Abstract
We describe a Massachusetts Bureau of Substance Abuse Services’ (BSAS) initiative to disseminate the office-based opioid treatment with buprenorphine (OBOT-B) Massachusetts Model from its development at Boston Medical Center (BMC) to its implementation at fourteen community health centers (CHCs) beginning in 2007. The Massachusetts Collaborative Care Model for the delivery of opioid agonist therapy with buprenorphine, in which nurses working with physicians play a central role in the evaluation and monitoring of patients, holds promise for the effective expansion of treatment for opioid use disorders. The training of and technical assistance for the OBOT nurses as well as a limited program assessment are described. Data spanning 6 years (2007 – 2013) reports patient demographics, prior treatment for opioid use disorders, history of overdose, housing, and employment. The expansion of OBOT to the fourteen CHCs increased the number of physicians who were “waivered” (i.e., enabling their prescribing of buprenorphine) by 375%, from 24 to 114, within 3 years. During this period the annual admissions of OBOT patients to CHCs markedly increased. Dissemination of the Massachusetts Model of the Office-Based Opioid Treatment with Buprenorphine employing a collaborative care model with a central role for nursing enabled implementation of effective treatment for patients with an opioid use disorder at community health centers throughout Massachusetts while effectively engaging primary care physicians in this endeavor.
Keywords: opioid agonist therapy, buprenorphine, waivered physicians, nurse care manager, access to treatment, opioid use disorder
1. Introduction
In the United States the number of people with opioid use disorders using prescription opioids increased in 2013 to 1.9 million and for heroin to 517,000. (Substance Abuse and Mental Health Services Administration, 2014b). The 2013 national mortality associated with opioid analgesic overdose exceeded 16,200 deaths (Center for Disease Control and Prevention, 2015). Effective pharmacotherapy exists for people with opioid use disorders but fewer than 24% receive any medication for their addiction. (SAMHSA Center for Behavioral Health Statistics and Quality, 2014).
The contrast of the magnitude of this public health problem with individuals’ receipt of efficacious therapy is striking (SAMHSA Center for Behavioral Health Statistics and Quality, 2014). Part of this inadequate response can be explained by the lack of clinical support and infrastructure resulting in difficulty finding a physician who can provide this care (Alford et al., 2011; Walley et al., 2008). Doctors feel that despite appropriate training, the delivery of opioid agonist treatment such as buprenorphine in an office-based setting is difficult (Walley et al., 2008). To address this dilemma, over a decade ago a primary care office-based opioid treatment (OBOT) Collaborative Care Model was created in an academic medical center (Alford et al., 2011). This OBOT approach has been referred to as the Massachusetts model by SAMHSA (Substance Abuse and Mental Health Services Administration, 2014a).
The expansion of the Drug Addiction Treatment Act 2000 allowed a “waivered” physician to prescribe buprenorphine in an office-based setting after a minimum of eight hours of training for up to 30 patients with opioid use disorders. The introduction of the “extended waiver” allowed physicians to apply for approval to treat up to 100 patients per physician after one-year experience with the initial waiver approval. In Massachusetts and nationwide, by 2014, less than 5% of physicians had received such training and were waivered; compounding this physician shortage is that historically, a sizable proportion of the waivered doctors do not prescribe buprenorphine (Kaiser Family Foundation, 2015). Studies on physicians’ willingness to treat such patients reveal several barriers to the office-based treatment of opioid use disorders (Barry et al., 2009; Kissin, McLeod, Sonnefeld, & Stanton, 2006; Turner, Laine, Lin, & Lynch, 2005; Walley et al., 2008) (Table 1).
Table 1.
Barriers to treatment and how the STATE OBOT program addressed them.
| Barriers | How STATE OBOT program addresses the barriers |
|---|---|
| Physician competing activities | NCMs meet with patients on a more regular basis and share some of the clinical responsibilities not required to be physician delivered. NCMs routinely confer with physicians regarding patient issues as the need arises. |
| Lack of support staff | State supported start up funding and integration of NCMs. Integration of Medical Assistants to work with NCMs. Education and engagement of non-medical staff. |
| Inadequate addiction expertise | TTA educates the staff on buprenorphine treatment through a day-long Buprenorphine-101 training. Continued support is provided as needed. Ongoing quarterly trainings for NCMs and Medical Assistants. Ongoing educational updates and sharing of information via email |
| Payment issues | FQHCs are able to bill for nursing visits at a comparable rate as they would for other licensed clinical providers. Program revenue provides funding for administrative costs. |
| Administrative obstacles | Education on disease and stigma TTA for administrative staff helps with the implementation. Assisted with systems for: tracking, reporting and visits. |
Recognizing these barriers to Office-Based Opioid Treatment with Buprenorphine (OBOT-B), in 2003, a multidisciplinary team at Boston Medical Center (BMC) created a new model of care to increase access to treatment: the Collaborative Care Model of OBOT, subsequently dubbed the Massachusetts Model (Alford et al., 2007; Alford et al., 2011) (Table 1). This program was supported by the Massachusetts Department of Public Health Bureau of Substance Abuse Services (BSAS), the administrative state agency that oversees addiction prevention, treatment and recovery support services. The OBOT-B Collaborative Care Model at BMC has grown to serve over 450 patients, with nineteen waivered primary care physicians, making this program one of the largest such primary care based programs in the country (Alford et al., 2007). This paper describes the expansion of the Massachusetts model into community health centers (CHCs) throughout the Commonwealth of Massachusetts.
Implementing such a model of care at CHCs has important potential advantages: enables distribution of treatment to a wide geographical area; promotes engagement of marginalized population; and utilizes a facilitative health care reimbursement model. The implementation of this model of care is best explained using the theoretical constructs outlined in the ADAPTS implementation science model (Table 2) (Knapp & Anaya, 2012). We report the process by which this implementation of the OBOT-B Massachusetts Model occurred in CHCs and some metrics of its effectiveness (e.g., annual active admissions, number of waivered physicians).
Table 2.
ADAPTS Implementation Science Model of the STATE OBOT-B program
| Step | Action |
|---|---|
| Assessment | Identified various barriers to physicians regarding providing the office-based treatment of opioid disorders in MA (Walley et al., 2008) BMC developed the Collaborative Care Model of OBOT and has shown success and need (wait list > 300 patients) Training and Technical Assistance needs assessment |
| Deliverables | Policy and procedures manual, visit templates, educational materials Training and Technical Assistance Increased number of prescribers and increased number of patients treated |
| Activate | Request-for-response was sent to 36 FQHCs in MA to encourage their applying for the grant Site champions: OBOT nurse and physicians |
| Pretraining | Each FQHC was given the option to make site-specific changes to the implementation to better integrate the program into their individual site |
| Training | STATE OBOT Training and Technical Assistance provides training and education to staff regarding OBOT-B and opioid use disorders and integration of buprenorphine treatment Provide ongoing training and support as needed |
| Sustainability | Ongoing support, updates, trainings and check-ins to maintain quality of care Educate about the billing for NCM services for financial sustainability |
2. Methods
2.1 State Technical Assistance Treatment Expansion (STATE) OBOT-B
Beginning in 2007, the OBOT-B Collaborative Care Model was implemented in CHCs in Massachusetts (STATE OBOT-B) (Alford et al., 2007; Alford et al., 2011). The goal of the STATE OBOT-B program was to incorporate the OBOT-B Collaborative Care Model into primary care in CHCs, expanding access to buprenorphine treatment. NCMs were hired at CHCs to provide waivered physicians with the clinical support to manage patients on buprenorphine with opioid use disorders. This OBOT-B model was designed to provide treatment to marginalized individuals living in the communities of the CHCs including the homeless, under-insured, uninsured, ethnic and racial minorities and those with co-occurring physical or mental health disorders.
The clinical model consists of four treatment stages: 1) screening and assessment of the patient’s appropriateness for office-based treatment; 2) medication induction under a Nurse Care Manager’s direct supervision; 3) stabilization; and 4) maintenance. The model adheres to recognized practice standards including SAMHSA’s Treatment Improvement Protocol 40 (Center for Substance Abuse Treatment, 2004) and evidence-based treatment guidance for nurses as noted in the Technical Assistance Publications Series 30 (Center for Substance Abuse Treatment, 2009), and practices as described by the Massachusetts Behavioral Health Partnership (Massachusetts Behavioral Health Partnership, 2010).
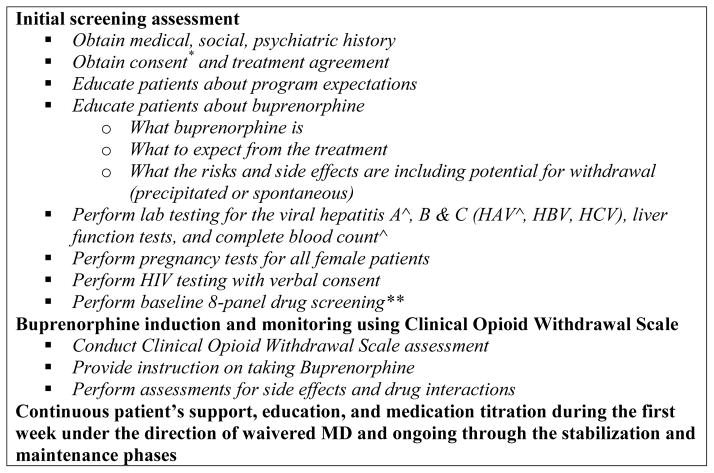
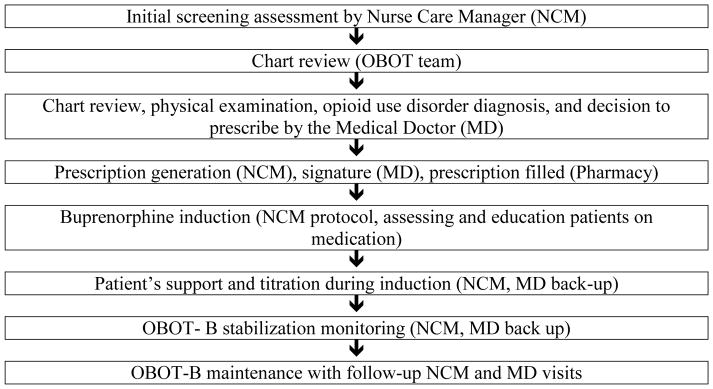
The Nurse Care Manager (NCM) is central to the OBOT-B Collaborative Care Model (Figure 2). The NCM is usually the initial contact for patients seeking OBOT-B treatment and acts as the primary liaison between the patient and the OBOT physician throughout the treatment process (Figure 1). The NCM performs the initial screening, after which a waivered physician sees the patient to confirm the patient’s opioid use disorder diagnosis and appropriateness for OBOT-B. The patient then schedules a medication induction visit with the NCM. Utilizing the Clinical Opioid Withdrawal Scale (Wesson & Ling, 2003), the NCM assesses the patient for withdrawal symptoms following a protocol, and supports the patient through the induction process under the orders of the waivered physician. The patient and the NCM remain in contact during the first day of induction for support, education, and titration under the direction of the waivered physician. With the NCM as the first point of contact, patients have access to the OBOT-B team for questions, issues or support during induction and as needed throughout treatment. During treatment stabilization, patients are followed closely with weekly or more frequent visits as well as telephone communication to provide support and education, assure adherence, and address other concerns the patient may have. Initially, patients are required to see the NCM weekly for follow-up visits with drug screening and verification of behavioral health counseling, which is provided by the CHC or a nearby addiction treatment clinic. Provided the patient continues to attend weekly counseling and drug screens are negative except for buprenorphine and other prescribed medications, the frequency of follow-up visits with the NCM decreases but can increase based on the patient’s needs. This model for treating opioid dependence in a CHC mirrors the model for treating other chronic diseases in that disease management is individualized and includes OBOT-B clinical contacts and referrals to specialized care based on patient need.
Figure 2.
Role of the nurse care manager (NCM) in the OBOT-B Collaborative Care Model.
* Treatment agreements and consents are often employed in the treatment of addiction to make explicit expectations of the patients and their involvement in the treatment process, which is consistent with the Clinical Guidelines in the Use of Buprenorphine Treatment Improvement Protocol 40 SAMHSA.
^ Lab tests done for the purposes of primary care of a patient with an opioid use disorder
** Drug screening is used to assess the patient’s ongoing treatment needs and allows the provider to have a conversation with the patient addressing the findings and assisting with appropriate treatment planning.
Figure 1.
Patient flow in the OBOT-B Collaborative Care Model.
The NCM keeps the physician informed at all times primarily via the electronic medical record in which all communications, results, clinical documentation and prescriptions are tracked. As care is provided within a primary care clinic, direct communication among the NCM, the waivered physician who is often but not always the primary care physician, and other clinical team members occurs as befits care of a chronic disease using a chronic care management model (McLellan, Lewis, O’Brien, & Kleber, 2000; Wagner et al., 2001). As not all primary care physicians prescribe buprenorphine, a waivered physician follows patients whose primary care physician is not waivered, for the buprenorphine treatment.
Originally, funding was provided for three years for each site, renewable twice for two additional years, enabling seven years of potential funding. Funding supported one full-time NCM at each site. The expectation was that each NCM would support the care of an active panel of 100 patients. Sites sought to achieve this desired patient caseload over time through a rolling admission process. This process required the induction of two to three new patients into the treatment program each week. To optimize the quality of performance among nurses and other staff involved in the program, a STATE OBOT-B Training and Technical Assistance (TTA) program provided special training and technical assistance. As the program expanded in 2011, its funder, BSAS, increased the caseload requirement to 125 patients per NCM with the addition of a medical assistant to support the NCM.
2.2 STATE OBOT-B Implementation Expectations
To receive STATE OBOT-B grant funding, CHCs, specifically targeting federally qualified health centers (FQHCs), committed to explicit implementation expectations. Specific program goals included the following: integrating buprenorphine treatment into primary care practice; increasing the number of waivered physicians in CHCs; providing accessible buprenorphine treatment to marginalized individuals; and developing expertise in the treatment of opioid use disorders among CHC physicians, nurses and other staff members. To maintain grant funding, CHCs needed to meet expectations including treating the requisite number of patients per NCM, engaging OBOT-B providers in training and technical assistance, reporting weekly program statistics and complying with site visits as requested by the STATE OBOT-B Program Director (PD) (author - CL).
2.3 STATE OBOT-B Training and Technical Assistance (TTA)
The STATE OBOT-B PD provided training and technical support, which included quarterly nurse trainings, telephone, and confidential email consultations, chart reviews and site visits at the CHCs as needed. In order to optimally support each CHC, the PD first conducted a qualitative assessment of each site via a structured interview to determine the providers’ training needs for effective OBOT-B model implementation. Themes that emerged from this assessment are summarized in Table 3.
Table 3.
Perceived TTA needs and how the STATE OBOT program addressed them
| Perceived TTA needs | How STATE OBOT program addresses the TTA needs |
|---|---|
| Addiction and buprenorphine treatment education | TTA educates the staff on buprenorphine treatment through a day-long Buprenorphine-101 training. Additionally, knowledge regarding working with individuals with substance use disorders is disseminated, and continued support is provided as needed. Ongoing quarterly trainings address pertinent addiction-related topics for OBOT program staff. |
| Program initiation and induction process | Education through formalized training and shadowing at BMC’s OBOT/buprenorphine clinic, hands-on support is provided at the CHC, ongoing telephone and email support is offered as needed. |
| Program integration into existing primary care setting | TTA assists program integration into the FQHCs and provides quality control of the programs. |
| Knowledge of Collaborative Care Model | Formalized training on the model and the policy and procedure manual is provided during initial day-long training. Additionally training is provided to all staff involved in the buprenorphine program. |
| NCM hiring and training | Assisted with the hiring process via posting through addiction RN list serve distributed to addiction nurses throughout the state. |
| Policy/procedure development | A buprenorphine treatment policy and procedure manual was developed. This manual was provided to the CHCs in a word document so that each CHC could make site-specific changes and incorporate it into their day-to-day practice. |
| Staff buy-in | CHC staff are provided trainings related to treating individuals with addiction, ongoing support is offered, ongoing communication with staff facilitates understanding, and site-meetings are scheduled as needed. |
| Stigma | Education about the disease of addiction is provided to all staff involved and health center wide if agreeable. Sites are supported ongoing when questions and issues arise. |
| Billing | Educating about utilization of an NCM in a FQHCs in billing. |
| Limited physician interest | CHC physicians are engaged through education about treating the disease of addiction and the incorporation of this treatment into a primary care setting. |
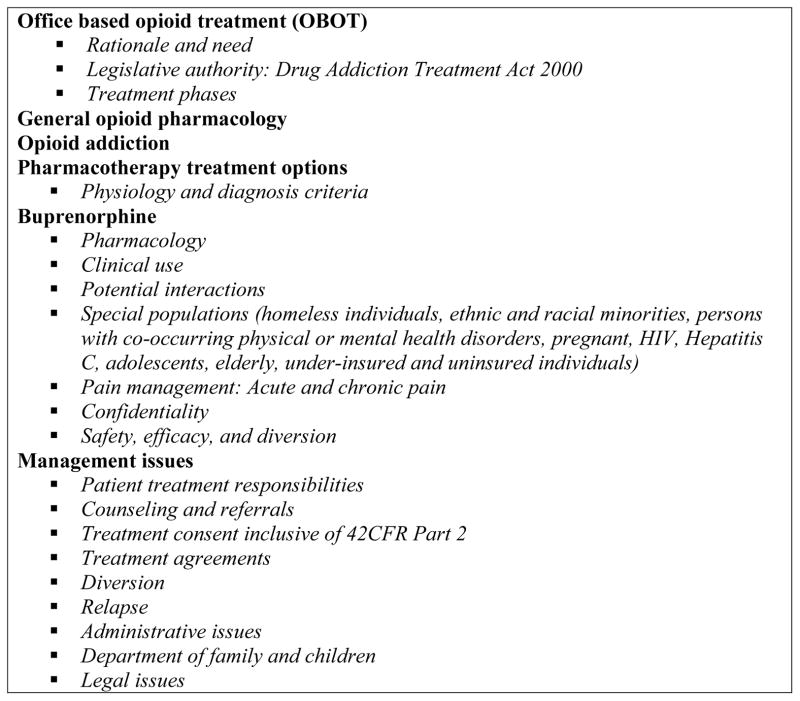
Mandatory eight-hour training sessions for the CHC NCMs prepared them for their role within the OBOT-B program. The development of these NCM trainings was based on the 8-hour physician waiver training, with more of an emphasis on day-to-day management issues (Figure 3). This core training course was supplemented by quarterly nurse trainings that addressed current and emerging management issues in addiction and OBOT-B treatment including pregnancy, pain, polysubstance use, psychiatric and medical co-morbidities including hepatitis C and HIV, retention, motivational interviewing, compassion fatigue, self-care, drug screening, relapse prevention, harm reduction and current drug trends. These trainings held in Boston also provided a networking opportunity and peer support for OBOT-B nurses at sites around the state given that they did not routinely work among other addiction colleagues. The trainings were designed to mitigate the sense of isolation and build an environment of collaboration and problem-solving among the NCMs. The NCM participants in the quarterly trainings evaluated the course upon completion, providing anonymous feedback.
Figure 3.
Topics covered in the OBOT NCM core training program.
Clerical, administrative, and non-nursing CHC personnel learned about addiction pharmacotherapy during on-site training with a specific emphasis on stigma of drug addiction. These trainings were provided to help on-site personnel fulfill responsibilities specific to OBOT-B, such as record keeping, confidentiality and collaborative quality care without judgmental or stigmatizing attitudes.
On-site technical assistance included supervision, training, and education to ensure that the following standards were maintained by NCMs: 1) proper incorporation of new knowledge and skills into OBOT-B nursing practice; 2) adherence to established clinical treatment guidelines for buprenorphine use; and 3) compliance of the record-keeping systems with the state and federal requirements. During the first year of OBOT-B implementation, the STATE OBOT-B PD visited each site to provide supervision and support. Site visits facilitated the transfer of knowledge and expertise, and ensured that OBOT-B operations met legal and contractual requirements (Rep Bliley, 2000; SAMHSA, 2015). The PD met with physicians, nurses, and administrative staff to assist in troubleshooting and system management issues. By joining the NCM at patient appointments, the PD helped to integrate academic training into clinical practice and to promote integration of practice standards and quality care.
Technical assistance was offered both on site and remotely. The NCMs had direct access to the STATE-OBOT-B PD by telephone and email as needed. Common needs were addressed in the first year of a CHC program’s initiation during established monthly telephone conference calls among the NCMs and STATE OBOT-B PD, in which general support, administrative and clinical updates, networking, and case discussions occurred. In subsequent years, such meetings were held less frequently based on the need of the CHC as assessed by both the PD and the CHC NCM; instead, more frequent phone and email consultations occurred. Additionally, the CHC NCMs were invited to BMC for clinical observation and hands-on training within the flagship’s OBOT-B clinical operations. Several sites at program initiation sent physicians, administrative staff and medical assistants to BMC for hands-on experience or group consultations.
2.4 STATE OBOT-B Program Assessment
CHCs completed reporting forms for the state funder assessing patients’ social and medical status including current living situation, employment, history of substance use, and mental health. Three types of patient assessments were performed: at enrollment upon program entry; quarterly for each active patient (i.e., patient who stays in the program and follows the prescribed treatment); and at disenrollment. The latter occurs when a patient is discharged, which occurs upon treatment completion or when the patient moves, dies, transfers to higher level of care or is lost to follow-up. This information was tracked by the STATE OBOT Program Manager (author - AB) and entered into the state data reporting system managed by the Massachusetts Executive Office of Health and Human Services. In addition, each site was required to submit to the Program Manager a weekly report with the following data: the number of active patients; the total number of patients treated to date; the number of new enrollments in the previous week; and the number of discharges in the previous week, with reasons for discharge. The data enabled ongoing program assessment about patient enrollment and retention. For technical assistance purposes, this information is shared quarterly with programs so that relative performance is transparent.
2.5 Demographics
Patient demographic and clinical data reflects admissions between August 2007 to December 2013 obtained by self-report at intake. Homeless individuals are those patients who described currently living on the streets or in a shelter.
2.6 CHC Outcomes of Interest
The primary STATE OBOT-B program outcome was the number of CHCs enrolled. Secondary outcomes included number of waivered physicians at participating CHCs, annual active admissions, defined as episodes of an individual initiating buprenorphine at the CHC and duration of treatment in the OBOT-B program.
3. Results
3.1. CHC STATE OBOT-B Expansion
Between 2007 and 2013, nineteen CHCs were enrolled into the STATE OBOT-B program. Five health centers returned the contract for the grant within the first 3–16 months. Specifically, these health centers demonstrated underutilization of grant funds, including inability to meet the grant caseload requirement (e.g., less than two patients per week) and difficulty adhering to weekly state reporting. The CHCs that opted out of grant participation also had some of the following administrative issues: lack of administrative support; challenges integrating addiction treatment into a CHC setting clinically and administratively; significant lag time integrating treatment; difficulty recruiting and maintaining a full-time NCM; and space restrictions. Fourteen CHCs remained in the STATE OBOT-B program.
Overall annual admissions into the STATE OBOT-B CHC programs increased from 178 in the latter 5 months of the first calendar year of the program (2007) to 1210 in last full year of complete data availability (2012) (Table 4). Prior to the implementation of the OBOT-B program, 24 physicians in the grant-supported CHCs were waivered to prescribe buprenorphine. Three years later, 114 physicians were waivered in these same CHCs. Chronic care for patients in OBOT-B increasingly became the standard. As of 2013, 67% of the patients across all CHC OBOT-B programs were in treatment for more than 12 months. This proportion of patients who were in treatment for more than 12 months increased steadily over the years with 32%, 56% and 65% receiving such treatment in 2010, 2011 and 2012, respectively. Of particular note, 7 of the 14 sites decided to expand program size beyond grant expectations; although the grant only required and funded one full-time NCM, these CHCs hired an additional NCM in order to treat more patients within their OBOT-B program.
Table 4.
Enrollment by calendar year from August 1st in 2007 through December in 31st in 2012.
| Calendar Year | Enrollments |
|---|---|
| 2007 | 178 (latter 5 months of the year) |
| 2008 | 1,499 |
| 2009 | 1,415 |
| 2010 | 1,307 |
| 2011 | 1,184 |
| 2012 | 1,210 |
The OBOT–B Collaborative Care Model also allowed the funded CHCs to provide a network for the state to assist those patients for whom their physician stopped prescribing buprenorphine. From the implementation of the program through the end of 2013, the STATE OBOT-B program supported patient transfers from fourteen physician practice closures and facilitated a relatively seamless transition of treatment.
3.2 Patient Demographic
Patient characteristics are described in Table 5. A majority of the admissions had each of the following demographic characteristics: white; males; and between the ages of 21 to 39 years. Additionally, 5% were African American, 23% were Hispanic and 7.5% were homeless. One-third of OBOT-B admissions were individuals employed at the time of enrollment (Table 5).
Table 5.
Demographic characteristics of admissions* enrolled (n=7722) from August 1st in 2007 through December 31st in 2013.
| Characteristic | Value (%)** |
|---|---|
| Gender | |
| Male | 63.4 |
| Female | 36.6 |
| Race | |
| White | 72.7 |
| Black or African American | 5.0 |
| Asian | <1.0 |
| Native Hawaiian/Other Pacific Islander | <1.0 |
| American Indian/Alaskan Native | <1.0 |
| Multi-Racial | 2.7 |
| Refused to answer | <1.0 |
| Other | 17.3 |
| Unknown | 1.2 |
| Ethnicity | |
| Hispanic | 23.1 |
| Non-Hispanic | 76.9 |
| Age (years) | |
| 20 and Under | 2.0 |
| 21 – 29 | 29.4 |
| 30 – 39 | 28.3 |
| 40 – 49 | 26.5 |
| 50 – 59 | 12.2 |
| Greater than 59 | 1.6 |
| Living status | |
| Homeless | 7.5 |
| Not homeless | 92.5 |
| Treatment at an opioid treatment facility prior to enrollment | |
| Yes | 56 |
| No | 44 |
| History of lifetime overdose | |
| Yes | 34 |
| No | 66 |
| Employment status | |
| Working, full-time | 20 |
| Working, part-time | 14 |
| Unemployed, looking for jobs | 44 |
| Unemployed, not looking for jobs | 22 |
- Admissions may reflect a particular individual more than once
- All characteristics are self-reported
3.4 TTA Nurse Feedback
NCMs involved in the quarterly training sessions rated the sessions using a 5-point Likert scale with 89% strongly agreed the training was helpful (i.e., a score of 5). The NCMs reported the training program allowed them to meet with their nurse colleagues, share ideas, build networks, learn new skills, enhance current knowledge, facilitate problem-solving and review cases. The nurses indicated that they found the activities “stimulating” and “inspiring”, and that they felt “supported, energized, and ready to try new things.”
4. Discussion
The STATE OBOT-B program utilizing the Massachusetts Collaborative Care Model substantially increased access to treatment for patients with opioid use disorders in CHCs. Within 3 years of implementing the program, the number of waivered physicians (i.e., credentialed to prescribe buprenorphine for opioid use disorders) and the number of annual patient admissions for buprenorphine treatment to the supported CHCs markedly increased. These results demonstrate that a substantial previously unmet demand for the treatment of opioid use disorders was beginning to be addressed by the STATE OBOT-B initiative.
The finding that more than two-thirds of OBOT-B patients in 2013 were in treatment for more than 12 months, a steady increase over time, reflect the maturation and effectiveness of the buprenorphine treatment program. Of note, this data reveals the percentage of program participants at a single point in time in the OBOT program that meet this metric and not a prospective view of the percentage of patients who enter and remain in the program for 12 months or more.
One previous study described delivery of buprenorphine within FQHCs. At 2 sites in Connecticut, programs were able to maintain 62% of the patients in treatment for at least 12 months (Haddad, Zelenev, & Altice, 2013). In the future, it will be important to assess individual outcomes such as patient 12-month retention in the Massachusetts Collaborative Care Model at CHCs.
The STATE OBOT-B program engaged minority populations into treatment (i.e., 5% African American and 23.1% Hispanic). The percentage of enrollments of African Americans and Hispanics in methadone treatment programs in Massachusetts in the same time period was 3.5% and 11.8%, respectively. These findings suggest that the STATE OBOT-B program provided access to minority populations.
The OBOT–B Collaborative Care Model has proven to be highly effective at expanding access to buprenorphine treatment. Implementing the model in the 14 CHCs has increased the uptake of opioid use disorder pharmacotherapy by integrating addiction treatment into the primary care office-based setting. Integrating this model with a central role for the NCM in the provision of OBOT-B enabled the physicians in these community settings to treat complex patients with opioid dependence in primary care practices in community health centers.
The OBOT-B Collaborative Care Model relies heavily on the care of NCMs, who provide complex care management for the patients under the direction of a waivered physician. The NCMs are dedicated full time to the care management of patients with opioid dependence, and thus are more accessible to promptly address urgent issues than the physicians. The model addresses one of the reasons that physicians are reluctant to prescribe buprenorphine, as they have busy primary care practices and the complexities of addiction are challenging with limited support. Although directly involved in patient care, the primary care physicians have limited time for care management, monitoring of urine drug tests, and other complexities of managing the needs of patients with addiction. The central role for nursing in this model of care enables these functions to get accomplished and is quite compatible with the existing staffing structures of many CHCs.
Another important aspect of the OBOT–B Collaborative Care Model is that the funded CHCs provide a network for the state to assist patients whose physicians stop prescribing buprenorphine. These patients are potentially placed at high risk for relapse unless they can access treatment without interruption. Under the statewide dissemination of the OBOT–B Collaborative Care Model, the patients from the 14 provider practice closures were transferred seamlessly to the funded CHCs to continue receiving appropriate treatment without interruption.
Sustainability is a concern for any new program. CHCs that are federally qualified health centers (FQHCs) are able to bill for nursing visits at a comparable rate as they would for other licensed clinical providers. The billable nature of OBOT-B services allows FQHCs to generate sufficient revenue for the nursing salary and other programmatic costs to sustain the OBOT-B Collaborative Care Model. A review of the OBOT-B program using a cost modeling analysis and reviewed by FQHCs’ CEOs and CFOs concluded that this model of care is sustainable over time (MassHealth, 2015; Substance Abuse and Mental Health Services Administration, 2014a). It takes approximately 40 cases per year, at 27 visits per patient per year to fund a full-time NCM position, adjusting for efficiency, and administrative cost. A typical NCM’s caseload was initially 100 patients, more than twice the number of cases required to fund the position; after the cost analysis, it was found that a NCM could support a higher caseload of 125 patients with the help of a full-time medical assistant. The additional revenue was used to fund ongoing education, technical support, administrative support, medical assistance, and/or training of more OBOT nurses and physicians to prevent treatment gaps.
The OBOT-B program is an integral part of an effort to engage patients into treatment in their communities throughout Massachusetts. This model has improved access to care for patients that would otherwise be unable to obtain addiction treatment due to the lack of providers or inability to pay. It was effectively implemented by addressing features supportive of effective implementation as noted in Table 2 and described in the ADAPTS theoretical model (Table 2) (Knapp & Anaya, 2012). Specifically the limited access to comprehensive care in the community setting was improved by providing two key structural enabling elements: 1) clinical support to physicians; and 2) training and technical support to nurses and others. As a consequence, it enabled the expansion and integration of opioid addiction treatment with buprenorphine into primary care practice in community settings.
The initiative of the OBOT-B Collaborative Care Model has been particularly successful in the engagement and retention of marginalized patients. Furthermore, the financial sustainability of the OBOT-B program using NCMs allowed seven FQHCs involved in the program to expand beyond grant funding to better serve the needs of their communities.
Given that the STATE OBOT-B program was only implemented in Massachusetts, a potential limitation of the study of this model could be that there might be unforeseen contextual factors that may hinder the adoption of the program into other states. Another limitation is that it has not considered other parameters used to measure the quality of services delivered by specific standard metrics such as patient specific program retention. Further program evaluation should examine the effectiveness of the STATE OBOT-B program for individual patients. The annual active admission data does not track individuals (i.e. a single patient could have multiple admissions). Thus it gives insight into the magnitude of patients with access to care but not how individual patients fared in treatment. Future study should examine treatment outcomes as well as factors that were associated with successful adoption of the STATE OBOT-B program. Such critical outcome data could be instrumental in facilitating appropriate adoption of the Massachusetts Model beyond that one state in the future.
5. Conclusion
Opioid use disorders are epidemic in the USA, as are the associated opioid overdose deaths. These problems compel us to develop strong and sustainable addiction treatment programs. The highly generalizable OBOT-B Collaborative Care Model developed in Massachusetts addresses key barriers to providing comprehensive health care in community settings by providing a structure in which to deliver multidisciplinary treatment to patients and clinical support to physicians prescribing buprenorphine. This model has enabled the expansion and integration of opioid treatment with buprenorphine into primary care practice settings utilizing a NCM model throughout an entire state within community health centers. This model has displayed successful clinical uptake as well as financial stability as a method of treatment for opioid addiction.
Acknowledgments
Research by one of the authors, Colleen LaBelle, has been funded in part by support from the Commonwealth of Massachusetts, grant number 0248908. Dr. Samet receives support from NIDA R25DA013582 to help support working with medical students on clinical addiction research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alford DP, LaBelle CT, Kretsch N, Bergeron A, Winter M, Botticelli M, Samet JH. Collaborative care of opioid-addicted patients in primary care using buprenorphine: Five-year experience. Archives of Internal Medicine. 2011;171(5):425–431. doi: 10.1001/archinternmed.2010.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alford DP, LaBelle CT, Richardson JM, O’Connell JJ, Hohl CA, Cheng DM, Samet JH. Treating homeless opioid dependent patients with buprenorphine in an office-based setting. Journal of General Internal Medicine. 2007;22(2):171–176. doi: 10.1007/s11606-006-0023-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry DT, Irwin KS, Jones ES, Becker WC, Tetrault JM, Sullivan LE, Fiellin DA. Integrating buprenorphine treatment into office-based practice: A qualitative study. Journal of General Internal Medicine. 2009;24(2):218–225. doi: 10.1007/s11606-008-0881-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Center for Disease Control and Prevention. Prescription drug overdose in the united states: Fact sheet. 2015 Retrieved from http://www.cdc.gov/homeandrecreationalsafety/overdose/facts.html.
- Center for Substance Abuse Treatment. Clinical guidelines for the use of buprenorphine in the treatment of opioid addiction. Rockville, MD: SAMHSA; 2004. No. DHHS Publication No. (SMA) 07-3939. [PubMed] [Google Scholar]
- Center for Substance Abuse Treatment. Buprenorphine: A guide for nurses. Rockville, MD: SAMHSA; 2009. No. DHHS Pub. No. (SMA) 09-4376. [Google Scholar]
- Drug addiction treatment act of 2000, Calendar No. 740, (2000).
- Haddad MS, Zelenev A, Altice FL. Integrating buprenorphine maintenance therapy into federally qualified health centers: Real-world substance abuse treatment outcomes. Drug and Alcohol Dependence. 2013;131(1):127–135. doi: 10.1016/j.drugalcdep.2012.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser Family Foundation. Total professionally active physicians. 2015 Retrieved from http://kff.org/other/state-indicator/total-active-physicians/
- Kissin W, McLeod C, Sonnefeld J, Stanton A. Experiences of a national sample of qualified addiction specialists who have and have not prescribed buprenorphine for opioid dependence. Journal of Addictive Diseases. 2006;25(4):91–103. doi: 10.1300/J069v25n04_09. [DOI] [PubMed] [Google Scholar]
- Knapp H, Anaya HD. Implementation science in the real world: a streamlined model. Journal for Healthcare Quality. 2012;34(6):27–35. doi: 10.1111/j.1945-1474.2012.00220.x. [DOI] [PubMed] [Google Scholar]
- Massachusetts Behavioral Health Partnership. Health New England be healthy provider manual. 2010 Retrieved from http://www.masspartnership.com/HNE/HNEProvManual.aspx.
- MassHeatlh. MassHealth Provider Manual Series Community Health Center Manual. MassHealth; 2015. [Google Scholar]
- McLellan AT, Lewis DC, O’Brien CP, Kleber HD. Drug dependence, a chronic medical illness: Implications for treatment, insurance, and outcomes evaluation. Jama. 2000;284(13):1689–1695. doi: 10.1001/jama.284.13.1689. [DOI] [PubMed] [Google Scholar]
- SAMHSA. Drug addiction treatment act of 2000. 2015 Retrieved from http://buprenorphine.samhsa.gov/fulllaw.html.
- SAMHSA Center for Behavioral Health Statistics and Quality. Treatment episode data set (TEDS) 2002 – 2012. National admissions to substance abuse treatment services. Rockville, MD: SAMHSA; 2014. No. BHSIS Series S-71, HHS Publication No. (SMA) 14-4850. [Google Scholar]
- Substance Abuse and Mental Health Services Administration. Medicaid coverage and financing of medications to treat alcohol and opioid use disorders. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2014a. No. HHS Publication No. SMA-14-4854. [Google Scholar]
- Substance Abuse and Mental Health Services Administration. Results from the 2013 national survey on drug use and health: Summary of national findings. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2014b. No. NSDUH Series H-48, HHS Publication No. (SMA) 14-4863. [Google Scholar]
- Turner BJ, Laine C, Lin Y, Lynch K. Barriers and facilitators to primary care or human immunodeficiency virus clinics providing methadone or buprenorphine for the management of opioid dependence. Archives of Internal Medicine. 2005;165(15):1769–1776. doi: 10.1001/archinte.165.15.1769. [DOI] [PubMed] [Google Scholar]
- Wagner EH, Austin BT, Davis C, Hindmarsh M, Schaefer J, Bonomi A. Improving chronic illness care: Translating evidence into action. Health Affairs (Project Hope) 2001;20(6):64–78. doi: 10.1377/hlthaff.20.6.64. [DOI] [PubMed] [Google Scholar]
- Walley AY, Alperen JK, Cheng DM, Botticelli M, Castro-Donlan C, Samet JH, Alford DP. Office-based management of opioid dependence with buprenorphine: Clinical practices and barriers. Journal of General Internal Medicine. 2008;23(9):1393–1398. doi: 10.1007/s11606-008-0686-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesson DR, Ling W. The clinical opiate withdrawal scale (COWS) Journal of Psychoactive Drugs. 2003;35(2):253–259. doi: 10.1080/02791072.2003.10400007. [DOI] [PubMed] [Google Scholar]