Abstract
Genetic modification of shoot and root morphology has potential to improve water and nutrient uptake of wheat crops in rainfed environments. Near-isogenic lines (NILs) varying for a tillering inhibition (tin) gene and representing multiple genetic backgrounds were phenotyped in contrasting, controlled environments for shoot and root growth. Leaf area, shoot and root biomass were similar until tillering, whereupon reduced tillering in tin-containing NILs produced reductions of up to 60% in total leaf area and biomass, and increases in total root length of up to 120% and root biomass to 145%. Together, the root-to-shoot ratio increased two-fold with the tin gene. The influence of tin on shoot and root growth was greatest in the cv. Banks genetic background, particularly in the biculm-selected NIL, and was typically strongest in cooler environments. A separate de-tillering study confirmed greater root-to-shoot ratios with regular tiller removal in non-tin-containing genotypes. In validating these observations in a rainfed field study, the tin allele had a negligible effect on seedling growth but was associated with significantly (P<0.05) reduced tiller number (–37%), leaf area index (–26%), and spike number (–35%) to reduce plant biomass (–19%) at anthesis. Root biomass, root-to-shoot ratio at early stem elongation, and root depth at maturity were all increased in tin-containing NILs. Soil water use was slowed in tin-containing NILs, resulting in greater water availability, greater stomatal conductance, cooler canopy temperatures, and maintenance of green leaf area during grain-filling. Together these effects contributed to increases in harvest index and grain yield. In both the controlled and field environments, the tin gene was commonly associated with increased root length and biomass, but the significant influence of genetic background and environment suggests careful assessment of tin-containing progeny in selection for genotypic increases in root growth.
Key words: Drought, phenotyping, root distribution, root length, root weight, tin, water use, wheat.
Introduction
Wheat crops are commonly grown in rainfed environments where variable rainfall and the occurrence of terminal water deficits limit biomass and grain yield. Developing novel germplasm and/or management practices must take account of the dominant stress development patterns in the target environment (Chenu et al., 2013). In many regions of the world, growth of winter cereals is heavily reliant on stored soil water (Richards et al., 2002; Chenu et al., 2013). Increasing water deficits around early stem elongation can limit tiller production to slow canopy development (Duggan et al., 2005a; Mitchell et al., 2013) while drought immediately prior to and during anthesis can reduce floret fertility to reduce grain number (Saini and Aspinall, 1981). Water limitation during grain-filling reduces assimilate supply to developing grains, reducing final yield and quality (van Herwaarden et al., 1998; Mitchell et al., 2013). As a consequence, the timing and severity of water deficit influence the final impact on grain yield, with some plasticity in yield possible through compensating processes such as changes in tiller or ear growth (Sadras and Rebetzke, 2013).
Rapid leaf area development and early ground cover is a breeding target in rainfed environments where crop water use arises largely from in-crop rainfall (Richards et al., 2002). However, in seasons or environments where water is limiting late in the growing season, excessive early leaf growth can exhaust available soil water prematurely, leaving inadequate moisture to sustain grain growth after anthesis (Passioura, 1976, 1977; Richards and Townley-Smith, 1987). Tiller number is a major determinant of leaf area and canopy development, and, at conventional sowing rates, as many as 15 tillers may be initiated on each plant (Berry et al., 2003; Duggan et al., 2005a). However, up to 75% of these tillers senesce before anthesis even at lower plant densities (Stapper and Fischer, 1990; Berry et al., 2003; Mitchell et al., 2013; Moeller et al., 2014) or where growing conditions are unfavourable (Duggan et al., 2005a, b). The death of tillers represents a loss in water and nutrients that cannot be fully recovered by translocation of stored reserves in well-watered (Berry et al., 2003) and water-limited (Geiger and Fondy, 1980) environments. Therefore, control of excessive tillering may be useful in slowing water use in environments where water is limited and terminal droughts are predominant.
Manual de-tillering of free-tillering wheat genotypes has been shown to slow water use, leaving more water for carbon assimilation during grain-filling (Jones and Kirby, 1977; Islam and Sedgely, 1981). A major tiller inhibition (tin) gene can restrict tiller production to as few as one, but more commonly 2–4 tillers per plant (Richards, 1988; Duggan et al., 2005a; Moeller et al., 2014). Early studies comparing wheat lines with and without the tin gene reported little difference in leaf area index (LAI) owing to compensatory increases in individual leaf size (Yunusa and Sedgley, 1992; Duggan et al., 2005a). More recently, tin-containing near-isogenic lines (NILs) were identified that reduce leaf area and slow water use, thereby increasing grain yield relative to free-tillering NILs under controlled water deficit conditions (Mitchell et al., 2012, 2013).
Genetic modification of cereal root systems is developing as a potential breeding objective in wheat varietal development programmes (Lopes and Reynolds, 2010; Wasson et al., 2012, 2014). Evidence for genetic potential for root modification is well established in studies targeting improved water and nutrient uptake (e.g. Christopher et al., 2013; Wasson et al., 2014), while field studies have demonstrated that capture of as little as 10mm post-anthesis water from the subsoil can increase wheat grain yield by 0.6 t ha–1 under terminal drought (Kirkegaard et al., 2007). The tin gene reduces tiller number, but has also been reported to have pleiotropic effects on other plant characteristics including increases in leaf and stem size, and reductions in specific leaf area (SLA) (Richards, 1988; Duggan et al., 2005b). Duggan et al. (2005b) also provided some evidence for small increases in the root-to-shoot ratio in tin-containing lines, while Mitchell et al. (2013) reported increased water use at different soil depths for tin-containing NILs. Similarly, wheat lines selected for reduced spike number in the UK had significantly increased water extraction compared with high spike number selections, especially below soil depths of 90cm (Innes et al., 1981). Thus it appears possible that genotypes containing the tin gene may simultaneously reduce excessive early above-ground biomass while maintaining or increasing root growth at depth. Characteristics that slow or defer water use without compromising yield potential, while increasing the capacity to access deeper water and soil nutrients during grain filing, are likely to improve wheat performance in storage-driven, rainfed environments.
The aims of this study were to confirm earlier reports of the association of the tin gene with an increased root-to-shoot ratio (Duggan et al., 2005b), and establish whether the resulting root and/or shoot changes contributed to improved agronomic performance under rainfed conditions in the field. Regular assessments were made of tin NILs (biculm and oligoculm tin, and free-tillering, non-tin) until mid-booting across a range of air temperatures in controlled glasshouse and outdoor conditions using multiple genetic backgrounds. The contrasting air and soil temperatures were selected to explore the potential impact of genotype×environment interaction and thereby assess the robustness of genic variation for root, shoot, and root-to-shoot partitioning in tin NILs. Responses were then assessed through validation of the same NILs in a rainfed field environment targeting greater soil water availability during the grain-filling stage.
Materials and methods
Reduced-tillering NILs
The influence of the tin reduced-tillering allele was investigated in BC3-derived NILs generated in genetically unrelated wheat backgrounds, cvs Banks and Kite (ӨAB=0.09, i.e. the probability of alleles at a random locus in Banks and Kite being identical by descent was <10%). The Banks NILs comprised three sister lines, Banks+tin ‘biculm’ (hereafter ‘B++’ producing 1–2 tillers per plant), Banks+tin ‘oligoculm’ (hereafter ‘B+’, 3–4 tillers per plant), and Banks–tin ‘free-tillering’ (hereafter ‘B–‘ with 6–15 tillers per plant) (Moeller et al., 2014). The Kite NILs comprised the Kite+tin ‘oligoculm’ (hereafter ‘K+’, 2–3 tillers per plant) and Kite–tin ‘free-tillering’ (hereafter ‘K–’ with 6–15 tillers per plant). All reduced-tillering tin lines contained the tin gene from the reduced-tillering donor, Moroccan wheat landrace ‘492’ (Atsmon et al., 1986). In addition, a BC1F5-derived, tin and non-tin pair in the Silverstar genetic background was included in the first year (2003) of assessments. All lines were genotyped to confirm the presence of the tin allele (Spielmeyer and Richards, 2004). Separate studies confirm small differences in flowering between NILs of <3 d (Moeller et al., 2014).
Shoot and root growth under controlled-environment conditions
Studies were conducted in both 2003 and 2004 in cooled and heated glasshouses producing mean temperatures of 13 °C and 20 °C, respectively. An additional cool, outdoor environment (mean temperature of 7 ºC) was included in 2004 to extend the potential range of sampled conditions. All seed were obtained from mother plants grown in the same glasshouse environment to avoid confounding genetic and seed source (maternal environment) effects. Seed were then sized between 40mg and 45mg, and 10 seed were randomly sampled from each line for embryo sizing using a stereomicroscope with a graduated ocular lens.
Seed used for each controlled-environment experiment were sampled from the same growing (glasshouse) environment and sized for consistency to within a range of 45–50mg. The resulting range in average kernel size was commonly small, averaging 3mg within each NIL set (data not shown). Embryo sizes, estimated on these graded seed as embryo (surface) area, were 4.96 (B++), 4.97 (B+), 5.00 (B–), 5.13 (K+), and 5.16 (K–) mm2. Differences in embryo area (and length and width; data not shown) were not statistically significant (P>0.05) within or between the Banks and Kite genetic backgrounds.
In all experiments, a single wheat plant was grown in 0.5 m long, 90mm diameter tubes for the first three harvests, and 1.5 m long, 90mm diameter PVC tubes for later harvests. A soil mix comprising 75% river sand and 25% potting compost was used in all studies. Adequate nutrients and water were provided to sustain wheat growth, and plants were regularly checked for pests. Genotypes were sown into a randomized complete block design containing three (2003) or four (2004) replicates for each harvest. Tubes were positioned on a regular 20×20cm grid and rotated weekly within each block. In 2003, up to seven samples were collected at regular intervals from 130 °Cd to 923 °Cd (mid-booting) whereas only three samples were collected in 2004 between 232 °Cd and 543 °Cd (early stem elongation). The Silverstar NILs were sampled on only two occasions, at weeks 3 and 7 after sowing.
At each harvest, shoots and roots were separated, and roots then washed from the soil on a 0.5mm mesh. Compost and/or sand were then carefully removed with forceps by close examination of the samples. Measurements were made of the numbers and lengths of seminal and branch roots on each plant using a WinRhizo® root scanner following their separation using a stereo-binocular microscope. Roots were then dried at 70 °C in an air-forced oven for 72h before weighing. Measurements were also made on shoots of the numbers of leaves and tillers (primary, secondary, and coleoptile), and mainstem and tiller leaf areas using a Delta-T® planimeter before drying and weighing as for roots. The root-to-shoot ratio was calculated as root dry weight÷shoot dry weight.
De-tillering effects on root growth
The free- and reduced-tillering Banks (B–, B+) and Kite (K–, K+) NILs, and the free-tillering variety Seri were seed-sized to a range of 40–45mg. Three seed were sown into 1.5 m long tubes containing the soil mix described above before moving into a brightly lit glasshouse set at temperatures of 15/10 °C (day/night). Tubes were positioned and rotated weekly as described above. Seedlings were thinned to one plant per tube. A random subset of the free-tillering lines were pruned weekly of all tillers (‘detillered’) while the remainder were left unpruned and their tillers allowed to grow. Watering was undertaken every 1–2 d, and nutrients were supplied during growth. Pruning was continued until the first mainstem for a given background reached anthesis, whereupon all tubes for this background were washed free of soil, and roots and shoots were separated and weighed. All line×pruning treatments were replicated four times.
Shoot and root validation in the field
The Banks and Kite NILs were sown into 10 row (rows spaced 0.18 m apart), 4 m long plots at Bethungra in southern NSW (34.76 °S, 147.85 °E, 297 m altitude). Bethungra is located in the high rainfall wheat zone with average annual rainfall of 530mm. The soil was a Red Kandosol comprising a silty loam for the upper 0.15–0.2 m grading to a pale-coloured medium clay at depth previously described in Kirkegaard et al. (2007). Land for this experiment had been an alfalfa (Medicago sativa L.) pasture for four years, and was sprayed with the herbicide glyphosate 10 months prior to the experiment, with cultivation (to 100mm depth) undertaken prior to sowing. The experiment was a randomized complete block design with four replicates, and was sown on 8 June 2004 at a target sowing density of 200 seed m–2 and sowing depth of 30mm. Two plots of each entry were also sown at a reduced density of 120 seed m–2. Nutrients were supplied at sowing as Starter 15® applied at 103kg ha–1, but no further N was required due to the previous alfalfa crop. The experiment received natural rainfall, and plots were maintained free of weeds and diseases with the application of appropriate herbicide and fungicide control measures.
Shoots and roots were sampled at 15, 29, 44, 58, and 72 days after sowing (DAS), corresponding to emergence and approximately 1.6, 2.9, 4.2, and 5.2 leaves on the mainstem, respectively. For the first sample, ~10 plants were dug from each plot by hammering and sampling from a metal-sided box 200×180mm in size to a depth of 200mm, and the roots and shoots separated. For samples at 29 and 44 DAS, polyvinyl chloride (PVC) columns 200mm long and 75mm in diameter were pushed into the soil in the row above plants shortly after emergence. Each sample contained one or two plants. For the 58 and 72 DAS sampling, shoots were sampled by cutting plants at ground level from two parallel, 0.5 m long sections of a row. Roots were sampled from the same areas by hammering and sampling with the metal-sided box 200×180mm in size for the upper 200mm, and then immediately below using a 90mm metal tube to a depth of 800mm. Root and shoot measurements were as for the outdoor and phytotron studies above but were expressed on a unit area basis. Deep soil cores (1.8 m long and 42mm ID) were removed from plots (four per plot) using a tractor-mounted hydraulic coring rig at maturity to determine rooting depth for the Banks-derived NIL plots only.
Soil water use was monitored regularly for each plot from sowing using neutron moisture readings with a local calibration (Kirkegaard et al., 2007). Plant development was recorded after Zadoks et al. (1974), and at anthesis a 0.18 m2 area was sampled in each plot for biomass (both plant densities). Estimates of canopy temperature (after Rebetzke et al., 2013b) and leaf diffusion resistance (later converted to leaf conductance after Rebetzke et al., 2003) were obtained for each plot mid-way through grain-filling. Numbers of green leaves per plant were recorded for five plants in each plot from mid grain-fill onward. At maturity, between 80 and 120 culms were hand-cut at ground level using a 40cm wide quadrat oriented across three-bordered rows. Samples were air-dried at 35 °C for 3 d and weighed before and after threshing, the numbers of fertile spikes counted, and the harvest index calculated as the ratio of grain to total above-ground biomass. Plots were end-trimmed to ~3.6 m length and the outside border rows removed before machine harvesting. Kernel weight was measured for a sample of 200 grains from each harvest index sample, and grain number (m–2) was calculated from kernel weight and plot yields. Nitrogen content at anthesis and maturity was determined from N concentrations at anthesis, and in mature grains using near-infrared spectroscopy (after Rebetzke et al., 2008a ).
Statistical analysis
Each environment was analysed separately, with the best spatial models being determined after first fitting the experimental design and then modelling the residual variation with autoregressive row and column terms. Significant spatial effects were then identified and plot residuals assessed for the need for fitting of other (e.g. linear) effects (Cullis et al., 1996). Specific contrasts reflecting the separate hypothesis of effect of presence or absence of tin, effect of tiller expression within tin (Banks NILs only), and the interaction of tin with genetic background were tested for statistical significance using mixed linear models in the SAS procedure MIXED (SAS, 2013). A second set of analyses were undertaken across environments and sample dates to assess the association for seminal and branch root lengths with total root length and biomass. Variance and covariance components were estimated by the method of restricted maximum likelihood using mixed linear models in the SAS procedure MIXED. Genotypic correlations and their approximate standard errors were then estimated after Holland (2006). Statistical significance under the null hypotheses was reported at a P-value (α) of 0.05 unless otherwise indicated.
Results
Controlled-environment studies
Tiller and leaf area development
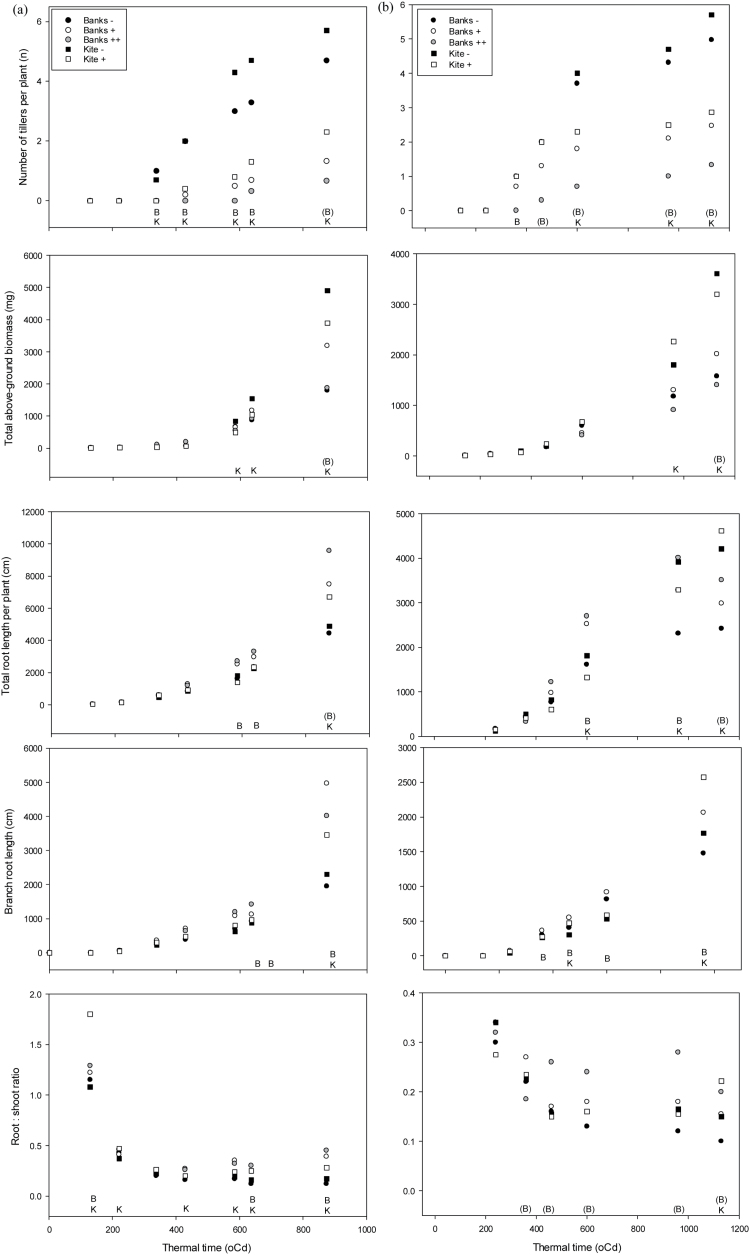
Tillers first appeared in both tin and non-tin NILs in Banks and Kite backgrounds at the 3.5-leaf stage (~350 °Cd) in cool and warm environments in both years (e.g. 2003 in Fig. 1; for 2004, see Supplementary Fig. S1 at JXB online). At final harvest, up to 10 tillers comprising mainstem, secondary, and occasionally tertiary tillers were observed on individual plants in both Banks and Kite non-tin NILs (data not shown). Coleoptile tillers were also commonly observed on plants, and particularly for the Kite genetic background where their frequency was >50% (data not shown). At final harvest, average numbers of tillers per plant for the non-tin, B–NIL was 4.6 and 3.9 for cool and warm 2003 environments (Fig. 1), and 4.1 and 3.2 for cool and warm 2004 environments, respectively (Supplementary Fig. S1). The influence of the tin gene on tiller reduction at final harvest was greatest for the Banks background and particularly for the biculm B++ (1–2 tillers per plant) and to a lesser extent oligoculm B+ (2–3 tillers per plant) NILs. Together, the reduction in tiller number was ~70% and 60% for B++ in cool and warm environments, and 40 and 25% for B+ in cool and warm environments, respectively. Differences in tiller number and size were small and not statistically significant between the tin-containing B++ and B+ NILs (e.g. Fig. 1 and Table 1). The numbers of tillers in the Kite background were commonly fewer for the oligoculm K+ (Fig. 1; Supplementary Fig. S1). In general, statistically significant differences in tiller number between Kite tin NILs were delayed until later harvests in warm environments. The influence of tin on tillering in the Silverstar genetic background was consistent with tiller reduction observed in the Banks NILs (Table 1).
Fig. 1.
Shoot and root growth for Banks (circles) and Kite (squares) tillering NILs (open symbol=+tin allele, shaded = biculm) assessed over multiple sampling dates in 2003 in (a) cool and (b) warm glasshouses. ‘B’ and ‘K’ denote statistical difference between the Banks and Kite tin and non-tin NILs, respectively, and ‘(B)’ indicates biculm and oligoculm tin-containing Banks NILs are statistically different at P=0.05.
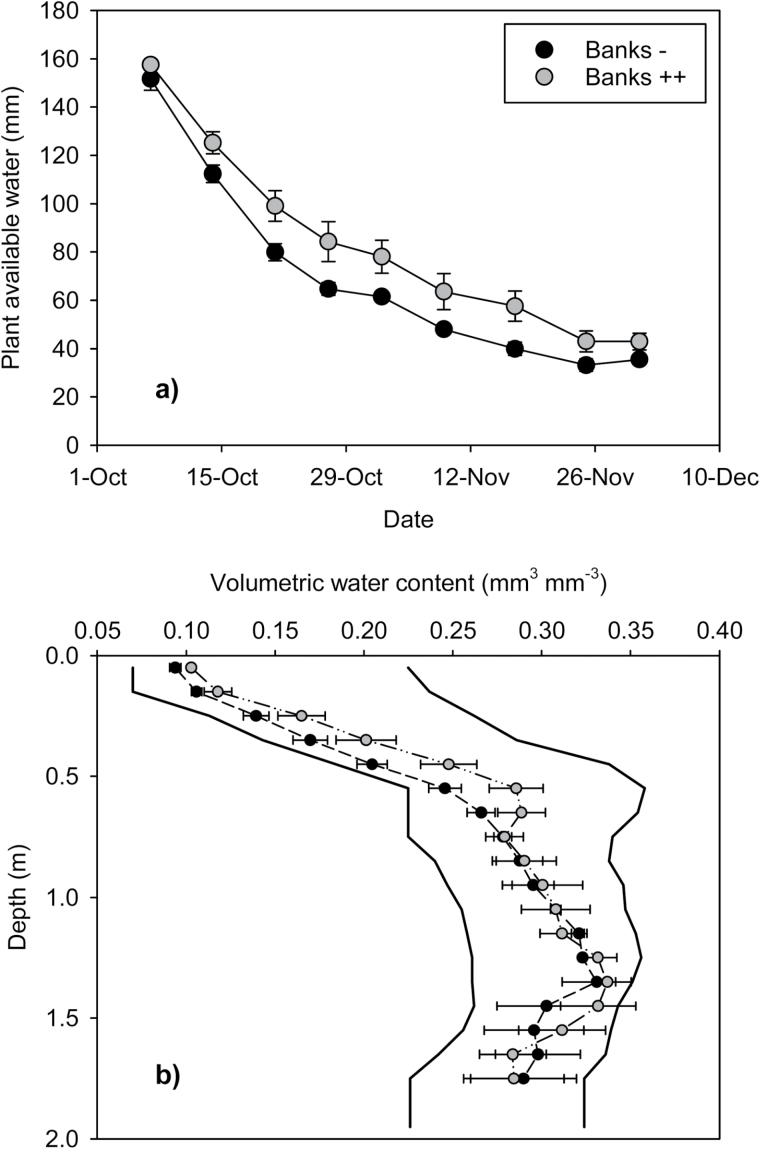
Fig. 4.
(a) Change in plant-available water in the soil (0–1.4 m) for two wheat Banks NILs (Banks– and Banks++) from the wettest profile around anthesis (7 October) until maturity (2 December) showing slower water use and higher soil water levels under Banks++, and (b) the profile of soil water on 21 October (point of maximum difference) for Banks– and Banks++. The solid lines in (b) show the drained upper limit and lower limit of water extraction, and the horizontal bars in both graphs are the SEM.
Table 1.
Selected shoot and root parameter means for Banks and Kite tiller inhibition NILs measured in cool and warm environments in 2003 and 2004
All environments were sampled at between 380 °Cd and 450 °Cd. Data are also given for Silverstar NILs sampled later at week 7 (585 °Cd and 570 °Cd for cool and warm environments, respectively).
| Environment | Entry | No. of leaves | Tillers per plant (n) | Tiller leaf area (cm2) | Tiller biomass per plant (mg) | Mainstem leaf area (cm2) | Total leaf area (cm2) | Specific leaf area (cm2 g–1) | Total shoot weight (mg) | No. of seminals per plant (n) |
|---|---|---|---|---|---|---|---|---|---|---|
| 2003 cool | Banks– | 4.2 | 2.0 | 2.1 | 69 | 46 | 48 | 439 | 217 | 4.7 |
| Banks+ | 4.3 | 0.3* | 0.1** | 2** | 48 | 48 | 391* | 191* | 5.0 | |
| Banks++ | 4.4 | 0.0* | 0.0** | 0** | 45 | 45 | 354** | 192* | 5.0 | |
| Kite– | 4.2 | 2.0 | 1.3 | 48 | 48 | 49 | 421 | 213 | 3.7 | |
| Kite+ | 4.2 | 0.4* | 0.3** | 13** | 61** | 61* | 409 | 228 | 3.3 | |
| Silverstar– | 5.9 | 5.3 | 115 | 588 | 127 | 242 | 356 | 1310 | 4.1 | |
| Silverstar+ | 6.2 | 1.7** | 32** | 118** | 124 | 156** | 310** | 768** | 4.9 | |
| 2003 warm | Banks– | 4.1 | 2.0 | 2.3 | 48 | 43 | 45 | 469 | 174 | 5.0 |
| Banks+ | 4.3 | 1.0* | 1.2* | 28* | 44 | 45 | 432* | 171 | 4.3* | |
| Banks++ | 4.3 | 0.0* | 0.0** | 0** | 49* | 49 | 362** | 175 | 5.0 | |
| Kite– | 4.6 | 2.0 | 2.9 | 67 | 53 | 56 | 431 | 239 | 4.3 | |
| Kite+ | 4.3 | 1.8 | 2.3* | 54 | 46* | 48* | 429 | 195** | 4.7 | |
| Silverstar– | 5.8 | 6.7 | 77 | 420 | 93 | 170 | 384 | 778 | 4.7 | |
| Silverstar+ | 6.0 | 2.7** | 84 | 483* | 114* | 198* | 332* | 1005** | 5.6 | |
| 2004 cool | Banks– | 4.1 | 2.0 | 0.5 | 73 | 33 | 34 | 374 | 200 | 5.3 |
| Banks+ | 4.4 | 1.0* | 0.2 | 71 | 37 | 37 | 328** | 237* | 5.3 | |
| Banks++ | 4.2 | 1.0* | 0.0* | 12** | 38 | 38 | 264** | 192 | 4.7* | |
| Kite– | 4.3 | 2.3 | 2.8 | 116 | 41 | 44* | 334 | 254 | 5.3 | |
| Kite+ | 4.2 | 2.0 | 1.9* | 81* | 49* | 51 | 293* | 325** | 4.8* | |
| 2004 warm | Banks– | 3.7 | 1.7 | 0.9 | 28 | 42 | 43 | 491 | 129 | 4.3 |
| Banks+ | 3.9 | 0.5* | 0.2* | 6 | 44 | 44 | 461** | 137 | 4.3 | |
| Banks++ | 3.8 | 0.0* | 0.1** | 4 | 40 | 40* | 412** | 141 | 4.5 | |
| Kite– | 3.8 | 1.8 | 0.7 | 24 | 50 | 51 | 435 | 159 | 4.3 | |
| Kite+ | 3.7 | 0.8* | 0.4 | 14 | 47 | 47* | 449 | 143* | 4.0 |
* and ** denote that restricted-tillering, tin NIL means are statistically different from non-tin, free-tillering sister NIL means at P=0.05 and 0.01, respectively. –, +, ++ refer to free tillering, oligoculm and biculm NILs, respectively.
The tin and non-tin NILs were not statistically different for leaf area or shoot biomass until soon after commencement of tillering (Fig. 1; Supplementary Fig. S1 at JXB online). Shoot morphological differences were subsequently assessed for NILs sampled for all genetic backgrounds at the same approximate developmental stage in all environments (~380–460 °Cd) (Table 1). At this stage, the number of mainstem leaves was similar for NILs and genetic backgrounds. Total leaf areas were commonly greater for the non-tin NILs, reflecting their greater tillering and tiller leaf areas. Among different tin NILs, the biculm B++ tended to produce smaller total leaf areas, reflecting production of fewer tillers and smaller tiller leaf area (Table 1). The SLAs were generally smaller for tin-containing NILs and particularly for the extreme reduced-tillering B++. Numbers of mainstem leaves, leaf areas, and SLA were maintained with later sampling, and were consistent across genetic backgrounds including the Silverstar background (Table 1). Consistent with leaf areas, seedling dry weights were similar for tin and non-tin NILs until early tillering, where reductions in tiller number and leaf area reduced total plant dry weights for tin-containing Banks, Silverstar, and sometimes Kite NILs (Fig. 1). In the Banks background, the B++ NIL was commonly smaller than B+ for total plant dry weight. Over all sampling dates in all controlled-environment experiments, genotypic increases in tiller number were associated with significant increases in total biomass (r g=0.52, P <0.01) and leaf areas (r g=0.62, P<0.01), and reductions in SLA (r g= –0.71, P<0.01). Biomass and leaf area were themselves strongly correlated (r g=0.87, P<0.01). Yet despite these relationships, tin-containing wheats tended to produce larger, heavier main stems containing large, thicker leaves to maintain reasonably large total plant dry weights (e.g. Table 1).
Root growth
Genotypic differences in total root length were typically small in early harvests, with differentiation between NILs becoming more evident at, or soon after, the first appearance of tillers (Fig. 1; Supplementary Fig. S1 at JXB online). At this time, the tin-containing NILs produced significantly greater root length in both Banks and Kite genetic backgrounds, and this difference was consistent in both cool and warm environments. The magnitude of difference in tin NILs appeared to increase by final harvest, particularly in 2003 (Fig. 1). The increase in total root length in the tin NILs reflected increasingly greater root branching (Fig. 1; Supplementary Fig. S1) and commonly increased seminal root length, while seminal root numbers were similar among NILs (Table 1). The biculm B++ tended to have greater total root length and to a lesser extent branch root length than oligoculm B+ in the warm environment (Fig. 1; Supplementary Fig. S1), while both Banks tin-containing NILs were similar in the cool, outdoor environment. Nodal root length differences were small until the final harvest, and were typically greater for the free-tillering NILs in all environments (data not shown). Root biomass increased for the tin-containing NILs and, together with the small reduction in shoot biomass, contributed to significantly greater root-to-shoot ratios in tin-containing NILs (Fig. 1; Table 1; Supplementary Fig. S1). Interestingly, the greater root-to-shoot ratios of the reduced-tillering lines was observed from very early stages of sampling (two leaves onward) and was maintained across the different environments.
Despite the fewer sampling dates for the Silverstar tin NILs, significant differences in mean root lengths of 2825cm and 2469cm, and root-to-shoot ratios of 0.29 and 0.19 were observed for Silverstar tin and non-tin NILs, respectively, at the 7 weeks sampling date in the cooler soil temperatures. This difference was maintained at warmer temperatures, with root lengths of 2020cm and 1634cm, and root-to-shoot ratios of 0.25 and 0.21, for tin and non-tin NILs, respectively.
Across sampling dates and NILs in all experiments, genotypic increases in total root biomass were closely associated with increases in total root length (r g=0.99, P<0.01) and root branching (r g=0.99, P<0.01), while total root length and branching were themselves strongly correlated (r g=0.98, P<0.01). Genotypic differences in total seminal root length were strongly correlated with total root weight (r g=0.92, P<0.01) and branch root length (r g=0.78, P<0.01) but only moderately correlated with total root length (r g=0.64, P<0.01).
De-tillering study
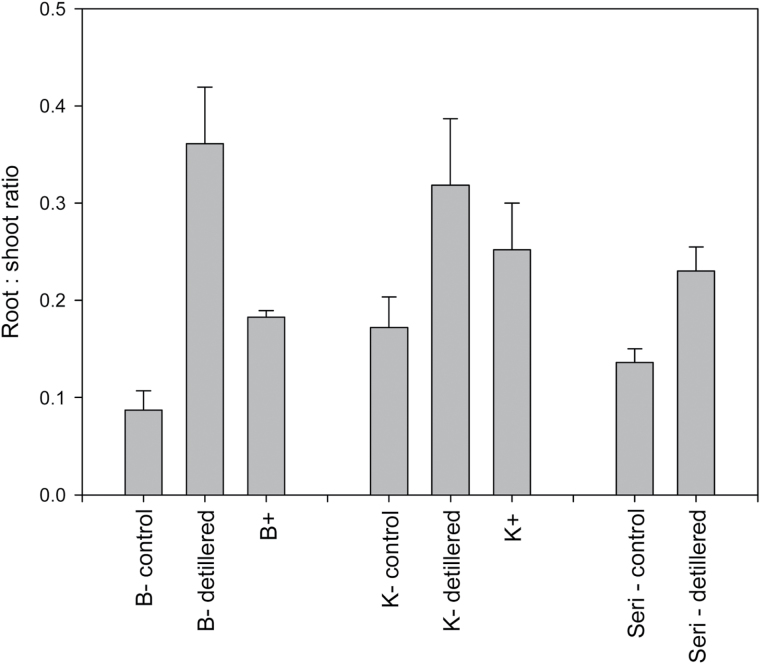
The free-tillering, tin, and de-tillered non-tin treatments averaged 4.7, 1.2, and 1.0 spikes per plant at anthesis, respectively. The continued removal of tillers was associated with a highly significant (P<0.01) reduction in numbers of spikes (–79%) and total shoot dry matter (–59%), greater individual stem weight (+36%), and a small but significant increase in root biomass (+35%) and increased root-to-shoot ratio (+232%) in de-tillered plants (Fig. 2). Similarly, increases in the root-to-shoot ratio were significantly greater for tin-containing Banks and Kite NILs, consistent with glasshouse and field experiments (compare Figs 1–3; Table 1; Supplementary Fig. S1 at JXB online). Compared with the influence of the tin gene in Banks and Kite, manual de-tillering was associated with significantly smaller shoot and root biomass, but greater root-to-shoot partitioning. Among backgrounds, smaller root-to-shoot partitioning in the Banks background was consistent with earlier tin NIL assessments. Across all genotype and de-tillering treatment means (n=8), increases in tiller number were negatively correlated (r p= –0.93, P<0.01) with reductions in the root-to-shoot ratio.
Fig. 2.
Root-to-shoot ratio at anthesis for three sets of lines including the free-tillering control, a detillering (‘detillered’) treatment and/or a near-isogenic (NIL) pair (B = Banks, K = Kite) containing the tin (‘+’) reduced-tillering gene and its non-tin (‘–‘) sister NIL. Standard error bars are given.
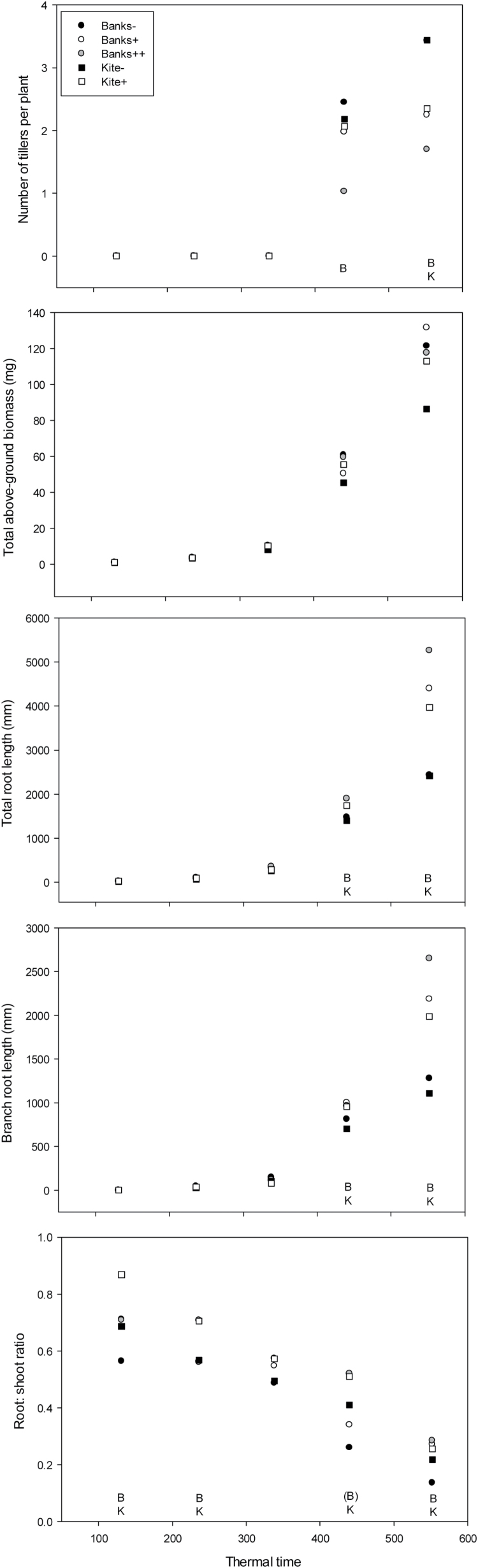
Fig. 3.
Shoot and root growth for Banks (circlels) and Kite (squares) tillering NILs (open symbol=+tin allele except Banks where the shaded circle is biculm expression) assessed over multiple sampling dates in the field in 2004. ‘B’ and ‘K’ denote statistical difference between the Banks and Kite tin and non-tin NILs, respectively, at P=0.05.
Field validation
The Bethungra field season was favourable for growth with good January–April, pre-sowing rainfall (70mm) and good in-crop rainfall through to maturity (375mm), consistent with expectation at this site. The 10 month fallow period prior to sowing together with the in-season rainfall ensured the profile was wet to depth, facilitating potential for deep root penetration. Air temperatures were mild, with daily averages of 8.3 °C for the June–September period up to anthesis, and 15.5 °C for the October–December grain-filling period. Averaged across the free-tillering Banks and Kite genotypes, grain yield was high (6.1 t ha–1), reflecting large average grain numbers (20 519 m–2) but smaller average grain size (30mg) (Table 4).
Table 4.
Means for agronomic traits measured on Banks and Kite tillering NILs assessed during grain-filling and maturity in the field in 2004
| Entry | Plant height (cm) | Stomatal conductancea (n) | Canopy temperature depressiona (°C) | No. of spikes (n m–2) | Grain yield (t ha–1) | Total biomass (t ha–1) | Harvest index | No. of grains (n m–2) | Grains per ear (n) | Kernel weight (mg) | Grain protein concentration (%) | Root depth (cm) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Banks– | 100 | 0.13 | 0 | 608 | 5.91 | 14.5 | 0.41 | 22 692 | 38 | 26 | 11.8 | 117 |
| Banks+ | 97 | 0.29** | –0.41 | 328*** | 6.90** | 15.0 | 0.45** | 19 714* | 60*** | 35*** | 13.1** | 147** |
| Banks++ | 96 | 0.38*** | –1.40** | 237*** | 6.59** | 14.1 | 0.46*** | 18 333** | 79*** | 36*** | 14.5*** | 135* |
| Kite– | 102 | 0.14 | –b | 536 | 6.22 | 15.9 | 0.39 | 18 353 | 34 | 34 | 14.5 | –b |
| Kite+ | 103 | 0.16 | –b | 447* | 6.19 | 16.3 | 0.38 | 14 420** | 33 | 43*** | 14.6 | –b |
a 7–10 d post-flowering averaged at three times: 9:00, 12:00, and 15:00h.
b Not sampled.
*, **, and *** denote that restricted-tillering, tin NIL means are statistically different from non-tin, free-tillering sister NIL means at P=0.10, 0.05, and 0.01, respectively. –, +, ++ refer to free tillering, oligoculm and biculm NILs, respectively.
Tiller, spike and leaf area development
Plant counts made soon after seedling emergence indicated no differences in the number of plants m–2 between individual lines (data not shown). The first tillers appeared at ~3.3 leaves, but differences in tiller number per plant were not apparent until the fourth harvest at ~450 °Cd (i.e. fourth leaf stage) (Fig. 3; Table 2). The Banks tin lines produced significantly fewer tillers per plant than B–, and the biculm B++ NIL produced significantly fewer tillers than the oligoculm B+. Differences in tillering in the Kite background were less pronounced owing to increased production of coleoptile tillers in the K+ plants (data not shown). By the fifth harvest (72 DAS, average leaf stage 5.2), both Banks and Kite tin lines produced significantly fewer tillers than non-tin sister lines (–43% and 37% for Banks and Kite, respectively, at 560 °Cd; Fig. 3.). Reductions in tillering were associated with significantly smaller shoot biomass for the B++ but not B+ NIL, and no difference in the biomass of the Kite NILs (Table 2).
Table 2.
Shoot and root characteristics for Banks and Kite tillering NILs at 58 DAS for field-assessed plants in 2004
| Entry | Shoot biomass (mg) | No. of tillers per plant (n) | Total root biomass (mg) | Total root length (mm) | Branch root length (mm) | Seminal root length (mm) | No. of seminal roots (n) | No. of nodal roots (n) | Root: shoot ratio | Root proportiona |
|---|---|---|---|---|---|---|---|---|---|---|
| Banks– | 60.6 | 2.5 | 15.6 | 1477 | 838 | 637 | 4.32 | 4.22 | 0.26 | 0.20 |
| Banks+ | 69.6 | 1.9* | 24.0** | 2053** | 1061** | 991** | 4.89** | 5.14** | 0.34* | 0.26* |
| Banks++ | 45.1** | 1.0** | 23.3** | 1903** | 965* | 939** | 4.83** | 5.09** | 0.52** | 0.34** |
| Kite– | 43.4 | 2.2 | 17.9 | 1353 | 681 | 673 | 4.65 | 4.00 | 0.41 | 0.29 |
| Kite+ | 56.0 | 2.0 | 28.5** | 2007** | 1079* | 929* | 5.11** | 4.44** | 0.51* | 0.34 |
a Proportion of total plant biomass that is root biomass
* and ** denote that restricted-tillering, tin NIL means are statistically different from non-tin, free-tillering sister NIL means at P=0.05 and 0.01, respectively. –, +, ++ refer to free tillering, oligoculm and biculm NILs, respectively.
Early leaf area and plant biomass were the same for all lines (Fig. 3). However, reduced tillering in the Banks tin NILs contributed to significant reductions in anthesis LAI (–40%), light interception (–12%), and biomass (–22%) (Table 3). This reduction was particularly large for B++ (e.g. 49% for LAI), whereas the reduced tillering in B+ contributed to significantly reduced LAI and light interception but not biomass. The Kite NILs were not statistically different for LAI and light interception at anthesis, but the tin-containing K+ NIL was reduced for biomass. At low sowing densities, reduced-tillering Banks and Kite lines produced significantly less anthesis biomass than their free-tillering sister NILs (Table 3). This reduction was relatively greater than for anthesis biomass at normal sowing density, with reductions in biomass of all tin-containing NILs of 19% and 33% at normal and low plant density treatments, respectively (Table 3). Increased light interception across all genotypes was significantly correlated with changes in anthesis biomass at normal (r p=0.95, P<0.05) and low (r p=0.95, P<0.05) plant densities, while anthesis biomass at low and normal plant densities was itself strongly correlated across genotypes (r p=0.96, P<0.05). Stem nitrogen concentration was greater for B++ but not B+, while leaf N concentration was greater for tin NILs in the Banks but not the Kite genetic backgrounds.
Table 3.
Leaf area index, light interception, biomass (including low density), and stem and leaf nitrogen concentration at anthesis for Banks and Kite tillering NILs assessed in the field in 2004
| Entry | Leaf area index (m2 m–2) | Light interception (%) | Anthesis biomass (normal density) (g m–2) | Anthesis biomass (low density) (g m–2) | Stem nitrogen concentration (%) | Leaf nitrogen concentration (%) |
|---|---|---|---|---|---|---|
| Banks– | 4.22 | 92 | 888 | 979 | 0.65 | 2.70 |
| Banks+ | 2.92** | 85** | 809* | 649** | 0.66 | 3.08** |
| Banks++ | 2.11*** | 77*** | 576*** | 449*** | 0.81*** | 2.99* |
| Kite– | 5.94 | 94 | 1095 | 1129 | 0.68 | 2.59 |
| Kite+ | 5.49 | 93 | 919* | 869** | 0.59 | 2.65 |
*, **, and *** denote that restricted-tillering, tin NIL means are statistically different from non-tin, free-tillering sister NIL means at P=0.10, 0.05, and 0.01, respectively. –, +, ++ refer to free tillering, oligoculm and biculm NILs, respectively.
Root growth
Differences in root growth in the field mirrored observations for these same lines under controlled-environment conditions. Total root length was the same for all lines until early tillering, after which the tin NILs produced significantly greater total root length (Fig. 3) and biomass (Table 2). Greater total root length reflected significant differences in seminal root length and extent of root branching between tin and non-tin NILs, and were maintained from tillering to final harvest at 72 DAS. By the last harvest, the tin-containing NILs produced approximately double the branched and total root length of the non-tin NILs. During the early stages of plant growth, tin-containing NILs produced significantly greater numbers of seminal roots but there was no difference in seminal root number between the B+ and B++ NILs (Table 2). Seminal root length varied significantly between tin and non-tin NILs, with the longest B–, B+, and B++ NILs averaging seminal root lengths of 146, 202, and 195cm, respectively. Nodal roots were recorded and were significantly greater in number for tin lines (Table 2). Only the Banks NILs were cored and measured for root depth at maturity. Here the tin-containing B+ and B++ NILs were significantly deeper at average depths of 147cm and 135cm, respectively, compared with 117cm for the non-tin B–NIL (Table 4). For all sample dates, the root-to-shoot ratio was significantly greater in the tin-containing NILs, and, as for the controlled-environment studies, the root-to-shoot ratio decreased progressively from sowing (Fig. 3).
Agronomic performance and water use
Differences in total tiller number increased with time, so that by maturity the B++ and B+ NILs produced 61% and 41% fewer spikes, respectively, than B– (Table 2). The difference in the Kite background was smaller, with a reduction of ~20% spikes for K+. Grain yields were not different between genetic backgrounds, averaging 6.47 t ha–1 and 6.20 t ha–1 for Banks and Kite, respectively (Table 4). Within backgrounds, the tin-containing Banks NILs produced significantly greater yield, whereas the Kite NILs were not different. The greater yield for tin in the Banks background was associated with a significant increase in harvest index, whereas total biomass was not different. Harvest index and biomass were the same for B++ and B+ NILs. Numbers of spikes and grains per m2 was commonly fewer, but numbers of grains per spike were greater for tin-containing Banks NILs. Reductions in numbers of grains were compensated by significant increases in average kernel weight. The Kite tin NILs produced significantly fewer spikes and fewer kernels of larger average size, whereas grains per spike were the same for both Kite NILs (Table 4). The B+ and B++ NILs were significantly greater for grain protein concentration and content than B–, whereas K– and K+ NILs were no different. The B++ NIL was significantly greater for grain protein concentration and content than B+.
The tin-containing NILs had significantly greater stomatal conductance early in grain-filling (7 d post-anthesis), but only the Banks tin NILs maintained high conductance through to physiological maturity (Supplementary Fig. S2 at JXB online). The greater conductance and thereby transpiration of B+ and B++ translated into significantly cooler and subsequently greater canopy temperature depression (CTD) for these NILs (Table 4). The tin-containing Banks NILs maintained significantly greater green leaf number during grain filling, particularly B++ with an average one green leaf per stem at physiological maturity (Supplementary Fig. S3). Despite the over-riding influence of tin in the Banks NILs, stomatal conductance, CTD, and green leaf number through grain filling were consistently greater for the biculm B++ than the oligoculm B+. Separate from improved root growth, water use was slowed by the presence of tin, and this was especially obvious during the post-anthesis drying period when water use patterns were less obscured by the frequent rainfall (Fig. 4a). From early October when the profiles in both B– and B++ had been filled, and total plant available water (PAW) was similar, water use was slower in B++ which preserved higher levels of PAW than B– throughout most of the grain-filling period (Fig. 4a). The increased soil water was present in the upper soil layers, with little significant difference below 1 m at least during mid-grain-fill (Fig. 4b). By maturity, most of the PAW had been used in both B– and B++ (Fig. 4a), but the deferred water use and higher soil water levels in B++ presumably served to maintain higher conductance and green leaf area during grain-fill.
Discussion
The Passioura identity (1977) defines grain yield in water-limited environments as integrating three physiological components: total water use and water use efficiency in building crop biomass, and then harvest index in final determination of yield. In wheat, robust and repeatable genetic variation has been established for traits contributing to greater water use efficiency (e.g. Richards et al., 2002) and harvest index (Duggan et al., 2005a; Rebetzke et al., 2008a ), while reduced heritability has limited the reliable identification of genetic variation for factors such as root architecture and size affecting capacity for greater water use. The potential to modify root morphology and growth (e.g. changes in root angle, diameter, and branching, root number, and root depth; e.g. Christopher et al., 2013) requires confirmation in the field (e.g. Wasson et al., 2012), while separate studies are required to establish related changes in water uptake, particularly deeper in the soil profile (Lopes and Reynolds, 2010). Validation of complex phenotypes such as shoot and root growth requires translation to the field before uptake and use in breeding programmes (Rebetzke et al., 2014). The studies reported herein summarize a continuum of activities extending from glasshouse to field, targeting assessment of the effects of the tin gene on root and shoot growth, and their impacts on the components of the Passioura identity.
Root growth
The current study has confirmed earlier observations (Duggan et al., 2005b) of the influence of a major tiller inhibition gene on partitioning of carbon to roots to increase post-tillering root biomass through increased root branching and subsequently greater total root length. The tin gene was associated with a longer, more branched, and a heavier root system from early in plant development, and this effect was consistent in controlled glasshouse and field studies, and, to a lesser extent, across genetic backgrounds. The increased root growth translated into deeper roots in the field and was greatest in cooler soils. Numbers and sizes of seminal roots were generally not different between tin and non-tin lines, while differences in nodal root length and biomass were commonly small. Together, the tin gene appeared to increase the number of first- and second-order laterals on the seminal axes.
Root growth was generally similar for all lines following emergence and during early seedling growth. Changes in root size between lines coincided with differentiation in tillering. The extent of root growth was approximately the same for oligoculm and biculm Banks NILs, while even small reductions in tillering in the Kite tin NIL was associated with greater root growth. The main root components changing with tin were seminal root lengths and their branches. Seminal root number was the same for tin in the controlled environments but was greater for tin wheats in the field study. Branch roots are finer and reportedly more effective in absorbing water and nutrients (Mackey, 1986), while a greater frequency of longer seminal roots presumably allows for greater exploration of the soil, particularly deeper soil layers, if water is available later in the season (Kirkegaard et al., 2007; Wasson et al., 2012). Root biomass was increased relative to shoot biomass in tin-containing wheats. Root-to-shoot ratios were also increased across multiple genetic backgrounds following manual de-tillering, and the size of the increase was consistent with the influence of tin on the root-to-shoot ratio in the same studies.
The greater root-to-shoot ratio of tin lines might be explained by a change in carbohydrate allocation to roots. An increased root-to-shoot ratio was very apparent at commencement of tillering. From this it was hypothesized that carbohydrates normally allocated to developing tillers were instead allocated to roots. Both genetic and management-like factors influencing spike size and/or number have demonstrated changes in shoot carbon to affect the root-to-shoot ratio and root length directly. These studies have included simple ear removal from before or at anthesis in both wheat and barley, and the monitoring of carbohydrate allocation from ears to roots to increase root biomass (e.g. Austin and Edrich, 1975; Koide and Ishihari, 1992). Other ear removal studies aimed at removal of competing sinks confirm a ubiquitous partitioning of carbon to other organs including roots (e.g. King et al., 1967; Gu and Marshall, 1988; Sharma-Natu and Ghildiyal, 1994). Early tiller removal or defoliation resulted in increased allocation of carbon to retained shoots to increase stem and leaf sheath thickness, increase leaf area, and reduce SLA (Gu and Marshall, 1988). These phenotypes are consistent with the tin phenotype observed for the Banks, Kite, and Silverstar backgrounds reported herein. In a separate genetics experiment, divergent selection into groups of related wheat lines contrasting for numbers of spikes at maturity was associated with increased water extraction below 90cm for the low spike number-selected group (Innes et al., 1981). It is unlikely that lines in that study varied for presence of the tin gene, but the observations confirm a resource allocation link between fewer spikes and the potential for more effective rooting at depth.
Shoot growth
Growing conditions early in the season are often conducive to the initiation and development of many tillers to produce large leaf areas and biomass (Berry et al., 2003; Duggan et al., 2005a; Rebetzke et al., 2008b ; Mitchell et al., 2013; Moeller et al., 2014). In the current study, shoot growth in seedlings was similar for tin and non-tin NILs, with reductions in leaf area and biomass not apparent in tin NILs until some time after commencement of tillering, and then more so in the extreme biculm tin types. Continued suppression of tillering resulted in generally reduced LAI and light interception, and reduced biomass at anthesis particularly at low plant densities where a lack of plasticity in the tin-containing NILs limited their ability to respond with more tillers and ultimately more spikes (Sadras and Rebetzke, 2013).
The influence of tin on tiller reduction was dependent on the genetic background and the thermal environment under assessment. Selection for fewer tillers in development of the B++ genotype was associated with reliably fewer tillers and final spike number, whereas the intermediate tillering B+ produced fewer tillers than the wild-type B– but was rarely as extreme in tiller reduction as its B++ biculm sibling. Similarly, with the tin gene in the Silverstar background, tillering was reliably less whereas tin expression in the Kite background was more variable in both cool and warm environments. Tiller expression in the cooler field experiment generally mirrored expression under cool controlled environment conditions. That is, differences in tin expression were maintained in the Banks backgrounds whereas tiller reduction was smaller in the Kite background, consistent with glasshouse assessments. Nonetheless, Kite tin NILs ultimately produced significantly fewer spikes in the field, consistent with the Banks B+ NIL. Selection for extreme reduction in tillering in B++ appears to have favoured a reliably extreme restricted tin tillering genotype, whereas selection for a more free-tillering tin genotype has produced NILs which are more like the non-tin, free-tillering NIL particularly early in the season. This was the case for both B+ and K+ NILs where differences in tiller and spike number were obvious from stem elongation onward, and particularly at anthesis.
Several studies have reported the influence of tillering on growth and yield of wheat in well-watered and water-limited environments alike. More recent studies have included assessment of the tin gene on reductions in tillering and final spike number in low-yielding environments (e.g. Duggan et al., 2005a; Mitchell et al., 2013; Moeller et al., 2014), and focusing on tiller development and correlated changes in tiller number, leaf area, and water use. Few studies have reported the influence of tin in favourable environments, although Gaju et al. (2009, 2014) and Mitchell et al. (2013) demonstrated small yield reductions for tin-containing recombinant inbred lines/double haploid lines under irrigation. The studies herein confirm other studies reporting the presence of tin reduced tillering in both controlled and a range of field environments in showing that fewer tillers translated into reductions in final spike number. Leaf area and anthesis biomass were also reduced. However, as reported here, the extent and timing of tiller inhibition with tin varied with genetic background, as did the magnitude of the impact on anthesis biomass and final spike number.
Previous studies have demonstrated the presence of the tin gene to be associated with changes in a number of other shoot characteristics (Duggan et al., 2005a). Along with tiller size, biomass, and leaf area, the SLA and stem size also increased. Changes in tillering mirrored changes in total leaf area and biomass, light interception, and anthesis biomass. The above-ground tin phenotypes observed in this study were consistent with the previous reports (e.g. Duggan et al., 2005a; Mitchell et al., 2012) and included reduced tiller and spike density; a higher proportion of productive tillers; thicker leaves and reduced SLA; a small reduction in above-ground biomass; increased numbers of kernels per spike; and larger average kernel size. In irrigated, high radiation environments aimed at very high yield potentials, Gaju et al. (2014) reported that the tin gene was associated with increased light interception from stem elongation to anthesis but reduced radiation use efficiency. In their study, high grain yields for tin-containing lines reflected increased carbon partitioning to spikes to increase grain number per spike and unit area despite significant reductions in spike number.
Links between modified root and shoot growth, water use, and grain yield in water-limited environments
The impact of any change in the root and shoot characteristics of plants on crop yield is ultimately determined by the specific pattern of water availability throughout the season, its impact on the timing and severity of water stress, and how these interact with the development and realization of season-specific yield potential. Our central hypothesis was that in storage-driven, agro-environments subject to increasing terminal stress, deferred water use from pre- to post-anthesis could be beneficial to yield. The tin gene offered opportunities to achieve this by simultaneously restricting pre-anthesis biomass growth (and hence water use) while increasing the capacity of the root systems to better exploit soil water during the post-anthesis phase through a deeper and more branched root system (e.g. Lopes and Reynolds, 2010). The extent to which links between modified root and shoot growth, water use, and grain yield can be demonstrated in field studies is confounded by the inherent variability in seasonal conditions, a fact that leads many to manage environments with irrigation and rain-out shelters (Mitchell et al., 2013; Rebetzke et al., 2013a) and/or supplement experimental studies with calibrated crop simulation models that capture seasonal variability. Lilley and Kirkegaard (2007) clearly showed how seasonal variability generated a wide range of expected benefit of deep soil water to wheat crops which averaged 37kg grain ha–1 mm–1, but ranged from 0 to 60kg ha–1 mm–1 according to seasonal rainfall distribution.
The seasonal conditions experienced in the field study reported herein did not feature the severe terminal stress commonly envisaged for storage-driven systems (e.g. Mitchell et al., 2013), but nonetheless evidence was provided that supported several aspects of the central hypothesis. The reduction in tillering and biomass growth up to anthesis was associated with conservation of soil water during the post-anthesis period (Fig. 4a, b). The reduction in tillering and biomass did not come at the expense of reduced yield potential, at least up to the high ~6.0 t ha–1 potential achieved at the site, because the tin-containing lines were able to compensate for reduced spike number by significantly increasing the numbers of grain per spike. As a result, yield potential (grain number) was not compromised, while water was preserved to assist the crop in filling the grains that had been set. The capacity to fill the grains was facilitated by two processes: first, the increased residual soil water available after anthesis which maintained green leaf area and photosynthesis (Supplementary Figs S2, S3 at JXB online), and the deeper and more branched root system increased the likelihood that photosynthesis could be maintained later into grain-filling to ensure larger grain; secondly, a feature of tin-containing lines is their higher levels of stem-soluble carbohydrates with capacity to remobilize these should current photosynthesis be restricted by drying soils (Rebetzke et al., 2008a ; Mitchell et al., 2013). Though much of this second process is expressed under more severe terminal stress than those experienced here (e.g. van Herwaarden et al., 1998; Rebetzke et al., 2008a ), previous experiments conducted under conditions of less water-limiting post-anthesis conditions similar to those experienced in this experiment identified tin lines that achieved relatively high grain yield and maintained a kernel number and weight advantage (e.g. Mitchell et al., 2012; Gaju et al., 2014). In fact, compensation for the decrease in spike density can result in similar or even higher grain yields for reduced-tillering tin lines (A.F. van Herwaarden, unpublished data). In some situations, even uniculm wheats have been reported to be higher yielding than free-tillering sister lines (Islam and Sedgley, 1981). Whan et al. (1988) also observed that reduced-tillering lines compensate for fewer numbers of spikes by increasing kernel number per ear and/or production of heavier kernels. Increased grain yield does not appear to be due to the tin gene itself but reflects the reduced-tillering response associated with this gene.
The soil water profiles at harvest were similar for the tin and non-tin lines so it appears that the deeper and more branched root system did not play a major role in the maintenance of green leaf area, and grain-filling in the tin lines. Rather the slower and deferred water use due to reduced shoot biomass prolonged green leaf duration and assimilation, and, together with the redistribution of soluble carbohydrates, facilitated grain-filling. We cannot be certain whether the increased grain nitrogen reflected increased N uptake with deeper, more extensive roots. Nevertheless, numerous experimental and simulation studies have demonstrated the benefits of a deeper and more effective root system in providing the potential to support higher yield through access to water late in the season (e.g. Lopes and Reynolds, 2010). Our study demonstrates that tin-containing lines can achieve deeper and more branched roots without the often assumed compromising of shoot growth and yield potential. Though not expressed under the seasonal conditions experienced here, the deeper and more extensively branched root system will inevitably benefit crop growth under conditions of more severe terminal stress than those experienced here (e.g. Mitchell et al., 2013).
It is well established that the tin gene has a major effect on tiller suppression and biomass accumulation but that tiller expression varies both between and within genetic backgrounds (Mitchell et al., 2013; Gaju et al., 2014). Herein, there was a 2-fold range in tiller number between B+ and B++ NILs in controlled and field studies. Similarly, the effect of tin on root growth and subsequent root-to-shoot ratios varied depending on background and somewhat independently of tiller number. Despite evidence for an association between tin and increased root growth, other factors appear to contribute to root growth (e.g. Bai et al., 2013; Liu et al., 2013), suggesting the need for careful assessment within tin-varying populations to target increased root growth.
Conclusions
The current study has demonstrated that the presence of the tin gene and its likely role in modifying tiller number has slowed and deferred water use, while maintaining yield potential (grain number), and contributed to changes in seminal and branch root length to increase total root length and biomass. The influence of tin in changing plant carbon dynamics is supported by de-tillering studies demonstrating altered carbon movement to increase root growth. Coupled with previous observations of improved water extraction with reduced tillering in tin (Mitchell et al., 2013) and non-tin (Innes et al., 1981) studies elsewhere, validation in the field confirms maintenance of transpiration and green leaf area, and changes in shoot and root growth combine to maintain favourable conditions for grain growth during grain-filling.
Supplementary data
Supplementary data are available at JXB online.
Figure S1. Shoot and root biomass for Banks and Kite tillering NILs assessed over multiple sampling dates in 2004 in cool outdoor and warm glasshouse conditions.
Figure S2. Mean leaf conductance (as 1/leaf porosity) scores for three dates post-anthesis for Banks and Kite tillering NILs.
Figure S3. Numbers of green leaves per spike estimated during and after mid grain-filling for the Banks tillering NILs evaluated in the field in 2004.
Acknowledgements
We would like to thank John Graham for excellent technical assistance with aspects of these studies, and Wolfgang Spielmeyer for providing seed of the Banks tin NILs. PJG is grateful to the Leverhulme Trust for a Study Abroad Fellowship that facilitated his participation in this work. Finally we would like to thank the two anonymous reviewers for their invaluable comments.
Glossary
Abbreviations:
- NIL
near-isogenic line
- SLA
specific leaf area
- tin
tiller inhibition.
References
- Atsmon D, Bush MG, Evans LT. 1986. Effects of environmental conditions on expression of the ‘Gigas’ characters in wheat. Australian Journal of Plant Physiology 13, 365–379. [Google Scholar]
- Austin RB, Edrich J. 1975. Effects of ear removal on photosynthesis, carbohydrate accumulation and on the distribution of assimilated 14C in wheat. Annals of Botany 39, 142–152. [Google Scholar]
- Bai C, Liang Y, Hawkesford MJ. 2013. Identification of QTLs associated with seedling root traits and their correlation with plant height in wheat. Journal of Experimental Botany 64, 1745–1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry PM, Spink JH, Foulkes MJ, Wade A. 2003. Quantifying the contributions and losses of dry matter from non surviving shoots in four cultivars of winter wheat. Field Crops Research 80, 111–121. [Google Scholar]
- Chenu K, Deihimfard R, Chapman SC. 2013. Large-scale characterization of drought pattern: a continent-wide modelling approach applied to the Australian wheatbelt—spatial and temporal trends. New Phytologist 198, 801–820. [DOI] [PubMed] [Google Scholar]
- Christopher J, Christopher M, Jennings R, Jones S, Fletcher S, Borrell A, Manschadi AM, Jordan D, Mace E, Hammer G. 2013. QTL for root angle and number in a population developed from bread wheats (Triticum aestivum) with contrasting adaptation to water-limited environments. Theoretical and Applied Genetics 126, 1563–1574. [DOI] [PubMed] [Google Scholar]
- Cullis BR, Thomson FM, Fisher JA, Gilmour AR, Thompson R. 1996. The analysis of the NSW wheat variety database 2. Variance component estimation. Theoretical and Applied Genetics 92, 28–39. [DOI] [PubMed] [Google Scholar]
- Duggan BL, Richards RA, van Herwaarden AF. 2005. b . Agronomic evaluation of a tiller inhibition (tin) gene in wheat. II. Growth and partitioning of assimilate. Australian Journal of Agricultural Research 56, 179–186. [Google Scholar]
- Duggan BL, Richards RA, van Herwaarden AF, Fettell NA. 2005. a . Agronomic evaluation of a tiller inhibition (tin) gene in wheat. I Effect on yield, yield components and grain protein. Australian Journal of Agricultural Research 56, 169–178. [Google Scholar]
- Gaju O, Reynolds MP, Sparkes DL, Foulkes MJ. 2009. Relationships between large-spike phenotype, grain number, and yield potential in spring wheat. Crop Science 49, 961–973. [Google Scholar]
- Gaju O, Reynolds MP, Sparkes DL, Mayes S, Ribas-Vargas G, Crossa J, Foulkes MJ. 2014. Relationships between physiological traits, grain number and yield potential in a wheat DH population of large spike phenotype. Field Crops Research 164, 126–135. [Google Scholar]
- Geiger DR, Fondy BR. 1980. Responses of export and partitioning to internal and environmental factors. New Phytologist 11, 767–787. [Google Scholar]
- Gu J, Marshall C. 1988. The effect of tiller removal and tiller defoliation on competition between the main shoot and tillers of spring barley. Annals of Applied Biology 112, 597–608. [Google Scholar]
- Holland JB. 2006. Estimating genotypic correlations and their standard errors using multivariate restricted maximum likelihood estimation with SAS Proc MIXED. Crop Science 46, 642–654. [Google Scholar]
- Innes P, Blackwell RD, Austin RB, Ford Margaret A. 1981. The effects of selection for number of ears on the yield and water economy of winter wheat. Journal of Agricultural Science 97, 523–532. [Google Scholar]
- Islam T, Sedgley R. 1981. Evidence for a ‘uniculm effect’ in spring wheat (Triticum aestivum L.) in a Mediterranean environment. Euphytica 30, 277–282. [Google Scholar]
- Jones HG, Kirby EJM. 1977. Effects of manipulation of number of tillers and water supply on grain yield in barley. Journal of Agricultural Science 88, 391–397. [Google Scholar]
- King RW, Wardlaw IF, Evans LT. 1967. Effect of assimilate utilization on photosynthetic rate in wheat. Planta 77, 261–276. [DOI] [PubMed] [Google Scholar]
- Kirkegaard J, Lilley J, Howe G, Graham J. 2007. Impact of subsoil water use on wheat yield. Australian Journal of Agricultural Research 58, 303–315. [Google Scholar]
- Koide K, Ishihari K. 1992. Effects of ear removal on dry matter production and its partitioning in wheat and barley. Japanese Journal of Crop Science 61, 651–658. [Google Scholar]
- Lilley JM, Kirkegaard JA. 2007. Seasonal variation in the value of subsoil water to wheat: simulation studies in southern New South Wales. Australian Journal of Agricultural Research 58, 1115–1128. [Google Scholar]
- Liu X, Li R, Chang X, Jing R. 2013. Mapping QTLs for seedling root traits in a doubled haploid wheat population under different water regimes. Euphytica 189, 51–66. [Google Scholar]
- Lopes MS, Reynolds MP. 2010. Partitioning of assimilates to deeper roots is associated with cooler canopies and increased yield under drought in wheat. Functional Plant Biology 37, 147–156. [Google Scholar]
- MacKey J. 1986. Shoot, root and shoot: root interrelations in cereals and the ideotype concept. In: Siddiquir KA, Faruqui AM, eds New genetical approaches to crop improvement . Pakistan, Atomic Energy Agricultural Research Centre, 811–833. [Google Scholar]
- Mitchell JH, Chapman SC, Rebetzke GJ, Bonnett DG, Fukai S. 2012. Evaluation of a reduced tillering (tin) gene in wheat lines grown across different production environments. Crop and Pasture Science 63, 128–141. [Google Scholar]
- Mitchell JH, Rebetzke GJ, Chapman SC, Fukai S. 2013. Evaluation of reduced-tillering (tin) wheat lines in terminal water deficit environments. Journal of Experimental Botany 64, 3439–3451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeller CM, Evers JB, Rebetzke GJ. 2014. Shoot branching at the meristem, plant, and population scales: which processes and which models to simulate plant plasticity. Frontiers in Plant Science 5, 617.25520724 [Google Scholar]
- Passioura JB. 1976. Physiology of grain yield of wheat growing on stored water. Australian Journal of Plant Physiology 3, 559–565. [Google Scholar]
- Passioura JB. 1977. Grain yield, harvest index and water use of wheat. Journal of the Australian Institute of Agricultural Science 43, 117–120. [Google Scholar]
- Rebetzke GJ, Biddulph B, Chenu K, Deery D, Mayer J, Moeller C, Bennett D, Rattey A. 2013. a . Development of a multisite, managed environment facility for targeted trait and germplasm evaluation. Functional Plant Biology 40, 1–13. [DOI] [PubMed] [Google Scholar]
- Rebetzke GJ, Condon AG, Richards RA, Farquhar GD. 2003. Gene action for leaf conductance in three wheat crosses. Australian Journal of Agricultural Research 54, 381–387. [Google Scholar]
- Rebetzke GJ, Condon AG, Rattey AR, Farquhar GD, Richards RA. 2013. b . Genomic regions for canopy temperature and their genetic association with stomatal conductance and grain yield in bread wheat (Triticum aestivum L.). Functional Plant Biology 40, 14–26. [DOI] [PubMed] [Google Scholar]
- Rebetzke GJ, Fischer RA, van Herwaarden AF, Bonnett DG, Chenu K, Rattey AR, Fettell NF. 2014. Plot size matters: interference from intergenotypic competition in plant phenotyping studies. Functional Plant Biology 41, 107–118. [DOI] [PubMed] [Google Scholar]
- Rebetzke GJ, van Herwaarden A, Jenkins C, Ruuska S, Tabe L, Lewis D, Weiss M, Fettell N, Richards RA. 2008a. Quantitative trait loci for water soluble carbohydrates and associations with agronomic traits in wheat. Australian Journal of Agricultural Research 59, 891–905. [Google Scholar]
- Rebetzke GJ, Lopez-Casteneda C, Botwright-Acuna T, Condon AG, Richards RA. 2008b. Inheritance of coleoptile tiller appearance and size in wheat. Australian Journal of Agricultural Research 59, 863–873. [Google Scholar]
- Richards RA. 1988. A tiller gene in wheat and its effects on plant growth. Australian Journal of Agricultural Research 39, 749–757. [Google Scholar]
- Richards RA, Rebetzke GJ, Condon AG, van Herwaarden AF. 2002. Breeding opportunities for increasing the efficiency of water use and crop yield in temperate cereals. Crop Science 42, 111–121. [DOI] [PubMed] [Google Scholar]
- Richards RA, Townley-Smith TF. 1987. Variation in leaf-area development and its effect on water-use, yield and harvest index of droughted wheat. Australian Journal of Agricultural Research 38, 983–992. [Google Scholar]
- Sadras VO, Rebetzke GJ. 2013. Plasticity in tillering is associated with plasticity in yield of wheat. Crop and Pasture Science 64, 234–243. [Google Scholar]
- Saini HS, Aspinall D. 1981. Effect of water deficit on sporogenesis in wheat (Triticum aestivum L.). Annals of Botany 48, 623–633. [Google Scholar]
- SAS Institute Inc. 2013. SAS/STAT ®13.1 user’s guide . Cary, NC: SAS Institute Inc. [Google Scholar]
- Sharma-Natu P, Ghildiyal MC. 1994. Photosynthesis and dry matter production in T. monococcum and T. aestivum wheat in response to ear removal. Journal of Agronomy and Crop Science 173, 218–224. [Google Scholar]
- Spielmeyer W, Richards RA. 2004. Comparative mapping of wheat chromosome 1AS which contains the tiller inhibition gene (tin) with rice chromosome 5S. Theoretical and Applied Genetics 109, 1303–1310. [DOI] [PubMed] [Google Scholar]
- Stapper M, Fisher RA. 1990. Genotype, sowing date and plant spacing influence on high-yielding irrigated wheat in southern New South Wales. I. Phasic development, canopy growth and spike production. Australian Journal of Agricultural Research 41, 997–1019. [Google Scholar]
- van Herwaarden AF, Angus JF, Richards RA, Farquhar GD. 1998. ‘Haying-off’, the negative grain yield response of dryland wheat to nitrogen fertiliser II. Carbohydrate and protein dynamics. Australian Journal of Agricultural Research 49, 1083–1093. [Google Scholar]
- Wasson AP, Richards RA, Chatrath R, Misra S, Sai Prasad SV, Saxena DC, Rebetzke GJ, Kirkegaard JA, Christopher J, Watt M. 2012. An ideotype breeding approach for root system traits to increase water uptake and yield in dry environments. Journal of Experimental Botany 63, 3485–3498. [DOI] [PubMed] [Google Scholar]
- Wasson A, Rebetzke GJ, Kirkegaard JA, Christopher J, Richards RA, Watt M. 2014. Soil coring at multiple field environments can directly quantify variation in deep root traits to select wheat genotypes for breeding. Journal of Experimental Botany 65, 6231–6249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whan BR, Delane R, Gilmour R. 1988. The potential of reduced tillering wheats in dry environments. In: Proceedings of the Seventh International Wheat Genetics Symposium . Cambridge, 907–11. [Google Scholar]
- Yunusa IAM, Sedgley RH. 1992. Reduced tillering spring wheats for heavy textured soils in a semiarid Mediterranean environment. Journal of Agronomy and Crop Science 168, 159–168. [Google Scholar]
- Zadoks JC, Chang TT, Konzak CF. 1974. A decimal code for the growth stages of cereals. Weed Research 14, 415–421. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.