
- Fendrych M., Synek L., Pecenková T., Drdová E.J., Sekeres J., de Rycke R., Nowack M.K., Zársky V. (2013). Visualization of the exocyst complex dynamics at the plasma membrane of Arabidopsis thaliana. Mol. Biol. Cell 24: 510–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk V., Kositsup B., Zhao C., Beers E.P. (2002). The Arabidopsis xylem peptidase XCP1 is a tracheary element vacuolar protein that may be a papain ortholog. Plant Physiol. 128: 84–94. [PMC free article] [PubMed] [Google Scholar]
- Gache V., Waridel P., Winter C., Juhem A., Schroeder M., Shevchenko A., Popov A.V. (2010). Xenopus meiotic microtubule-associated interactome. PLoS One 5: e9248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardiner J.C., Harper J.D., Weerakoon N.D., Collings D.A., Ritchie S., Gilroy S., Cyr R.J., Marc J. (2001). A 90-kD phospholipase D from tobacco binds to microtubules and the plasma membrane. Plant Cell 13: 2143–2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gindhart J.G. Jr., Goldstein L.S. (1996). Tetratrico peptide repeats are present in the kinesin light chain. Trends Biochem. Sci. 21: 52–53. [PubMed] [Google Scholar]
- Goto Y., Asada T. (2007). Excessive expression of the plant kinesin TBK5 converts cortical and perinuclear microtubules into a radial array emanating from a single focus. Plant Cell Physiol. 48: 753–761. [DOI] [PubMed] [Google Scholar]
- Gutierrez R., Lindeboom J.J., Paredez A.R., Emons A.M., Ehrhardt D.W. (2009). Arabidopsis cortical microtubules position cellulose synthase delivery to the plasma membrane and interact with cellulose synthase trafficking compartments. Nat. Cell Biol. 11: 797–806. [DOI] [PubMed] [Google Scholar]
- Gupton S.L., Collings D.A., Allen N.S. (2006). Endoplasmic reticulum targeted GFP reveals ER organization in tobacco NT-1 cells during cell division. Plant Physiol. Biochem. 44: 95–105. [DOI] [PubMed] [Google Scholar]
- Hamada T., Igarashi H., Taguchi R., Fujiwara M., Fukao Y., Shimmen T., Yokota E., Sonobe S. (2009). The putative RNA-processing protein, THO2, is a microtubule-associated protein in tobacco. Plant Cell Physiol. 50: 801–811. [DOI] [PubMed] [Google Scholar]
- Hamada T., Igarashi H., Yao M., Hashimoto T., Shimmen T., Sonobe S. (2006). Purification and characterization of plant dynamin from tobacco BY-2 cells. Plant Cell Physiol. 47: 1175–1181. [DOI] [PubMed] [Google Scholar]
- Hamada T., Nagasaki-Takeuchi N., Kato T., Fujiwara M., Sonobe S., Fukao Y., Hashimoto T. (2013). Purification and characterization of novel microtubule-associated proteins from Arabidopsis cell suspension cultures. Plant Physiol. 163: 1804–1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada T., Tominaga M., Fukaya T., Nakamura M., Nakano A., Watanabe Y., Hashimoto T., Baskin T.I. (2012). RNA processing bodies, peroxisomes, Golgi bodies, mitochondria, and endoplasmic reticulum tubule junctions frequently pause at cortical microtubules. Plant Cell Physiol. 53: 699–708. [DOI] [PubMed] [Google Scholar]
- Hamada T., Ueda H., Kawase T., Hara-Nishimura I. (2014). Microtubules contribute to tubule elongation and anchoring of endoplasmic reticulum, resulting in high network complexity in Arabidopsis. Plant Physiol. 166: 1869–1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hepler P.K., Newcomb E.H. (1964). Microtubules and fibrils in the cytoplasm of coleus cells undergoing secondary wall deposition. J. Cell Biol. 20: 529–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirakawa Y., Shinohara H., Kondo Y., Inoue A., Nakanomyo I., Ogawa M., Sawa S., Ohashi-Ito K., Matsubayashi Y., Fukuda H. (2008). Non-cell-autonomous control of vascular stem cell fate by a CLE peptide/receptor system. Proc. Natl. Acad. Sci. USA 105: 15208–15213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano K., Kotake T., Kamihara K., Tsuna K., Aohara T., Kaneko Y., Takatsuji H., Tsumuraya Y., Kawasaki S. (2010). Rice BRITTLE CULM 3 (BC3) encodes a classical dynamin OsDRP2B essential for proper secondary cell wall synthesis. Planta 232: 95–108. [DOI] [PubMed] [Google Scholar]
- Hong Z., Geisler-Lee C.J., Zhang Z., Verma D.P. (2003). Phragmoplastin dynamics: multiple forms, microtubule association and their roles in cell plate formation in plants. Plant Mol. Biol. 53: 297–312. [DOI] [PubMed] [Google Scholar]
- Hubbert C., Guardiola A., Shao R., Kawaguchi Y., Ito A., Nixon A., Yoshida M., Wang X.F., Yao T.P. (2002). HDAC6 is a microtubule-associated deacetylase. Nature 417: 455–458. [DOI] [PubMed] [Google Scholar]
- Hugdahl J.D., Bokros C.L., Morejohn L.C. (1995). End-to-end annealing of plant microtubules by the p86 subunit of eukaryotic initiation factor-(iso)4F. Plant Cell 7: 2129–2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes J.R., Meireles A.M., Fisher K.H., Garcia A., Antrobus P.R., Wainman A., Zitzmann N., Deane C., Ohkura H., Wakefield J.G. (2008). A microtubule interactome: complexes with roles in cell cycle and mitosis. PLoS Biol. 6: e98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igarashi H., Orii H., Mori H., Shimmen T., Sonobe S. (2000). Isolation of a novel 190 kDa protein from tobacco BY-2 cells: possible involvement in the interaction between actin filaments and microtubules. Plant Cell Physiol. 41: 920–931. [DOI] [PubMed] [Google Scholar]
- Jacobs J., Roe J.L. (2005). SKS6, a multicopper oxidase-like gene, participates in cotyledon vascular patterning during Arabidopsis thaliana development. Planta 222: 652–666. [DOI] [PubMed] [Google Scholar]
- Kim Y.M., Han Y.J., Hwang O.J., Lee S.S., Shin A.Y., Kim S.Y., Kim J.I. (2012). Overexpression of Arabidopsis translationally controlled tumor protein gene AtTCTP enhances drought tolerance with rapid ABA-induced stomatal closure. Mol. Cells 33: 617–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korolev A.V., Buschmann H., Doonan J.H., Lloyd C.W. (2007). AtMAP70-5, a divergent member of the MAP70 family of microtubule-associated proteins, is required for anisotropic cell growth in Arabidopsis. J. Cell Sci. 120: 2241–2247. [DOI] [PubMed] [Google Scholar]
- Korolev A.V., Chan J., Naldrett M.J., Doonan J.H., Lloyd C.W. (2005). Identification of a novel family of 70 kDa microtubule-associated proteins in Arabidopsis cells. Plant J. 42: 547–555. [DOI] [PubMed] [Google Scholar]
- Kozielski F., Riaz T., DeBonis S., Koehler C.J., Kroening M., Panse I., Strozynski M., Donaldson I.M., Thiede B. (2011). Proteome analysis of microtubule-associated proteins and their interacting partners from mammalian brain. Amino Acids 41: 363–385. [DOI] [PubMed] [Google Scholar]
- Krtková J., Zimmermann A., Schwarzerová K., Nick P. (2012). Hsp90 binds microtubules and is involved in the reorganization of the microtubular network in angiosperms. J. Plant Physiol. 169: 1329–1339. [DOI] [PubMed] [Google Scholar]
- Kubo M., Udagawa M., Nishikubo N., Horiguchi G., Yamaguchi M., Ito J., Mimura T., Fukuda H., Demura T. (2005). Transcription switches for protoxylem and metaxylem vessel formation. Genes Dev. 19: 1855–1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumagai H., Sakai H. (1983). A porcine brain protein (35 K protein) which bundles microtubules and its identification as glyceraldehyde 3-phosphate dehydrogenase. J. Biochem. 93: 1259–1269. [DOI] [PubMed] [Google Scholar]
- Lee Y.R., Liu B. (2004). Cytoskeletal motors in Arabidopsis. Sixty-one kinesins and seventeen myosins. Plant Physiol. 136: 3877–3883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei L., Li S., Du J., Bashline L., Gu Y. (2013). Cellulose synthase INTERACTIVE3 regulates cellulose biosynthesis in both a microtubule-dependent and microtubule-independent manner in Arabidopsis. Plant Cell 25: 4912–4923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leroux M.R., Hartl F.U. (2000). Protein folding: versatility of the cytosolic chaperonin TRiC/CCT. Curr. Biol. 10: R260–R264. [DOI] [PubMed] [Google Scholar]
- Li R., Xiong G., Zhou Y. (2010). Membrane trafficking mediated by OsDRP2B is specific for cellulose biosynthesis. Plant Signal. Behav. 5: 1483–1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S., Chen M., Yu D., Ren S., Sun S., Liu L., Ketelaar T., Emons A.M., Liu C.M. (2013). EXO70A1-mediated vesicle trafficking is critical for tracheary element development in Arabidopsis. Plant Cell 25: 1774–1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S., Lei L., Somerville C.R., Gu Y. (2012). Cellulose synthase interactive protein 1 (CSI1) links microtubules and cellulose synthase complexes. Proc. Natl. Acad. Sci. USA 109: 185–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda K., Nakata T., Noda Y., Sato-Yoshitake R., Hirokawa N. (1992). Interaction of dynamin with microtubules: its structure and GTPase activity investigated by using highly purified dynamin. Mol. Biol. Cell 3: 1181–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malpighi M. (1675). Anatome Plantarum. (London: J. Martyn; ). [Google Scholar]
- Mao G., Chan J., Calder G., Doonan J.H., Lloyd C.W. (2005). Modulated targeting of GFP-AtMAP65-1 to central spindle microtubules during division. Plant J. 43: 469–478. [DOI] [PubMed] [Google Scholar]
- Mei Y., Gao H.B., Yuan M., Xue H.W. (2012). The Arabidopsis ARCP protein, CSI1, which is required for microtubule stability, is necessary for root and anther development. Plant Cell 24: 1066–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ménard D., Pesquet E. (2015). Cellular interactions during tracheary elements formation and function. Curr. Opin. Plant Biol. 23: 109–115. [DOI] [PubMed] [Google Scholar]
- Møller S.G., McPherson M.J. (1998). Developmental expression and biochemical analysis of the Arabidopsis atao1 gene encoding an H2O2-generating diamine oxidase. Plant J. 13: 781–791. [DOI] [PubMed] [Google Scholar]
- Nakamura M., Ehrhardt D.W., Hashimoto T. (2010). Microtubule and katanin-dependent dynamics of microtubule nucleation complexes in the acentrosomal Arabidopsis cortical array. Nat. Cell Biol. 12: 1064–1070. [DOI] [PubMed] [Google Scholar]
- O’Connell P.A., Pinto D.M., Chisholm K.A., MacRae T.H. (2006). Characterization of the microtubule proteome during post-diapause development of Artemia franciscana. Biochim. Biophys. Acta 1764: 920–928. [DOI] [PubMed] [Google Scholar]
- Oda Y., Fukuda H. (2012). Secondary cell wall patterning during xylem differentiation. Curr. Opin. Plant Biol. 15: 38–44. [DOI] [PubMed] [Google Scholar]
- Oda Y., Fukuda H. (2013). Rho of plant GTPase signaling regulates the behavior of Arabidopsis kinesin-13A to establish secondary cell wall patterns. Plant Cell 25: 4439–4450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oda Y., Iida Y., Kondo Y., Fukuda H. (2010). Wood cell-wall structure requires local 2D-microtubule disassembly by a novel plasma membrane-anchored protein. Curr. Biol. 20: 1197–1202. [DOI] [PubMed] [Google Scholar]
- Oda Y., Iida Y., Nagashima Y., Sugiyama Y., Fukuda H. (2015). Novel coiled-coil proteins regulate exocyst association with cortical microtubules in xylem cells via the conserved oligomeric golgi-complex 2 protein. Plant Cell Physiol. 56: 277–286. [DOI] [PubMed] [Google Scholar]
- Parrotta L., Cresti M., Cai G. (2013). Heat-shock protein 70 binds microtubules and interacts with kinesin in tobacco pollen tubes. Cytoskeleton (Hoboken) 70: 522–537. [DOI] [PubMed] [Google Scholar]
- Patel P.C., Fisher K.H., Yang E.C., Deane C.M., Harrison R.E. (2009). Proteomic analysis of microtubule-associated proteins during macrophage activation. Mol. Cell. Proteomics 8: 2500–2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesquet E., Korolev A.V., Calder G., Lloyd C.W. (2010). The microtubule-associated protein AtMAP70-5 regulates secondary wall patterning in Arabidopsis wood cells. Curr. Biol. 20: 744–749. [DOI] [PubMed] [Google Scholar]
- Pesquet E., Korolev A.V., Calder G., Lloyd C.W. (2011). Mechanisms for shaping, orienting, positioning and patterning plant secondary cell walls. Plant Signal. Behav. 6: 843–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesquet E., Lloyd C. (2011). Microtubules, MAPs and cylem formation. In The Plant Cytoskeleton, Advances in Plant Biology, Liu B., ed (New York: Springer; ), pp. 277–306. [Google Scholar]
- Pesquet E., et al. (2013). Non-cell-autonomous postmortem lignification of tracheary elements in Zinnia elegans. Plant Cell 25: 1314–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrášek J., Freudenreich A., Heuing A., Opatrný Z., Nick P. (1998). Heat-shock protein 90 is associated with microtubules in tobacco cells. Protoplasma 202: 161–174. [Google Scholar]
- Pignocchi C., Doonan J.H. (2011). Interaction of a 14-3-3 protein with the plant microtubule-associated protein EDE1. Ann. Bot. (Lond.) 107: 1103–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy A.S., Day I.S. (2001). Kinesins in the Arabidopsis genome: a comparative analysis among eukaryotes. BMC Genomics 2: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royle S.J. (2012). The role of clathrin in mitotic spindle organisation. J. Cell Sci. 125: 19–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryser U., Schorderet M., Zhao G.F., Studer D., Ruel K., Hauf G., Keller B. (1997). Structural cell-wall proteins in protoxylem development: evidence for a repair process mediated by a glycine-rich protein. Plant J. 12: 97–111. [DOI] [PubMed] [Google Scholar]
- Sambade A., Findlay K., Schäffner A.R., Lloyd C.W., Buschmann H. (2014). Actin-dependent and -independent functions of cortical microtubules in the differentiation of Arabidopsis leaf trichomes. Plant Cell 26: 1629–1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampathkumar A., Gutierrez R., McFarlane H.E., Bringmann M., Lindeboom J., Emons A.M., Samuels L., Ketelaar T., Ehrhardt D.W., Persson S. (2013). Patterning and lifetime of plasma membrane-localized cellulose synthase is dependent on actin organization in Arabidopsis interphase cells. Plant Physiol. 162: 675–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampathkumar A., Lindeboom J.J., Debolt S., Gutierrez R., Ehrhardt D.W., Ketelaar T., Persson S. (2011). Live cell imaging reveals structural associations between the actin and microtubule cytoskeleton in Arabidopsis. Plant Cell 23: 2302–2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shevchenko A., Wilm M., Vorm O., Mann M. (1996). Mass spectrometric sequencing of proteins silver-stained polyacrylamide gels. Anal. Chem. 68: 850–858. [DOI] [PubMed] [Google Scholar]
- Smertenko A.P., Chang H.Y., Wagner V., Kaloriti D., Fenyk S., Sonobe S., Lloyd C., Hauser M.T., Hussey P.J. (2004). The Arabidopsis microtubule-associated protein AtMAP65-1: molecular analysis of its microtubule bundling activity. Plant Cell 16: 2035–2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorek N., Sorek H., Kijac A., Szemenyei H.J., Bauer S., Hématy K., Wemmer D.E., Somerville C.R. (2014). The Arabidopsis COBRA protein facilitates cellulose crystallization at the plasma membrane. J. Biol. Chem. 289: 34911–34920. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Soyano T., Thitamadee S., Machida Y., Chua N.H. (2008). ASYMMETRIC LEAVES2-LIKE19/LATERAL ORGAN BOUNDARIES DOMAIN30 and ASL20/LBD18 regulate tracheary element differentiation in Arabidopsis. Plant Cell 20: 3359–3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szyk A., Deaconescu A.M., Piszczek G., Roll-Mecak A. (2011). Tubulin tyrosine ligase structure reveals adaptation of an ancient fold to bind and modify tubulin. Nat. Struct. Mol. Biol. 18: 1250–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno Y., Ishikawa T., Watanabe K., Terakura S., Iwakawa H., Okada K., Machida C., Machida Y. (2007). Histone deacetylases and ASYMMETRIC LEAVES2 are involved in the establishment of polarity in leaves of Arabidopsis. Plant Cell 19: 445–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wightman R., Turner S.R. (2008). The roles of the cytoskeleton during cellulose deposition at the secondary cell wall. Plant J. 54: 794–805. [DOI] [PubMed] [Google Scholar]
- Xiong G., Li R., Qian Q., Song X., Liu X., Yu Y., Zeng D., Wan J., Li J., Zhou Y. (2010). The rice dynamin-related protein DRP2B mediates membrane trafficking, and thereby plays a critical role in secondary cell wall cellulose biosynthesis. Plant J. 64: 56–70. [DOI] [PubMed] [Google Scholar]
- Zeng C.J., Lee Y.R., Liu B. (2009). The WD40 repeat protein NEDD1 functions in microtubule organization during cell division in Arabidopsis thaliana. Plant Cell 21: 1129–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu C., Dixit R. (2012). Functions of the Arabidopsis kinesin superfamily of microtubule-based motor proteins. Protoplasma 249: 887–899. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.