Abstract
Background
Prediabetes affects 1 in 3 Americans. Both intensive lifestyle intervention and metformin can prevent or delay progression to diabetes. Over the past decade, lifestyle interventions have been translated across various settings, but little is known about the translation of evidence surrounding metformin use.
Objective
To examine metformin prescription for diabetes prevention and patient characteristics that may affect metformin prescription.
Design
Retrospective cohort analysis over a 3-year period.
Setting
Employer groups that purchased health plans from the nation’s largest private insurer.
Participants
A national sample of 17 352 working-age adults with prediabetes insured for 3 continuous years between 2010 and 2012.
Measurements
Percentage of health plan enrollees with prediabetes who were prescribed metformin.
Results
Only 3.7% of patients with prediabetes were prescribed metformin over the 3-year study window. After adjustment for age, income, and education, the predicted probability of metformin prescription was almost 2 times higher among women and obese patients and more than 1.5 times higher among patients with 2 or more comorbid conditions.
Limitation
Missing data on lifestyle interventions, possible mis-classification of prediabetes and metformin use, and inability to define eligible patients exactly as defined in the American Diabetes Association guidelines.
Conclusion
Evidence shows that metformin is rarely prescribed for diabetes prevention in working-age adults. Future studies are needed to understand potential barriers to wider adoption of this safe, tolerable, evidence-based, and cost-effective prediabetes therapy.
Primary Funding Source
Centers for Disease Control and Prevention (Division of Diabetes Translation) and the National Institute of Diabetes and Digestive and Kidney Diseases.
Diabetes prevention is an important national health goal. The number of persons with prediabetes, which has increased to more than 1 in 3 U.S. adults (1, 2), shows the urgent need for effective action leading to prevention. However, the means through which diabetes prevention can best be achieved on an individual as well as population level remains unclear.
For more than 10 years, the literature has provided strong evidence to support the use of both intensive lifestyle intervention and metformin to help prevent diabetes among persons at increased risk because of prediabetes. In 2002, the DPP (Diabetes Prevention Program) showed that lifestyle intervention and metformin reduced the incidence of diabetes by 58% and 31%, respectively, compared with placebo over 2.8 years (3). These findings were supported by several other randomized studies and were shown to persist for up to 10 years in longitudinal observational studies (3–8). The 16-week intensive lifestyle intervention in the DPP was associated with the largest cumulative risk reduction, which prompted many translational studies (9–11). However, efforts to translate DPP-based lifestyle interventions have been associated with various levels of uptake and reach (9–12).
In contrast, little is known about the translation of the evidence supporting metformin use to prevent diabetes. Such evidence is strongest for those at increased risk for progression to diabetes, including persons younger than 60 years, those with a body mass index (BMI) of 35 kg/m2 or greater, or those with a history of gestational diabetes (3, 6, 8, 13). Beginning in 2008, the annual “Standards in Medical Care in Diabetes” guidelines from the American Diabetes Association recommended metformin use for diabetes prevention in patients at very high risk who meet the aforementioned criteria and added that metformin use “may be considered” in those with impaired glucose tolerance, impaired fasting glucose level, or a hemoglobin A1c level of 5.7% to 6.4% (13).
Despite inclusion in national guidelines for more than 6 years (13) and proven long-term tolerability, safety, and cost-effectiveness (14), the prescription of metformin in the real-world clinical approach to diabetes prevention remains unclear. The only published study to include incidence of metformin use among patients with prediabetes found that fewer than 0.1% were prescribed metformin (15). However, these data were collected from an integrated health delivery system that may not accurately reflect wider practice patterns and were reported for only 1 time point within 6 months of prediabetes identification. Further, this study began in 2006, which was 2 years before metformin use was first emphasized in national guideline recommendations for diabetes prevention (13, 16).
The goal of our analysis was to characterize metformin prescriptions in a sample of insured, working-age adults with prediabetes from all 50 states. We also explored the association between specific patient characteristics and the receipt of metformin. We hypothesized that despite the existence of practice guidelines supporting its use, metformin is rarely prescribed for diabetes prevention.
Methods
We examined data from 2010 to 2012 from United-Healthcare (UHC), the nation’s largest private insurer (17), using a retrospective cohort analysis of metformin prescription among adults with prediabetes over a 3-year period.
Setting and Participants
Participants were employees and covered dependents aged 19 to 58 years at baseline and enrolled in UHC benefit plans for 3 continuous years. The study window was from 2010 to 2012. Data from year 1 (2010) were used to define the sample with prediabetes and exclude persons with diabetes.
All participants had diagnoses of prediabetes at year 1, defined as any of the following: 2 or more International Classification of Diseases, Ninth Revision (ICD-9), diagnostic codes of 790.2× from an inpatient or out-patient claim; last hemoglobin A1c level of 5.7% to 6.4%; last fasting plasma glucose level of 5.55 to 6.94 mmol/L (100 to 125 mg/dL); or last 2-hour plasma glucose level of 7.77 to 11.04 mmol/L (140 to 199 mg/dL) on an oral glucose tolerance test. Patients with a diagnosis of diabetes in year 1 were excluded from the sample. Diabetes was defined as any of the following: 1 or more ICD-9 diagnostic codes of 250.xx from an inpatient or outpatient claim; hemoglobin A1c level of 6.5% or greater; fasting plasma glucose level greater than 6.94 mmol/L (>125 mg/dL); 2-hour plasma glucose level of 11.1 mmol/L (200 mg/dL) or greater on an oral glucose tolerance test; or 1 or more prescription claims for insulin or an antiglycemic medication other than metformin.
Data from 183 UHC employer groups with sufficient administrative and laboratory data to identify employees with prediabetes and pharmacy claims over the 3-year study window were available as part of a larger design study on health benefits (Appendix, available at www.annals.org) (18) (Moin T, Steers WN, Ettner SL, Duru OK, Turk N, Neugebauer R, et al. The association of a diabetes-specific health plan with ER and in-patient hospital utilization: a natural experiment for translation in diabetes [NEXT-D]. In preparation.). These 183 groups were identified from a larger set of 1357 employer groups that purchased benefit plans from UHC between 2009 and 2010. Compared with the larger pool of 1174 groups, these 183 groups tended to be larger, had slightly higher proportions of patients with chronic conditions, and had slightly more Hispanic employees but were similar in terms of other racial/ethnic distributions, mean employee income, proportion of female employees, and proprietary estimates of benefit generosity provided by the health plan.
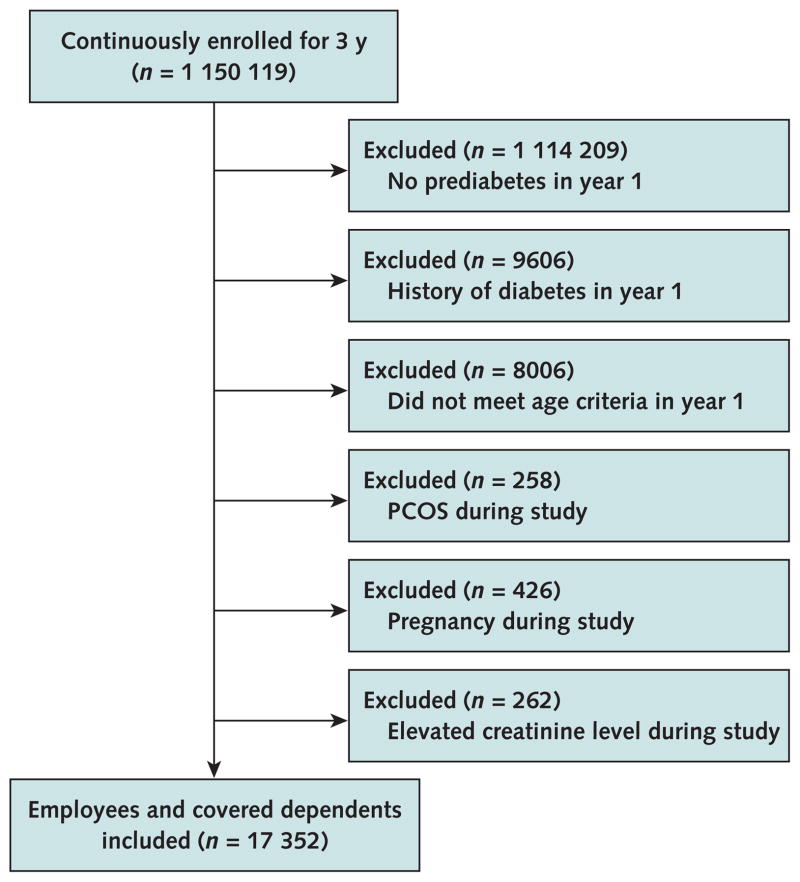
Among the 183 employer groups, there were 35 910 employees or covered dependents who were continuously enrolled with UHC for 3 years and had prediabetes in year 1. We excluded patients with a history of diabetes in year 1 (n = 9606), those who were not aged 19 to 58 years in year 1 (n = 8006) because national guidelines highlight evidence for metformin use in patients younger than 60 years, and women with a history of the polycystic ovary syndrome (n = 258) because metformin can be prescribed for reasons other than diabetes prevention in this group (for example, oligomenorrhea and infertility) (19–21). We also excluded those who were pregnant (n = 426) because metformin is classified under U.S. Food and Drug Administration pregnancy category B, as well as those with an elevated creatinine level (defined as ≥132.6 μmol/L [1.5 mg/dL] for men and ≥123.8 μmol/L [1.4 mg/dL] for women [n = 262]) because renal insufficiency is a contraindication to metformin use. The final analytic sample comprised 17 352 patients with prediabetes (Figure). We then identified a dominant provider for each patient by using the most frequent National Provider Identifier number in inpatient or out-patient claims during the 3-year study window.
Figure.
Study flow diagram.
The study window was 3 y (2010 to 2012). Prediabetes was defined as any of the following: ≥2 ICD-9 diagnostic codes of 790.2× from an inpatient or outpatient claim, last hemoglobin A1c level of 5.7% to 6.4%, last fasting plasma glucose level of 5.55 to 6.94 mmol/L (100 to 125 mg/dL), or last 2-h plasma glucose level of 7.77 to 11.04 mmol/L (140 to 199 mg/dL) on an oral glucose tolerance test. Pregnancy was defined as ≥1 pregnancies during the study. Elevated creatinine level was defined as ≥132.6 μmol/L (≥1.5 mg/dL) for men and ≥123.8 μmol/L (≥1.4 mg/dL) for women. ICD-9 = International Classification of Diseases, Ninth Revision; PCOS = the polycystic ovary syndrome.
Primary Outcome
The primary outcome was a dichotomous indicator for metformin prescription among adult employees and covered dependents with prediabetes based on UHC prescription claims data. Metformin prescription was defined as any prescription claim for metformin in the 3-year study window. For patients who developed diabetes during years 2 and 3 (2011 and 2012), only metformin prescriptions before diabetes identification were included.
Covariates
The age and sex of patients were obtained from UHC eligibility files. Education, income, and race/ethnicity were estimated by UHC using a proprietary algorithm that incorporated geographic locators (that is, ZIP codes of record); consumer survey information; census income distribution data; and first, middle, and last names. Comorbid conditions, including history of the polycystic ovary syndrome, pregnancy, gestational diabetes, and obesity (BMI ≥30 kg/m2), were defined as 1 or more ICD-9–related diagnoses from inpatient or outpatient claims.
Statistical Analysis
We used a multivariate logistic regression model to test the association between specific patient characteristics and metformin prescription during the 3-year study window. We adjusted the model for age, sex, race, income, education, diagnosis of obesity, and number of comorbid conditions at baseline. Age and obesity were included in the model because the evidence for metformin use is greatest in those younger than 60 years or with a BMI greater than 35 kg/m2. We included race because certain groups (such as African Americans) have a higher risk for diabetes, and this may affect willingness or motivation to prescribe metformin for prevention. Sex was included because women are more likely to use health services overall, which may affect the likelihood of receiving a prescription, such as metformin. The number of comorbid conditions may also affect the use of health services and willingness to use metformin (that is, willingness may be higher for one who is already accustomed to taking medications but lower for one who has never been prescribed medications in the past). Last, we included estimates of income and education because these are proxies for health literacy and financial resources. We conducted multiple imputation by a chained equations approach to address missing data in the race/ethnicity (5%), education (1%), and income (8%) estimates. The resulting estimations were combined across 10 imputed data sets by using Rubin rules (22, 23). Multiple imputation was done in STATA using the user-written “ice” command, and the “mi estimate” command was then used to estimate and combine the primary outcome of interest across 10 imputed data sets. The STATA “margins” command was used to obtain predicted probabilities of metformin prescription over the 3-year study window.
The academic team members analyzed all data independently and retained sole authority over all publication-related decisions throughout the study. All analyses were done using SAS, version 9.3 (SAS Institute), and STATA, version 12.1 (StataCorp). The study was approved by the Institutional Review Board at the University of California, Los Angeles.
Role of the Funding Source
This study was jointly funded by the Centers for Disease Control and Prevention (Division of Diabetes Translation) and the National Institute of Diabetes and Digestive and Kidney Diseases as part of the Natural Experiments for the Translation of Diabetes (NEXT-D) study (grant number U58DP002722-05). The funding sources had no role in the design, conduct, or reporting of the study or the decision to submit the manuscript for publication.
Results
We analyzed data from 17 352 adults aged 19 to 58 years with prediabetes who were continuously enrolled in UHC benefit plans from 2010 to 2012. Forty-five percent were women, and 65% were white (Table 1). A total of 13 743 dominant providers submitted visit claims for this cohort during the 3-year study window. Only 3.7% (n = 647) of patients had a prescription claim for metformin during the study (Appendix Table 1, available at www.annals.org, provides the prevalence of metformin prescription by relative distribution of prediabetes inclusion criteria). However, among the smaller subset of patients with a BMI greater than 35 kg/m2 (n = 391) or gestational diabetes (n = 121)—the group for which the American Diabetes Association guideline places the most emphasis on treating prediabetes with metformin—the prevalence of metformin prescription was 7.8%.
Table 1.
Baseline (2010) Demographic Characteristics of Patients With Prediabetes Continuously Insured Over 3 Years*
| Characteristic | Enrollees With Prediabetes (n = 17 352), n (%) |
|---|---|
| Female | 7884 (45) |
|
| |
| Age group | |
| 19 to <35 y | 1385 (8) |
|
| |
| 35 to <45 y | 4062 (23) |
|
| |
| 45 to <55 y | 8068 (47) |
|
| |
| 55–58 y | 3837 (22) |
|
| |
| Race/ethnicity | |
| White | 11 209 (65) |
|
| |
| Hispanic | 2557 (15) |
|
| |
| African American | 1635 (9) |
|
| |
| Asian | 1021 (6) |
|
| |
| Other | 64 (0) |
|
| |
| Missing | 866 (5) |
|
| |
| Annual household income | |
| <$30 000 | 593 (3) |
|
| |
| $30 000–$49 000 | 2529 (15) |
|
| |
| $50 000–$74 000 | 4331 (25) |
|
| |
| ≥$75 000 | 8454 (49) |
|
| |
| Missing | 1445 (8) |
| Education level | |
|
| |
| High school or less | 4363 (25) |
|
| |
| Some college | 9076 (52) |
|
| |
| Bachelor’s degree or higher | 3750 (22) |
|
| |
| Missing | 163 (1) |
|
| |
| Obesity† | 1341 (8) |
|
| |
| Number of comorbid conditions | |
| 0 | 4115 (24) |
|
| |
| 1 | 5440 (31) |
|
| |
| 2 | 4688 (27) |
|
| |
| ≥3 | 3109 (18) |
Race/ethnicity, education, and income were estimated by a proprietary algorithm. We conducted multiple imputation by a chained equations approach to address missing data in the race/ethnicity, education, and income estimates.
International Classification of Diseases, Ninth Revision, classification for body mass index ≥30 kg/m2.
We found that the adjusted predicted probability of metformin prescription was almost twice as high among women (4.8%) than among men (2.8%) ( P < 0.001) and among obese patients (6.6%) compared with nonobese patients (3.5%) (P < 0.001) and was 1.5 times as high among patients with 2 or more comorbid conditions (4.2%) versus those with none (2.8%) (P = 0.001) (Table 2).
Table 2.
Adjusted Predicted Probability of Metformin Prescription Over the 3-Year Study Window*
| Characteristic | Predicted Probability of Metformin Prescription (n = 17 352), % | P Value |
|---|---|---|
| Sex | ||
|
| ||
| Male | 2.8 (reference) | |
|
| ||
| Female | 4.8 | <0.001 |
| Age group | ||
|
| ||
| 19 to <35 y | 3.5 (reference) | |
|
| ||
| 35 to <45 y | 3.8 | 0.65 |
|
| ||
| 45 to <55 y | 3.8 | 0.70 |
|
| ||
| 55–58 y | 3.6 | 0.91 |
| Race/ethnicity | ||
|
| ||
| White | 3.8 (reference) | |
|
| ||
| Hispanic | 3.8 | 0.94 |
|
| ||
| African American | 2.9 | 0.060 |
|
| ||
| Asian | 3.2 | 0.36 |
|
| ||
| Other | 8.2 | 0.089 |
|
| ||
| Annual household income | ||
| <$30 000 | 2.5 (reference) | |
|
| ||
| $30 000–$49 000 | 3.8 | 0.118 |
|
| ||
| $50 000–$74 000 | 4.1 | 0.052 |
|
| ||
| ≥$75 000 | 3.6 | 0.156 |
|
| ||
| Education level | ||
| High school or less | 3.8 (reference) | |
|
| ||
| Some college | 3.6 | 0.54 |
|
| ||
| Bachelor’s degree or higher | 3.9 | 0.94 |
| Obesity† | ||
|
| ||
| No | 3.5 (reference) | |
|
| ||
| Yes | 6.6 | <0.001 |
| Number of comorbid conditions | ||
|
| ||
| 0 | 2.8 (reference) | |
|
| ||
| 1 | 3.4 | 0.119 |
|
| ||
| 2 | 4.2 | 0.001 |
|
| ||
| ≥3 | 4.9 | <0.001 |
Adjusted for sex, age, race/ethnicity, income, education, diagnosis of obesity, and number of comorbid conditions at baseline. Race/ ethnicity, education, and income were estimated by a proprietary algorithm. We conducted multiple imputation by a chained equations approach to address missing data in the race/ethnicity (5%), education (1%), and income (8%) estimates.
International Classification of Diseases, Ninth Revision, classification for body mass index ≥30 kg/m2.
We did 4 sensitivity analyses (Appendix Table 2, available at www.annals.org). First, we applied a more stringent diagnostic criterion of 2 or more ICD-9 codes to define diabetes. With this approach, the prevalence of metformin prescription was 4.8% over 3 years, but there were no changes in the significance of the association of metformin prescription with patient-level characteristics. Second, we included metformin prescription before identification of diabetes in all 3 years (vs. years 2 and 3), and this increased the prevalence of metformin prescription to 4.4% over 3 years. Third, we included women with a history of the polycystic ovary syndrome, which also increased the prevalence of metformin prescription to 4.4% over 3 years. Last, we tried to address the lack of available data on the use of lifestyle interventions. Although systematic data on uptake of DPP-based lifestyle interventions were not available across all plans included in this analysis, the uptake of DPP lifestyle interventions has ranged from 2% to 25% for health plans that contract with UHC to provide these programs (Bandapati S, Chapman-Smith L, Keckhafer A. Personal communication). Thus, we conservatively assumed that 25% of the entire sample was participating in lifestyle interventions, which would imply that the prevalence of metformin prescription was still only 5.0% in the remaining sample.
Discussion
Our study of a national sample of insured, working-age adults found that only 3.7% of patients with prediabetes (1 in 27) were prescribed metformin over a 3-year period. The prevalence of metformin prescription was higher (7.8%) among a smaller subset of patients with a history of gestational diabetes or a BMI greater than 35 kg/m2. However, this means that fewer than 1 in 12 of these high-risk patients, specifically identified by national guidelines, received metformin. The predicted probability of metformin prescription was higher for women, obese patients, and those with more comorbid conditions.
Our findings highlight concern about translation of decade-old evidence supporting the use of metformin for diabetes prevention. Although we observed a metformin prescription prevalence of 3.7%, which is higher than the prevalence of less than 0.1% previously reported by Schmittdiel and colleagues (15), it still represents underuse of a highly effective prevention strategy. Schmittdiel and colleagues only reported metformin use within 6 months of prediabetes identification, but our study examined the prevalence of metformin prescription over a 3-year study window. In addition, Schmittdiel and colleagues examined electronic medical record data between 2006 and 2010, but our study is, to our knowledge, the first to specifically examine the prevalence of metformin prescription after the American Diabetes Association guideline changes in 2008 and the first to include a national sample of working-age adults younger than 60 years, in whom indications for metformin use may be strongest (24).
From a public health perspective, the lack of translation of a safe, evidence-based therapy for a highly prevalent condition is problematic. Studies have shown that lifestyle intervention is cost-effective, whereas metformin can be marginally cost-saving (25). Our study targeted insured persons with prediabetes who were receiving care, and metformin use of 3.7% seems especially low for this group. It is unlikely that many patients in our cohort were participating in intensive lifestyle programs. If we assumed that 25% of patients in the sample were actively participating in lifestyle interventions, the prevalence of metformin prescription was still only 5.0% in the remaining sample. The ideal scenario would be for everyone at risk for diabetes to pursue lifestyle interventions. However, given the resources needed to effectively deliver intensive lifestyle programs and the variable uptake reported in translational studies (9–12), patients should, at a minimum, be educated about the potential benefits of metformin and should, ideally, also be offered this option as preventive treatment for diabetes. This protocol may be feasible to implement on a wide scale given its relative accessibility, safety, and cost-effectiveness (14, 25, 26), and it is accessible under most prescription drug plans. It may also be a particularly attractive option for persons with significant time commitments that make lifestyle change challenging and may allow them to fully engage in evidence-based diabetes prevention strategies.
The reasons for low metformin use are not entirely clear, and future studies should examine an array of patient-, provider-, and organization-level factors that may contribute to underuse. For providers, barriers may include lack of knowledge about the DPP or related evidence. Even when the randomized clinical trial evidence is fully realized, there is little guidance for the application of these findings in real-world settings. Metformin is not approved by the U.S. Food and Drug Administration for prediabetes, which may increase hesitancy to prescribe it “off label” in this context (27). We identified 13 743 dominant physicians for this cohort of 17 352 patients, so metformin underuse is probably widespread among physicians rather than being limited to a small group of providers caring for many patients.
In addition, both providers and patients may place higher priority on other medical needs or be reluctant to “medicalize” prediabetes (15). Patients and providers may also lack awareness of prediabetes, specifically the natural disease course that can result in incident diabetes in 30% of patients within 3 to 4 years (28) and the associated risks (for example, microvascular complications) (3). However, obesity is a well-recognized risk factor for diabetes, and this may have contributed to the increased probability of metformin prescription seen in this group. Patients with more comorbid conditions may also be at increased risk for diabetes. They are also probably more accustomed to taking daily medications, so the threshold to start metformin (that is, to add 1 more pill) may be lower or they may be more motivated to prevent another comorbid condition. These observations, although based on a small sample, suggest that there is awareness of risk on some level, but further studies should examine how prediabetes risk perception and awareness affect patient and provider approaches to management. In general, further studies are also needed to fully understand all possible barriers surrounding metformin use in patients at highest risk so that successful interventions can be developed.
To reap the full public health benefits of the considerable research investment in diabetes prevention to date (29), we need truly informed and shared decision making between patients and providers in real-world settings. Optimal prediabetes management should be based on individualized risk assessment and patient preferences. The evidence for metformin use is strongest for patients younger than 60 years, those with a BMI greater than 35 kg/m2, or those with a history of gestational diabetes (13). Expert panels have also advocated that metformin use be considered in patients with an impaired fasting glucose level and impaired glucose tolerance if they also have a family history of diabetes in first-degree relatives, elevated triglyceride levels, reduced high-density lipoprotein cholesterol levels, hypertension, or more severe or progressive hyperglycemia (either a hemoglobin A1c level >6.0% or progression of an underlying disease, as evidenced by an increase in fasting plasma glucose, hemoglobin A1c, or 2-hour postprandial glucose level) (30). Potential strategies to increase awareness and promote informed decision making among this at-risk population could include clinical decision-making tools, physician-directed and performance-based incentive programs, or media campaigns to increase public awareness of prediabetes and its consequences if left untreated.
Our findings should be interpreted with a few limitations in mind. First, we did not have access to data on participation in lifestyle interventions, which are considered first-line therapy for diabetes prevention. However, a sensitivity analysis using estimates of uptake of lifestyle interventions did not substantially change our estimated prevalence of metformin prescription. Second, because this was a claims-based analysis, possible misclassification of prediabetes and metformin use may have occurred (for example, metformin use may not have been captured if no prescription claim was generated because an enrollee paid out of pocket or if a prescription was written by a provider but never filled by the patient). Third, our analysis focused on commercially insured adults and may not be generalizable to uninsured or older patients. However, our focus on working-age adults is important because prediabetes affects more than 1 in 3 adults older than 20 years and the evidence for metformin use is strongest for those younger than 60 years (3, 6, 30). Last, our definition of eligible patients differed from the definition in the American Diabetes Association national guidelines (13). Underdetection of women who may have had gestational diabetes more than 3 years ago and lack of actual BMI measurements make estimates in these high-risk groups more challenging. However, the prevalence of metformin prescription was only 7.8% in the small subset of patients identified as having a history of gestational diabetes or a BMI greater than 35 kg/m2 based on claims data. Thus, fewer than 1 in 12 high-risk patients specifically identified by national guidelines received metformin, which suggests room for improved translation of the evidence surrounding metformin use for diabetes prevention.
In conclusion, our findings indicate that metformin is rarely prescribed for diabetes prevention despite a strong evidence base in the literature for more than 10 years and inclusion in practice guidelines for more than 6 years. This is a potential gap in the approach to prediabetes management and a significant missed opportunity for diabetes prevention in patients at highest risk. Further studies are needed to understand the root causes of this potential gap and possible interventions to help promote the translation of known evidence into real-world practice.
EDITORS’ NOTES.
Context
Randomized trial evidence shows that lifestyle changes or metformin can delay the progression of prediabetes to overt diabetes. Efforts to modify lifestyle interventions to include diabetes prevention have had various levels of uptake, but little is known about the uptake of metformin for this purpose.
Contribution
This study examined metformin use in a large sample of insured U.S. adults with prediabetes and found that only 3.7% were prescribed metformin over a 3-year period.
Implication
Metformin seems to be used infrequently to prevent the development of overt diabetes in patients with prediabetes.
Acknowledgments
The authors thank Mr. Robert Luchs and Mr. Charlie Chan for their help with obtaining the data used in this analysis, Ms. Lindsay Kimbro for her administrative and project management support, and all NEXT-D (Natural Experiments for the Translation of Diabetes) collaborators for their support.
Grant Support: This study was jointly funded by the CDC (Division of Diabetes Translation) and the National Institute of Diabetes and Digestive and Kidney Diseases as part of the NEXT-D study (grant U58DP002722-05). Dr. Moin received support from the Veterans Affairs (VA) Office of Academic Affiliations through the VA Health Services Research and Development Advanced Fellowship Program (TPM65-010) of the VA Greater Los Angeles Health System from 2011 to 2014. Dr. Mangione received support from the University of California, Los Angeles (UCLA)/Drew Center for Health Improvement of Minority Elderly (under NIH/National Institute on Aging grant P30-AG021684) and the NIH/National Center for Advancing Translational Sciences and UCLA Clinical and Translational Science Institute (grant UL1TR000124). Dr. Mangione holds the Barbara A. Levey and Gerald S. Levey Endowed Chair in Medicine, which partially supported her work. Dr. Duru is supported in part by the UCLA/Drew Center for Health Improvement of Minority Elderly (under NIH/National Institute on Aging grant P30-AG021684) and an NIH career development award (K08-AG033360).
Appendix: Methods
Data from 183 UHC employer groups were available as part of a larger design study on health benefits constructed to evaluate an innovative, disease-specific health plan known as the Diabetes Health Plan (DHP). The goal of the larger study was to examine the association of the DHP with outcomes, such as medication adherence and utilization of health services, by comparing data from employer groups that purchased the DHP with comparable employer groups that purchased standard benefit plans during the same time.
Among the employer groups purchasing standard benefit plans, 1357 contracted with UHC for pharmacy benefits and were in similar industries and of similar size compared with employer groups that had purchased the DHP. Because randomization was not possible in this natural experiment, we analyzed an employer-level propensity score to identify employer groups offering standard plans that were most comparable to DHP groups (20, 21). We compared groups on the basis of employer size, mean income, proportion of female employees, proportion of employees with a chronic condition, and proprietary information on the generosity of benefits provided by the health plan. Propensity score modeling yielded 339 groups most comparable to the DHP groups (that is, within the region of common support) (22, 23). Among these, 233 had sufficient administrative and laboratory data to identify employees with prediabetes. We excluded groups that did not have available pharmacy claims over the entire 3-year study window (n = 13), were in the Mid-Atlantic region (where no DHP employer groups were located [n = 22]), had more than 90% of employees enrolled in high-deductible health plans (n = 8), had fewer than 20 employees with diabetes or prediabetes (n = 2), or had terminated their contract with UHC within the study period (n = 5), which left a final sample of 183 employer groups as the control.
Our sample can probably be generalized to larger employer groups (>500 employees), but we have no reason to believe that the prevalence of metformin prescriptions would vary by size of an employer group.
Appendix Table 1.
Prevalence of Metformin Prescription by Relative Distribution of Prediabetes Inclusion Criteria*
| Prediabetes Inclusion Criteria | Patients Identified, n | Patients Prescribed Metformin, n | Prevalence of Metformin Prescription, % |
|---|---|---|---|
| ≥2 ICD-9 codes of 790.2x | 2361 | 243 | 10.3 |
| Hemoglobin A1c level of 5.7%–6.4% | 5459 | 268 | 4.9 |
| Fasting plasma glucose level of 5.55 to 6.94 mmol/L (100–125 mg/dL) | 11 516 | 274 | 2.4 |
| 2-h plasma glucose level of 7.77 to 11.04 mmol/L (140–199 mg/dL) on oral glucose tolerance test | 50 | 7 | 14.0 |
| Any | 17 352 | 647 | 3.7 |
ICD-9 = International Classification of Diseases, Ninth Revision.
The last laboratory values in a given year were used, and some patients met >1 inclusion criterion; therefore, the sum of the values in the “Patients Identified” column is >17 352, and the sum of the values in the “Patients Prescribed Metformin” column is >647.
Appendix Table 2.
Results of Sensitivity Analyses
| Description of Sensitivity Analysis | Metformin Prescriptions (Revised Numerator), n | Cohort (Revised Denominator), n | Prevalence of Metformin Prescription, % |
|---|---|---|---|
| Apply a diabetes definition with ≥2 ICD-9 codes (250.xx) during the 3-y study window* | 868 | 18 114 | 4.8 |
| Include metformin prescriptions before diabetes identification throughout 3-y study window | 820 | 18 687 | 4.4 |
| Include women with history of PCOS (n = 227) | 123 received metformin; 123 + 647 = 770 | 17 579† | 4.4 |
| Assume 25% of patients in cohort are participating in lifestyle interventions (n = 4338) | 647 (unchanged) | 13 014 | 5.0 |
| Results of main analyses for comparison | 647 | 17 352 | 3.7 |
ICD-9 = International Classification of Diseases, Ninth Revision; PCOS = the polycystic ovary syndrome.
Used to exclude patients with diabetes in year 1 (2010) and those who developed diabetes in years 2 and 3 (2011 and 2012). For patients who developed diabetes during years 2 and 3, only metformin prescriptions before diabetes identification were included.
There were 258 women with PCOS, but we excluded the 31 who were also pregnant during the 3-y study window, leaving 227 eligible women with PCOS for the sensitivity analysis (17 352 + 227 = 17 579 [new denominator]).
Footnotes
Disclaimer: The findings and conclusions in this manuscript are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention (CDC) or the National Institutes of Health (NIH). Drs. Moin and Mangione had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Disclosures: Disclosures can be viewed at www.acponline.org/authors/icmje/ConflictOfInterestForms.do?msNum=M14-1773.
Reproducible Research Statement: Study protocol: A version with redacted confidential information is available to approved persons through written agreement with the authors (e-mail, tmoin@mednet.ucla.edu). Statistical code: Available through written agreement with the authors (e-mail, tmoin@mednet.ucla.edu). Data set: Not available due to confidentiality agreements between the academic and health plan partners.
Author Contributions: Conception and design: T. Moin, S. Ettner, S. Ho, C.M. Mangione.
Analysis and interpretation of the data: T. Moin, J. Li, O.K. Duru, S. Ettner, N. Turk, A. Keckhafer, S. Ho, C.M. Mangione. Drafting of the article: T. Moin.
Critical revision of the article for important intellectual content: T. Moin, J. Li, O.K. Duru, S. Ettner, A. Keckhafer, S. Ho, C.M. Mangione.
Final approval of the article: T. Moin, O.K. Duru, S. Ettner, A. Keckhafer, C.M. Mangione.
Provision of study materials or patients: T. Moin, A. Keckhafer, S. Ho.
Statistical expertise: S. Ettner, N. Turk.
Obtaining of funding: O.K. Duru, S. Ettner, C.M. Mangione. Administrative, technical, or logistic support: S. Ho, C.M. Mangione.
Collection and assembly of data: J. Li, N. Turk, A. Keckhafer, S. Ho.
References
- 1.Centers for Disease Control and Prevention. National Diabetes Fact Sheet: National Estimates and General Information on Diabetes and Prediabetes in the United States, 2011. Atlanta, GA: Centers for Disease Control and Prevention; 2011. [on 19 February 2015]. Accessed at www.cdc.gov/diabetes/pubs/pdf/ndfs_2011.pdf. [Google Scholar]
- 2.Centers for Disease Control and Prevention. Prediabetes: Could It Be You? Atlanta, GA: Centers for Disease Control and Prevention; 2014. [on 4 February 2015]. Accessed at www.cdc.gov/diabetes/pubs/statsreport14/prediabetes-infographic.pdf. [Google Scholar]
- 3.Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, et al. Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lindström J, Louheranta A, Mannelin M, Rastas M, Salminen V, Eriksson J, et al. Finnish Diabetes Prevention Study Group. The Finnish Diabetes Prevention Study (DPS): Lifestyle intervention and 3-year results on diet and physical activity. Diabetes Care. 2003;26:3230–6. doi: 10.2337/diacare.26.12.3230. [DOI] [PubMed] [Google Scholar]
- 5.Lindström J, Ilanne-Parikka P, Peltonen M, Aunola S, Eriksson JG, Hemiö K, et al. Finnish Diabetes Prevention Study Group. Sustained reduction in the incidence of type 2 diabetes by lifestyle intervention: follow-up of the Finnish Diabetes Prevention Study. Lancet. 2006;368:1673–9. doi: 10.1016/S0140-6736(06)69701-8. [DOI] [PubMed] [Google Scholar]
- 6.Ramachandran A, Snehalatha C, Mary S, Mukesh B, Bhaskar AD, Vijay V Indian Diabetes Prevention Programme (IDPP) The Indian Diabetes Prevention Programme shows that lifestyle modification and metformin prevent type 2 diabetes in Asian Indian subjects with impaired glucose tolerance (IDPP-1) Diabetologia. 2006;49:289–97. doi: 10.1007/s00125-005-0097-z. [DOI] [PubMed] [Google Scholar]
- 7.Li G, Zhang P, Wang J, Gregg EW, Yang W, Gong Q, et al. The long-term effect of lifestyle interventions to prevent diabetes in the China Da Qing Diabetes Prevention Study: a 20-year follow-up study. Lancet. 2008;371:1783–9. doi: 10.1016/S0140-6736(08)60766-7. [DOI] [PubMed] [Google Scholar]
- 8.Knowler WC, Fowler SE, Hamman RF, Christophi CA, Hoffman HJ, Brenneman AT, et al. Diabetes Prevention Program Research Group. 10-year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. Lancet. 2009;374:1677–86. doi: 10.1016/S0140-6736(09)61457-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ali MK, Echouffo-Tcheugui J, Williamson DF. How effective were lifestyle interventions in real-world settings that were modeled on the Diabetes Prevention Program? Health Aff (Millwood) 2012;31:67–75. doi: 10.1377/hlthaff.2011.1009. [DOI] [PubMed] [Google Scholar]
- 10.Cardona-Morrell M, Rychetnik L, Morrell SL, Espinel PT, Bauman A. Reduction of diabetes risk in routine clinical practice: are physical activity and nutrition interventions feasible and are the outcomes from reference trials replicable? A systematic review and meta-analysis. BMC Public Health. 2010;10:653. doi: 10.1186/1471-2458-10-653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dunkley AJ, Bodicoat DH, Greaves CJ, Russell C, Yates T, Davies MJ, et al. Diabetes prevention in the real world: effectiveness of pragmatic lifestyle interventions for the prevention of type 2 diabetes and of the impact of adherence to guideline recommendations: a systematic review and meta-analysis. Diabetes Care. 2014;37:922–33. doi: 10.2337/dc13-2195. [DOI] [PubMed] [Google Scholar]
- 12.Kahn R, Davidson MB. The reality of type 2 diabetes prevention. Diabetes Care. 2014;37:943–9. doi: 10.2337/dc13-1954. [DOI] [PubMed] [Google Scholar]
- 13.American Diabetes Association. Standards of medical care in diabetes—2014. Diabetes Care. 2014;37(Suppl 1):S14–80. doi: 10.2337/dc14-S014. [DOI] [PubMed] [Google Scholar]
- 14.Diabetes Prevention Program Research Group. Long-term safety, tolerability, and weight loss associated with metformin in the Diabetes Prevention Program Outcomes Study. Diabetes Care. 2012;35:731–7. doi: 10.2337/dc11-1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schmittdiel JA, Adams SR, Segal J, Griffin MR, Roumie CL, Ohnsorg K, et al. Novel use and utility of integrated electronic health records to assess rates of prediabetes recognition and treatment: brief report from an integrated electronic health records pilot study. Diabetes Care. 2014;37:565–8. doi: 10.2337/dc13-1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.American Diabetes Association. Standards of medical care in diabetes—2008. Diabetes Care. 2008;31(Suppl 1):S12–54. doi: 10.2337/dc08-S012. [DOI] [PubMed] [Google Scholar]
- 17.Heilbrunn E. Top Health Insurance Companies. New York: U.S. News & World Report; 2013. [on 28 December 2013]. Accessed at http://health.usnews.com/health-news/health-insurance/articles/2013/12/16/top-health-insurance-companies. [Google Scholar]
- 18.Duru OK, Mangione CM, Chan C, Keckhafer A, Kimbro L, Kirvan KA, et al. Evaluation of the Diabetes Health Plan to improve diabetes care and prevention. Prev Chronic Dis. 2013;10:E16. doi: 10.5888/pcd10.120150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xiao J, Chen S, Zhang C, Chang S. The effectiveness of metformin ovulation induction treatment in patients with PCOS: a systematic review and meta-analysis. Gynecol Endocrinol. 2012;28:956–60. doi: 10.3109/09513590.2012.705368. [DOI] [PubMed] [Google Scholar]
- 20.Baran S, Api M, Goksedef BP, Cetin A. Comparison of metformin and clomiphene citrate therapy for induction of ovulation in the polycystic ovary syndrome. Arch Gynecol Obstet. 2010;282:439–43. doi: 10.1007/s00404-010-1497-y. [DOI] [PubMed] [Google Scholar]
- 21.Mathur R, Alexander CJ, Yano J, Trivax B, Azziz R. Use of metformin in polycystic ovary syndrome. Am J Obstet Gynecol. 2008;199:596–609. doi: 10.1016/j.ajog.2008.09.010. [DOI] [PubMed] [Google Scholar]
- 22.White IR, Royston P, Wood AM. Multiple imputation using chained equations: issues and guidance for practice. Stat Med. 2011;30:377–99. doi: 10.1002/sim.4067. [DOI] [PubMed] [Google Scholar]
- 23.Rubin DB. Multiple imputation after 18+ years. J Am Stat Assoc. 1996;91:473–89. doi: 10.1080/01621459.1996.10476908. [DOI] [Google Scholar]
- 24.Crandall J, Schade D, Ma Y, Fujimoto WY, Barrett-Connor E, Fowler S, et al. Diabetes Prevention Program Research Group. The influence of age on the effects of lifestyle modification and metformin in prevention of diabetes. J Gerontol A Biol Sci Med Sci. 2006;61:1075–81. doi: 10.1093/gerona/61.10.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Diabetes Prevention Program Research Group. The 10-year cost-effectiveness of lifestyle intervention or metformin for diabetes prevention: an intent-to-treat analysis of the DPP/DPPOS. Diabetes Care. 2012;35:723–30. doi: 10.2337/dc11-1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ramachandran A, Snehalatha C, Yamuna A, Mary S, Ping Z. Cost-effectiveness of the interventions in the primary prevention of diabetes among Asian Indians: within-trial results of the Indian Diabetes Prevention Programme (IDPP) Diabetes Care. 2007;30:2548–52. doi: 10.2337/dc07-0150. [DOI] [PubMed] [Google Scholar]
- 27.Fradkin JE, Roberts BT, Rodgers GP. What’s preventing us from preventing type 2 diabetes? N Engl J Med. 2012;367:1177–9. doi: 10.1056/NEJMp1208169. [DOI] [PubMed] [Google Scholar]
- 28.Nichols GA, Hillier TA, Brown JB. Progression from newly acquired impaired fasting glucose to type 2 diabetes. Diabetes Care. 2007;30:228–33. doi: 10.2337/dc06-1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lenfant C. Shattuck lecture—clinical research to clinical practice—lost in translation? N Engl J Med. 2003;349:868–74. doi: 10.1056/NEJMsa035507. [DOI] [PubMed] [Google Scholar]
- 30.Nathan DM, Davidson MB, DeFronzo RA, Heine RJ, Henry RR, Pratley R, et al. American Diabetes Association. Impaired fasting glucose and impaired glucose tolerance: implications for care. Diabetes Care. 2007;30:753–9. doi: 10.2337/dc07-9920. [DOI] [PubMed] [Google Scholar]