Abstract
Although somatic mutations in exon 2 of the mediator complex subunit 12 (MED12) gene have been reported previously in uterine fibroids in women from Finland, South Africa, and North America, the status of these mutations was not reported in the Southern United States women. The aim of this study is to determine the MED12 somatic mutations in uterine fibroids of women from Southern Unites States, which will help to better understand the contribution of MED12 mutations in fibroid tumor biology. Herein, we determined the frequency of MED12 gene exon 2 somatic mutations in 143 fibroid tumors from a total of 135 women from the Southern United States and in 50 samples of the adjacent myometrium using PCR amplification and Sanger sequencing. We observed that the MED12 gene is mutated in 64.33 % (92/143) of uterine fibroid cases in the exon 2 (including deletion mutations). These mutations include 107T > G (4.3 %), 130G > C (2.8 %), 130G > A (7.0 %), 130G > T (2.8 %), 131G > C (2.1 %), 131G > A (20.2 %), and 131G > T (2.1 %). Interestingly, we identified four novel mutations in these patients: 107 T > C (12.8 %), 105A > T (2.1 %), 122T > A (2.1 %), and 92T > A (2.1 %). As expected, we did not observe any mutations in the normal myometrium. Moreover, we found a higher rate of deletion mutations (17.5 %, 25/143) in the above fibroid tumors. Our results clearly demonstrate that the MED12 gene exon 2 is frequently mutated in human uterine fibroids in Southern United States women. These results highlight the molecular pathogenesis of human uterine fibroids with the central role of MED12 somatic mutations.
Keywords: Uterine fibroids, Leiomyoma, MED12, Somatic mutations, Pathogenesis
Introduction
Uterine fibroids (leiomyoma) are the most common benign non-cancerous tumors in woman of reproductive age (Baird et al. 2003). Fibroids are tumors of the smooth muscle of the uterus; they cause a variety of symptoms, including pelvic pain, abnormal uterine bleeding, and infertility (Farhi et al. 1995; Wilcox et al. 1994). The medical term for a fibroid is leiomyoma, which is a type of myoma or mesenchymal tumor mass. Uterine fibroids are the most common benign pelvic tumors that affect many women in the United States (Eldar-Geva et al. 1998; Farhi et al. 1995; Houston et al. 2001; Stewart 2001). These growths occur in up to 50 % of all women and are one of leading causes of hysterectomy in the United States. An estimated 600,000 hysterectomies are performed in the United States annually, at a cost of more than 5 billion dollars annually, and at least one-third of these procedures are for fibroids. Although surgery is the major option for fibroid treatment, the available effective non-surgical treatment options are very limited due to their significant side effects (Friedman et al. 1994; Stewart et al. 1998).
Uterine fibroids are monoclonal tumors of the smooth muscle cells of the uterus, which are estrogen- and progesterone-dependent tumors and are typically regress with the onset of menopause. Uterine fibroids do not affect all ethnicities equally. African American women have a higher risk of developing uterine fibroids than Caucasian women (Baird et al. 2003). They also have an earlier age at onset, with larger, numerous, and more rapidly growing fibroids (Huyck et al. 2008; Marshall et al. 1997). Although several reports have recently demonstrated that the MED12 gene is frequently mutated in at least 70 % of the cases of uterine fibroids in different populations, including Finnish (Caucasian), northern USA regions, and South African patients (Makinen et al. 2011a, b; Markowski et al. 2012; McGuire et al. 2012), the frequency of these mutations in exon 2 of the MED12 gene in women from the Southern parts of the United States is still unknown. The aim of this study is to determine the status of MED12 somatic mutations in the Southern United States women, which will help to better understand the role of MED12 somatic mutations in the pathogenesis of human uterine fibroids. Moreover, understanding the role of MED12 somatic mutations in the pathogenesis of human uterine fibroids will help to develop non-surgical therapeutic options for the treatment of uterine fibroids.
MED12 is known as a mediator complex subunit 12 (MED12) gene, which consists of 26 subunit transcriptional regulators that bridge the DNA regulatory sequences to the RNA polymerase II initiation complex (Taatjes 2010). Somatic mutations in exon 2 of the MED12 gene have recently been reported by several studies (Makinen et al. 2011a, b; Markowski et al. 2012; McGuire et al. 2012). The MED12 gene has been known to play roles in gene-specific transcription processes, and it is also involved in many developmental processes (Taatjes 2010). MED12 is localized on chromosome Xq13.1. It is highly conserved in all eukaryotes, and is required for the transcription of almost all of the genes in yeast. Germline mutations of MED12 gene have been reported in the Opitz–Kaveggia and Lujan–Fryns syndromes, which are characterized by congenital anomalies and intellectual disability (Taatjes 2010). Evidence has showed that MED12 performs both general and gene-specific roles to regulate gene expression (Graham et al. 2010; Taatjes 2010). Nevertheless, the explanation for the high occurrence of MED12 gene mutations and the functional roles of these mutations in the tumorigenesis of uterine fibroids remain unknown. One study has recently demonstrated that uterine fibroids with MED12 gene mutations expressed higher levels of WNT4 mRNA, thus suggesting a possible role of MED12 mutations in the functional activation of the WNT pathway (Markowski et al. 2012). In addition, it has been linked to the direct interaction of MED12 with β-catenin, which is required for the cellular response to WNT signaling (Markowski et al. 2012).
In this study, we examined the frequency of the mutations in exon 2 of the MED12 gene in the uterine fibroids in women from the Southern parts of the United States and confirmed the status of the MED12 gene’s somatic mutations in human uterine fibroids. Herein, we screened a total of 143 uterine fibroid tumors from 135 individual subjects for these mutation analyses. Our findings demonstrated that somatic mutations in exon 2 of the MED12 gene may be important in the pathogenesis of uterine fibroids in Southern American women as well as women worldwide.
Materials and methods
Patient recruitment and materials
To study the genetic basis of uterine fibroids, we determined the mutation status in exon 2 of the MED12 gene in women from the Southern parts of the United States. Uterine fibroids and the adjacent myometrial tissues were collected from individuals who underwent abdominal, vaginal and laparoscopic surgery to remove their confirmed uterine fibroids. We used this large collection of uterine fibroids from women with surgically and pathologically confirmed uterine fibroids from various hospitals in Texas and Tennessee. These tissues were collected from different ethnic groups, including African American, Caucasian and Hispanic patients attending clinics in Texas and Tennessee, under approved Institutional Review Board protocols. The age range of the subjects was from 35 to 53 years old, with an average of 44 years, and their uterine fibroid sizes varied from 2.5-cm to 14.0-cm diameters. The patients were not administered any hormone supplements for at least 3 months before the hysterectomy was performed. Other sample characteristics are described in our recent publication (Halder et al. 2013). The collected tissues were snap-frozen and stored at −80 °C until further analysis. We analyzed somatic mutations in exon 2 of the MED12 gene in 143 collected uterine fibroids from 135 women from the Southern United States and 50 adjacent normal myometrium tissues that were available for use in this study.
Tissue samples and genomic DNA extraction
To isolate the genomic DNA, 2–3 mm3 of each frozen fibroid tumor was cut into small pieces, homogenized in tissue lysis buffer, and treated with proteinase K to help in protein degradation and tissue lysis. Genomic DNA was extracted using the QIAamp DNA Mini Kit (Qiagen) according to the manufacturer’s instructions. To evaluate whether MED12 mutations were present in the adjacent normal myometrium, a total of 50 adjacent normal myometrium tissue samples were selected for DNA isolation, as described above. The myometrial tissues were sampled within 1 cm from each tumor. DNA concentrations were determined using a NanoVue system (GE Healthcare, USA).
PCR amplification and Sanger sequencing
DNA amplification and sequencing analyses were performed at Vanderbilt University’s sequencing core facilities. The DNA fragment was amplified with AmpliTaq-Gold® DNA polymerase (Applied Biosystems, Foster City, CA) using sense and anti-sense primers that targeted exon 2 of the MED12 gene and produced a 125-bp polymerase chain reaction (PCR) product as described previously (Makinen et al. 2011b). Information for the MED12 gene-specific primers and the PCR amplification of the genomic DNA were obtained from the published literature (Makinen et al. 2011b). MED12 gene-specific primer sequences for the amplification of genomic DNA were sense 5′-GCCCTTTCACCTTGTTCCTT-3′ and anti-sense 5′-TGTCCCTATAAGTCTTCCCAACC-3′. These primers were purchased from Qiagen (Valencia, CA). In an AirClean 600 PCR Workstation hood, a 25-μl mastermix including genomic DNA (0.04 % volume), forward and reverse primers (each 0.04 % volume), ABI’s Amplitaq Gold DNA Polymerase (50 % volume), and water (remaining volume) was made for each reaction and subjected to the following thermal cycling conditions on a MJ Research Tetrad thermal cycler: denaturing at 95 °C for 10 min for 30 cycles, including 95 °C for 30 s, 60 °C for 30 s, 72 °C for 30 s, and extension at 72 °C for 10 min; then, the reaction was finally soaked at 4 °C. Following PCR amplification, the products were purified using 3 μl of ExoSAP-IT PCR product clean-up (Corporation, Cleveland, OH) at a 1:1 dilution with water combined with 7 μl of the amplified DNA and heated according to its protocol. The samples were then diluted with water to a concentration of 30 ng/μl, and then the purified PCR products were bidirectionally direct sequenced using the Sanger method, ending with capillary electrophoresis on an ABI 3730xl Automatic DNA Analyzer (Applied Biosystems, Foster City, CA). The PCR products were sequenced using the BigDye Terminator v.3.1 Kit (Applied Biosystems) using the original PCR primers specific to MED12 gene exon 2, according to the manufacturer’s instructions. The sequence graphs were analyzed manually for the MED12 gene exon 2 somatic mutations.
Results
MED12 gene somatic mutations are associated with fibroid pathogenesis in Southern United States women
Our sequence analyses showed that exon 2 in the MED12 gene is mutated/deleted in 63.63 % (92/143) of fibroid tumors, as summarized in Table 1. These mutations include 107T > G (1.4 %, 2/143), 128A > C (1.4 %, 2/143), 130G > C (2.8 %, 4/143), 130G > A (7.0 %, 10/143), 130G > T (2.8 %, 4/143), 131G > C (1.4 %, 2/143), 131G > A (20.2 %, 29/143), and 131G > T (1.4 %, 2/143). Interestingly, we also found several unique mutations in this population, and these unique mutations were 92T > A (0.7 %, 1/143), 105A > T (0.7 %, 1/143), 107T > C (4.2 %, 6/143), 108G > C (0.7 %, 1/143), 108G > T (1.4 %, 2/143), and 122T > A (0.7 %, 1/143), compared with the Finnish population (Table 1). We have analyzed the status of somatic mutations particularly in the exon 2 of the MED12 gene. The primer set we used for PCR analyses which can amplify only exon 2 region of the MED12 gene, but not the exon 1, and thus the 92T > A mutation is located in exon 2 of the MED12 gene. In addition, all other unique mutations were also present in exon 2 of the MED12 gene in uterine fibroids, while no mutation was observed in exon 2 of the MED12 gene in the adjacent normal myometrium. Moreover, we found a higher rate of deletion mutations (17.5 %, 25/143) in exon 2 of the MED12 gene in this population (Table 1). All of the fibroid tumors displayed only one mutation, and all of these mutations were heterozygous and presented in exon 2 of the MED12 gene. We presented the chromatographs for more common mutations in uterine fibroids of women from the Southern United States (Supplemental data). We found that in most of the cases of MED12 gene mutations, the nucleotides 130 and 131 in the exon 2 were affected. Moreover, the G > A transitions of codon 44 at nucleotides 130 and 131 were predominant in MED12 mutation-positive tumors (Table 1). Among these mutations, 131G > A base substitution (43.28 %, 29/67) was followed by 130G > A (14.92 %, 10/67), and 130G > C and 130G > T (5.97 %, 4/67) base substitutions (Table 1). On the other hand, the mutation at the 107 nucleotide substitution (107T > C) occurred considerably more frequently than other unique mutations at nucleotides 92, 105, 108, and 122 (8.95 versus 2.98 or 1.49 %) among all of the mutation-positive tumors (Table 1).
Table 1.
Summary of the distinct pattern of somatic mutations in exon 2 of the MED12 gene in uterine fibroids in women from the Southern parts of the United States
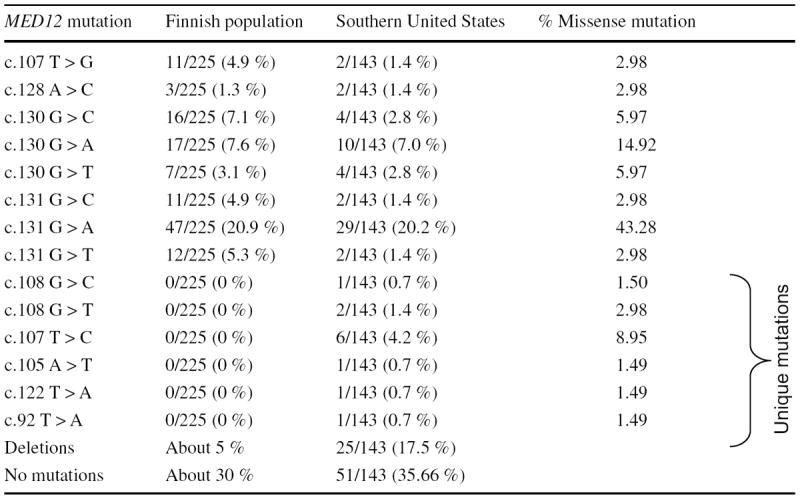
|
Mutation pattern in uterine fibroids was also compared between women from Finnish and Southern United States. Percentage of the missense mutation in each type is calculated out of the total number of fibroid tumors from Southern United States women harboring missense mutations. No mutation was found in normal myometrium tissues adjacent to uterine fibroids
To verify the absence of any mutations in the normal myometrium adjacent to the tumors, we sequenced a total of 50 DNA samples for the MED12 gene exon 2 mutation analyses, because those adjacent myometrium tissues were available for this study, as indicated above. As expected, no mutation in exon 2 of the MED12 gene was detected in any of these DNA samples. These results suggest that MED12 gene somatic mutations were present only in the uterine fibroids while these mutations were absent in the adjacent normal myometrial smooth muscle cells.
To further test whether differences exist between the frequency of mutation-positive fibroids in women from different ethnic groups, such as African Americans, Caucasian, and Hispanic, we further analyzed our data, and it is presented in Table 2. We observed that the nucleotide substitution mutation at 131G > A is higher in African Americans than in Caucasian or Hispanic women (61.3 versus 22.5, or 16.1 %) (Table 2). However, the number of the tumor samples for this mutation was limited to establish a statistical significance. A larger number of samples may be required to establish whether there is an association between specific somatic mutations with ethnicity. We also found that 130G > A and 130G > T substitution mutations were similarly present in the African American and Caucasian groups, at a frequency rate of approximately 50 % (Table 2). In addition, we found slightly higher frequency of 131G > A nucleotide substitution compared with 130G > A (61.3 versus 50 %) in African American women. Together, our results suggest that somatic mutations in exon 2 of the MED12 gene are common in the Southern United States women.
Table 2.
MED12 mutation status in different ethnic groups
| MED12 mutation | African American (68) | Caucasian (43) | Hispanic (24) |
|---|---|---|---|
| c.130G > A | 5/10 (50 %) | 4/10 (40 %) | 1/10 (10 %) |
| c.130G > T | 2/4 (50 %) | 2/4 (50 %) | 0/4 (0 %) |
| c.130G > C | 4/4 (100 %) | 0/4 (0 %) | 0/4 (0 %) |
| c.131G > A | 19/31 (61.3 %) | 7/31 (22.5 %) | 5/31 (16.1 %) |
| c.131G > T | 1/1 (100 %) | 0/1 (0 %) | 0/1 (0 %) |
| c.131G > C | 2/2 (100 %) | 0/2 (0 %) | 0/2 (0 %) |
| c.107T > C | 2/4 (50 %) | 1/4 (25 %) | 1/4 (25 %) |
| c.107T > G | 0/2 (0 %) | 2/2 (100 %) | 0/2 (0 %) |
| c.108G > C | 0/1 (0 %) | 0/1 (0 %) | 1/1 (100 %) |
| c.108G > T | 0/2 (0 %) | 0/2 (0 %) | 2/2 (100 %) |
| Deletions | 10/24 (41.6 %) | 10/24 (41.6 %) | 3/24 (12.5 %) |
| No mutations | 23/53 (43.3 %) | 17/53 (32.1 %) | 11/53 (20.7 %) |
The mutation status in exon 2 of the MED12 gene in the different ethnic groups including African American, Caucasian, and Hispanic from the Southern United States is presented
Discussion
Uterine fibroid, a quite common benign disease that rarely results in death, is a major cause of infertility, abortion, and hysterectomy (Farhi et al. 1995; Wilcox et al. 1994). It has been demonstrated by several studies that somatic mutations in the MED12 gene are associated with uterine fibroid tumorigenicity. Recently, somatic mutations in exon 2 of the MED12 gene have been identified in 70, 58.8, 67.6, and 52.2 % cases of uterine fibroids from different populations, including Finnish, South African, and North American women (Je et al. 2012; Makinen et al. 2011a, b; Markowski et al. 2012; McGuire et al. 2012). The recent findings of MED12 gene somatic mutations suggest a possibility that targeting mutations could be used in better understanding of the molecular mechanisms of pathogenesis of human uterine fibroids and can be used to develop non-surgical treatment strategy of uterine fibroids (Je et al. 2012).
In the current study, we focused on mutation analyses of exon 2 of the MED12 gene in a large number of uterine fibroids collected from the Southern United States women. Although a number of recent studies showed MED12 mutations in different populations including Finnish, South African, and North Americans, as well as Korean women (Je et al. 2012; Makinen et al. 2011a, b; Markowski et al. 2012; McGuire et al. 2012), the association of these mutations in exon 2 of the MED12 gene with the pathogenesis of uterine fibroids in Southern American women has yet to be established. Therefore, the current study was designed to verify the somatic mutations in exon 2 of the MED12 gene using PCR amplification and the direct sequencing method. Genomic DNA was isolated from a set of 143 uterine fibroid tumors from a total of 135 Southern United States women and 50 myometrial tissues that were adjacent to tumors and then amplified by PCR using the MED12 gene-specific forward and reverse primers as described in “Materials and methods”. Our results showed that at least 64.33 % (92/143) of the uterine fibroids from our Southern United States population showed MED12 gene somatic mutations (including deletion mutations), while no mutations were observed in the normal adjacent myometrium (Table 1). These results indicate that mutations in exon 2 of the MED12 gene are also common in the Southern United States women. Several reports also recently demonstrated the presence of MED12 gene exon 2 somatic mutations in uterine fibroid cases (Je et al. 2012; Makinen et al. 2011a, b; Markowski et al. 2012; McGuire et al. 2012). Our findings indicate that somatic mutations in exon 2 of the MED12 gene are also common in uterine fibroids in women from Southern United States. Moreover, we observed that none of the normal adjacent myometrium samples from the same patients showed evidence of mutations by direct DNA sequencing, which indicates that the MED12 mutations had developed somatically and presented only in uterine fibroids. Previous study also demonstrated that somatic mutations in the exon 2 of the MED12 gene are only present in uterine fibroids, but not in the adjacent normal myometrium (Makinen et al. 2011b). We also observed that all of the tumors showed only one mutation in exon 2 of the MED12 gene, and all of these genomic mutations are heterozygous in nature, which was also reported previously (Makinen et al. 2011b).
Race and ethnicity are important risk factors for the development of human uterine fibroids. Studies have shown that African Americans have a threefold to fourfold higher risk for uterine fibroids than Caucasian women (Baird et al. 2003; Kjerulff et al. 1996). The reasons for this ethnic variation of uterine fibroids are unknown. Uterine fibroids are characterized as estrogen- and progesterone-dependent tumors, and we have previously demonstrated that African American women have a higher prevalence of the estrogen receptor alpha (ER-α) variant than white women (Al-Hendy and Salama 2006); thus, this variant is known to be associated with an increasing risk of uterine fibroids in ethnicities.
Studies have shown that the MED12 gene somatic mutations are associated with uterine fibroids in women from Finland, South Africa, North America, and Korea (Je et al. 2012; Makinen et al. 2011a, b; Markowski et al. 2012; McGuire et al. 2012). Our data also showed higher incidence of MED12 somatic mutations in Southern United States women (Table 1). Despite the high incidence of MED12 somatic mutations in uterine fibroids, at least 30 % of uterine fibroids in Southern United States women are devoid of MED12 somatic mutations, which may suggest that other factors might also play roles in uterine fibroid pathogenesis. A recent study with whole exome sequencing of MED12 mutation-positive and MED12 mutation-negative uterine fibroids showed no additional gene mutations, and has demonstrated that other factors such as somatic structural rearrangements, epigenetic changes, and intronic variants are likely to have a particular impact on the development of MED12 wild-type uterine fibroid lesions (Makinen et al. 2014). For example, chromosomal translocations can lead to the overexpression of high mobility group AT-hook 2 (HMGA2) (Gross et al. 2003), which are particularly associated with MED12 mutation-negative uterine fibroids (Markowski et al. 2012). Uterine fibroids can also associate with heterozygous germline mutations in fumarate hydratase (FH) that encodes an enzyme known as fumarase (Tomlinson et al. 2002). Recurrent chromosomal translocation of the recombination repair gene RAD51B also produces premature truncated transcript in uterine fibroids (Ingraham et al. 1999). In addition, a recent study with gene expression analyses of MED12 mutation-positive fibroid tumors and their adjacent matched myometrium showed that at least three signaling pathways, including WNT signaling, are altered (Makinen et al. 2011b). Makinen et al. (2011b) have suggested a role of MED12 gene mutations in uterine fibroids through the activation of the WNT/β-catenin pathway. Moreover, it has been shown that MED12 is implicated in the transcription activation of WNT target genes by interacting with β-catenin (Kim et al. 2006; Rocha et al. 2010). In addition, WNT4 has been shown to be overexpressed in uterine fibroids with MED12 gene exon 2 mutations, and it is suggested that WNT4 is a possibly relevant downstream effector of the mutated MED12 gene (Markowski et al. 2012). On the other hand, it has also been demonstrated that estrogen can rapidly induce WNT4 expression in both an estrogen receptor-dependent and an estrogen-independent manner in cell culture systems (Hou et al. 2004; Miyakoshi et al. 2009). Therefore, it is reasonable to think that both mutated MED12 gene and estrogen can directly activate targets in the WNT signaling pathway. In our preliminary observation, we found upregulation of WNT4 protein expression in MED12 mutation-positive uterine fibroids compared with the matched adjacent normal myometrium. We also observed that uterine fibroids harboring MED12 gene exon 2 somatic mutations showed a higher expression of β-catenin (our unpublished data). One report indicated that the constitutive expression of activating β-catenin in uterine smooth muscle cells leads to the development of leiomyoma-like tumors in a mouse model (Tanwar et al. 2009). Thus, it is reasonable that the activation of β-catenin by the WNT signaling pathway may be a potential mechanism by which MED12 gene somatic mutations cause uterine fibroid pathogenesis.
In this study, our findings demonstrate that uterine fibroids from the Southern United States women harbor similar mutations in the MED12 gene exon 2 as Finnish women (Table 1). Our study confirmed the exon 2 mutation status of the MED12 gene in uterine fibroids despite different ethnic backgrounds. In addition, we found that the mutations at 130 and 131 nucleotides are very common in all populations, irrespective of ethnicity (Table 2). These findings suggest that mutations in the 130 and 131 nucleotides may play a major functional role in the MED12 gene in uterine fibroid pathogenesis. It has been stated previously that there was no direct association between specific mutations with ethnicity. Consistent to the previous report, our data also did not show direct correlation with specific mutations versus ethnicity. Interestingly, we found several unique mutations in uterine fibroids in the Southern United States women compared with the Finnish women (Table 1), suggesting that these unique mutations possibly have roles in fibroid pathogenesis. Thus, it is important to determine whether these mutations, particularly 130G > A and 131G > A which are the major mutations in all populations studied, have functional roles in the pathogenesis of human uterine fibroids.
In conclusion, we found that the MED12 gene was mutated in 64.4 % of the uterine fibroids in women from the Southern United States. We also found that the majority of uterine fibroids possess 131G > A (43.28 %) and 130G > A (14.92 %) mutations irrespective of ethnicity. Moreover, we found several unique mutations in above population. Thus, somatic mutations in exon 2 of the MED12 gene are associated with fibroid pathogenesis in the Southern United States women. Future study will be required to understand the functions of these mutations in the development and progression of human uterine fibroids. A detailed understanding of the functions of MED12 gene somatic mutations may be helpful to determine the molecular mechanism of fibroid developments, which can further help in developing non-surgical medical treatment options for uterine fibroids.
Supplementary Material
Acknowledgments
We would like to thank the Vanderbilt DNA core facility for custom sequencing of exon 2 of the MED12 gene. This study was supported primarily by the Research Centers in Minority Institutions (RCMI) pilot grant 2G12RR003032-26 (to S. K. H), and National Institute of Health (NIH)/R01 HD046228 (to A. A. H.).
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s00438-014-0938-x) contains supplementary material, which is available to authorized users.
Conflict of interest The authors in this paper have no conflict of interest or nothing to disclose.
References
- Al-Hendy A, Salama SA. Ethnic distribution of estrogen receptor-alpha polymorphism is associated with a higher prevalence of uterine leiomyomas in black Americans. Fertil Steril. 2006;86:686–693. doi: 10.1016/j.fertnstert.2006.01.052. [DOI] [PubMed] [Google Scholar]
- Baird DD, Dunson DB, Hill MC, Cousins D, Schectman JM. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol. 2003;188:100–107. doi: 10.1067/mob.2003.99. [DOI] [PubMed] [Google Scholar]
- Eldar-Geva T, Meagher S, Healy DL, MacLachlan V, Breheny S, Wood C. Effect of intramural, subserosal, and submucosal uterine fibroids on the outcome of assisted reproductive technology treatment. Fertil Steril. 1998;70:687–691. doi: 10.1016/s0015-0282(98)00265-9. [DOI] [PubMed] [Google Scholar]
- Farhi J, Ashkenazi J, Feldberg D, Dicker D, Orvieto R, Ben Rafael Z. Effect of uterine leiomyomata on the results of in vitro fertilization treatment. Hum Reprod. 1995;10:2576–2578. doi: 10.1093/oxfordjournals.humrep.a135748. [DOI] [PubMed] [Google Scholar]
- Friedman AJ, Daly M, Juneau-Norcross M, Gleason R, Rein MS, LeBoff M. Long-term medical therapy for leiomyomata uteri: a prospective, randomized study of leuprolide acetate depot plus either oestrogen-progestin or progestin ‘add-back’ for 2 years. Hum Reprod. 1994;9:1618–1625. doi: 10.1093/oxfordjournals.humrep.a138762. [DOI] [PubMed] [Google Scholar]
- Graham JM, Jr, Clark RD, Moeschler JB, Rogers RC. Behavioral features in young adults with FG syndrome (Opitz-Kaveggia syndrome) Am J M Genet Part C Semin Med Genet. 2010;154C:477–485. doi: 10.1002/ajmg.c.30284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross KL, Neskey DM, Manchanda N, Weremowicz S, Kleinman MS, Nowak RA, Ligon AH, Rogalla P, Drechsler K, Bullerdiek J, Morton CC. HMGA2 expression in uterine leiomyomata and myometrium: quantitative analysis and tissue culture studies. Genes Chromosom Cancer. 2003;38:68–79. doi: 10.1002/gcc.10240. [DOI] [PubMed] [Google Scholar]
- Halder SK, Osteen KG, Al-Hendy A. 1,25-dihydroxyvitamin d3 reduces extracellular matrix-associated protein expression in human uterine fibroid cells. Biol Reprod. 2013;89:150. doi: 10.1095/biolreprod.113.107714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou X, Tan Y, Li M, Dey SK, Das SK. Canonical Wnt signaling is critical to estrogen-mediated uterine growth. Mol Endocrinol. 2004;18:3035–3049. doi: 10.1210/me.2004-0259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houston KD, Hunter DS, Hodges LC, Walker CL. Uterine leiomyomas: mechanisms of tumorigenesis. Toxicol Pathol. 2001;29:100–104. doi: 10.1080/019262301301418900. [DOI] [PubMed] [Google Scholar]
- Huyck KL, Panhuysen CI, Cuenco KT, Zhang J, Goldhammer H, Jones ES, Somasundaram P, Lynch AM, Harlow BL, Lee H, Stewart EA, Morton CC. The impact of race as a risk factor for symptom severity and age at diagnosis of uterine leiomyomata among affected sisters. Am J Obstet Gynecol. 2008;198(168):e161–e169. doi: 10.1016/j.ajog.2007.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingraham SE, Lynch RA, Kathiresan S, Buckler AJ, Menon AG. hREC2, a RAD51-like gene, is disrupted by t(12;14) (q15;q24.1) in a uterine leiomyoma. Cancer Genet Cytogenet. 1999;115:56–61. doi: 10.1016/s0165-4608(99)00070-9. [DOI] [PubMed] [Google Scholar]
- Je EM, Kim MR, Min KO, Yoo NJ, Lee SH. Mutational analysis of MED12 exon 2 in uterine leiomyoma and other common tumors. Int J Cancer J Int Cancer. 2012;131:E1044–E1047. doi: 10.1002/ijc.27610. [DOI] [PubMed] [Google Scholar]
- Kim S, Xu X, Hecht A, Boyer TG. Mediator is a transducer of Wnt/beta-catenin signaling. J Biol Chem. 2006;281:14066–14075. doi: 10.1074/jbc.M602696200. [DOI] [PubMed] [Google Scholar]
- Kjerulff KH, Langenberg P, Seidman JD, Stolley PD, Guzinski GM. Uterine leiomyomas. Racial differences in severity, symptoms and age at diagnosis. J Reprod Med. 1996;41:483–490. [PubMed] [Google Scholar]
- Makinen N, Heinonen HR, Moore S, Tomlinson IP, van der Spuy ZM, Aaltonen LA. MED12 exon 2 mutations are common in uterine leiomyomas from South African patients. Oncotarget. 2011a;2:966–969. doi: 10.18632/oncotarget.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makinen N, Mehine M, Tolvanen J, Kaasinen E, Li Y, Lehtonen HJ, Gentile M, Yan J, Enge M, Taipale M, Aavikko M, Katainen R, Virolainen E, Böhling T, Koski TA, Launonen V, Sjöberg J, Taipale J, Vahteristo P, Aaltonen LA. MED12, the mediator complex subunit 12 gene, is mutated at high frequency in uterine leiomyomas. Science. 2011b;334:252–255. doi: 10.1126/science.1208930. [DOI] [PubMed] [Google Scholar]
- Makinen N, Vahteristo P, Butzow R, Sjoberg J, Aaltonen LA. Exomic landscape of MED12 mutation-negative and -positive uterine leiomyomas. Int J Cancer. 2014;134:1008–1012. doi: 10.1002/ijc.28410. [DOI] [PubMed] [Google Scholar]
- Markowski DN, Bartnitzke S, Loning T, Drieschner N, Helmke BM, Bullerdiek J. MED12 mutations in uterine fibroids–their relationship to cytogenetic subgroups. Int J Cancer J Int Cancer. 2012;131:1528–1536. doi: 10.1002/ijc.27424. [DOI] [PubMed] [Google Scholar]
- Marshall LM, Spiegelman D, Barbieri RL, Goldman MB, Manson JE, Colditz GA, Willett WC, Hunter DJ. Variation in the incidence of uterine leiomyoma among premenopausal women by age and race. Obstet Gynecol. 1997;90:967–973. doi: 10.1016/s0029-7844(97)00534-6. [DOI] [PubMed] [Google Scholar]
- McGuire MM, Yatsenko A, Hoffner L, Jones M, Surti U, Rajkovic A. Whole exome sequencing in a random sample of North American women with leiomyomas identifies MED12 mutations in majority of uterine leiomyomas. PLoS One. 2012;7:e33251. doi: 10.1371/journal.pone.0033251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyakoshi T, Kajiya H, Miyajima K, Takei M, Tobita M, Takekoshi S, Osamura RY. The expression of Wnt4 is regulated by estrogen via an estrogen receptor alpha-dependent pathway in rat pituitary growth hormone-producing cells. Acta Histochem Cytochem. 2009;42:205–213. doi: 10.1267/ahc.09033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha PP, Scholze M, Bleiss W, Schrewe H. Med12 is essential for early mouse development and for canonical Wnt and Wnt/PCP signaling. Development. 2010;137:2723–2731. doi: 10.1242/dev.053660. [DOI] [PubMed] [Google Scholar]
- Stewart EA. Uterine fibroids. Lancet. 2001;357:293–298. doi: 10.1016/S0140-6736(00)03622-9. [DOI] [PubMed] [Google Scholar]
- Stewart EA, Rhoades AR, Nowak RA. Leuprolide acetate-treated leiomyomas retain their relative overexpression of collagen type I and collagen type III messenger ribonucleic acid. J Soc Gynecol Investig. 1998;5:44–47. doi: 10.1016/s1071-5576(97)00097-x. [DOI] [PubMed] [Google Scholar]
- Taatjes DJ. The human Mediator complex: a versatile, genome-wide regulator of transcription. Trends Biochem Sci. 2010;35:315–322. doi: 10.1016/j.tibs.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanwar PS, Lee HJ, Zhang L, Zukerberg LR, Taketo MM, Rueda BR, Teixeira JM. Constitutive activation of Beta-catenin in uterine stroma and smooth muscle leads to the development of mesenchymal tumors in mice. Biol Reprod. 2009;81:545–552. doi: 10.1095/biolreprod.108.075648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomlinson IP, Alam NA, Rowan AJ, Barclay E, Jaeger EE, Kelsell D, Leigh I, Gorman P, Lamlum H, Rahman S, Roylance RR, Olpin S, Bevan S, Barker K, Hearle N, Houlston RS, Kiuru M, Lehtonen R, Karhu A, Vilkki S, Laiho P, Eklund C, Vierimaa O, Aittomäki K, Hietala M, Sistonen P, Paetau A, Salovaara R, Herva R, Launonen V, Aaltonen LA. Germline mutations in FH predispose to dominantly inherited uterine fibroids, skin leiomyomata and papillary renal cell cancer. Nat Genet. 2002;30:406–410. doi: 10.1038/ng849. [DOI] [PubMed] [Google Scholar]
- Wilcox LS, Koonin LM, Pokras R, Strauss LT, Xia Z, Peterson HB. Hysterectomy in the United States, 1988–1990. Obstet Gynecol. 1994;83:549–555. doi: 10.1097/00006250-199404000-00011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


