Abstract
Purpose of review
Innate lymphoid cells (ILCs) are a newly-identified population of immune cells prevalent in, but not limited to, mucosal tissues that not only play a significant role in immune homeostasis and host defense, but also in disease pathogenesis. This review highlights the importance of type 3 ILCs (ILC3s) and their interactions with the intestinal microflora, both in maintaining gut health and in the development of inflammatory bowel disease (IBD).
Recent findings
Distinct lineages of ILCs are defined based on the presence of cell surface proteins, secretion of effector cytokines, and expression of master transcription factors that determine their differentiation and inflammatory behavior. These ILC subgroups mirror corresponding CD4+ T-cell subsets, with which they share many phenotypic, morphologic, and functional attributes. ILC3s, in particular, through direct and indirect interactions with the gut microbiota, have been identified to promote protection and maintenance of epithelial integrity, but also to regulate intestinal inflammation and fibrosis, such as that observed in IBD.
Summary
Gut mucosal ILCs respond to environmental cues, such as diet and microflora composition, which can shape downstream immune function. As such, ILCs represent attractive targets for the development of therapeutic modalities to maintain gut health and to potentially treat IBD.
Keywords: Innate lymphoid cells (ILCs), gut microbiota, inflammatory bowel disease (IBD), mucosal immunity
Introduction
IBD, primarily encompassing Crohn’s disease (CD) and ulcerative colitis (UC), describes a chronic and relapsing inflammatory condition of the gastrointestinal (GI) tract attributed to the combinatorial effects of immune dysregulation and environmental factors in genetically-predisposed individuals. For several years, dysregulation of the adaptive immune system, mainly of CD4+ T-cells, was believed to be paramount for the development of IBD. More recently, aberrancies in gut mucosal innate immunity have been shown to play a primary, initiating role. In this context, newly-identified innate immune cells, termed ILCs, have been described that play a pivotal role in both mucosal immune homeostasis, as well as in the development of IBD. This review will discuss the involvement of intestinal ILCs, with emphasis on ILC3s, in gut health and disease, and their interactions with the microbiome during IBD.
ILC development and lineages
As their name suggests, ILCs are of lymphoid origin, derived from the common lymphoid progenitor (CLP), and rely on interleukin (IL)-2Rγc signaling. ILCs are distinguished by the absence of features found in other immune cells, such as the lack of recombined antigen-specific receptors typically found on B- and T-cells, and of phenotypic markers associated with monocytes, dendritic cells (DCs), granulocytes, and B- and T-cells (1). ILCs can be found throughout the anatomy of both mice and humans, but are mostly enriched in mucosal barrier surfaces of the respiratory and GI tracts, and skin (1–3). Similar to how memory T-cells, natural killer (NK)T-cells, and γδT-cells are activated, ILCs are stimulated by stress signals, the cytokine milieu of the tissue environment, and microbes, rather than by antigens. ILCs are early effectors during an immune response and secrete cytokines classically associated with T-helper cells, and can therefore potentially mediate type 1, 2, and 3 immunity that control intracellular pathogens, helminths, and extracellular microbes, respectively.
ILC subsets
Previously referred to by a variety of names, such as ‘natural helper cells,’ ‘nuocytes,’ and ‘innate helper cells,’ ILC nomenclature has been designed to recognize its growing diversity (4). ILCs are currently divided into subsets, based on the expression of lineage-defining transcription factors. These different subsets, their associated cytokine profiles, and the transcription factors that direct their lineages and regulate their functions are reviewed in detail elsewhere (5). ILC1s, which include NK cells, secrete interferon (IFN)γ and tumor necrosis factor (TNF) in response to IL-12, IL-15, and IL-18, and their function is regulated by the transcription factor, T-bet (6). The second group, ILC2s, originally coined ‘nuocytes,’ is dependent on the transcription factors, GATA-3 and Bcl11b (7, 8), and produces the Th2 cytokines, IL-5 and IL-13, in response to IL-25, IL-33, and thymic stromal lymphopoietin (TSLP), as well as to helminth infection and allergic inflammation (9–12). ILC2s are also distinguished by the expression of ST2 (IL-33R), c-kit, and KLRG1. Thirdly, the ILC3 lineage is controlled by the transcription factor, RORγt, and is characterized by production of IL-17 and IL-22, and the expression of c-kit and IL-23R. They can be further subdivided by expression of natural cytotoxicity receptors (NCRs), wherein NCR+ ILC3s secrete IL-22 and NCR− ILC3s, which include lymphoid tissue inducer (LTi) cells, express both IL-22 and IL-17 (13).
ILCs in the intestines
ILC1, ILC2, and ILC3 subsets have been characterized in the gut and gut-associated lymph nodes of both mice and humans during homeostasis, as well as during disease (14–16), and also in response to bacterial and parasitic infections (17–19). ILCs were first described in human mucosa-associated lymphoid tissues and initially defined as an NK cell subset that secretes IL-22 in response to IL-23 (20).
ILCs are rarely present in the steady state. In fact, ILCs expressing NCRs constitute only 5% of lymphocytes in human colon and small intestines (21, 22). They are even less common in naïve mice, where NCR-expressing ILCs represent only 1% of hematopoietic cells in the colon, 2% in the small intestines, and less than 1% of intraepithelial lymphocytes (23, 24).
In mice intestines, ILCs are categorized into three groups: 1) T-bet+ IFNγ-producing ILC1s, 2) RORγt+ IL-22-secreting ILC3s, and 3) a subset that appears to be transitional between RORγt+ ILC3s and T-bet+ ILC1s (25). Another unique subpopulation of NCR+ ILC1s is thought to inhabit the gut epithelium (26). Human intestinal ILCs resemble the subsets described in mice. These comprise Nkp44+ (a human NCR) CD103+ intraepithelial cells (26), Nkp44+ RORγt+ ILC3s, Nkp44− c-kit− ILC1s, and Nkp44− c-kit+ RORγt+ NCR− ILC3s (27).
Interestingly, plasticity between ILC subsets resembles that of CD4+ T-cell lineages. NCR− ILC3s in the gut express RORγt and secrete IL-17 and/or IL-22. A subset of NCR− ILC3s co-expresses T-bet, inducing secretion of IFNγ and mirroring Th1 cells (28–30). These NCR− ILC3s are capable of producing inflammatory cytokines consistent with generating immunity against intestinal pathogens. ILC2s comprise approximately 5% of small intestinal lymphoid cells and are able to recruit and maintain eosinophils through IL-5 (31). ILC2 function in the human GI tract has not been reported, but as reported in murine studies, they may influence protection against helminth infection (32, 33).
ILCs and IBD
Several mouse models suggest that all ILC subsets contribute to intestinal inflammation to varying degrees. One of the first studies describes that in a Helicobacter hepaticus-induced IBD model, an increase in colonic IL-17- and IFNγ-producing RORγt+ CD127+ NCR− ILC3s drives intestinal inflammation and depletion of these cells leads to abrogation of disease (30). Studies using Tbx21−/−Rag2−/− UC (TRUC) mice support these observations. TRUC mice develop microbiota-dependent colitis characteristic of UC and display an increase in colonic IL-17- and IL-22-producing NCR− ILC3s. Either depleting ILCs themselves or ablating Nod2/Ripk signaling in DCs, which activates ILCs, abrogates colitis (28, 29).
Interestingly, ILC3s have also been detected in CD and UC patients, with isolated colonic ILCs expressing increased IL-17, IL-22, RORC, aryl hydrocarbon receptor (AHR), and IL-23R (34). Overall, current studies suggest that NCR− ILC3s are pathogenic in the context of IBD. However, the same is not true for ILC3s that express NCR. In fact, studies show that IBD patients have decreased IL-22-secreting NCR+ ILC3s that is associated with CD (22, 27). NCR+ ILC3s are key producers of IL-22, which is critical in maintaining intestinal epithelial barrier integrity, and a reduced number of these cells may predispose these individuals to dampened gut mucosal protection. Similarly, during acute Citrobacter rodentium infection, blocking IL-22 or depleting IL-22+ ILCs aggravates disease outcome (35, 36). As such, the possibility exists that during chronic intestinal inflammation, a decrease in IL-22+ ILC3s is unfavorable for IBD patients due to the lack of appropriate epithelial barrier function.
Increased intestinal ILC1s, both in the epithelial and lamina propria (LP) compartments, have also been reported in experimental IBD models (25, 26). These ILC1s express Nkp46 and a similar Nkp44+ ILC1 counterpart is also increased in CD patients (26, 27), suggesting that ILC1s may have a pathogenic role in CD. Interestingly, a role for ILC2s during IBD has not yet been defined. A study, however, investigating collagen deposition in human intestinal tissues showed an increase in infiltrating IL-13-expressing killer cell immunoglobulin-like receptor (KIR)+ cells in the muscularis of fibrotic CD patients compared to controls. These IL-13R+ KIR+ ILCs are reported to promote fibrosis via IL-13, preventing matrix protein degradation in myofibroblasts and inducing disproportionate collagen deposition (37), suggesting the potential role of ILC2s in inducing inflammation-associated fibrosis in CD.
ILC interactions with the commensal flora
In contrast to intestinal DCs and macrophages, direct interaction between ILCs and commensal microbes has not yet been proven, and at present, expression of toll-like receptors (TLRs) on ILCs has not been reported. Evidence exists, however, that ILCs are able to act in response to microbiota through communication with both epithelial cells and intestinal mononuclear phagocytes via cytokines/cytokine signaling.
Regulation of ILC function by the microbiome
Studies show that human RORγt+ ILCs can indirectly respond to TLR2 agonists by secreting IL-2, which in turn induces IL-22 (38). Similarly, flagellin activation of TLR5 in LP mononuclear phagocytes leads to IL-23 production and augments IL-22 by RORγt+ ILCs. Increased IL-22 can lead to subsequent expression of antimicrobial peptides, such as Reg3g, by intestinal epithelial cells (IECs) (39–41), thus promoting gut mucosal protection.
IL-1β expression by intestinal macrophages in response to the gut microflora also induces IL-22 from RORγt+ ILCs (42, 43). IL-1β can stimulate granulocyte macrophage colony stimulating factor (GM-CSF) from ILC3s, and IL-10 and retinoic acid from resident DCs and macrophages, which support the proliferation of intestinal T-regulatory cells (Tregs). Blocking GM-CSF by ILC3s abolishes tolerance to food antigen; however, it is unknown whether Treg-associated tolerance towards the microbiome is affected. Therefore, homeostasis can also be regulated by interactions between ILCs and intestinal mononuclear phagocytes (44). In fact, CX3CR1+ mononuclear phagocytes develop in the intestine after commensal colonization and are critical for IL-22 production by RORγt+ ILCs (45, 46). In contrast, microbiota-stimulated IL-25 from IECs can decrease constitutive IL-22 production by RORγt+ ILCs (47). In the absence of symbiotic microbiota, epithelial-derived IL-25 is decreased. Besides TLRs, NK cells and NCR+ RORγt+ ILCs may directly sense commensal flora through NCRs, such as Nkp44 and Nkp46, which can be stimulated by elements derived from commensal bacteria (48, 49).
Recent studies have shown that certain phytochemicals derived from vegetables can influence ILC survival and cytokine secretion through expression of AHR (50–52). Indirectly, the microbiota can modulate generation of the tryptophan-derived AHR ligand, indole-3-aldehyde, which can promote ILC3 function. Lactobacillus spp. utilize tryptophan and control the availability of its byproduct that augments IL-22 production by ILC3s (53). It is well documented that AHR promotes Th17 differentiation, as well as the maintenance and function of ILC3s (54). However, recent investigation of AHR-deficient mice show that these mice actually possess increased numbers of intestinal Th17 cells, as well as decreased IL-22 from ILC3s, that is permissive for outgrowth of Th17-skewing segmented filamentous bacteria (SFB) (55). Taken together, these data suggest that commensal bacteria and their metabolic products have the ability to regulate RORγt+ ILCs.
Commensal bacteria can colonize barrier surfaces and directly interact with IECs. Germ-free or antibiotic-treated mice show decreased IEC-derived IL-7 (25, 56), a key cytokine in the homeostasis and function of ILC2s and ILC3s. In fact, microbiota-dependent IL-7 production from IECs is necessary for ILC3 RORγt expression (25), whereas lack of IFNγ signaling in IECs dampens IL-7 (56); thus, implicating commensal bacteria in regulating both IFNγ-producing NK cells and IL-7-dependent ILCs. The expression of IL-1 family members, such as IL-18, IL-33, and IL-1β can also stimulate responses from ILC1s, ILC2s, and ILC3s, respectively (43, 57, 58); however, a direct influence by commensal bacteria has not been well-characterized.
Given the proximity of commensal bacteria, IECs, and ILCs, it is logical to assume that the gut microbiota may affect ILC development. However, current studies show that the microbiota is not required for development of most ILC subsets, such as NK cells and GATA3+ ILC2s (12, 59, 60). Nonetheless, the numbers of NCR+ RORγt+ ILC3s are decreased, and a lack of IL-22 and Rorc expression is observed in the small intestinal LP of germ-free and antibiotic-treated mice (23, 25, 35). In contrast, and under these same conditions, normal development proceeds in all RORγt+ ILC3s (47, 61–63). In fact, LTi cells and secondary lymphoid structures are still generated in the fetus before birth (64). After birth however, the formation of lymphoid follicles is impaired in germ-free mice, suggesting a compromise in function of LTi-like RORγt+ ILC subsets (65, 66). As such, the effect of the microbiome on ILC development is still controversial and further investigation is warranted.
Even in the absence of microbial colonization, around the third trimester of gestation in mice, ILC3s are present in the fetal gut. Compared to ILCs in the adult gut, these cells produce less IL-22, indicating that IL-22 production depends on the presence of the microbiome (67). Upon binding to the IL-22R expressed on IECs, IL-22 activates STAT3 and induces antimicrobial peptides, such as Ref3g and Reg3b, in a Citrobacter rodentium infection model (36, 68, 69). In IBD patients, ILC3s in contact with the intestinal microbiota in the afferent limb of the gut express more cytokines than those not interacting with the microbiome, in the efferent limb of the gut. It appears that this functional difference is dependent on stimulation of ILC3s by CX3CR1high CD14high mononuclear phagocytes that have been activated by microbes (70).
Together, these studies suggest that the gut microbiota can shape the development and function of ILCs, primarily through indirect interactions with myeloid and epithelial cell populations, and describe a well-developed network among ILCs, IECs, and the gut microbiota.
Regulation of the microbiota by ILCs
ILCs can also reciprocally regulate the commensal flora through various mechanisms. For example, cytokines derived from ILCs can influence the composition and compartmentalization of the intestinal microbiota. T-bet deficient ILC1s are impaired in producing IFNγ, and mice lacking T-bet develop colitis dependent on IL-17-expressing RORγt+ ILCs and dysbiosis of Helicobacter typhlonius (28). Moreover, ILC2s may represent key players in controlling the anatomical restraint of commensal microbiota when the epithelial barrier is compromised. While not yet proven within the GI tract, ILC2s within the respiratory tract produce amphiregulin, which maintains epithelial barrier function and restores lung epithelium in the event of airway damage due to infection (12). Additional studies are required to demonstrate that ILC1s and ILC2s can directly regulate the commensal microbiota.
Emerging evidence, however, suggests that RORγt+ ILC3s have the ability to regulate bacterial populations in the intestine. In the healthy intestine, RORγt+ ILC3s are major producers of IL-22 (36, 47, 63). In the absence of RORγt+ ILC3s, an increase in serum IgG titers specific for intestinal commensals occurs (71), indicating potential disruption in epithelial barrier integrity and spread of commensal bacteria to peripheral tissues. Following dextran sodium sulfate (DSS) administration to RORγt-deficient mice that induces intestinal epithelial damage, generation of hyperactive B-cells results, promoting colitis (71). As such, RORγt+ ILC3s may be necessary to inhibit intestinal damage induced by pathogenic B-cells.
CX3CR1+ phagocytes are also important for IL-22 production by RORγt+ ILC3 cells. Studies on mice lacking CX3CR1+ phagocytes show an increased presence of commensal bacteria in mesenteric lymph nodes (MLNs) and greater susceptibility to DSS-induced colitis (45, 72). Blocking IL-22 or depleting ILCs results in outgrowth of Alcaligenes (normally found in Peyer’s patches and MLNs) into the liver and spleen, and induces systemic inflammation (63), suggesting that ILCs indeed play a role in anatomical containment of commensal bacteria. ILC3-derived IL-22 can also act on the intestinal epithelium to induce expression of tissue protective mucins and antimicrobial peptides, such as RegIIIβ, RegIIIγ, S100A8 and S100A9 (73). Systemic inflammation is ameliorated after introduction of IL-22 and mice lacking Muc2 or RegIIIγ show a lack of spatial segregation of commensal bacteria, suggesting an important, albeit indirect, role for ILCs in limiting bacterial outgrowth and inflammation (74, 75). Similarly, S100A8 and S100A9 bind to calprotectin to inhibit proliferation of commensal bacteria and support containment of these bacteria (63). Finally, consistent with the results in mice, Alcaligenes-specific responses are detected in CD patients (63).
Conclusion
ILC3s are a subset of ILCs that are enriched in the mucosa of experimental models of IBD as well as in inflammatory lesions of IBD patients. Because of their prime location, ILC3s are optimally positioned to instantaneously react to both environmental and inflammatory signals received from the gut microbiota, IECs, and other immune cells within the GI tract. Their role in IBD appears to be complex, and not yet fully elucidated, but is greatly influenced by the intestinal cytokine milieu, as well as interactions with the commensal flora and with both hematopoietic and non-hematopoietic mucosal cells. Although several studies cited within this review were performed prior to the identification of ILCs (indeed, early papers referred to ILCs as a subset of NK cells), they support a clear role, particularly for ILC3s, during homeostatic and disease states that is influenced by interactions with the intestinal microbiota (summarized in Figure 1). Nonetheless, future studies are imperative to further define lineage development of gut ILC subsets, to determine the role of other ILC subsets in IBD, and to better describe the reciprocal relationship between mucosal ILCs and the gut microenvironment, and the implications of these interactions during gut health and disease.
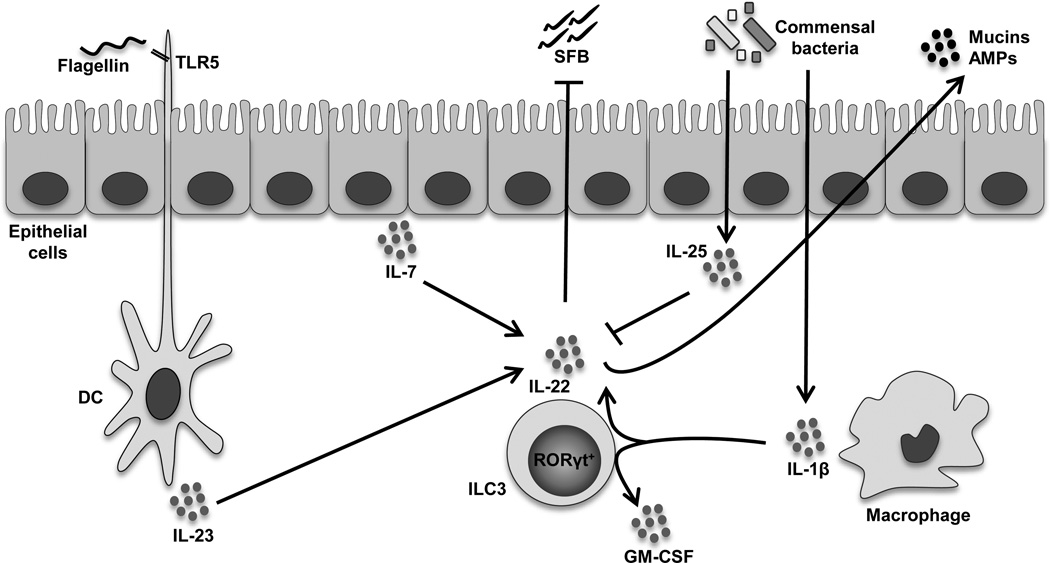
Fig. 1. Interactions between type 3 innate lymphoid cells (ILC3s) and the gut microbiota.
Emerging evidence suggests that crosstalk between ILC3s and components of the intestinal microflora has the ability to support both maintenance of gut homeostasis, as well as induce chronic intestinal inflammation. Central to this process is IL-22, which promotes gut health by inducing the production of epithelial-derived antimicrobial peptides (AMPs) and mucins. RORγt+ ILC3s are major producers of IL-22 in response to microbial products, mucosal antigen presenting cells, as well as the local cytokine milieu. Flagellin, the principal component of bacterial flagella, induces TLR5 in dendritic cells (DCs) and promotes IL-23 secretion, which enhances IL-22 from ILC3s. IECs produce IL-7 in response to elements of the gut microbiota, which also stimulates IL-22 from ILC3s. Intestinal macrophage-derived IL-1β has the ability to induce both IL-22 and GM-CSF from ILC3s to support dietary antigenic tolerance. However, commensal bacteria may also induce IECs to produce IL-25, decreasing IL-22 production. In the absence of ILC3-derived IL-22, segmented filamentous bacteria (SFB) demonstrate unrestricted growth, which encourages pathogenic Th17 immune responses that can promote colitis in mice.
Key points.
ILCs are a novel population of innate lymphocytes that are selectively enriched at mucosal sites, comprise subpopulations that are distinct phenotypically and functionally, and are involved in both the maintenance and loss of homeostasis at mucosal surfaces, such as in the GI tract.
Type 3 ILCs (ILC3s) are major producers of IL-22 in response to microbial products, mucosal antigen presenting cells, as well as the local cytokine milieu, and appear to possess effector function in mouse models of IBD.
Gut-derived ILC3s expressing natural cytotoxicity receptors (NCRs) may be protective by promoting epithelial integrity and barrier function, while NCR- ILC3s may be pathogenic by inducing intestinal inflammation.
Dysregulation in the quantity and composition of ILC subsets is observed in IBD patients compared to controls.
Interactions between ILC3s and the gut microbiota reciprocally regulate each other’s functions and greatly influence the role each play during intestinal health and disease.
Acknowledgements
Disclosure of funding: This work was funded by grants from the National Institutes of Health (DK056762, DK091222, and AI102269) and a Pilot & Feasibility Award (CA150964) to TTP
Footnotes
Conflict of interest
None declared.
References and recommended reading
*of special interest
**of outstanding interest
- 1.Spits H, Cupedo T. Innate lymphoid cells: emerging insights in development, lineage relationships, and function. Annu Rev Immunol. 2012;30:647–675. doi: 10.1146/annurev-immunol-020711-075053. [DOI] [PubMed] [Google Scholar]
- 2.Spits H, Di Santo JP. The expanding family of innate lymphoid cells: regulators and effectors of immunity and tissue remodeling. Nat Immunol. 2011;12(1):21–27. doi: 10.1038/ni.1962. [DOI] [PubMed] [Google Scholar]
- 3.Veldhoen M, Withers DR. Immunology. Innate lymphoid cell relations. Science. 2010;330(6004):594–595. doi: 10.1126/science.1198298. [DOI] [PubMed] [Google Scholar]
- 4.Spits H, Artis D, Colonna M, Diefenbach A, Di Santo JP, Eberl G, et al. Innate lymphoid cells-a proposal for uniform nomenclature. Nat Rev Immunol. 2013;13(2):145–149. doi: 10.1038/nri3365. [DOI] [PubMed] [Google Scholar]
- 5. Eberl G, Colonna M, Di Santo JP, McKenzie AN. Innate lymphoid cells. Innate lymphoid cells: a new paradigm in immunology. Science. 2015;348(6237):aaa6566. doi: 10.1126/science.aaa6566.. This recent review thoroughly discusses the different ILC subsets, their corresponding cytokine profiles, and the transcription factors that regulate their function.
- 6.Klose CS, Flach M, Mohle L, Rogell L, Hoyler T, Ebert K, et al. Differentiation of type 1 ILCs from a common progenitor to all helper-like innate lymphoid cell lineages. Cell. 2014;157(2):340–356. doi: 10.1016/j.cell.2014.03.030. [DOI] [PubMed] [Google Scholar]
- 7.Walker JA, Oliphant CJ, Englezakis A, Yu Y, Clare S, Rodewald HR, et al. Bcl11b is essential for group 2 innate lymphoid cell development. J Exp Med. 2015;212(6):875–882. doi: 10.1084/jem.20142224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yu Y, Wang C, Clare S, Wang J, Lee SC, Brandt C, et al. The transcription factor Bcl11b is specifically expressed in group 2 innate lymphoid cells and is essential for their development. J Exp Med. 2015;212(6):865–874. doi: 10.1084/jem.20142318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoyler T, Klose CS, Souabni A, Turqueti-Neves A, Pfeifer D, Rawlins EL, et al. The transcription factor GATA-3 controls cell fate and maintenance of type 2 innate lymphoid cells. Immunity. 2012;37(4):634–648. doi: 10.1016/j.immuni.2012.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim BS, Siracusa MC, Saenz SA, Noti M, Monticelli LA, Sonnenberg GF, et al. TSLP elicits IL-33-independent innate lymphoid cell responses to promote skin inflammation. Sci Transl Med. 2013;5(170):170ra16. doi: 10.1126/scitranslmed.3005374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chang YJ, Kim HY, Albacker LA, Baumgarth N, McKenzie AN, Smith DE, et al. Innate lymphoid cells mediate influenza-induced airway hyper-reactivity independently of adaptive immunity. Nat Immunol. 2011;12(7):631–638. doi: 10.1038/ni.2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Monticelli LA, Sonnenberg GF, Abt MC, Alenghat T, Ziegler CG, Doering TA, et al. Innate lymphoid cells promote lung-tissue homeostasis after infection with influenza virus. Nat Immunol. 2011;12(11):1045–1054. doi: 10.1031/ni.2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Takatori H, Kanno Y, Watford WT, Tato CM, Weiss G, Ivanov II, et al. Lymphoid tissue inducer-like cells are an innate source of IL-17 and IL-22. J Exp Med. 2009;206(1):35–41. doi: 10.1084/jem.20072713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ciccia F, Guggino G, Rizzo A, Saieva L, Peralta S, Giardina A, et al. Type 3 innate lymphoid cells producing IL-17 and IL-22 are expanded in the gut, in the peripheral blood, synovial fluid and bone marrow of patients with ankylosing spondylitis. Ann Rheum Dis. 2015 Apr 22; doi: 10.1136/annrheumdis-2014-206323. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 15.Mackley EC, Houston S, Marriott CL, Halford EE, Lucas B, Cerovic V, et al. CCR7-dependent trafficking of RORgamma(+) ILCs creates a unique microenvironment within mucosal draining lymph nodes. Nat Commun. 2015;6:5862. doi: 10.1038/ncomms6862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bando JK, Liang HE, Locksley RM. Identification and distribution of developing innate lymphoid cells in the fetal mouse intestine. Nat Immunol. 2015;16(2):153–160. doi: 10.1038/ni.3057.. This study identifies precursors of LTi cells, a type of ILC3, that develop in the fetal mouse intestine before organogenesis of Peyer's patches, where these cells populate and from where gut-derived ILC populations are derived.
- 17. Guo X, Liang Y, Zhang Y, Lasorella A, Kee BL, Fu YX. Innate lymphoid cells control early colonization resistance against intestinal pathogens through ID2-dependent regulation of the microbiota. Immunity. 2015;42(4):731–743. doi: 10.1016/j.immuni.2015.03.012.. This study demonstrates that expression of the transcription factor, ID2, in ILC3s is important in regulating the microbiota and protecting against Citrobacter rodentium infection, highlighting an immune surveillance function of ILC3s.
- 18.Geiger TL, Abt MC, Gasteiger G, Firth MA, O'Connor MH, Geary CD, et al. Nfil3 is crucial for development of innate lymphoid cells and host protection against intestinal pathogens. J Exp Med. 2014;211(9):1723–1731. doi: 10.1084/jem.20140212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Guo X, Qiu J, Tu T, Yang X, Deng L, Anders RA, et al. Induction of innate lymphoid cell-derived interleukin-22 by the transcription factor STAT3 mediates protection against intestinal infection. Immunity. 2014;40(1):25–39. doi: 10.1016/j.immuni.2013.10.021.. This study highlights the requirement of STAT3 for IL-2 production from ILC3s and for defense against intestinal pathogens.
- 20.Cella M, Fuchs A, Vermi W, Facchetti F, Otero K, Lennerz JK, et al. A human natural killer cell subset provides an innate source of IL-22 for mucosal immunity. Nature. 2009;457(7230):722–725. doi: 10.1038/nature07537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chinen H, Matsuoka K, Sato T, Kamada N, Okamoto S, Hisamatsu T, et al. Lamina propria c-kit(+) immune precursors reside in human adult intestine and differentiate into natural killer cells. Gastroenterol. 2007;133(2):559–573. doi: 10.1053/j.gastro.2007.05.017. [DOI] [PubMed] [Google Scholar]
- 22.Takayama T, Kamada N, Chinen H, Okamoto S, Kitazume MT, Chang J, et al. Imbalance of NKp44(+)NKp46(−) and NKp44(−)NKp46(+) natural killer cells in the intestinal mucosa of patients with Crohn's disease. Gastroenterol. 2010;139(3):882–892. doi: 10.1053/j.gastro.2010.05.040. [DOI] [PubMed] [Google Scholar]
- 23.Sanos SL, Bui VL, Mortha A, Oberle K, Heners C, Johner C, et al. RORgammat and commensal microflora are required for the differentiation of mucosal interleukin 22-producing NKp46+ cells. Nat Immunol. 2009;10(1):83–91. doi: 10.1038/ni.1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hall LJ, Murphy CT, Quinlan A, Hurley G, Shanahan F, Nally K, et al. Natural killer cells protect mice from DSS-induced colitis by regulating neutrophil function via the NKG2A receptor. Mucosal Immunol. 2013;6(5):1016–1026. doi: 10.1038/mi.2012.140. [DOI] [PubMed] [Google Scholar]
- 25.Vonarbourg C, Mortha A, Bui VL, Hernandez PP, Kiss EA, Hoyler T, et al. Regulated expression of nuclear receptor RORgammat confers distinct functional fates to NK cell receptor-expressing RORgammat(+) innate lymphocytes. Immunity. 2010;33(5):736–751. doi: 10.1016/j.immuni.2010.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fuchs A, Vermi W, Lee JS, Lonardi S, Gilfillan S, Newberry RD, et al. Intraepithelial type 1 innate lymphoid cells are a unique subset of IL-12- and IL-15-responsive IFN-gamma-producing cells. Immunity. 2013;38(4):769–781. doi: 10.1016/j.immuni.2013.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bernink JH, Peters CP, Munneke M, te Velde AA, Meijer SL, Weijer K, et al. Human type 1 innate lymphoid cells accumulate in inflamed mucosal tissues. Nat Immunol. 2013;14(3):221–229. doi: 10.1038/ni.2534. [DOI] [PubMed] [Google Scholar]
- 28. Powell N, Walker AW, Stolarczyk E, Canavan JB, Gokmen MR, Marks E, et al. The transcription factor T-bet regulates intestinal inflammation mediated by interleukin-7 receptor+ innate lymphoid cells. Immunity. 2012;37(4):674–684. doi: 10.1016/j.immuni.2012.09.008.. This study establishes that ILCs can mediate colitis through IL-17 and that T-bet modulates the interactions among mucosal DCs, ILCs, and the microbiome that drive IL-17 production from ILCs.
- 29. Ermann J, Staton T, Glickman JN, de Waal Malefyt R, Glimcher LH. Nod/Ripk2 signaling in dendritic cells activates IL-17A-secreting innate lymphoid cells and drives colitis in T-bet−/−.Rag2−/− (TRUC) mice. Proc Natl Acad Sci U S A. 2014;111(25):E2559–E2566. doi: 10.1073/pnas.1408540111.. This study demonstrates that Nod/Ripk2 signaling is important for DC production of cytokines in response to the microbiota, which stimulates IL-17 production from ILCs to mediate colitis.
- 30. Buonocore S, Ahern PP, Uhlig HH, Ivanov II, Littman DR, Maloy KJ, et al. Innate lymphoid cells drive interleukin-23-dependent innate intestinal pathology. Nature. 2010;464(7293):1371–1375. doi: 10.1038/nature08949.. This study was the first to implicate ILCs in mediating intestinal inflammation, such as that found in IBD.
- 31.Nussbaum JC, Van Dyken SJ, von Moltke J, Cheng LE, Mohapatra A, Molofsky AB, et al. Type 2 innate lymphoid cells control eosinophil homeostasis. Nature. 2013;502(7470):245–248. doi: 10.1038/nature12526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fallon PG, Ballantyne SJ, Mangan NE, Barlow JL, Dasvarma A, Hewett DR, et al. Identification of an interleukin (IL)-25-dependent cell population that provides IL-4, IL-5, and IL-13 at the onset of helminth expulsion. J Exp Med. 2006;203(4):1105–1116. doi: 10.1084/jem.20051615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Neill DR, Wong SH, Bellosi A, Flynn RJ, Daly M, Langford TK, et al. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature. 2010;464(7293):1367–1370. doi: 10.1038/nature08900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Geremia A, Arancibia-Carcamo CV, Fleming MP, Rust N, Singh B, Mortensen NJ, et al. IL-23-responsive innate lymphoid cells are increased in inflammatory bowel disease. J Exp Med. 2011;208(6):1127–1133. doi: 10.1084/jem.20101712.. Similar to citation #31, this was one of the first studies to described the role of ILCs in IBD.
- 35. Satoh-Takayama N, Vosshenrich CA, Lesjean-Pottier S, Sawa S, Lochner M, Rattis F, et al. Microbial flora drives interleukin 22 production in intestinal NKp46+ cells that provide innate mucosal immune defense. Immunity. 2008;29(6):958–970. doi: 10.1016/j.immuni.2008.11.001.. This study was the first to demonstrate interactions between the microbiome and ILCs.
- 36.Sonnenberg GF, Monticelli LA, Elloso MM, Fouser LA, Artis D. CD4(+) lymphoid tissue-inducer cells promote innate immunity in the gut. Immunity. 2011;34(1):122–134. doi: 10.1016/j.immuni.2010.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bailey JR, Bland PW, Tarlton JF, Peters I, Moorghen M, Sylvester PA, et al. IL-13 promotes collagen accumulation in Crohn's disease fibrosis by down-regulation of fibroblast MMP synthesis: a role for innate lymphoid cells? PLoS One. 2012;7(12):e52332. doi: 10.1371/journal.pone.0052332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Crellin NK, Trifari S, Kaplan CD, Satoh-Takayama N, Di Santo JP, Spits H. Regulation of cytokine secretion in human CD127(+) LTi-like innate lymphoid cells by Toll-like receptor 2. Immunity. 2010;33(5):752–764. doi: 10.1016/j.immuni.2010.10.012. [DOI] [PubMed] [Google Scholar]
- 39. Kinnebrew MA, Buffie CG, Diehl GE, Zenewicz LA, Leiner I, Hohl TM, et al. Interleukin 23 production by intestinal CD103(+)CD11b(+) dendritic cells in response to bacterial flagellin enhances mucosal innate immune defense. Immunity. 2012;36(2):276–287. doi: 10.1016/j.immuni.2011.12.011.. This study shows that upon exposure to flagellin, LP DCs produce IL-23 that drives IL-22-dependent production of antimicrobial peptides in the gut.
- 40.Kinnebrew MA, Ubeda C, Zenewicz LA, Smith N, Flavell RA, Pamer EG. Bacterial flagellin stimulates Toll-like receptor 5-dependent defense against vancomycin-resistant Enterococcus infection. J Infect Dis. 2010;201(4):534–543. doi: 10.1086/650203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Van Maele L, Carnoy C, Cayet D, Songhet P, Dumoutier L, Ferrero I, et al. TLR5 signaling stimulates the innate production of IL-17 and IL-22 by CD3(neg)CD127+ immune cells in spleen and mucosa. J Immunol. 2010;185(2):1177–1185. doi: 10.4049/jimmunol.1000115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shaw MH, Kamada N, Kim YG, Nunez G. Microbiota-induced IL-1beta, but not IL-6, is critical for the development of steady-state TH17 cells in the intestine. J Exp Med. 2012;209(2):251–258. doi: 10.1084/jem.20111703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hughes T, Becknell B, Freud AG, McClory S, Briercheck E, Yu J, et al. Interleukin-1beta selectively expands and sustains interleukin-22+ immature human natural killer cells in secondary lymphoid tissue. Immunity. 2010;32(6):803–814. doi: 10.1016/j.immuni.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Mortha A, Chudnovskiy A, Hashimoto D, Bogunovic M, Spencer SP, Belkaid Y, et al. Microbiota-dependent crosstalk between macrophages and ILC3 promotes intestinal homeostasis. Science. 2014;343(6178):1249288. doi: 10.1126/science.1249288.. This study shows that IL-22 is a critical mediator by which ILC3s are able to communicate with other cells in maintaining homeostasis in the gut.
- 45.Manta C, Heupel E, Radulovic K, Rossini V, Garbi N, Riedel CU, et al. CX(3)CR1(+) macrophages support IL-22 production by innate lymphoid cells during infection with Citrobacter rodentium. Mucosal Immunol. 2013;6(1):177–188. doi: 10.1038/mi.2012.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Niess JH, Adler G. Enteric flora expands gut lamina propria CX3CR1+ dendritic cells supporting inflammatory immune responses under normal and inflammatory conditions. J Immunol. 2010;184(4):2026–2037. doi: 10.4049/jimmunol.0901936. [DOI] [PubMed] [Google Scholar]
- 47. Sawa S, Lochner M, Satoh-Takayama N, Dulauroy S, Berard M, Kleinschek M, et al. RORgammat+ innate lymphoid cells regulate intestinal homeostasis by integrating negative signals from the symbiotic microbiota. Nat Immunol. 2011;12(4):320–326. doi: 10.1038/ni.2002.. This study provides evidence that a balance between IL-22 production from intestinal RORgammaγt+ ILCs and IL-25 expression by IECs in response to the gut microbiota regulates intestinal homeostasis.
- 48.Chaushu S, Wilensky A, Gur C, Shapira L, Elboim M, Halftek G, et al. Direct recognition of Fusobacterium nucleatum by the NK cell natural cytotoxicity receptor NKp46 aggravates periodontal disease. PLoS Pathog. 2012;8(3):e1002601. doi: 10.1371/journal.ppat.1002601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Esin S, Batoni G, Counoupas C, Stringaro A, Brancatisano FL, Colone M, et al. Direct binding of human NK cell natural cytotoxicity receptor NKp44 to the surfaces of mycobacteria and other bacteria. Infect Immun. 2008;76(4):1719–1727. doi: 10.1128/IAI.00870-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li Y, Innocentin S, Withers DR, Roberts NA, Gallagher AR, Grigorieva EF, et al. Exogenous stimuli maintain intraepithelial lymphocytes via aryl hydrocarbon receptor activation. Cell. 2011;147(3):629–640. doi: 10.1016/j.cell.2011.09.025. [DOI] [PubMed] [Google Scholar]
- 51.Lee JS, Cella M, McDonald KG, Garlanda C, Kennedy GD, Nukaya M, et al. AHR drives the development of gut ILC22 cells and postnatal lymphoid tissues via pathways dependent on and independent of Notch. Nat Immunol. 2012;13(2):144–151. doi: 10.1038/ni.2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Qiu J, Heller JJ, Guo X, Chen ZM, Fish K, Fu YX, et al. The aryl hydrocarbon receptor regulates gut immunity through modulation of innate lymphoid cells. Immunity. 2012;36(1):92–104. doi: 10.1016/j.immuni.2011.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zelante T, Iannitti RG, Cunha C, De Luca A, Giovannini G, Pieraccini G, et al. Tryptophan catabolites from microbiota engage aryl hydrocarbon receptor and balance mucosal reactivity via interleukin-22. Immunity. 2013;39(2):372–385. doi: 10.1016/j.immuni.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 54.Lee JS, Cella M, Colonna M. AHR and the transcriptional regulation of Type-17/22 ILC. Front Immunol. 2012;3:10. doi: 10.3389/fimmu.2012.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Qiu J, Guo X, Chen ZM, He L, Sonnenberg GF, Artis D, et al. Group 3 innate lymphoid cells inhibit T-cell-mediated intestinal inflammation through aryl hydrocarbon receptor signaling and regulation of microflora. Immunity. 2013;39(2):386–399. doi: 10.1016/j.immuni.2013.08.002.. This study describes the direct modulation of both pathogenic Th17 cells and the commensal flora by ILC3s through AHR signaling.
- 56.Shalapour S, Deiser K, Sercan O, Tuckermann J, Minnich K, Willimsky G, et al. Commensal microflora and interferon-gamma promote steady-state interleukin-7 production in vivo. Eur J Immunol. 2010;40(9):2391–2400. doi: 10.1002/eji.201040441. [DOI] [PubMed] [Google Scholar]
- 57.Moro K, Yamada T, Tanabe M, Takeuchi T, Ikawa T, Kawamoto H, et al. Innate production of T(H)2 cytokines by adipose tissue-associated c-Kit(+)Sca-1(+) lymphoid cells. Nature. 2010;463(7280):540–544. doi: 10.1038/nature08636. [DOI] [PubMed] [Google Scholar]
- 58.Schulthess J, Meresse B, Ramiro-Puig E, Montcuquet N, Darche S, Begue B, et al. Interleukin-15-dependent NKp46+ innate lymphoid cells control intestinal inflammation by recruiting inflammatory monocytes. Immunity. 2012;37(1):108–121. doi: 10.1016/j.immuni.2012.05.013. [DOI] [PubMed] [Google Scholar]
- 59.Ganal SC, Sanos SL, Kallfass C, Oberle K, Johner C, Kirschning C, et al. Priming of natural killer cells by nonmucosal mononuclear phagocytes requires instructive signals from commensal microbiota. Immunity. 2012;37(1):171–186. doi: 10.1016/j.immuni.2012.05.020. [DOI] [PubMed] [Google Scholar]
- 60.Sonnenberg GF, Artis D. Innate lymphoid cell interactions with microbiota: implications for intestinal health and disease. Immunity. 2012;37(4):601–610. doi: 10.1016/j.immuni.2012.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Reynders A, Yessaad N, Vu Manh TP, Dalod M, Fenis A, Aubry C, et al. Identity, regulation and in vivo function of gut NKp46+RORgammat+ and NKp46+RORgammat-lymphoid cells. EMBO J. 2011;30(14):2934–2947. doi: 10.1038/emboj.2011.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sawa S, Cherrier M, Lochner M, Satoh-Takayama N, Fehling HJ, Langa F, et al. Lineage relationship analysis of RORgammat+ innate lymphoid cells. Science. 2010;330(6004):665–669. doi: 10.1126/science.1194597. [DOI] [PubMed] [Google Scholar]
- 63. Sonnenberg GF, Monticelli LA, Alenghat T, Fung TC, Hutnick NA, Kunisawa J, et al. Innate lymphoid cells promote anatomical containment of lymphoid-resident commensal bacteria. Science. 2012;336(6086):1321–1325. doi: 10.1126/science.1222551.. This study emphasizes that ILC-derived IL-22 is required to prevent dissemination of commensal flora and systemic inflammation associated with CD.
- 64.van de Pavert SA, Mebius RE. New insights into the development of lymphoid tissues. Nat Rev Immunol. 2010;10(9):664–674. doi: 10.1038/nri2832. [DOI] [PubMed] [Google Scholar]
- 65.Tsuji M, Suzuki K, Kitamura H, Maruya M, Kinoshita K, Ivanov II, et al. Requirement for lymphoid tissue-inducer cells in isolated follicle formation and T cell-independent immunoglobulin A generation in the gut. Immunity. 2008;29(2):261–271. doi: 10.1016/j.immuni.2008.05.014. [DOI] [PubMed] [Google Scholar]
- 66.Bouskra D, Brezillon C, Berard M, Werts C, Varona R, Boneca IG, et al. Lymphoid tissue genesis induced by commensals through NOD1 regulates intestinal homeostasis. Nature. 2008;456(7221):507–510. doi: 10.1038/nature07450. [DOI] [PubMed] [Google Scholar]
- 67.Hoorweg K, Peters CP, Cornelissen F, Aparicio-Domingo P, Papazian N, Kazemier G, et al. Functional differences between human NKp44(−) and NKp44(+) RORC(+) innate lymphoid cells. Front Immunol. 2012;3:72. doi: 10.3389/fimmu.2012.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zheng Y, Valdez PA, Danilenko DM, Hu Y, Sa SM, Gong Q, et al. Interleukin-22 mediates early host defense against attaching and effacing bacterial pathogens. Nat Med. 2008;14(3):282–289. doi: 10.1038/nm1720. [DOI] [PubMed] [Google Scholar]
- 69.Pickert G, Neufert C, Leppkes M, Zheng Y, Wittkopf N, Warntjen M, et al. STAT3 links IL-22 signaling in intestinal epithelial cells to mucosal wound healing. J Exp Med. 2009;206(7):1465–1472. doi: 10.1084/jem.20082683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Longman RS, Diehl GE, Victorio DA, Huh JR, Galan C, Miraldi ER, et al. CX(3)CR1(+) mononuclear phagocytes support colitis-associated innate lymphoid cell production of IL-22. J Exp Med. 2014;211(8):1571–1583. doi: 10.1084/jem.20140678.. This study highlights the role of mononuclear phagocytes in integrating signals from the microbiome to promote IL-22 production by ILC3s during colitis.
- 71.Lochner M, Ohnmacht C, Presley L, Bruhns P, Si-Tahar M, Sawa S, et al. Microbiota-induced tertiary lymphoid tissues aggravate inflammatory disease in the absence of RORgamma t and LTi cells. J Exp Med. 2011;208(1):125–134. doi: 10.1084/jem.20100052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Medina-Contreras O, Geem D, Laur O, Williams IR, Lira SA, Nusrat A, et al. CX3CR1 regulates intestinal macrophage homeostasis, bacterial translocation, and colitogenic Th17 responses in mice. J Clin Invest. 2011;121(12):4787–4795. doi: 10.1172/JCI59150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sonnenberg GF, Fouser LA, Artis D. Border patrol: regulation of immunity, inflammation and tissue homeostasis at barrier surfaces by IL-22. Nat Immunol. 2011;12(5):383–390. doi: 10.1038/ni.2025. [DOI] [PubMed] [Google Scholar]
- 74.Johansson ME, Phillipson M, Petersson J, Velcich A, Holm L, Hansson GC. The inner of the two Muc2 mucin-dependent mucus layers in colon is devoid of bacteria. Proc Natl Acad Sci U S A. 2008;105(39):15064–15069. doi: 10.1073/pnas.0803124105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Vaishnava S, Yamamoto M, Severson KM, Ruhn KA, Yu X, Koren O, et al. The antibacterial lectin RegIIIgamma promotes the spatial segregation of microbiota and host in the intestine. Science. 2011;334(6053):255–258. doi: 10.1126/science.1209791. [DOI] [PMC free article] [PubMed] [Google Scholar]