Abstract
Purpose
To explore the association between peripapillary atrophy (PPA) area and conversion from ocular hypertension (OHT) to glaucoma.
Design
Prospective, longitudinal cohort study of cases and controls.
Participants
Age- and follow-up time-matched 279 eyes with OHT that converted to glaucoma and 279 eyes with OHT that did not convert to glaucoma.
Methods
Initial and last acceptable optic disc photos were analyzed. Disc, α- and β-zone PPA were traced independently by two trained readers and their areas were measured with Photoshop. The α- and β-zone areas were expressed as a percent of optic disc area.
Main Outcome measures
α- and β-zone PPA size over time.
Results
Intraclass correlations (ICC) demonstrated that readers had good agreement on disc area (ICC = 0.97) and β-zone (ICC = 0.82), but not α-zone (ICC = 0.48). The ß-zone, as a percent of disc area, increased in size (P < 0.001) both in eyes with incident POAG (mean=10.6%, SD = 22.6%) and matched controls (mean = 10.1%, SD = 33.7), over follow-up (mean = 12.3yrs). The increase in size did not differ between cases and controls (P = 0.82). β-zone enlargement was not correlated with follow-up time (P = 0.39).
Conclusions
The results did not show a difference in size of β-zone at baseline between eyes that proceed to develop glaucoma and those that do not. Moreover, β-zone enlarges equally in case and control eyes during follow-up.
Introduction
Peripapillary atrophy (PPA) is one of the parameters that are often taken into consideration in diagnosing open-angle glaucoma (OAG).1, 2 The association between PPA and OAG has been extensively investigated. Although there are a few studies to the contrary,3-5 most cross-sectional6-13 and prospective studies14, 15 have found that PPA is more frequent and larger in patients with OAG than those without OAG. Other longitudinal studies have demonstrated that PPA enlarges in some eyes as glaucoma progresses,7, 15-19 as well in some eyes with age.15 It has also been reported that there is a significant association between the location of PPA and that of the most marked visual field loss20-22 and that the extent of PPA significantly correlates with the degree of optic disc damage and visual field defects.20, 23, 24
There have only been a few reports on the prevalence of PPA in patients with ocular hypertension (OHT),25, 26 and the issue of whether the presence and/or progression of PPA is a risk factor for conversion from OHT to OAG remains uncertain.5, 27-31 However, many of these past investigations included relatively small numbers of patients, had poorly defined inclusion and exclusion criteria, and were cross-sectional rather than longitudinal. These limitations have also made it difficult to make comparisons across studies and highlight the need for more data to clarify the association between PPA progression in OHT and conversion to glaucoma. Because not all eyes with OHT develop OAG,32-35 it is important to identify other factors such as structural changes to the optic disc that may help determine the risk of conversion from OHT to glaucoma. Evaluation of a large, prospectively collected data on PPA in OHT patients carries the potential to improve our understanding of the association of PPA and changes in PPA in OHT with conversion to glaucoma. The Ocular Hypertension Treatment Study (OHTS), which has provided the largest data set of prospectively collected information on patients with OHT, is well suited to study the association of PPA progression in this group of subjects with the onset of glaucoma. The OHTS data files contain detailed demographic and clinical information including evaluations of serial stereoscopic optic disc photographs taken annually and results of visual field testing performed every six months. The present study was designed to assess digitized photographs to explore whether PPA enlarges over time during the course of OHT and whether enlargement of PPA is associated with conversion to glaucoma.
Materials and Methods
Study Population
The patients enrolled in this study participated in the OHTS, whose protocol has been presented elsewhere.35 The institutional review boards at all clinical sites approved their respective informed consent statements and procedures. The design of the OHTS followed the tenets of the Declaration of Helsinki.36 This OHTS data set files includes information of 3200 eyes from 1600 subjects studied from February 1994 to December 2009, with over 300 eyes that converted from OHT to glaucoma through 2008 (personal communication, Mae Gordon, OHTS, January 2009). Determination of conversion to POAG in the OHTS was defined as the development of a reproducible visual field abnormality and/or a reproducible optic disc change consistent with glaucoma in one or both eyes that was attributed to POAG by an endpoint committee masked to randomized treatment assignment. Definitions for visual field abnormality and optic disc deterioration are detailed elsewhere.32
This is a nested case-control study. Cases included 279 eyes of 279 participants with OHT that converted to POAG and controls were 279 eyes of 222 participants with OHT that did not meet conversion criteria in either eye. Case follow-up photographs were those collected at the last follow-up study visit. Control follow-up photographs were selected from eyes of participants who did not convert to glaucoma and matched with respect to eye laterality, participant's age within 5 years, and study follow-up visit within 6 months. Controls and cases were matched by the same follow-up visit in all but 2 (1%) matches and by the same age (88% of matches), within one year of age (9% of matches), or within 2 to 5 years of age (3% of matches).
Optic Disc Slide Scanning and Digitization
Optic disc photographs in the OHTS were acquired at annual visits for both eyes after pupil dilation. All images were captured using 35mm film-based technology. The images were then mounted in 2-inch × 2-inch slide format, labeled with individual anonymous codes to protect confidentiality, and stored at the Optic Disc Reading Center (ODRC) of the OHTS at the Bascom Palmer Eye Institute, University of Miami School of Medicine. Standard 35-mm Fujifilm 100 ASA (Fuji, Japan) was used for film capture, because of its good image quality. Funding from the National Eye Institute supported the creation of a digital archive of all stereoscopic disc photographs collected during the OHTS. All original photographic transparencies of all subjects’ visits were then digitized in RGB format using a Nikon Super CoolScan 5000ED scanner with SilverFast Ai software (LaserSoft Imaging Inc., Sarasota, Florida, USA) and saved in Tagged Image File Format (TIFF). If the disc photos were taken with a sequential fundus camera, left and right images were scanned individually, cropped with Adobe Photoshop 3.0 (Adobe System, Inc., Mountain View, CA), placed side-by-side, and then saved as a single image of the stereoscopic pair. Disc photos taken with a simultaneous fundus camera were scanned and saved with no image manipulation. All images were labeled after the scanning process and moved to server storage.
Scanned Optic Disc Image Evaluation and PPA Margin Delineation
The baseline and study follow-up visit digital stereoscopic optic disc photographs of eyes with glaucoma and matched controls were retrieved from the server and presented on an interactive battery-free pen and liquid crystal display unit (WACOM Cintiq 12WX, Vancouver, WA) for evaluation. Stereoscopic disc photographs with poor image quality (for example, due to cataract or technical reasons) that prevented reliable outlining of the disc margin or the boundaries of the peripapillary zones α and β37 were excluded and replaced by the one taken immediately after (if a baseline photograph) or immediately before (if a follow-up photograph). Each stereoscopic photograph was evaluated independently by two readers (ES, RV) from the ODRC of the OHTS using a hand-held stereoscope (Screen-VU, Portland, OR) to view the images stereoscopically on the liquid crystal display. The ODRC readers underwent extensive training in identification of optic disc structures and tracing by a senior investigator (DRA).
The drawing tool (pen) was calibrated before each tracing session and standardized in terms of tip size (in pixels) and hardness. The structures to be quantified (optic disc, peripapillary zones α and β areas) were outlined on the inside edge so that the thickness of the trace would be incorporated in the total delineated area. Images were evaluated in a masked fashion without knowledge of the clinical diagnosis or other clinical information.
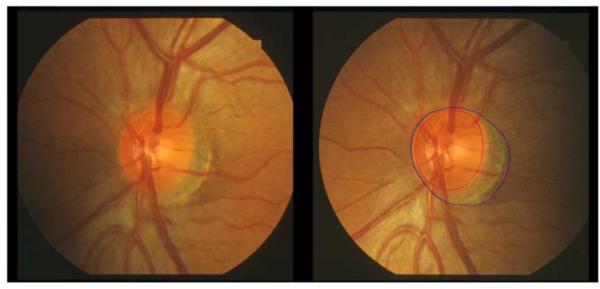
The border of the optic disc was defined as the inner margin of the peripapillary scleral ring of Elschnig, recognized as a white band seen in part of or all around the circumference of the optic disc, or by the boundary between disc tissue and retinal pigment epithelium when it obscured the scleral ring. PPA was differentiated into α- and β-zones as described by Jonas et al.37 Alpha-zone was defined as an irregular area of hypopigmentation and hyperpigmentation adjacent to the scleral ring or located on the outer side of β-zone if present. Beta-zone, when present, extended from the scleral ring and was characterized by the absent retinal pigment epithelium, making visible the sclera or the choroid with its large vessels. The peripapillary scleral ring was included in the measurements of β-zone (Figure 1). Because the width of the scleral ring was usually very thin, any error introduced by adding the scleral ring area to β-zone was considered to be inconsequential. For each stereoscopic image, readers outlined successively three concentric regions: the edge of the optic disc, the area occupied by the optic disc and PPA β-zone, and finally the region including optic disc and PPA β- and α-zones on the right side of the stereo pair, unless the left side provided a better quality image.
Figure 1.
Left eye optic disc photographs of one of the study participants without (left) and with delineation of the disc in red, PPA α-zone in green, and PPA β-zone combined with scleral ring in blue. In this case, PPA α and β-zone margins coincide nasally.
Planimetric Analysis
Measuring tools of Adobe Photoshop were used to generate the areas automatically, in pixels, on an outlined image. For each disc image, the software calculated the areas corresponding to disc area, along the inner edge of the scleral ring, if visible, a second area that included the optic disc with any visible scleral rim and the β-zone (if present), and a third area that included the previous plus the α-zone (if present).
The α- and β-zone areas (obtained by subtraction of the three traced outlines) were normalized to the optic disc area by expressing it as a percent of the disc area to minimize the effects of the difference in camera magnification and/or refraction-related errors.
Statistical Analysis
Paired t-tests were used to compare the spherical equivalent, optic disc area in pixels and peripapillary atrophy areas in pixels and in percent of optic disc. Participant age was compared with t-test and gender was compared with chi-square test. The intraclass correlation coefficient (ICC) was used to assess between reader agreement for disc, β- and α-zones areas in pixels and β- and α-zones as a percent of optic disc area. In an ancillary analysis, generalized estimating equations with exchangeable covariance structure and robust estimates were used to account for use of the contralateral eye as a control in more than one matched pair in the comparison of follow up minus baseline zone areas as a percent of disc areas. A p-value of 0.05 or less was considered statistically significant.
Results
Characteristics of the case and control groups at the baseline visit are summarized in Table 1. Mean age was identical due to study design. Mean spherical equivalent was also comparable between the two groups (paired t-test). None of the eyes in the two groups had a spherical equivalent refractive error more myopic than -9D. Sixteen (5.7%) converted by both optic disc and visual field; 153 (54.8%) converted by optic disc criteria first, and sixteen (39.4%) of the 279 cases converted by visual field criteria first.
Table 1.
Participant Demographic and Ocular Characteristics at Baseline Visit
| Progressive OHT (n = 279) | Non-progressive OHT (n = 222) | P Value | |
|---|---|---|---|
| Mean age (±SD), years | 58 (9) | 58 (9) | 0.99 |
| Sex, male (%) | 157 (56) | 117 (53) | 0.48 |
| Mean spherical equivalent (±SD), D | −0.56 (2.5) | −0.37 (2.4) | 0.36 |
OHT: ocular hypertension; SD: standard deviation, D: diopter
Reproducibility of Measurements
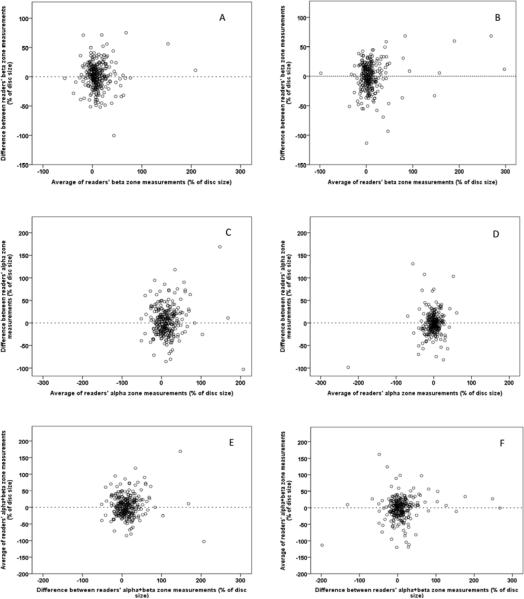
Intra-class correlation coefficient (ICC) was used to assess between reader agreement for disc, β- and α-zones areas in pixels and β- and α-zones as a percent of optic disc area. Table 2 presents the between reader reproducibility of baseline and follow-up optic disc, PPA β- and α-zone area measurements for cases and controls based on all images measured. Agreement was assessed before conversion from pixels to percent of disc area to assess of reproducibility of disc measurements and to ensure that the conversion had not adversely impacted reproducibility of overall measurements. The scale of agreement strength as proposed by Fleiss is as follows: values greater than 0.75 represent excellent agreement beyond chance, values between 0.40 and 0.75 represent fair to moderate agreement, and values below 0.40 represent poor agreement.38 Between reader ICCs were excellent for both baseline and follow-up PPA β-zone measurements and moderate for PPA α-zone measurements. In this study, ICC of between reader PPA change over time as a percent of disc area were 0.72 (95% CI: 0.67 – 0.75) for β-zone, considered good reproducibility; and 0.51 (95% CI: 0.45 – 0.57) for α-zone, considered fair to moderate. Examination of Bland-Altman plots revealed no systematic differences between readers with respect to size of PPA changes (Figure 2).
Table 2.
Between Reader Reproducibility of Optic Disc and Peripapillary Atrophy Areas Before and After Conversion to Percent of Disc Area
| Pre-conversion ICC (95% CI) (in pixels) | Post-conversion ICC (95% CI) (in % of disc area) | |
|---|---|---|
| Optic disc | 0.967 (0.86-0.99) | N/A |
| β-zone | 0.831 (0.67-0.90) | 0.823 (0.77-0.86) |
| α-zone | 0.582 (0.53-0.63) | 0.483 (0.40-0.55) |
CI: confidence intervals
Figure 2.
Bland-Altman plots of the difference (% of disc size) between the two readers against their averages. Inter-reader agreement on measurements of change in β-zone (A and B), α zone (C and D), and α + β zones (E and F). Plots on the left are for eyes with non-progressive OHT and plots on right are for eyes that converted to POAG.
Baseline PPA Measurements as a Percent of Disc Area
Baseline β-zone areas averaged 49.9% (SD: 24.3%, range: 20.1 – 143.6%) in cases and 47.4% (SD: 20.3%, range: 19.2 – 246.6%) in controls (p=0.18, paired t-test). Baseline α-zone areas averaged 19.1% (SD: 21.0%, range: 0 – 101.1%) in cases and 20.4% (SD: 15.8%, range: 0 – 275.3%) in controls (p=0.41, paired t-test). At baseline, 24 (9%) of cases and 22 (8%) of controls had no measurable α-zone, which did not differ significantly between the groups (p=0.88, McNemar's test, data not shown in the Table). Beta-zone was present in all cases and controls, except in one control eye, because Elschnig's scleral ring was incorporated to β-zone measurements. Thus, the β-zone measurements, when small, represent measurements of the scleral rim area only without any true β-zone in which retinal pigment epithelium did not completely cover peripapillary choroid. We did not attempt to distinguish these two anatomic structures, as the scleral rim is small and does not disrupt calculations of any β-zone increase with time.
PPA Change over Time as a Percent of Disc Area
The study follow-up visit at which disc photos used in this analysis were collected ranged from 12 to 168 months, with median of 156 months. The 10th and 90th percentiles were 120 and 162 months, respectively, so most follow-up times were within a 3.5 year range of each other, from 10 to 13.5 years. Over this time, β-zone increased by 10% in both cases that converted to OAG (p < 0.001, paired t-test) and unconverted controls (P < 0.001, paired t-test) (Table 3). Change in β-zone area (follow-up minus baseline) in cases and controls were not statistically different (P = 0.82, paired t-test) (Figure 3). ). The p-value was similar when generalized estimating equations were used in an ancillary analysis (P = 0.87). There was a highly statistically significant difference (P < 0.001) in mean α-zone areas expressed as a percent of disc area between the two groups (Table 3). There was similarly a statistically significant difference between the mean change of α-zone from baseline to follow-up (3.9% difference P = 0.021, paired t-test). An ancillary analysis using generalized estimating equations produced a similar result (P = 0.018). However, this difference included a small increase in cases and an apparent small reduction of α-zone area in the non-converting controls. The range of the differences was from −60.0% to +242.0% (SD of 27.9% around the mean change of 3.9%), so with such an overlap, the small mean change cannot be considered of much importance. Analysis of the combined α- and β-zones change over follow-up also failed to show differences between converters to OAG and non-converter controls (P = 0.1, paired t-test).
Table 3.
Mean β-zone and α-zone Areas (as a percent of disc area) at Baseline, Follow-up, and Difference (follow-up minus baseline), in the glaucoma conversion and control groups.
| Groups | Mean B-zone area (±SD) | Mean α-zone area (±SD) | |
|---|---|---|---|
| Percentage of disc area st baseline | Control | 49.9 (24.3) | 19.1 |
| Glaucoma | 47.4 (20.3) | 20.4 | |
| P* Value | 0.18 | 0.41 | |
| Percentage of disc area at follow-up | Control | 59.8 (48.3) | 17.5 (13.4) |
| Glaucoma | 57.7 (29.6) | 22.4 (17.8) | |
| P* Value | 0.51 | <0.001 | |
| Follow-up minus baseline area as % of disc area | Control | 10.1 (33.7) | −1.70 (21.0) |
| Glaucoma | 10.5 (22.6) | 2.20 (17.3) | |
| P* Value | 0.82 | 0.021 |
SD: standard deviation
paired t-test
Figure 3.
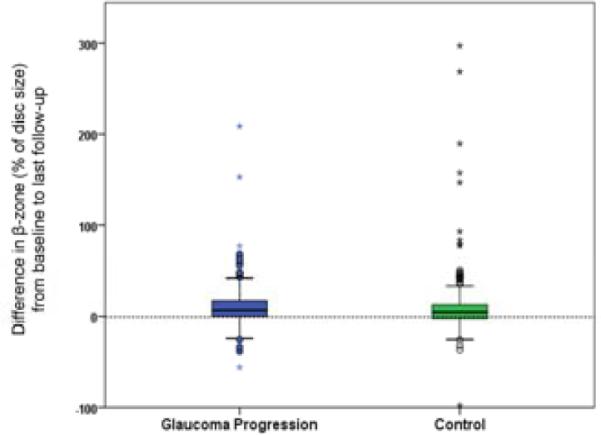
Differences between follow-up and baseline β-zone areas (% of disc size) in case and control eyes.
Cases that converted by disc criteria (with or without visual field conversion) averaged an 11.5 ± 23.6% β-zone enlargement as a percent of the disc area as opposed to 7.1 ± 18.5% β-zone enlargement in controls; the difference was not statistically significant (P = 0.17, t-test; P = 0.18 ANCOVA accounting for months of follow-up). Whether conversion was by disc or visual field criteria also did not influence the difference in β-zone enlargement rate in cases versus controls (P = 0.19, repeated measures ANOVA test of case/control status with evaluation for potential interaction between β-zone enlargement and study protocol determination of conversion by disc or field criteria). In cases, β-zone enlargement from baseline to last follow-up did not correlate with the time interval (range: 0 to 156 months) from determination of endpoint to the date at which the follow-up disc photograph was obtained (Pearson r = −0.04, P = 0.57).
Discussion
Because of the uncertainty of the relationship between PPA and the development of glaucoma, the opportunity for a longitudinal study with large number of subjects was the impetus for designing the present study. Although PPA has been associated with glaucoma in cross-sectional studies, it is unknown whether PPA or its worsening precedes the development of primary open angle glaucoma, coincident with it, or unrelated in time. In addition, the issue of whether PPA is larger in OHT eyes that subsequently convert to glaucoma compared to PPA in non-converting eyes has been suggested,25 but not definitively explored. Determining whether PPA size at baseline is associated with a higher risk of later conversion of OHT to OAG is of clinical importance, as it may allow closer monitoring or earlier treatment of OHT patients who are deemed to be at risk. Alternatively, PPA may enlarge in concert with development and progression of glaucomatous cupping, which is of interest in understanding the pathogenesis of each. The OHTS data set is well suited for the study of these questions, because of a large overall sample size, long-term prospective follow-up, and rigorous criteria for POAG endpoints.
We found that both at baseline and follow-up, the ICCs were excellent for PPA β-zone and moderate for α-zone measurements in these eyes matched and paired with each other. There was no significant difference between cases and controls in baseline or follow-up of either the PPA β-zone or α-zone. The magnitude of change in β-zone area over time was similar in cases and controls. In contrast, the change in α-zone area was statistically significantly, different between the two groups, but small and highly variable. To see whether there was evidence that β-zone enlargement occurred after conversion to glaucoma, we also assessed the strength of correlation between β-zone enlargement and months between conversion and last follow up in the cases. No correlation was found. Possibly abnormal IOP and a large β-zone each represent a risk factor, but in combination with other causative risk factors in a quantified manner not yet known, but none of which are necessary if other factors dominate.
Determination of optic disc borders is somewhat subjective, which potentially, brings a certain degree of variability into the measurements. Yet, we found excellent between reader agreements for both PPA zones, which implies that measurement variability did not impact our results. Tuulonen et al39 evaluated the variability of baseline PPA measurements (in mm2) in 23 eyes of 23 OAG patients and age-matched controls obtained by manual planimetry and reported between-observer Pearson correlation values of 0.97 for optic disc area, 0.91 for β-zone area, and 0.63 for α-zone area. The reported agreement is comparable to the current study.
Unlike some previous studies, we chose to estimate α- and β-zone measurements as percent of optic disc area rather than absolute areas because optic disc photographs were taken with different cameras having different magnifications, and because the clinical parameters needed to calculate the absolute areas were not available. Nevertheless, assuming disc area to be constant over time, a change in the PPA (α- or β-zones) area-to-disc ratio should be related to the change in PPA area. Alpha zones are more difficult to delineate than β-zone ones (especially with media changes over time) and changes are consequently quite variable within each of the study groups. The ICC is only moderate, and the smaller, but statistically significant, reduction in the non-converter controls (Table 3) is difficult to interpret.
Enlargement of PPA, in particular β-zone, has been correlated with progressive glaucomatous damage in several studies with various methodology. Rockwood and Anderson15 in a retrospective optic disc stereophotograph review, found qualitatively noticeable increase in PPA size as a whole in 21% of eyes with progressive glaucoma versus 4% of eyes with non-progressive glaucoma and eyes with ocular hypertension that did not become glaucomatous over a 12-month follow-up period. Interestingly, they also noted that the changes in PPA observed in eyes with progressive glaucoma were both too small and too rare to explain the high prevalence and large size of PPA in eyes with glaucoma. They speculated that eyes with inherited large β-zone may be more susceptible to the development of glaucoma, a view later shared by Healey et al40 following their investigation on the inheritance of PPA. Tezel et al31 analyzed serial optic disc photographs of 350 OHT eyes with 10-year follow-up and observed PPA enlargement in 9.9% of non-progressive eyes and in 49% of eyes that eventually developed OAG. In another retrospective study19 performed in 75 glaucomatous eyes with PPA at baseline and a minimum follow-up of 4 years, 93% of the 28 eyes where progression of PPA was observed showed progressive optic disc damage or visual field loss. Budde and Jonas16 in a longitudinal large series of OAG, OHT, and normal eyes, described a progressive enlargement of PPA β-zone in 2.7% of glaucomatous eyes and in 0.3% of OHT eyes. In eyes with OAG, PPA β -zone enlargement was more frequently observed in eyes with progressive than those with stable glaucoma. On the contrary, after comparing both baseline and progression rates of PPA area and neuroretinal rim area measured with scanning confocal laser tomography every 6 months for 8.6 years in 94 patients with OAG and 7.1 years in 54 normal control subjects, See et al4 reported similar baseline and follow-up PPA areas in the two groups. In addition, they found no correlation between rates of global PPA area and neuroretinal rim area progression both in both groups. Also in agreement with our findings, Quigley and colleagues5 did not find a significant difference in the prevalence of PPA between OHT patients who progressed and who did not progress to overt glaucoma after a 5-year follow up. Because the clinical value of PPA with regard to the diagnosis of OAG remains controversial, Ehrlich and Radcliffe41 used generalized linear models to determine if clinical PPA assessment improves the prediction of glaucoma hemifield test (GHT) beyond that of standard assessment of variables such as age, central corneal thickness, IOP and cup-to-disc ratio. The results indicated that adding PPA parameters to a model already containing these commonly assessed variables does not significantly improve the ability to discriminate between OAG and no glaucoma status.
The similar rates of PPA β-zone enlargement in converters and in non-converters found in our series of OHT subjects suggest a lack of relationship between increase in PPA β-zone area and development of glaucomatous damage. This discrepancy compared to the earlier studies described in the previous paragraph may be due to differences in study design (i.e. cross-sectional or longitudinal), follow-up length, sample size, border delineation methods (i.e. manual or automated), PPA size assessment (i.e. qualitative or quantitative), and definition of glaucoma progression. Our findings are based on manual planimetry performed by two independent, masked, and consistent readers on digitized optic disc stereoscopic photographs analyzed with a computerized measuring tool. In some eyes the PPA β-zone was large and easily detected, but in many others it was small with poorly definable borders, increasing the chance of being overlooked without the detailed evaluation and standardized delineation performed in this study. Surprisingly, none of the earlier studies has clearly acknowledged the difficulty associated with delineating PPA boundaries in some eyes, which has led to the wrong belief that outlining PPA boundaries is always relatively easy. With our thorough disc assessment and tracing technique we observed a mean 10% PPA β-zone area enlargement on follow-up photographs in both cases and controls. Of the 28 eyes with quantitative PPA progression described by Uchida et al,19 7 (25%) were not detected by qualitative assessment by observers. The average increase in PPA area not detected by qualitative analysis was 8.2% versus quantitatively detectable increase of 18.3%. We also found diversity between groups in PPA α-zone measurements on follow-up, which we believe generated a difference in α-zone enlargement between cases and controls, and an apparent small reduction in α-zone area in non-converters. Interpretation of this finding is limited since PPA α-zone measurements had a less than optimal reproducibility, as in other studies.
As far as we know, we have studied the largest and longest prospectively collected series of optic disc photographs in OHT patients. Some variability in measurements is introduced by manual delineation of optic disc and α- and β-zone margins on stereoscopic disc photographs, but it seems relatively small. New technologies such as computerized optic nerve head analyzers may provide more objective and even more consistent delineation of optic disc and PPA margins, and perhaps the ability to detect even smaller changes in the area of PPA more reliably. For example, spectral domain optical coherence tomography (SD-OCT) reconstructs “in vivo” three-dimensional optic disc and peripapillary structures by detecting termination of the various retinal layers.42-45 Lee et al42 used SD-OCT to evaluate the cross-sectional configuration of α and β-zones in 120 normal eyes, ., all of which showed an α-zone and 75% presented a β-zone on optic disc photographs. Specific OCT findings corresponding to α- and β-zone, respectively, were the gradual thinning of retinal layers immediately related to Bruch's membrane (inner segment-outer segment junction of the photoreceptors and the external limiting membrane) found in 87% of the eyes, and absence of the retinal pigment epithelial layer observed in 100% of the 90 eyes in which β-zone was visible. Kim et al43 analyzed the continuity of Bruch's membrane in 161 OAG eyes presenting β-zone and observed intact Bruch's membrane in 76 eyes (47%). This group was significantly older compared to groups presenting discontinuous or absent Bruch's membrane, suggesting that PPA presenting an intact Bruch's membrane could be an age-related atrophic change, and that the pathogenesis of PPA may be diverse. We found an increase in PPA size over time in converters to OAG and controls, indicating that PPA enlargement may be due to other factors such as age. This observation corroborates the findings of histologic46 and SD-OCT studies.47
In conclusion, based on results of previous studies and the findings of our series, it seems that the value of measuring PPA size over time in subjects with OHT for the diagnosis of conversion to glaucoma is still uncertain. Although PPA is seen in patients with glaucoma, clinical decisions should not be based on the presence of and/or change in PPA alone. In OHT subjects, PPA enlargement may occur independently of glaucoma conversion, and systematically with time, suggesting that a substantial part of this phenomenon may be age-related.
Supplementary Material
Précis.
Longitudinal measurement of peripapillary atrophy (PPA) size, particularly β-zone, is an unreliable mean for diagnosing the conversion of ocular hypertension to glaucoma because PPA may enlarge to the same degree in converters and non-converters.
Acknowledgments
Supported by National Institutes of Health Grants NIH-R21 EY019954 and NIHP30 EY014801, Bethesda, MD, and an unrestricted research grant from Research to Prevent Blindness, New York, NY.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presented at the American Academy of Ophthalmology Annual Meeting, November 10-13, 2012, Chicago, IL.
References
- 1.Fingeret M, Medeiros FA, Susanna R, Jr, Weinreb RN. Five rules to evaluate the optic disc and retinal nerve fiber layer for glaucoma. Optometry. 2005;76:661–8. doi: 10.1016/j.optm.2005.08.029. [DOI] [PubMed] [Google Scholar]
- 2.Susanna R, Jr, Vessani RM. New findings in the evaluation of the optic disc in glaucoma diagnosis. Curr Opin Ophthalmol. 2007;18:122–8. doi: 10.1097/ICU.0b013e328040bfe0. [DOI] [PubMed] [Google Scholar]
- 3.Derick RJ, Pasquale LR, Pease ME, Quigley HA. A clinical study of peripapillary crescents of the optic disc in chronic experimental glaucoma in monkey eyes. Arch Ophthalmol. 1994;112:846–50. doi: 10.1001/archopht.1994.01090180146049. [DOI] [PubMed] [Google Scholar]
- 4.See JL, Nicolela MT, Chauhan BC. Rates of neuroretinal rim and peripapillary atrophy area change: a comparative study of glaucoma patients and normal controls. Ophthalmology. 2009;116:840–7. doi: 10.1016/j.ophtha.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 5.Quigley HA, Katz J, Derick RJ, et al. An evaluation of optic disc and nerve fiber layer examinations in monitoring progression of early glaucoma damage. Ophthalmology. 1992;99:19–28. doi: 10.1016/s0161-6420(92)32018-4. [DOI] [PubMed] [Google Scholar]
- 6.Jonas JB, Martus P, Horn FK, et al. Predictive factors of the optic nerve head for development or progression of glaucomatous visual field loss. Invest Ophthalmol Vis Sci. 2004;45:2613–8. doi: 10.1167/iovs.03-1274. [DOI] [PubMed] [Google Scholar]
- 7.Park KH, Tomita G, Liou SY, Kitazawa Y. Correlation between peripapillary atrophy and optic nerve damage in normal-tension glaucoma. Ophthalmology. 1996;103:1899–906. doi: 10.1016/s0161-6420(96)30409-0. [DOI] [PubMed] [Google Scholar]
- 8.Quigley HA, Pease ME. Change in the optic disc and nerve fiber layer estimated with the glaucoma-scope in monkey eyes. J Glaucoma. 1996;5:106–16. [PubMed] [Google Scholar]
- 9.Jonas JB, Fernandez MC, Naumann GO. Glaucomatous parapapillary atrophy. Occurrence and correlations. Arch Ophthalmol. 1992;110:214–22. doi: 10.1001/archopht.1992.01080140070030. [DOI] [PubMed] [Google Scholar]
- 10.Uhm KB, Lee DY, Kim JT, Hong C. Peripapillary atrophy in normal and primary open-angle glaucoma. Korean J Ophthalmol. 1998;12:37–50. doi: 10.3341/kjo.1998.12.1.37. [DOI] [PubMed] [Google Scholar]
- 11.Tuulonen A, Airaksinen PJ, Erola E, et al. The Finnish evidence-based guideline for open-angle glaucoma. Acta Ophthalmol Scand. 2003;81:3–18. doi: 10.1034/j.1600-0420.2003.00021.x. [DOI] [PubMed] [Google Scholar]
- 12.Varma R, Ying-Lai M, Francis BA, et al. Los Angeles Latino Eye Study Group. Prevalence of open-angle glaucoma and ocular hypertension in Latinos: the Los Angeles Latino Eye Study. Ophthalmology. 2004;111:1439–48. doi: 10.1016/j.ophtha.2004.01.025. [DOI] [PubMed] [Google Scholar]
- 13.Xu L, Wang Y, Yang H, Jonas JB. Differences in parapapillary atrophy between glaucomatous and normal eyes: the Beijing Eye Study. Am J Ophthalmol. 2007;144:541–6. doi: 10.1016/j.ajo.2007.05.038. [DOI] [PubMed] [Google Scholar]
- 14.Hayreh SS, Jonas JB, Zimmerman MB. Parapapillary chorioretinal atrophy in chronic high-pressure experimental glaucoma in rhesus monkeys. Invest Ophthalmol Vis Sci. 1998;39:2296–303. [PubMed] [Google Scholar]
- 15.Rockwood EJ, Anderson DR. Acquired peripapillary changes and progression in glaucoma. Graefes Arch Clin Exp Ophthalmol. 1988;226:510–5. doi: 10.1007/BF02169197. [DOI] [PubMed] [Google Scholar]
- 16.Budde WM, Jonas JB. Enlargement of parapapillary atrophy in follow-up of chronic open-angle glaucoma. Am J Ophthalmol. 2004;137:646–54. doi: 10.1016/j.ajo.2003.11.021. [DOI] [PubMed] [Google Scholar]
- 17.Kwon YH, Kim YI, Pereira ML, et al. Rate of optic disc cup progression in treated primary open-angle glaucoma. J Glaucoma. 2003;12:409–16. doi: 10.1097/00061198-200310000-00003. [DOI] [PubMed] [Google Scholar]
- 18.Tezel G, Kass MA, Kolker AE, Wax MB. Comparative optic disc analysis in normal pressure glaucoma, primary open-angle glaucoma, and ocular hypertension. Ophthalmology. 1996;103:2105–13. doi: 10.1016/s0161-6420(96)30382-5. [DOI] [PubMed] [Google Scholar]
- 19.Uchida H, Ugurlu S, Caprioli J. Increasing peripapillary atrophy is associated with progressive glaucoma. Ophthalmology. 1998;105:1541–5. doi: 10.1016/S0161-6420(98)98044-7. [DOI] [PubMed] [Google Scholar]
- 20.Anderson DR. Correlation of the peripapillary anatomy with the disc damage and field abnormalities in glaucoma. Doc Ophthalmol Proc Ser. 1983;35:1–10. [Google Scholar]
- 21.Heijl A, Samander C. Peripapillary atrophy and glaucomatous visual field defects. Doc Ophthalmol Proc Ser. 1985;42:403–7. [Google Scholar]
- 22.Jonas JB, Naumann GO. Parapapillary chorioretinal atrophy in normal and glaucoma eyes. II. Correlations. Invest Ophthalmol Vis Sci. 1989;30:919–26. [PubMed] [Google Scholar]
- 23.Jonas JB. Clinical implications of peripapillary atrophy in glaucoma. Curr Opin Ophthalmol. 2005;16:84–8. doi: 10.1097/01.icu.0000156135.20570.30. [DOI] [PubMed] [Google Scholar]
- 24.Tsai CS, Zangwill L, Sample PA, et al. Correlation of peripapillary retinal height and visual field in glaucoma and normal subjects. J. Glaucoma. 1995;4:110–6. [PubMed] [Google Scholar]
- 25.Kasner O, Feuer WJ, Anderson DR. Possibly reduced prevalence of peripapillary crescents in ocular hypertension. Can J Ophthalmol. 1989;24:211–5. [PubMed] [Google Scholar]
- 26.Buus DR, Anderson DR. Peripapillary crescents and halos in normal-tension glaucoma and ocular hypertension. Ophthalmology. 1989;96:16–9. doi: 10.1016/s0161-6420(89)32930-7. [DOI] [PubMed] [Google Scholar]
- 27.Airaksinen PJ, Tuulonen A, Alanko HI. Prediction of development of glaucoma in ocular hypertensive patients. In: Krieglstein GK, editor. Glaucoma Update IV. Springer-Verlag; Berlin: 1991. pp. 183–6. [Google Scholar]
- 28.Motolko M, Drance SM. Features of the optic disc in preglaucomatous eyes. Arch Ophthalmol. 1981;99:1992–4. doi: 10.1001/archopht.1981.03930020868010. [DOI] [PubMed] [Google Scholar]
- 29.Stewart WC, Connor AB, Wang XH. Anatomic features of the optic disc and risk of progression in ocular hypertension. Acta Ophthalmol Scand. 1995;73:237–41. doi: 10.1111/j.1600-0420.1995.tb00275.x. [DOI] [PubMed] [Google Scholar]
- 30.Tezel G, Kolker AE, Kass MA, et al. Parapapillary chorioretinal atrophy in patients with ocular hypertension. I. An evaluation as a predictive factor for the development of glaucomatous damage. Arch Ophthalmol. 1997;115:1503–8. doi: 10.1001/archopht.1997.01100160673001. [DOI] [PubMed] [Google Scholar]
- 31.Tezel G, Kolker AE, Wax MB, et al. Parapapillary chorioretinal atrophy in patients with ocular hypertension. II. An evaluation of progressive changes. Arch Ophthalmol. 1997;115:1509–14. doi: 10.1001/archopht.1997.01100160679003. [DOI] [PubMed] [Google Scholar]
- 32.Kass MA, Heuer DK, Higginbotham EJ, et al. Ocular Hypertension Treatment Study Group. The Ocular Hypertension Treatment Study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120:701–13. doi: 10.1001/archopht.120.6.701. [DOI] [PubMed] [Google Scholar]
- 33.Kitazawa Y, Horie T, Aoki S, et al. Untreated ocular hypertension. A long-term prospective study. Arch Ophthalmol. 1977;95:1180–4. doi: 10.1001/archopht.1977.04450070078004. [DOI] [PubMed] [Google Scholar]
- 34.Linner E. Ocular hypertension. I. The clinical course during ten years without therapy. Aqueous humour dynamics. Acta Ophthalmol (Copenh) 1976;54:707–20. doi: 10.1111/j.1755-3768.1976.tb01790.x. [DOI] [PubMed] [Google Scholar]
- 35.Lundberg L, Wettrell K, Linner E. Ocular hypertension. A prospective twenty-year follow-up study. Acta Ophthalmol (Copenh) 1987;65:705–8. doi: 10.1111/j.1755-3768.1987.tb07067.x. [DOI] [PubMed] [Google Scholar]
- 36.Gordon MO, Kass MA. The Ocular Hypertension Study: design and baseline characteristics of the participants. Arch Ophthalmol. 1999;117:573–83. doi: 10.1001/archopht.117.5.573. [DOI] [PubMed] [Google Scholar]
- 37.Jonas JB, Nguyen XN, Gusek GC, Naumann GO. Parapapillary chorioretinal atrophy in normal and glaucoma eyes. I. Morphometric data. Invest Ophthalmol Vis Sci. 1989;30:908–18. [PubMed] [Google Scholar]
- 38.Fleiss JL. Statistical Methods for Rates and Proportions. Wiley; New York: 1981. p. 218. [Google Scholar]
- 39.Tuulonen A, Jonas JB, Valimaki S, et al. Interobserver variation in the measurements of peripapillary atrophy in glaucoma. Ophthalmology. 1996;103:535–41. doi: 10.1016/s0161-6420(96)30661-1. [DOI] [PubMed] [Google Scholar]
- 40.Healey PR, Mitchell P, Gilbert CE, et al. The inheritance of peripapillary atrophy. Invest Ophthalmol Vis Sci. 2007;48:2529–34. doi: 10.1167/iovs.06-0714. [DOI] [PubMed] [Google Scholar]
- 41.Ehrlich JR, Radcliffe NM. The role of clinical parapapillary atrophy evaluation in the diagnosis of open angle glaucoma. Clin Ophthalmol. 2010;4:971–6. doi: 10.2147/opth.s12420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee KY, Tomidokoro A, Sakata R, et al. Cross-sectional anatomic configurations of peripapillary atrophy evaluated with spectral domain-optical coherence tomography. Invest Ophthalmol Vis Sci. 2010;51:666–71. doi: 10.1167/iovs.09-3663. [DOI] [PubMed] [Google Scholar]
- 43.Kim M, Kim TW, Weinreb RN, Lee EJ. Differentiation of parapapillary atrophy using spectral-domain optical coherence tomography. Ophthalmology. 2013;120:1790–7. doi: 10.1016/j.ophtha.2013.02.011. [DOI] [PubMed] [Google Scholar]
- 44.Manjunath V, Shah H, Fujimoto JG, Duker JS. Analysis of peripapillary atrophy using spectral domain optical coherence tomography. Ophthalmology. 2011;118:531–6. doi: 10.1016/j.ophtha.2010.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Park SC, De Moraes CG, Tello C, et al. In-vivo microstructural anatomy of beta-zone parapapillary atrophy in glaucoma. Invest Ophthalmol Vis Sci. 2010;51:6408–13. doi: 10.1167/iovs.09-5100. [DOI] [PubMed] [Google Scholar]
- 46.Curcio CA, Saunders PL, Younger PW, Malek G. Peripapillary chorioretinal atrophy: Bruch's membrane changes and photoreceptor loss. Ophthalmology. 2000;107:334–43. doi: 10.1016/s0161-6420(99)00037-8. [DOI] [PubMed] [Google Scholar]
- 47.Spaide RF. Age-related choroidal atrophy. Am J Ophthalmol. 2009;147:801–10. doi: 10.1016/j.ajo.2008.12.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.