Highlight
The major flowering time genes cloned to date regulate photoperiod and vernalization response. We identified a deletion containing genes regulating earliness per se, which fine tune flowering in hexaploid wheat.
Key words: Arabidopsis thaliana, Brachypodium distachyon, Eps-D1, flowering, Triticum aestivum, Triticum monococcum, wheat.
Abstract
Earliness per se (Eps) genes account for the variation in flowering time when vernalization and photoperiod requirements are satisfied. Genomics and bioinformatics approaches were used to describe allelic variation for 40 Triticum aestivum genes predicted, by synteny with Brachypodium distachyon, to be in the 1DL Eps region. Re-sequencing 1DL genes revealed that varieties carrying early heading alleles at this locus, Spark and Cadenza, carry a subtelomeric deletion including several genes. The equivalent region in Rialto and Avalon is intact. A bimodal distribution in the segregating Spark X Rialto single seed descent (SSD) populations enabled the 1DL QTL to be defined as a discrete Mendelian factor, which we named Eps-D1. Near isogenic lines (NILs) and NIL derived key recombinants between markers flanking Eps-D1 suggest that the 1DL deletion contains the gene(s) underlying Eps-D1. The deletion spans the equivalent of the Triticum monoccocum Eps-A m 1 locus, and hence includes MODIFIER OF TRANSCRIPTION 1 (MOT1) and FTSH PROTEASE 4 (FTSH4), the candidates for Eps-A m 1. The deletion also contains T. aestivum EARLY FLOWERING 3-D1 (TaELF3-D1) a homologue of the Arabidopsis thaliana circadian clock gene EARLY FLOWERING 3. Eps-D1 is possibly a homologue of Eps-B1 on chromosome 1BL. NILs carrying the Eps-D1 deletion have significantly reduced total TaELF3 expression and altered TaGIGANTEA (TaGI) expression compared with wild type. Altered TaGI expression is consistent with an ELF3 mutant, hence we propose TaELF3-D1 as the more likely candidate for Eps-D1. This is the first direct fine mapping of Eps effect in bread wheat.
Introduction
Humans consume most of their calories from wheat and 20% of the dietary protein for developing countries is obtained from wheat (Reynolds et al., 2012), which makes wheat strategic for global food security. Wheat is widely consumed across the globe because it can be grown in a wide range of environments (Slafer and Rawson, 1994). The major problem is that even though wheat yields are increasing, the yields are not keeping up with demand (Coff et al., 2008; Gupta et al., 2008; Lopes et al., 2012). The challenge is to bridge the gap between wheat demand and wheat production (Dixon et al., 2009; Rosegrant and Agcaoli, 2010). A better understanding of the mechanism of Eps leading to breeding by design for adaptive traits will be an important part of this process.
One of the important processes that controls grain number and quality, and hence yield, is the genetic and environmental control of the timing of flowering (Fjellheim et al., 2014). Three major classes of genes control the timing of flowering. The photoperiod genes enable plants to perceive changes in day length while the vernalization genes enable response to exposure to extended periods of cold. In this way photoperiod and vernaliation genes control adaptation to mega environments. The third class is Eps genes, which control flowering time when both photoperiod and vernalization requirements are met (Slafer and Rawson, 1994), and act in fine tuning flowering time within mega environments (Zikhali and Griffiths, 2015).
The major photoperiod and vernalization genes of bread wheat have been positionally cloned (Yan et al., 2003, 2004, 2006; Turner et al., 2005), but the identification of Eps genes has remained elusive in bread wheat. However, two Triticeae Eps loci have been cloned to date. One in Triticum monococcum on chromosome 3A (Eps-3A m) for which the candidate gene is an orthologue of the Arabidopsis thaliana LUX ARRHYTHMO/ PHYTOCLOCK 1 [(LUX/PCL1, which acts by disturbing the circadian clock (Shindo et al., 2002; Gawrosnski and Schnurbusch, 2012; Mizuno et al., 2012; Gawrosnski et al., 2014)]. The other is the Hordeum vulgare EPS2 on chromosome 2H, which is orthologous with the wheat group 2 loci (Laurie et al., 1995; Laurie, 1997). The candidate gene for EPS2 is a homologue of the Antirrrhunum gene CENRORADIALIS (CEN) designated HvCEN (Comadran et al., 2012).
In barley, the EARLY FLOWERING 3 gene (Boden et al., 2014)—referred to also as EARLY MATURITY 8 (eam8) (Faure et al., 2012) or Praematurum-a (Mat-a) (Zakhrabekova et al., 2012)—has been shown to play a major role in flowering time. Faure et al. (2012) and Zakhrabekova et al. (2012) both showed that mutants of this gene have an impaired circadian clock. The most recent report (Boden et al., 2014) went further and showed that the wild type EARLY FLOWERING 3 prevents flowering under short days by preventing gibberellin production and suppressing FLOWERING LOCUS T1 (FT1) expression.
Further effort to clone Eps genes in T. monococcum include the thermo-sensitive earliness per se (Eps-A m 1) locus (Bullrich et al., 2002) for which two possible candidates—MODIFIER OF TRANSCRIPTION 1 (MOT1) and FTSH PROTEASE 4 (FTSH4)—have been suggested (Faricelli et al., 2010). These studies are of particular relevance to the current study. The T. aestivum homologues of MOT1 and FTSH4 will hereafter be referred to as TaMOT1 (generic) and TaFTSH4 (generic) and -A1, -B1 as well as -D1 suffix will designate the three homoeologues, respectively.
The Eps effect is an important adaptive trait but the genes controlling it are not well understood. For example, the Eps-A m 1 and the Eps-3A m loci are both thermo-sensitive and determine the number of spikelets as well as the number of grains per spike, in addition to regulating flowering time (Bullrich et al., 2002 Lewis et al., 2008; Gawrosnski et al., 2014). One possible reason why Eps genes have not been well studied is that they were often mapped in crosses segregating for Ppd and Vrn, which often mask their effects.
Here we describe the high resolution mapping of an Eps quantitative trait locus (QTL) on the long arm of wheat chromosome 1 (1DL), which we named Eps-D1 that was originally identified using doubled haploid populations (Griffiths et al., 2009). We recently validated the 1DL QTL as an Eps effect using near isogenic lines (NILs) (Zikhali et al., 2014) and now we show, using NILs and recombinants, that a chromosomal deletion which contains several genes including the wheat homologue of the Arabidopsis circadian clock gene Early Flowering 3 (ELF3) is the likely cause of the Eps-D1 effect.
Materials and methods
Plant growth: doubled haploid populations
Ninety-six lines each from three independent doubled haploid (DH) populations of crosses between Spark X Rialto, Avalon X Cadenza, and Malacca X Hereward, were grown in 1L pots. The growth conditions were as decribed for Spark X Rialto NILs in our earlier report (Zikhali et al., 2014). For each of the 96 DH lines from the three populations, nine seeds were sown and germinated between 15–20 °C for 2 weeks. The nine seedlings of each line were then separated into three photoperiod treatments (each treatment had three plants of each line from the three DH populations). All treatments were sown on 21 December 2011 and germinated in a heated glasshouse up to 9 January 2012. The heating was then turned off to allow natural vernalization to commence on 10 January 2012 and heating was kept off up to 6 March 2012 to allow 8 weeks of vernalization. From 17 February 2012, all the benches with the three treatments were adjusted to move into the shed at 1700 hours (hrs) and out of the shed at 0700 hrs to give all treatments 10h of sunlight up to 6 March 2012 when vernalization ended. After the 8 weeks vernalization, one of the three treatments remained exposing plants to short days (SD, 10h light and 14h of darkness) by moving the plants out of the shed at 0700 hrs and then moving them back into the shed at 1700 hrs. The other two photoperiod treatments were adjusted to move out of the shed at 0600 hrs and back into the shed at 1800 hrs giving these bences 12h of natural light. Then additional lighing in the shed was used to give long days (LD, 16h light) and very long days (VLD, 20h light). Additional lighting was provided using 4h and 8h artificial white light using eight tungsten bulbs spaced 0.9 m apart delivering 1 μmol s–1 m–1 to augment the LD and VLD, respectively. The temperature was adjusted to 13–18 °C in glasshouse conditions using heaters. Heading date was measured on the leading tiller at Zadoks growth stage 55 (Zadoks et al., 1974). The heading date scores were then used to carry out QTL confidence interval mapping (CIM) analysis using the R software (vs. 3.02) programme (R Core Team, 2013).
Single seed descent populations
The single seed descent (SSD) populations were derived from a cross between Spark and Rialto. The same Spark X Rialto DH lines SR9 and SR23 used to develop the NILs in our earlier report (Zikhali et al., 2014) were used to develop the SSD populations. The only difference between the NILs and SSD lines is that after screening the self fertilized heterozygotes from backcross 2 (BC2), the homozygotes at the same markers—Xcfd63, Xgdm111, and Xbarc62—were selected for the NILs (Zikhali et al., 2014) while, for the SSD population, the heterozygotes at the three markers were selected for self-fertilization to form the recombinant SSD populations.
A population of 266 Backcross 2 F3 (BC2F3) individuals from four maintained independent populations was grown using SSD, up to BC2F5. Cross pollination was avoided in the four populations by covering spikes with cellophane bags before anthesis. When the SSD lines reached BC2F5, four plants were grown in randomized blocks for each SSD line in 1L pots and vernalized under short days (10h light) for 8 weeks at 6–10 °C in a controlled environment room. After the 8 weeks vernalization, the plants were then grown at 16h light with 4h supplementary light.
Identification and design of additional molecular markers on 1DL
The Xbarc62 marker (Song et al., 2005) was the most distal on 1DL for the Spark X Rialto DH population and was at the peak of the 1DL QTL. We therefore determined the physical position of Xbarc62 on 1DL in order to develop more markers to fine map the 1DL Eps effect. We retrieved from the National Centre for Biotechnology Information (NCBI) database the sequence BV211449 (Song et al., 2005) used to develop the Xbarc62 simple sequence repeat (SSR) marker. We then used the sequence to conduct homology searches (Altschul et al., 1990) of the unassembled reads of the Chinese Spring 454 sequence database (Brenchley et al., 2012). The BV211449 sequence matched the 3′ untranslated region (3′UTR) of the T. aestivum Early Flowering 3 (TaELF3) (Higgins et al., 2010) Genbank accession number AK332315 (Kawaura et al., 2009). We also aligned the BV211449 sequence, which contains the ATCT repeat (Xbarc62) (Song et al., 2005), with sequences from part of exon 4 and the 3′UTR of Aegiolps tauschii ELF3 (AtELF3), Triticum urartu ELF3 (TuELF3), and of the A, B, and D homoeologues of the Chinese Spring TaELF3 gene.
We then used synteny between wheat and Brachypodium (Faricelli et al., 2010; Higgins et al., 2010; Faure et al., 2012; Zakhrabekova et al., 2012) to design 1DL specific markers on both the distal and proximal side of Xbarc62.
Assembly of the three wheat homoeologues for each syntenic gene
We used two approaches to retrieve the wheat homoeologues of the Brachypodium syntenic genes. The first one made use of the flow sorted chromosome arm sequence database (IWGSC). We used BLAST homology searches (Altschul et al., 1990) of the flow sorted chromosome arm sequence database using full length mRNA sequences of the Brachypodium syntenic genes as the query except for TaELF3, where the wheat mRNA GenBank accession number AK332315 was used as the query sequence. In some cases, sections of genes were missing from the chromosome arm sequence database and hence we used our second approach where we homology searched the unassembled reads of the Chinese Spring sequence database (Brenchley et al., 2012) as for the chromosome arm survey sequence database. We then assembled the three wheat homologues for each of the 24 genes using Vector NTI sequence alignment tool as described for T. aestivum FLOWERING LOCUS T 3 (TaFT3) in our earlier report (Zikhali et al., 2014).
Genome specific primer development
The primers were designed to have 100% match with 1DL sequences and to end with a 1DL specific base. The primers were also designed to contain as many mismatches with the 1AL and 1BL copies so as to exclude the 1AL and 1BL copies during PCR amplification. Markers were designed by re-sequencing 24 wheat genes on 1DL that are syntenous with B. distachyon chromosome 2 genes. The 1DL specific primers selectively amplified overlapping portions of 1DL gene copies while competitively excluding the A and B copies of the genes. At least one non-specific (amplified from all the three genomes) primer pair was designed for each gene and this was used as a control. We also designed 1DL specific primers for the region covering part of the BV211449 sequence (Xbarc62) and used these together with the primers developed by Song et al. (2005) for the same region to amplify PCR amplicons from Aegilops tauschii and the wheat varieties Chinese Spring, Savannah, Badger, Cadenza, Rialto, Avalon, and Charger.
Amplification and sequencing of genes on 1DL
Amplicons were obtained from genomic DNA using standard PCR protocol and PCR reaction conditions, and detected by agarose electrophoresis as described in our earlier report (Zikhali et al., 2014) for the 24 syntenous genes on 1DL. The amplicons were directly sequenced for Rialto, Avalon, Savannah, Charger, Spark, Badger, and Cadenza using ABI Big Dye Mix v3.1 (Applied Biosystems Inc.) under the manufacturer’s conditions, with products resolved on an ABI 3730 capillary electrophoresis instrument. For the genes TaBradi2g14290 (TaELF3) and TaBradi2g14340 (TaMOT1), we sequenced full length genomic sequences for the D copies of TaELF3 and TaMOT1 as well as the TaELF3-A1 and TaELF3-B1 copies. For TaBradi2g14330 (TaFTSH4), we sequenced most of the sequences except about 700 bases, which incuded exon 1 and part of intron 1 for both the B and D copies.
Scoring single nucleotide polymorphisms in TaFT3-D1
Scoring of KASP single nucleotide polymorphisms (SNPs) to detect recombinants for the Spark X Rialto BC2F5 SSD populations was done as described by Zikhali et al. (2014) for TaFT3-D1. We also designed KASP assays (LGC) for: TaBradi2g14790, which we also used to identify recombinants in the Spark X Rialto SSD population; TaELF3-D1, which we mapped to Savannah X Rialto 1DL; and TaELF3-B1, which we mapped on the Avalon X Cadenza 1BL. We used the same KASP reagents as described for Vrn-A1 (Diaz et al., 2012) and for TaFT3-D1 (Zikhali et al., 2014). Primer combinations for TaBradi2g14790, TaELF3-B1, TaELF3-D1, and TaMOT1-D1 are shown in Supplementary Table S1 (available at JXB online).
Genotyping Spark X Rialto NILs and SSD populations
The Spark X Rialto NILs and SSD populations were genotyped using the markers that we developed on 1DL as well as SSR markers Xgdm111 and Xcfd63. We also scored the presence/absence of PCR amplicons on agarose gels for NILs, SSD, and DH population. Histograms were then used to classify lines as early or late. The markers also enabled us to identify recombinants between Spark and Rialto alleles on 1DL in the SSD populations.
Comparative genomics exploiting synteny between Brachypodium and wheat
We used the wheat (IWGSC-based) pseudomolecule v3.3 (JIC) database and synteny between wheat and B. distachyon to determine the gene order on 1AL, 1BL, and 1DL. We used blast homology searches of the wheat (IWGSC-based) pseudomolecule v3.3 (JIC) using sequences linked to markers on 1DL and 1BL and retrieved the positions of these markers on each of the pseudomolecules. We then used the pseudo molecule positions to align the QTL on 1DL for Spark X Rialto, Avalon X Cadenza, and Savannah X Rialto, as well as the QTL on 1BL for Avalon X Cadenza with the 1AL region. We also aligned the Eps-Am1 (Bullrich et al., 2002; Faricelli et al., 2010) locus with the QTLs on the distal group 1 chromosomes.
Gene expression
Expression studies and analysis of TaGI and TaELF3 were carried out as described by Shaw et al. (2012) except that norm2 (Forward primer AGCGATTTCCAGCTGCCTTC and reverse primer TGCGAAGAGGCCAGTCAGTC) was used as the expression reference instead of RP15. All above ground parts of 3-week-old plants grown under long days (16h light) at 16–18 °C in the light and 13–15 °C in the dark period were ground using a pestle and mortar. Two NIL pairs (NIL1 and NIL4, and NIL16 and NIL18) were selected for gene expression because these had the highest Rialto background relative to the other NILs (Zikhali et al. 2014). Samples were collected at 3 weeks at 3h intervals through a 24h period. Optimal genome specific qPCR assays could not be developed due to a relative paucity of homoeologous SNPs in the coding region, hence our limitation to total TaELF3 expression using generic qPCR primers (Forward primer GTGGGATCGACAGACCTC and reverse primer CGACGCGTTCCTTCC).
Genbank accession numbers for the genes sequenced in this study
1. Triticum aestivum Early Flowering 3-D1 (TaELF3-D1)
Rialto (KR055808), Avalon (KR055809), Charger (KR055810), Savannah (KR055811)
2. T. aestivum Early Flowering 3-B1 (TaELF3-B1)
Cadenza (KR082515), Spark (KR082517), Rialto (KR082518), Badger (KR082519), Savannah (KR082520), Avalon (KR082521), Charger (KR082522)
3. T. aestivum Early Flowering 3-A1 (TaELF3-A1)
Spark (KR082526), Savannah (KR082527), Rialto (KR082528), Avalon (KR082529), Cadenza (KR082530)
4. T. aestivum MODIFIER OF TRANSCRIPTION 1-D1 (TaMOT1-D1)
Rialto (KR082499), Avalon (KR082500), Charger (KR082501), Savannah (KR082502)
5. T. aestivum MODIFIER OF TRANSCRIPTION 1-B1 (TaMOT1-B1)
Charger (KR082507), Rialto (KR082508), Spark (KR082509), Avalon (KR082510),
Cadenza (KR082511), Badger (KR082512), Savannah (KR082513)
6. T. aestivum FTSH PROTEASE 4-D1 (TaFTSH4-D1)
Rialto (KR082535), Avalon (KR082536), Charger (KR082537), Savannah (KR082538)
7. T. aestivum FTSH PROTEASE 4-B1 (TaFTSH4-B1)
Avalon (KR082543), Charger (KR082544), Spark (KR082548), Badger (KR082549), Cadenza (KR082550), Rialto (KR082551), Savannah (KR082552)
Results
Satisfactory vernalization and photoperiod requirements in bread wheat
QTL profiles for DH populations show that the 1DL heading date QTL is an Eps effect because we detected QTLs after fully vernalizing the plants under LD and VLD for both DH populations but no significant QTL was detected under SD for both DH populations (Supplementary Fig. S1A,B available at JXB online). We detected QTLs for Spark X Rialto and Avalon X Cadenza (Supplementary Fig. S1A,B) at the same locus on the distal group 1 chromosome as was observed in the field (Griffiths et al., 2009), and through QTL validation using NILs (Zikhali et al., 2014). Since we did not detect a QTL for Malacca X Hereward on 5A, we concluded that 8 weeks of vernalization satisfied the vernalization requirement—given that a QTL with LOD score >22 was observed and shown to be due to copy number variation at the Vrn-A1 when this DH population is vernalized for 4 weeks (Diaz et al., 2012).
Position of Xbarc62 on 1DL
The Xbarc62 sequence is in the 3′-UTR of the TaELF3-D1 gene and the ATCT SSR repeat detected by Xbarc62 is 254b from the TaELF3-D1 stop codon (Supplementary Fig. S2). The Xbarc62 primers described by Song et al. (2005), are not 1DL specific as evidenced by the production of two PCR amplicons (129 and 118 bases) when these primers were used to amplify Chinese Spring (CS), Savannah (Sav), Rialto (Ria), Avalon (Ava), and Charger (Cha) but a single product (129b) from A. tauschii (Tau) (Fig. 1b). Sequencing revealed that the 118b size fragment (Fig. 1b), which is absent from A. tauschii, was from the A genome (1AL) copy (data not shown). When we used the 1DL specific primers, a single product (185b) was amplified from A. tauschii, Chinese Spring (CS), Savannah (Sav), Rialto (Ria), Avalon (Ava), and Charger (Cha) but there was no amplification from Spark, Badger, and Cadenza (Fig. 1b).
Fig. 1.
(A) Position of the Xbarc62 SSR 254 bases downstream of the stop codon in the 3′UTR of the Chinese Spring TaELF-D1 gene. The solid rectangles numbered 1–4 are the exons of the TaELF3-D1 gene. The eight arrows in the dotted rectangle represent the eight ATCT repeats detected by the Xbarc62 SSR marker (Song et al., 2005). The two unshaded rectangles are miniature inverted transposable elements (MITEs) with similar sequences with sizes 305 and 308 bases, respectively. The start and stop represent the beginning (ATG) and end (TGA) respectively of the ORF. (B) Deletion of the Xbarc62 locus in Spark (Spa), Badger (Bad), and Cadenza (Cad) as shown by the absence of the 129b (1) and 185b (3) size fragments when using the primers designed by Song et al. (2005) and the 1DL specific primers (this study), respectively. The 118b (2) fragment size is from the TaELF3-A1 copy (see Supplementary Fig. S2 at JXB online).
The Xbarc62 SSR marker does not amplify a D copy amplicon in Spark, Badger, and Cadenza and is scored as a null (data not shown). This suggested a deletion or other disturbance of the sequences in these varieties. The Xbarc62 primers by Song et al. (2005) amplify from both the A and D copies because they do not distinguish the two homoeologues (Supplementary Fig. S2 at JXB online). We showed this by aligning the GenBank accession BV211449 sequence, which contains the ATCT Xbarc62 repeat, with sequences from A. tauschii ELF3 (AtELF3), T. urartu ELF3 (TuELF3), and the three wheat homoeologues of the TaELF3 gene (Supplementary Fig. S2).
Having shown that the Xbarc62 SSR marker was linked to the TaELF3-D1 gene (Supplementary Fig. S2), we designed primers specific for coding sequence from TaELF3-D1. Spark, Badger, and Cadenza did not yield PCR amplicons while Rialto, Avalon, Savannah, and Charger did. The same pattern was also observed for PCR primers based on the Brachypodium syntenic genes flanking TaELF3, suggesting that there was a deletion that includes several genes for Spark and Cadenza while Rialto, Avalon, Savannah, and Charger are intact in this region (Fig. 2).
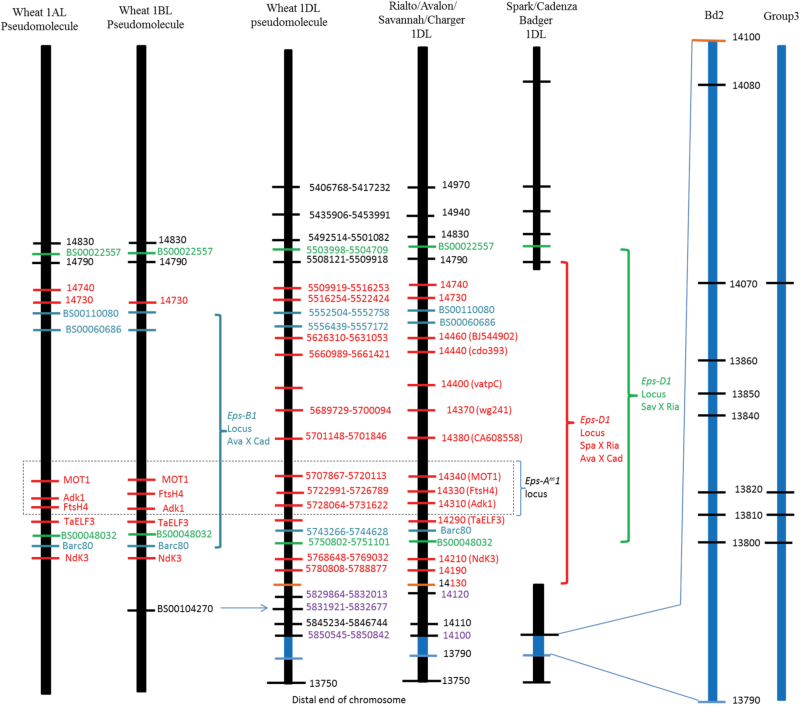
Fig. 2.
Schematic presentation of the Eps-B1 locus for Avalon X Cadenza (QTL confidence interval between SSR marker XBarc80 and KASPar markers XBS0011080 and XBS00060686, Eps-D1 (deletion of several genes on 1DL of Spark), Eps-D1 for Savannah X Rialto (no deletion) QTL confidence interval between KASPar markers XBS00048032 and XBS00022557. The dotted rectangle marks the Eps-Am1 locus and the two proposed candidates MOT1 and FTSH4 (Faricelli et al., 2010). The blue and green horizontal numbers and letters indicate SSR marker (XBarc80) and KASPar markers beginning with BS. The black vertical bars indicate the 1AL, 1BL, and 1DL wheat pseudomolecules from the wheat (IWGSC-based) pseudomolecule v3.3 (JIC) database and the region is collinear with Brachypodium chromosome 2. The red or black numbers on the right of Rialto 1DL next to the red or black horizontal bars, respectively, indicate the Brachypodium chromosome 2 gene numbers syntenic with wheat 1DL. The red numbers and letters on the right of Rialto/Avalon/Savannah/Charger 1DL indicate the genes deleted from Spark while the black numbers show those outside the 1DL deletion. The purple coloured numbers on the right side of wheat 1DL psuedomolecule and Rialto/Avalon/Savannah/Charger 1DL denote the genes between TaBradi2g14130 and TaBradi2g13790 that have not been checked if they are in the deletion but are likely to be outside the 1DL deletion. The gap on the vertical bar labelled Spark/Cadenza/Badger 1DL indicates the 1DL deletion. The blue vertical bars (Bd2 and group 3) are used to illustrate that all the eight brachypodium genes (13800, 13810, 13820, 13840, 13850, 13860, 14070, and 14080) between the predicted genes Bradi2g13790 (designated 13790 on Fig. 2) and 14130 do not match the wheat group 1 genes but four of these genes match the group 3 chromosome genes. The gene TaBradi2g14130 (14130) is labelled in black and red because part of this gene was amplified and sequenced from Spark while the rest of the gene seems to be deleted.
Defining the Eps-D1 (1DL deletion) and Eps-B1 loci
Based on the synteny with Brachypodium and the wheat 1DL pseudomolecule, there is no gene on wheat 1DL between TaBradi2g14790 and TaBradi2g14740 (Fig. 2; Supplementary Table S2 at JXB online). The gene TaBradi2g14790 is outside the 1DL deletion on the proximal side (Figs 2 and 3). The marker segregation shown on Fig. 3A is due to an insertion in the TaBradi2g14790 gene for Spark. We developed a KASP assay for TaBradi2g14790 that we used to identify recombinants. Since all the 10 PCR primers that span TaBradi2g14740 do not amplify from Spark (Supplementary Fig. S3.2H at JXB online), this suggests the proximal deletion breakpoint is in the inter-genomic region between TaBradi2g14790 and TaBradi2g14740. The distal deletion breakpoint may be in the gene TaBradi2g14130, given that we sequenced part of this gene but the rest seems deleted from Spark.
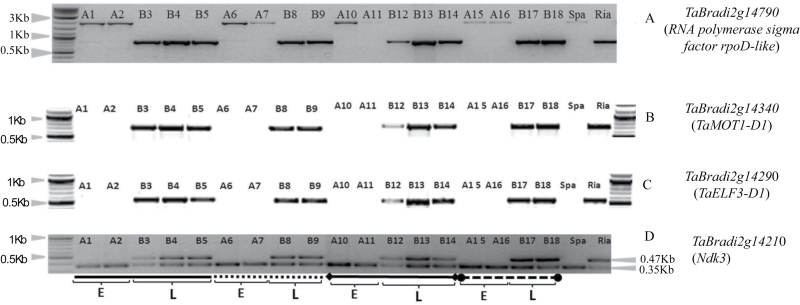
Fig. 3.
The genotypes of the Spark X Rialto 1DL NILs using markers TaBradi2g14790 (A), TaBradi2g14340 (B), TaBradi2g14290 (C), and TaBradi2g14210 (D). The letters A and B, which are followed by numbers, indicate the NILs carrying the Spark and Rialto allele, respectively. Spa and Ria are Spark and Rialto wheat cultivars, respectively. The solid horizontal line from A1 to B5 designates the first NIL pair, the dotted horizontal line (A6–B9) designates the second NIL pair, the solid horizontal line flanked by diamond shapes (A10–B14) is the third NIL pair and the dashed horizontal line flanked by circles (A15–B18) is the fourth NIL pair. The letters E and L at the bottom of the Figure indicate early flowering (E) and late flowering (L), respectively, of the NILs. The fragment size markers are shown in kilobases (kb). We showed the flowering time differences both in the field and controlled environments for these NILs in our earlier report (Zikhali et al., 2014).
The 1DL subtelomeric deletion spans the equivalent of the entire Eps-A m 1 locus (Faricelli et al., 2010; Fig. 2), which makes TaMOT1-D1 and TaFTSH4-D1 potential candidates for Eps-D1. The deletion also contains a homologue of the Arabidopsis circadian clock gene ELF3 designated TaELF3-D1 (Higgins et al., 2010). It has been suggested but not proven that Eps-A m 1 was orthologous with QTLs on T. aestivum group 1 chromosomes (Griffiths et al., 2009), and our results show that the QTLs on 1DL (Spark X Rialto, Avalon X Cadenza, Savannah X Rialto) and 1BL (Avalon X Cadenza) have QTL confidence intervals that span the region equivalent to the entire Eps-A m 1 locus (Fig. 2; Supplementary Fig. S4A, B).
The TaELF3-D1 gene was not assigned a position on the wheat 1DL pseudomolecule (Fig. 2) but we placed it between XBarc80 and Adk1 on Rialto 1DL because we mapped this gene between markers XBS00048032 and XBS00022557 (markers shown in green in Fig. 2) in Savannah X Rilato 1DL (Supplementary Fig. S4B at JXB online). We also mapped TaELF3-B1 between XBarc80 and XBS00110080/XBS00060686 in Avalon X Cadenza (Supplementary Fig. S4B). Although both XBS00110080/XBS00060686 were not assigned on 1BL pseudomolecule (Fig. 2), these markers were placed distal to TaBradi2g14730 because both are distal to TaBradi2g14730 on 1AL and 1DL pseudomolecules. The TaELF3-A1 and TaELF3-B1 genes are between markers XBS00048032 and Adk1 on both 1AL and 1BL pseudomolecules (Fig. 2). Since Adk1, XBarc80, and XBS00048032 were all assigned positions on the 1AL, 1BL, and 1DL pseudomolecules, the TaELF3-D 1 position on 1DL was determined to be between XBarc80 and Adk1 (Fig. 2).
Eps-D1 NIL genotypes
We showed previously that the 1DL NILs carrying the Spark alleles at this locus were all early flowering relative to those with the Rialto allele, both in the field and controlled environments (Zikhali et al., 2014). Our genotyping results for the same 1DL NILs (Fig. 3) suggest that the deletion is the likely cause of the flowering time differences between the NIL pairs. The expected amplicon size for TaBradi2g14210 was the 470bp amplicon, which is absent from Spark, and the 1DL NILs with the Spark allele (Fig. 3D). The 0.35kb amplicon was sequenced for Spark and did not match the group 1 chromosomes while the 470bp amplicon was sequenced and matched the 1DL gene copy. This suggested that the TaBradi2g14210 gene is also deleted in Spark.
Phenotyping and genotyping the Eps-D1 BC2F5 populations
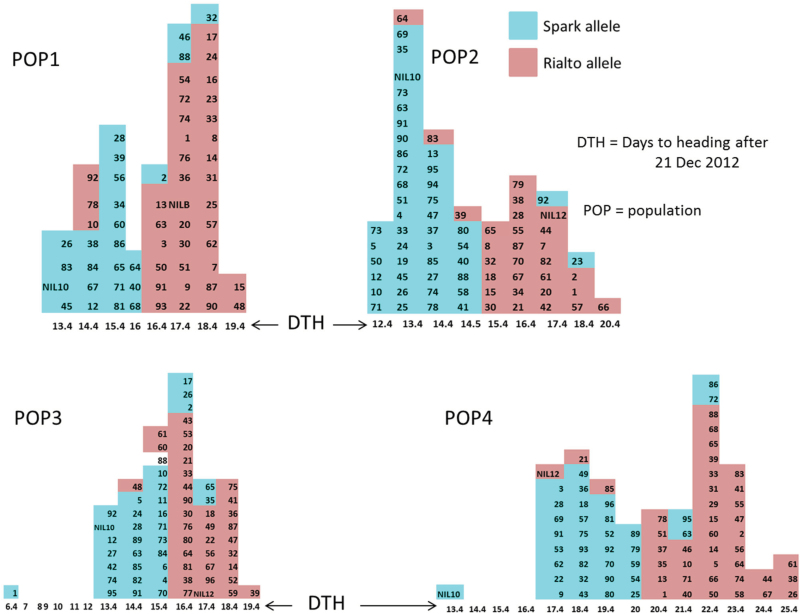
We observed a bimodal distribution of lines carrying the deletion and those with an intact portion of the chromosome for SSD populations 1, 2, and 4 (POP1, POP2, and POP4) (Fig. 4). The division of population 3 (POP3) into early and late groups becomes apparent when classified according to deletion genotype (Fig. 4). Most lines carrying the deletion have an early flowering phenotype and this was consistent with the 1DL NILs, which we used to validate this QTL in an earlier report (Zikhali et al., 2014).
Fig. 4.
Days to ear emergence measured at growth stage 55 (Zadoks et al., 1974) of four independent Spark X Rialto Backcross 2 (BC2) SSD populations (POP1–POP4) scored at F5 (BC2F5). The two NILs, NIL10 and NIL12, carrying the Spark and Rialto 1DL alleles, respectively, were used as controls and grown together with SSD populations. Key: DTH was measured at growth stage 55 (Zadoks et al., 1974) shown by the arrows pointing at the uncoloured numbers. The coloured numbers are the individual SSD lines. POP1, POP2, POP3, and POP4 are the four Spark X rialto 1DL SSD populations at BC2F5. Spark and Rialto alleles are the 1DL deletion and intact chromosomes, respectively.
The following lines POP1 (10, 78, 92 and 2, 32, 46 and 88), POP2 (39, 64 and 83 23, 65 and 92), POP3 (13 (48, 60, 61, 2, 17, 26, 35, 65), and POP4 (21, 85, 63, 72, 86 and 95) run against the trend and are hereafter referred to as outliers. The genotypes of the outliers are shown on Supplementary Fig. S5. Line P1_78 has a Rialto 1DL arm, while lines P2_23 and P3_65 have Spark 1DL arms with no recombination, hence these three outliers are not due to recombination on 1DL (Supplementary Fig. S5). The flowering time shift observed for POP4, which flowers 5 d later than the other populations, as well as the very early flowering observed for line 1 from POP3 (Fig. 4), suggest that there could be a genetic background effect—given that our SSD populations were self-fertilized up to BC2F5 with expected Spark background of 12.25%. It is interesting to note that the gene responsible for the observed delay in heading date in POP4 relative to POP1, POP2, and POP3 is independent of the 1DL Eps effect (Fig. 4) and just delays the flowering of the whole population by 5 d relative to the other three populations (Fig. 4).
Recombinants suggest the 1DL deletion may contain the candidate for Eps-D1
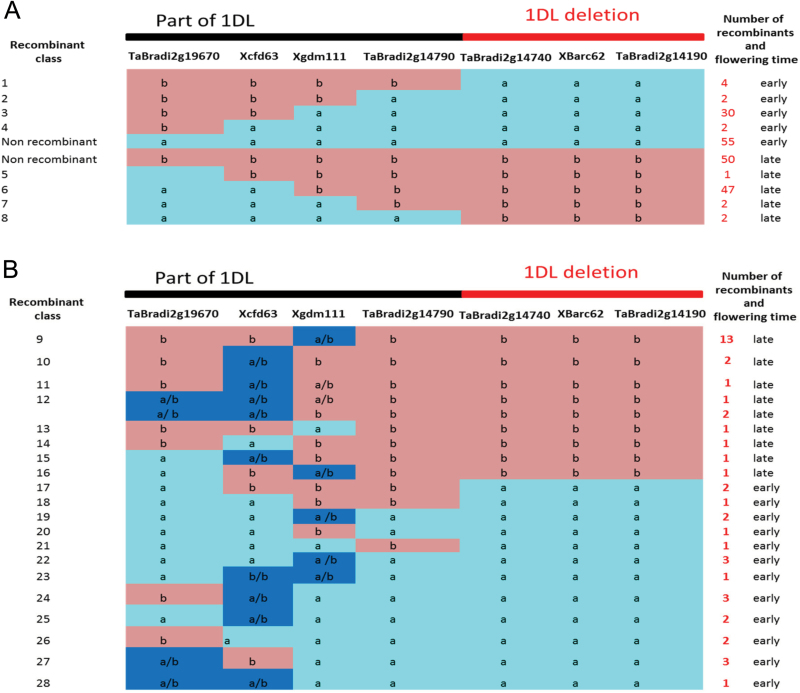
One hundred and thirty-five lines that are recombinants on 1DL (Fig. 5A,B) suggest that the Eps-D1 effect is distal to the marker TaBradi2g14790, which Brachypodium gene content would predict to be the last intact gene in the proximal side of the deletion (Fig. 2). The distal end of the deletion is near the telomeric region and we were unable to identify recombinants on the distal end of the deletion.
Fig. 5.
(A) Single recombinants at the 1DL QTL interval that segregate for late flowering and early flowering in the BC2F5 SSD lines of a cross between Spark and Rialto. Recombinant classes 1–4 are early flowering while recombinant classes 5–8 are late flowering. The data show that the earliness allele is distal to the TaBradi2g14790 marker. (B) Recombinants containing heterozygous regions as well as double recombinants at the 1DL QTL interval that segregate for late flowering and early flowering in the BC2F5 SSD lines of a cross between Spark and Rialto. Recombinant classes 9–14 are late flowering while recombinant classes 15–28 are early flowering. Key a=Spark 1DL Eps-D1 allele, b=Rialto 1DL allele and a/b represents heterozygous for a and b. The data also shows that the earliness allele is linked with the 1DL deletion.
Prioritization of candidates for Eps-D1 by exploiting homoeologous relationships
It is not possible to genetically dissect the putative Eps-D1 deletion and so all of the genes within it have equal standing as candidate genes. We decided to use homoeologous relationships and other populations with QTL in equivalent locations to try to prioritize the list of candidates. A QTL on 1BL identified in Avalon X Cadenza, which we named Eps-B1, is likely to be homoeologous with Eps-D1 (Fig. 2). In addition, Eps-D1 is orthologous with Eps-A m 1 (Faricelli et al., 2010) since the equivalent of the entire Eps-A m 1 confidence interval is spanned by the Eps-D1 deletion (Fig. 2). We also detected a QTL for Savannah X Rialto DH population in the region that spans the Eps-D1 deletion and Eps-A m 1 equivalent region; even though neither of these varieties carries the deletion (Fig. 2). We used these homoeologous relationships to try to prioritize/rank candidates. Out of the 33 syntenic genes in the 1DL deletion (Supplementary Table S2) we prioritized TaMOT1-D1, TaFTSH4-D1—based on previous studies in T. monococcum (Faricelli et al., 2010), and TaELF3-D1 genes as possible candidates for Eps-D1 and Eps-B1. The TaELF3-D1 gene was prioritized because its homologues have been reported to affect flowering time in Arabidopsis (Dixon et al., 2011), Oryza sativa (Matsubara et al., 2012), Hodeum valgare (Faure et al., 2012; Zakhabekova et al., 2012), Zea mays (Bate and Aukerman, 2011), and the legumes lentil and pea (Weller et al., 2012). Sequencing the homoeologous copies of these three genes in the wheat cultivars Avalon, Rialto, Charger, Savannah, Spark, Cadenza, and Badger revealed the mutations outlined below. For TaELF3-D1, two SNPs which are silent mutations in exon 2 and exon 4 (at positions 1746 and 3343 from the start codon) for Savannah (early allele) distinguishes it from the wild type (Table 1; Supplementary Fig. S6A). We designed a KASP assay for the TaELF3-D1 exon 4 SNP (Table 1; Supplementary Fig. S6A), which we used to map the gene in the Savannah X Rialto DH population (Supplementary Fig. S4B). For TaELF3-B1, where Avalon carries an early allele, a SNP in exon 4 (position 3432 from the start codon for Avalon) changes a conserved glycine to serine (Supplementary Fig. S7) and distinguishes Avalon from the wild type (Table 1; Supplementary Fig. S6B). We designed a KASP assay for the TaELF3-B1 and used it to map the gene in the Avalon X Cadenza DH population (Supplementary Fig. S4A).
Table 1.
Mutations at TaELF3-D1, TaELF3-B1, TaMOT1-D1, TaMOT1-B1, TaFTSH4-D1, and TaFTSH4-B1 and their association with heading date phenotype at the Eps-D1 and Eps-B1 loci
No mutation in the ORF means there was no SNP deletion or insertion from the start codon (ATG to the stop codon TGA or TAG). Spark/Cadenza or Rialto/Avalon means both varieties Spark and Cadenza or Rialto and Avalon have the same mutations for the three genes TaELF3-D1, TaMOT1-D1, and TaFTSH4-D1 except for TaMOT1-D1 where Rialto only has a SNP in exon 6.
| Variety | Gene | Polymorphism | Allele effect |
|---|---|---|---|
| Spark/ | TaELF3-D1 | Whole gene deleted | Early heading |
| Cadenza | TaMOT1-D1 | Whole gene deleted | |
| TaFTSH4-D1 | Whole gene deleted | ||
| Savannah | TaELF3-D1 | Silent SNPs in exons 2 & 4 | Early heading |
| TaMOT1-D1 | No mutation in the ORF | ||
| TaFTSH4-D1 | No mutation in the ORF | ||
| Rialto/ | TaELF3-D1 | No mutation in the ORF | Late heading |
| Avalon | TaMOT1-D1 | 24 ATT repeats (intron1) SNP intron 6 (Rialto) | |
| TaFTSH4-D1 | |||
| Exon 7 SNP changes serine to leucine | |||
| Avalon | TaELF3-B1 | Exon 4 SNP changes glycine to serine | Early heading |
| TaMOT1-B1 | No mutation in the ORF | ||
| TaFTSH4-B1 | No mutation in the ORF | ||
| Cadenza | TaELF3-B1 | No mutation in the ORF | Late heading |
| TaMOT1-B1 | No mutation in the ORF | ||
| TaFTSH4-B1 | Premature stop codon due to 17b deletion | ||
| in exon 3 |
For TaELF3-A1, seven mutations were observed along the gene (Supplementary Fig. S6C). The first is a GGATT SSR repeat with Spark having 9 of these GGATT repeats, Savannah has 8, Chinese Spring has 7, and the wild type including T. urartu has 6 repeats. Spark has a C/T SNP that distinguishes it from the wild type at position 614 (Supplementary Fig. S6C). Positions 1359, 1407, 1507, and 2798 have SNPs that result in silent mutations and distinguish Spark/Savannah from wild type, Savannah from wild type, Spark/Savannah from wild type, and Spark from wild type, respectively (Supplementary Fig. S6C). The SNP at position 2703 in exon 4 of TaELF3-A1 changes the wild type glycine to tryptophan for Spark/Savannah (Supplementary Fig. S6C). In spite of the SNPs on TaELF3-A1, we did not detect a QTL on 1AL in the populations we used. Chinese Spring and all the wheat varieties we used in this study have an approximately 1.25 and 0.95kb deletion in the intergenic region between TaELF3-A1 and the upstream gene TaBradi2g14280 relative to the B and D copies respectively.
For TaMOT1-D1, two mutations were observed; the first starting at position 93 (intron 1) from the start codon where Avalon/Rialto have 24 ATT repeats and the wild type has 22, and a SNP at position 2702 (intron 6) which distinguishes Rialto from the wild type (Supplementary Fig. S6D). We were unable to sequence the whole of exon 1 and part of intron 1 but sequenced the rest of the gene for both TaFTSH4-D1 and TaFTSH4-B1. For TaFTSH4-D1, a SNP at position 3955 changes a conserved serine to leucine for Rialto/Avalon (Table 1; Supplementary Fig. S6E). For TaFTSH4-B1, a 17 base deletion in exon 3 introduces a premature stop codon for Spark, Badger, Cadenza, Rialto, and Savannah, and this distinguishes them from Charger and Avalon, which have an intact exon 3 (Table 1; Supplementary Fig. S6F). There were no mutations detected in the coding sequence in the TaMOT1-B1 gene for all the varieties we sequenced.
TaGIGANTEA (TaGI) and TaELF3 expression analysis
Because we considered the deletion of TaELF3-D1 within Eps-D1 locus to be a very likely cause of early flowering in Spark, we undertook expression analysis of TaELF3 to show that loss of the D copy did indeed result in reduced total expression, discounting compensation from other genomes or non-expression of the intact copy. We also looked at expression of TaGI as TaELF3-D1 homologues are known to be direct repressors of GI in a number of species. Figure 6 shows that in both NIL pairs GI expression was increased with the presence Spark Eps-D1 allele relative to Rialto later in the light period. This pattern was observed for all three homoeologues in two independent NIL genetic backgrounds (Fig. 6). For TaELF3, total expression was consistently higher with the presence of the Eps-D1 Rialto allele in NIL4. For NIL pair 16/18 Spark alleles were associated with reduced TaELF3 expression except for time-points 5 and 7.
Fig. 6.
Expression patterns of the evening—loop gene TaGI A, B, and D homoeologues and that of the TaELF3 genes (generic primer amplifying all three A, B and D homoeologues) in the Eps-D1 mutants (NIL1 and NIL16) (red diamonds and lines) and the wild type (NIL4 and NIL18) (black circles and lines). The vertical axis is the relative expression of the genes against the house keeping gene norm2. Plants were grown under long days (16h light and 8h darkness) for 21 d and sampled at 3h intervals beginning at 0300 hrs which was 3h after turning on the lights, except samples taken at 2100 hrs (21), which were sampled 2h 45min after the seventh sample (18) to ensure that these samples were taken during darkness. Lights were turned off at 1600 hrs such that three samples were taken during the dark period indicated by the black solid bar on the horizontal axis. The stars indicate significant difference in expression ***P<0.001, **P<0.01 and *P<0.05 using the Student’s t-test for each time point. The error bars are the standard error of the mean of two technical replicates derived from a pool of three plants.
Discussion
This study shows that bread wheat Eps genes, still segregating in the elite gene pool, can be isolated as Mendelian factors and fine mapped. This has enabled us to define Eps-D1 and show that a deletion on 1DL is responsible for the early flowering phenotype of Spark. This means that Eps genes can be defined and positionally cloned in the same way as the first generation of flowering time genes Ppd (Turner et al., 2005) and Vrn (Yan et al., 2003) genes were. This study used elite winter wheat varieties Spark, Rialto, Avalon Cadenza, and Savannah, and we show, using NILs and SSD populations that it is possible to fine map these effects directly in hexaploid wheat.
Lin et al. (2008) and Griffiths et al. (2009) both speculated that the QTLs on the distal end of group1 chromosomes were homoeologues of the T. monococcum Eps-A m 1 (Bullrich et al., 2002; Valarik et al., 2006; Faricelli et al., 2010). Results from this study show that the Eps-A m 1 is at the same locus as the T. aestivum Eps-D1 and Eps-B1 locus given that the QTL confidence interval for Eps-A m 1 is in the 1DL deletion that this study showed to be tightly linked with the heading date Eps QTL (Figs 2–5).
Probable candidates for the Eps gene in the 1DL deletion
The results from this study suggest that the Eps-D1 effect is likely due to a deletion that includes several genes. All of the genes in the deletion are potential candidates for the Eps effect and the possibility of more than one gene being responsible is not ruled out. Deletion mutations of large portions of chromosomes (Shitsukawa et al., 2007; Distelfeld and Dubcovsky, 2010), single genes (Faure et al., 2012), or portions of genes (Yan et al., 2003; Fu et al., 2005; Wilhelm et al., 2009) have been shown to cause variation in flowering time.
Possibility that MODIFIER OF TRANSCRIPTION 1 (MOT1) is the candidate
However, among the deleted genes there are some candidates which stand out. These are MODIFIER OF TRANSCRIPTION 1 (MOT1) and FTSH PROTEASE 4 (FTSH4)—the suggested candidates for the Eps-A m 1 (Faricelli et al., 2010).
The parental lines Spark and Cadenza carry the 1DL deletion (Fig. 2) which makes MOT1-D1 a potential candidate for Eps-D1 given that the same QTL is observed at the same locus for the Spark X Rialto and Avalon X Cadenza DH populations (Supplementary Fig. S1). However, neither Savannah nor Rialto carries the 1DL deletion but a flowering QTL was observed at the same locus as that of Spark X Rialto and Avalon X Cadenza DH populations (Fig. 2; Supplementary Fig. S4B). Sequencing the TaMOT1-D1 gene revealed no mutations in all the 28 exons but two (introns 1 and 6) mutations differentiate Rialto from Savannah (Table 1). Given that both Rialto and Avalon have the ATT repeat mutation in TaMOT1-D1, intron 1 can only be the causal mutation if it is a gain of function mutation because this gene is deleted for both Spark and Cadenza which segregate with Rialto and Avalon, respectively, for the 1DL effect. The SNP in intron 6 is unlikely to be the cause given that it is only present in Rialto but absent from Avalon.
FTSH PROTEASE 4 (FTSH4) as possible candidate
The other gene that was suggested as a candidate for Eps-A m 1 is FTSH4. The Arabidopsis FTSH4 mutants flower a week later than wild type (Gibala et al., 2009). However, in T. monococcum, there was no amino acid substitution in the predicted FTSH4 protein sequences of the lines segregating for the Eps-A m 1 (Faricelli et al., 2010). Again there were no significant FTSH4 transcript differences between NILs segregating for Eps-A m 1 (Faricelli et al., 2010). In this study, sequencing TaFTSH4-D1 revealed that Rialto/Avalon and Savannah segregate for a mutation that changes a conserved serine to leucine in Rialto and Avalon (Table 1; Supplementary Fig. S6A).
From Table 1, it seems FTSH4 is a flowering promoter—given that loss of the gene TaFTSH4-B1 in Cadenza is linked with late phenotype while the wild type FTSH4-B1 in Avalon is linked with the early phenotype. In, Rialto, TaFTSH4-D1 mutation is linked with the late phenotype while Savannah has no mutation and is linked with an early phenotype (Table 1). These results from the two homoeologues of FTSH4 are consistent with FTSH4 being a flowering promoter—as in Arabidopsis (Gibala et al., 2009)—and make FTSH4-B1 and FTSH4-D1 likely candidates for Eps-B1 and Eps-D1. However, when we also consider that TaFTSH4-D1 and TaELF3-D1 are both deleted from Spark and Cadenza, and the deletion is linked with the early phenotype (Figs 2–5; Table 1), the candidature of TaFTSH4-D1 is questionable as its loss should result in late flowering.
For the Eps-B1 locus, sequencing of the TaFTSH4-B1 gene revealed that Cadenza has a deletion in exon 3, which introduces a premature stop codon, while Avalon has got an intact exon 3 (Table 1. However, even though Charger and Badger segregate for the same mutation as Avalon and Cadenza on 1BL, no Eps-B1 QTL was detected both in the field (Griffiths et al., 2009) and controlled environments for the Charger X Cadenza DH population. This brings to question the possibility that TaFTSH4-B1 is a candidate for Eps-B1 although it is possible that a background effect could be masking the effect in the Charger X Badger population.
It should also be pointed out here that the study in Arabidopsis showed that FTSH4 loss did not affect growth under adequate photoperiod but only affected late rosette development under limiting photoperiod when the plants were grown in short days (Gibala et al., 2009). This was different from the effect caused by Eps-D1 and Eps-B1, where the effect was stronger under long day conditions but not detectable under short days for both the Spark X Rialto and Avalon X Cadenza DH populations (Supplementary Fig. S1).
Possibility that TaELF3-D1 is the candidate for Eps-D1
In this study, TaELF3-D1, a circadian clock gene, is suggested as a possible candidate for Eps-D1 in addition to MOT1-D1 and FTSH4-D1 because it is contained within the subtelomeric deletion and has been shown to affect flowering in a number of species. The ELF3 gene is a repressor of flowering time in at least six species and one would predict that loss of function mutations like premature stop codons or deletion of the entire gene would result in an early flowering phenotype for wheat. Our results using NILs (Fig. 3; Zikhali et al., 2014) and SSD populations (Figs 4 and 5) for crosses between Spark and Rialto show that the deletion is linked with an early flowering phenotype and this is consistent with loss of ELF3 hence TaELF3-D1 is a likely candidate for Eps-D1. Here we offer support at the expression level for TaELF3-D1 as a candidate, because TaELF3 total expression is reduced when the early Spark allele is present and this is accompanied by increased expression of TaGI (Fig. 6). The Spark allele has the 1DL deletion, which includes TaELF3-D1, and since there is no TaELF3-D1 contributed by the Spark allele, we attribute the significant difference in the total TaELF3 expression to this deletion. Again our results suggest that there is no compensation from the other two genomes since the difference in total TaELF3 expression is significantly different in two independent NIL pairs (Fig. 6). All the three homoeologues of TaGI have higher expression later during the day in the Eps-D1 mutants relative to the wild type as would be expected for an ELF3 mutant since ELF3 is a repressor of GI (Higgins et al., 2010; Dixon et al., 2011; Faure et al., 2012; Zakhrabekova et al., 2012). Further studies will be needed to determine why the loss of only one genome leads to early flowering, but at this stage we can only speculate that this may be a dosage effect.
A patent in maize (Bate and Aukerman, 2011) showed that manipulating ELF3 can help to increase maize yield. The patent shows that over-expressing the Zea mays ELF3 (ZmELF3) gene enabled the plants to be grown at high density. This was because over-expressing ZmELF3 suppressed the shade avoidance response by enabling plants to tolerate limited light. The functioning of the ZmELF3 in delaying flowering is consistent with observations in our study where loss of TaELF3-D1 by deletion of the gene (Spark and Cadenza) results in early flowering relative to the plants with an intact gene which are later flowering. Furthermore, Boden et al. (2014) showed that the barley EARLY FLOWERING 3 gene suppresses gibberellin biosynthesis and that plants with constitutive gibberellin biosynthesis flowered earlier than mutants. Again this result is consistent with results from this study which suggest that Eps-D1 is due to a floral repressor, given that the 1DL deletion is associated with early flowering (Figs 2–5).
It has not escaped our attention that Eps-D1 is due to a deletion that includes several genes, so it is quite possible that another flowering repressor gene or a micro RNA could be among the deleted genes and act in addition to the candidates we characterized in this study. For Savannah X Rialto Eps-D1 locus (Supplementary Fig. S4B), no candidate mutations in the gene were observed but there were two silent mutations in exons 3 and 4 in Savannah (Supplementary Fig. S6A). Epigenetic control for TaELF3-D1 or other mutations in the promoter that we did not sequence may account for the Savannah X Rialto Eps-D1 effect. Further studies can be done in future to test this hypothesis.
Possibility that TaELF3-B1 is the candidate for Eps-B1
Sequencing TaELF3-B1 also showed that this gene is a plausible candidate for Eps-B1 because Avalon has a mutation that changes a conserved glycine to serine in exon 4 (Supplementary Fig. S6A). The mutant TaELF3-B1 (Avalon) would be predicted to result in early flowering relative to the wild type Cadenza and our results suggest that this is the case (Table 1).
However, Charger and Badger are polymorphic for the 1DL deletion (Figs 1B and 2) but there was no Eps-D1 QTL detected for this population in the field (Griffiths et al., 2009) and in the controlled environments in this study, as was observed for Eps-B1. We had expected this population to behave like the Spark X Rialto and Avalon X Cadenza DH populations, which segregate for Eps-D1 (Supplementary Fig. S1). Given that TaMOT1-D1, TaFTSH4-D1, and TaELF3-D1 are all deleted from Badger, we tested the hypothesis that the candidate gene among the three would have a loss of function mutation for Charger that would account for the unavailability of the Eps-D1 effect in the Charger X Badger population by sequencing the three genes for Charger. However, we did not detect mutations in Charger for all the three genes in their coding sequences. It is possible that there could be promoter mutations that we did not sequence in the three genes that may account for why Charger X Badger does not segregate for Eps-D1. It is also possible that Charger X Badger population behaves like the outliers for Eps-D1 (Supplementary Fig. S5). Further studies in future may resolve this anomaly.
Apart from the anomaly observed for Charger, which affects the candidature of all the three genes we prioritized, TaELF3-D1, TaMOT1-D1, and TaFTSH4-D1, we ranked TaELF3-D1 higher than TaFTSH4-D1 and TaMOT1-D1 as a possible candidate for Eps-D1 because higher expression of TaGI later during the day in the NILs carrying the Eps-D1 deletion and reduced total TaELF3 expression coupled with early flowering relative to the wild type is consistent with an ELF3 mutant. In addition to that, the mutations at TaELF3 explain the phenotypes at both Eps-D1 and Eps-B1, while mutations at both TaMOT1 and TaFTSH4 at best explain the phenotype at one locus.
Supplementary data
Supplementary data are available at JXB online.
Fig. S1. Eps-D1 QTLs.
Fig. S2. Position of the Xbarc62 SSR marker on 1DL.
Fig. S3. Agarose gel pictures showing the genes used to define the 1DL deletion.
Fig. S4. Chromosomal location of Eps-B1 QTL and Savannah X Rialto Eps-D1 QTL.
Fig. S5. The genotypes of the outliers in the Eps-D1 region
Fig. S6. Schematic diagrams showing the positions of SNPs for the genes TaELF3-D1, TaELF3-B1, TaELF3-A1, TaMOT1-D1, TaFTSH4-D1, and TaFTSH4-B1.
Fig. S7. Alignment of ELF3 proteins from different species showing conserved amino acid change in TaELF3-B1.
Table S1. The 40 syntenous B. distachyon genes used to define the 1DL deletion.
Table S2. KASP primer combinations for TaBradi2g14790, TaELF3-B1, TaELF3-D1 and TaMOT1-D1
Acknowledgements
The research leading to these results has received funding from the European Union Seventh Framework Programme (FP7/2007–2013) under grant agreement no. 289842 (ADAPTAWHEAT). Genetic diversity and yield stability for increased resilience against climate change in the UK (BB/H012176/1). MZ was partly supported during a Rotation PhD sponsored by the John Innes Foundation, John Innes Centre and the Sainsbury Laboratory.
MZ and SG conceived, designed, and performed the experiments, and wrote the paper; MZ, SG, and LUW analysed the data.
References
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. 1990. Basic Local Alignment Search Tool. Journal of Molecular Biology 215, 403–410. [DOI] [PubMed] [Google Scholar]
- Bate NJ, Aurkerman MJ. 2011. Composition and methods for increasing plant tolerance to high population density. United States Patent. Patent number 7,868,224 B2. Date of Patent 11 January 2011.
- Boden SA, Weiss D, Ross JJ, Davies NW, Trevaskis B, Chandler PM, Swain SM. 2014. EARLY FLOWERING 3 regulates flowering in spring barley by mediating gibberellin production and FLOWERING LOCUS T expression. The Plant Cell 26, 1557–1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenchley R, Spannagl M, Pfeifer M, et al. 2012. Analysis of the bread wheat genome using whole-genome shotgun sequencing. Nature , 491, 705–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullrich L, Appendino ML, Tranquilli G, Lewis S, Dubcovsky J. 2002. Mapping a thermo-sensitive earliness per se gene on Triticum monococcum chromosome 1Am . Theoretical and Applied Genetics 105, 585–593. [DOI] [PubMed] [Google Scholar]
- Coff C, Barling D, Korthals M, Nielsen T. 2008. Ethical Traceability and Communicating Food . Springer, Netherlands. [Google Scholar]
- Comadran J, Kilian B, Russel J, et al. 2012. Natural variation in a homologue of Antirrhinum CENRORADIALIS contributed to spring growth habit and environmental adaptation in cultivated barley. Nature Genetics 44, 1388–1392. [DOI] [PubMed] [Google Scholar]
- Dı′az A, Zikhali M, Turner AS, Isaac P, Laurie DA. 2012. Copy number variation affecting the Photoperiod-B1 and Vernalization-A1 genes is associated with altered flowering time in wheat (Triticum aestivum). PLoS One 7, e33234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Distelfeld A, Dubcovsky J. 2010. Characterization of the maintained vegetative phase deletions from diploid wheat and their effect on VRN2 and FT transcript levels. Molecular Genetics and Genomics 283, 223–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon J, Braun HJ, Kosina P, Crouch J. 2009. Wheat Facts and Futures 2009 . CIMMYT, Mexico, DF. [Google Scholar]
- Dixon LE, Knox K, Kozma-Bognar L, Southern MM, Pokhildo A, Millar A. 2011. Temporal repression of core circadian genes is mediated through EARLY FLOWERING 3 in Arabidopsis. Current Biology 21, 120–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faricelli ME, Valarik M, Dubcovsky J. 2010. Control of flowering time and spike development in cereals: the earliness per se Eps-1 region in wheat, rice, and Brachypodium. Functional & Integrative Genomics , 10, 293–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faure S, Turner AS, Gruszka D, Christodoulou V, Davis SJ, von Korff M, Laurie DA. 2012. Mutation at the circadian clock gene Early maturity 8 adapts domesticated barley (Hordeum vulgare) to short growing seasons. Proceedings of the National Academy of Sciences USA , 109, 8328–8333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fjellheim S, Boden S, Trevaskis B. 2014. The role of seasonal flowering responses in adaptation of grasses to temperate climates. Frontiers in Plant Science 5(431) doi: 10.3389/fpls.2014.00431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu D, Szucs P, Yan L, Helguera M, Skinner JS, von Zitzewitz J, Hayes PM, Dubcovsky J. 2005. Large deletions within the first intron in VRN-1 are associated with spring growth habit in barley and wheat. Molecular Genetics and Genomics 273, 54–65. [DOI] [PubMed] [Google Scholar]
- Gawrosnski P, Schnurbusch T. 2012. High-density mapping of the earliness per se-3Am (Eps-3Am) locus in diploid einkorn wheat and its relation to the syntenic regions in rice and Brachypodium distachyon L. Molecular Breeding 30, 1097–1108. [Google Scholar]
- Gawroński P, Ariyadasa R, Himmelbach A, et al. 2014. A distorted circadian clock causes early flowering and temperature-dependent variation in spike development in the Eps-3A m mutant of einkorn wheat. Genetics 196, 1253–1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibala M, Kicia M, Sakamoto W, Gola EM, Kubrakiewicz J, Smakowska E, Janska H. 2009. The lack of mitochondrial AtFtsH4 protease alters Arabidopsis leaf morphology at the late stage of rosette development under short-day photoperiod. The Plant Journal 59, 685–699. [DOI] [PubMed] [Google Scholar]
- Griffiths S, Simmonds J, Leverington M, et al. 2009. Meta- QTL analysis of the genetic control of ear emergence in elite European winter wheat germplasm. Theoretical and Applied Genetics 119, 383–395. [DOI] [PubMed] [Google Scholar]
- Gupta PK, Mir RR, Mohan A, Kumar J. 2008. Wheat Genomics: Present Status and Future Prospects. International Journal of Plant Genomics , doi:10.1155/2008/896451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins JA, Bailey PC, Laurie DA. 2010. Comparative Genomics of Flowering Time Pathways Using Brachypodium distachyon as a Model for the Temperate Grasses. PLoS ONE 5, e10065. doi:10.1371/journal.pone.0010065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaura K, Mochida K, Enju A, et al. 2009. Assessment of adaptive evolution between wheat and rice as deduced from the full-length cDNA sequence data and the expression patterns of common wheat. BMC Genomics 10, 271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurie DA, Pratchett N, Bezant JH, Snape JW. 1995. RFLP mapping of five major genes and eight quantitative trait loci controlling flowering time in a winter X spring barley Hordeum vulgare L. cross. Genome 38, 575–585. [DOI] [PubMed] [Google Scholar]
- Laurie DA. 1997. Comparative genetics of flowering time. Plant Molecular Biology 35, 167–177. [PubMed] [Google Scholar]
- Lewis S, Faricelli ME, Appendino ML, Valarik M, Dubcovsky J. 2008. The chromosome region including the earliness per se locus Eps-Am1 affects the duration of early developmental phases and spikelet number in diploid wheat. Journal of Experimental Botany 59, 3593–3607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin F, Xue SL, Tian DG, Li CJ, Cao Y, Zhang ZZ, Zhang CQ, Ma Q. 2008. Mapping chromosomal regions affecting flowering time in a spring wheat RIL population. Euphytica 164, 769–777. [Google Scholar]
- Lopes M, Reynolds MP, Manes Y, Singh RP, Crossa J, Braun HJ. 2012. Genetic yield gains and changes in associated traits of CIMMYT spring bread wheat in a ‘Historic’ set representing 30 years of breeding. Crop Science 52, 1123–1131. [Google Scholar]
- Matsubara K, Tanaka EO, Hori K, Ebana K, Ando T, Yano M. 2012. Natural variation in Hd17, a homolog of Arabidopsis ELF3 that is involved in rice photoperiod flowering. Plant Cell Physiology 53, 709–716. [DOI] [PubMed] [Google Scholar]
- Mizuno N, Nitta M, Sato K, Nasuda S. 2012. A wheat homologue of PHYTOCLOCK 1 is a candidate gene conferring the early heading phenotype to einkorn wheat. Genes & Genetic Systems 87, 357–367. [DOI] [PubMed] [Google Scholar]
- R Core Team. 2013. R: a language and environment for statistical computing . R Foundation for Statistical Computing, Vienna, Austria. [Google Scholar]
- Reynolds M, Foulkes J, Furbank R, Griffiths G, King J, Murchie E, Parry M, Slafer G. 2012. Achieving yield gains in wheat. Plant, Cell and Environment 35, 1799–1823. [DOI] [PubMed] [Google Scholar]
- Rosegrant MW, Agcaoili M. 2010. Global Food Demand, Supply, and Price Prospects to 2010 . International Food Policy Research Institute, Washington, DC, USA. [Google Scholar]
- Shaw LM, Turner AS, Laurie DA. 2012. The impact of photoperiod insensitive Ppd-1a mutations on the photoperiod pathway across the three genomes of hexaploid wheat (Triticum aestivum). The Plant Journal 71, 71–84. [DOI] [PubMed] [Google Scholar]
- Shindo C, Sasakuma T, Watanabe N, Noda K. 2002. Two-gene systems of vernalization requirement and narrow-sense earliness in einkorn wheat. Genome 45, 563–569. [DOI] [PubMed] [Google Scholar]
- Shitsukawa N, Ikari C, Shimada S, et al. 2007. The einkorn wheat (Triticum monococcum) mutant, maintained vegetative phase, is caused by a deletion in the VRN1 gene. Genes and Genetic Systems 82, 167–170. [DOI] [PubMed] [Google Scholar]
- Slafer GA, Rawson HM. 1994. Sensitivity of wheat phasic development to major environmrntal Factors: A re-examination of some assumptions made by physiologists and modellers. Australian Journal of Plant Physiology 21, 393–426. [Google Scholar]
- Song QJ, Shi JR, Singh S, Fickus EW, Costa JM, Lewis J, Gill BS, Ward R, Cregan PB. 2005. Development and mapping of microsatellite (SSR) markers in wheat. Theoretical and Applied Genetics 110, 550–560. [DOI] [PubMed] [Google Scholar]
- Turner A, Beales J, Faure S, Dunford RP, Laurie DA. 2005. The pseudo-response regulator Ppd-H1 provides adaptation to photoperiod in barley. Science 310, 1031–1034. [DOI] [PubMed] [Google Scholar]
- Valarik M, Linkiewicz AM, Dubcovsky J. 2006. A microcolinearity study at the earliness per se gene Eps-A m 1 region reveals an ancient duplication that preceded the wheat-rice divergence. Theoretical and Applied Genetics 112, 945–957. [DOI] [PubMed] [Google Scholar]
- Weller JL, Liew LC, Hecht VFG, et al. 2012. A conserved molecular basis for photoperiod adaptation in two temperate legumes. Proceedings of the National Academy of Sciences USA 109, 21158–21163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm EP, Turner AS, Laurie DA. 2009. Photoperiod insensitive Ppd-A1a mutations in tetraploid wheat (Triticum durum Desf.) Theoretical and Applied Genetics 118, 285–294. [DOI] [PubMed] [Google Scholar]
- Yan L, Loukoianov A, Tranquilli G, Helguera M, Fahima T, Dubcovsky J. 2003. Positional cloning of the wheat vernalization gene VRN1 . Proceedings of the National Academy of Sciences USA 100, 6263–6268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan L, Loukoianov A, Blechl A, Tranquilli G, Ramakrishna W, SanMiguel P, Bennetzen JL, Echenique V, Dubcovsky J. 2004. The wheat VRN2 gene is a Flowering repressor down-regulated by vernalization. Science 303, 1640–1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan L, Fu D, Li C, Blechl A, Tranquilli G, Bonafede M, Sanchez A, Valarik M, Dubcovsky J. 2006. The wheat and barley vernalization gene VRN3 is an orthologue of FT . Proceedings of the National Academy of Sciences USA 103, 19581–19586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zadoks JC, Chang TT, Konzak CF. 1974. A decimal code for the growth stages of cereals. Weed Research 14, 415–421. [Google Scholar]
- Zakhrabekova S, Gough SP, Braumann I, et al. 2012. Induced mutations in circadian clock regulator Mat—a facilitated short-season adaptation and range extension in cultivated barley. Proceedings of the National Academy of Sciences, USA 109, 4326–4331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zikhali M, Leverington-Waite M, Fish L, Simmonds J, Orford S, Wingen LU, Goram R, Gosman N, Bentley A, Griffiths S. 2014. Validation of a 1DL earliness per se (Eps) flowering QTL in bread wheat (Triticum aestivum). Molecular Breeding 34, 1023–2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zikhali M., Griffiths S. 2015. The effect of Earliness per se (Eps) genes on flowering time in bread wheat. In: Ogihara, Yasunari, Takumi, Shigeo, Handa, Hirokazu, eds. Advances in Wheat Genetics: From Genome to Field, Proceedings of the 12th International Wheat Genetics Symposium . Springer, Japan, 339–345. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.