Highlight
MSH1 participates in fertility reversion and male sterility by mediating mitochondrial genomic substoichiometric shifting of ORF220 and altering expression of anther development-associated genes in Brassica juncea.
Key words: Cytoplasmic male sterility, DNA recombination, mitochondrial DNA, MSH1, pollen, substoichiometric shifting.
Abstract
Cytoplasmic male sterility (CMS) has consistently been associated with the expression of mitochondrial open reading frames (ORFs) that arise from genomic rearrangements. Spontaneous fertility reversion in CMS has been observed in several cases, but a clear understanding of fertility reversion controlled by nuclear genetic influences has been lacking. Here, we identified spontaneous fertile revertant lines for Brassica juncea CMS cytoplasm in which the mitochondrial genome has undergone substoichiometric shifting (SSS) to suppress ORF220 copy number. We placed ORF220, with or without a mitochondrial targeting presequence, under the control of the CaMV35S and AP3 promoters in Arabidopsis to confirm that ORF220 causes male sterility when mitochondrially localized. We found that copy number of the ORF220 gene was altered under conditions that suppress MSH1, a nuclear gene that controls illegitimate recombination in plant mitochondria. MSH1-RNAi lines with increased ORF220 copy number were male sterile compared with wild type. We found that a wide range of genes involved in anther development were up- and down-regulated in revertant and MSH1-RNAi lines, respectively. The system that we have developed offers valuable future insight into the interplay of MSH1 and SSS in CMS induction and fertility reversion as a mediator of nuclear–mitochondrial crosstalk.
Introduction
Cytoplasmic male sterility (CMS) is a maternally inherited trait that prevents the production of functional pollen. The phenomenon, observed in >150 plant species with several conserved features in common, comprises one of very few systems of nuclear–mitochondrial genetic interaction amenable to study in higher plants. CMS has been associated with expression of novel mitochondrial open reading frames (ORFs) that arise by rearrangement of mitochondrial genomes (Hanson and Bentolila, 2004; Woodson and Chory, 2008). Fertility restorer (Rf) genes—pentatricopeptide repeat (PPR) proteins in most cases—are examples of nuclear genes that can alter mitochondrial CMS-associated gene expression. The PPR proteins usually operate at post-transcriptional levels, by RNA editing, processing, and polyadenylation, as well as post-translationally (Hanson and Bentolila, 2004; Schmitz-Linneweber and Small, 2008). In the system of Wild Abortive CMS in rice, for example, restorer genes Rf4 and Rf3 suppress the CMS-associated WA352 gene at transcriptional and translational levels, respectively (Luo et al., 2013).
Spontaneous fertility reversion in CMS has been seen in several plant species, and serves as an alternative means in nature to overcome mitochondrially encoded male sterility (Bellaoui et al., 1998; Arrieta-Montiel et al., 2001). Spontaneous fertility reversion in CMS is generally characterized by mitochondrial genomic substoichiometric shifting (SSS) (Arrieta-Montiel and Mackenzie, 2010), with the frequency of these genomic changes influenced by nuclear genetic background (Mackenzie et al., 1988; Small et al., 1988). An individual nuclear gene has been shown to reproducibly direct particular mitochondrial rearrangement events in common bean (Mackenzie and Chase, 1990), and natural or induced nuclear gene mutation can cause fertility reversion in carrot (Chahal et al., 1998) and rice (Shen et al., 1996). Tissue culture conditions can also give rise to fertility reversion in petunia and maize, again in association with mitochondrial genomic changes (Clark et al., 1988; Small et al., 1988; Fauron et al., 1990). However, the nuclear genes controlling mitochondrial genomic recombination to effect fertility reversion have not been identified in most cases.
The plant mitochondrial genome is known to undergo high frequency recombination and to comprise a multipartite organization (Arrieta-Montiel and Mackenzie, 2010; Marechal and Brisson, 2010). Asymmetric DNA exchange at small repeats appears to influence the stoichiometry of subgenomic mtDNA molecules—a phenomenon termed SSS (Small et al., 1989). This recombination is influenced by nuclear genes, including RecA3 and MSH1, which suppress ectopic mitochondrial recombination (Abdelnoor et al., 2003; Shedge et al., 2007). In Arabidopsis msh1, over 47 recombination repeat pairs become differentially active in the mitochondrial genome (Davila et al., 2011). Disruption of MSH1 has been shown to result not only in mitochondrial SSS, but also the appearance of CMS in several crops (Sandhu et al., 2007). Mitochondrial genome recombination plays an important role in plant mitochondrial genome evolution (Small et al., 1989; Chang et al., 2011b), generating novel mitotypes (Chen et al., 2011), and environmental adaptation (Shedge et al., 2010; Xu et al., 2011).
We previously developed a CMS line of Brassica juncea and identified the CMS-associated locus ORF220 in the mitochondrial genome (Yang et al., 2010). In this study, we identified fertile revertant lines in CMS B. juncea. We then established a link between MSH1 and mitochondrial genome rearrangements, effecting ORF220 SSS in association with fertility reversion. We suggest that the MSH1–mitochondrial interaction in plants may participate in the reversible male sterility–fertility transitions involved in gynodioecious reproductive systems.
Materials and methods
Plant materials
CMS and its fertile maintainer lines of B. juncea were used for identification of revertant lines and development of MSH1-RNAi lines. Wild type (WT) Arabidopsis (Arabidopsis thaliana) (Col-0) was used for transformation of ORF220 with and without a mitochondrial-targeting peptide under 35S (CaMV 35S) and AP3 (APETALA3) promoters. The mitochondrial targeting peptide was amplified from a previous construction plasmid (He et al., 1996). WT Arabidopsis (Col-0) was used for amplification of AP3 promoter sequences. A fertile isogenic maintainer line of B. juncea was used to generate the MSH1-RNAi line.
Mitochondrial genome assembly
Total DNA was isolated from fresh leaves of CMS and REV19 lines using a cetyl trimethylammonium bromide (CTAB) protocol. Total genomic DNA was prepared in paired-end libraries, tagged and sequenced on the Illumina Hiseq2500 platform. High quality reads were mapped to the B. juncea mitochondrial genome sequence (Genbank: KJ461445) using SAOP2, and paired mapping reads were extracted for mitochondrial genome assembly. These reads were assembled into scaffolds with the Velvet program (Zerbino and Birney, 2008).
DNA gel blotting and SSS of ORF220
Total genomic DNA samples were extracted from leaves for DNA gel blotting and SSS analysis of ORF220. For blotting, total genomic DNA samples were digested with HindIII endonuclease (Takara, Japan). Digested DNA samples were separated by electrophoresis for 24h, and were transferred and fixed to HyBond N+ nylon membrane (Amersham, Sweden) by capillary method. The ORF220 probe was prepared by PCR with the DIG probe synthesis kit (Roche, Switzerland). Hybridization was performed by standard pre-hybridization, probe denaturation, and hybridization in solution. The hybridization signal was detected using DIG High Prime DNA Labeling and Detection Starter II kit (Roche, Switzerland).
SSS of ORF220 was monitored by varying PCR amplification cycles. The PCR reaction was performed in a total volume of 50 μl containing 5 μl 10× Ex Taq Buffer (Mg2+ plus), 4 μl 10mM dNTP, 10 pmol of forward and reverse primers, 200ng of template DNA and 0.25 μl Ex TaqTM DNA Polymerase. The amount of template DNA was adjusted to be equal in each sample. The PCR solutions were incubated for 5min at 94 °C, and then run for 25, 30, and 35 cycles, respectively, at 94 °C for 30s, 50 °C for 30s, and 72 °C for 60 s, followed by final extension at 72 °C for 10min. The amplifications were separated by electrophoresis on 1% agarose gels. The primers used for ORF220 SSS assays are listed in Supplementary Table S1 at JXB online.
Expression analysis of ORF220
RT-PCR and real-time RT-PCR were used for transcriptional expression of ORF220. Protein gel blotting was employed to investigate translational expression of ORF220. ORF220 polyclonal antibodies were prepared by BGI Protein (BGI, China). Plant total proteins were extracted from floral buds using Plant Protein Extraction kit (BestBio, China). Plant proteins were separated in 5% and 12% gradient acrylamide gels, and were transferred to PVDF membrane and immunoblotted with anti-ORF220 polyclonal antibodies. The membrane was then combined with Enhanced Luminol Reagent and Oxidizing Reagent substrates. The signals were detected by FUJIFILM LAS-300 Luminescent Image Analyzer (FUJIFILM, Japan). The primers used for ORF220 assay are listed in Supplementary Table S1 at JXB online.
ORF220 construction and transformation in Arabidopsis
cDNA of ORF220 and the mitochondrial presequence were amplified according to our previous publication (Yang et al., 2010). The ORF220 and mt-ORF220 constructions were inserted into pMDC83 binary expression vector to generate ORF220-pMDC83 by gateway protocols (Curtis and Grossniklaus, 2003). Four constructions of ORF220 plasmid were introduced into Agrobacterium tumefaciens strain (GV1301) and the floral dipping method was used to introduce the ORF220 construction to Arabidopsis (Clough and Bent, 1998). PCR and RT-PCR amplifications of ORF220 confirmed successful transformations. WT and transgenic Arabidopsis T2 lines were used in this study. The primers used for ORF220 constructions are listed in Supplementary Table S1 at JXB online.
MSH1-RNAi line construction
The MSH1-RNAi construction was prepared identically with that reported in tomato previously (Sandhu et al., 2007). The MSH1-RNAi construct was made by cloning part of MSH1 (AT3G24320) domain VI in the RNAi vector pFGC1008 (Kerschen et al., 2004) using primers MSH1RNAI-AscI-F: AGTCGGCGCGCCATTGAGCCTGAAGCAATAGAATGTC; MSH1RNAI-SwaI-R: AGTCATTTAAATGAGGACGTTCC GAAATTACGGTGC; MSH1RNAI-SpeI-F: AGTCACTAGTATT GAGCCTGAAGCAATAGAATGTC; MSH1RNAI-BamHI-R: AG TCGGATCCGAGGACGTTCCGAAATTACGGTGC. Presence of MSH1 inserts and correct orientation was confirmed by PCR as well as sequencing using primers MSH1RNAI-AscI-F with Gus-5out: AGAGGTTAAAGCCGACAGCA for the left fragment and MSH1RNAI-SpeI-F with Gus-3out: AAGCAACGCGTAAACTCGAC for the right fragment. The MSH1-RNAi plasmid was then introduced into Agrobacterium tumefaciens strain GV1301. The transformation procedure generating the MSH1-RNAi line of B. juncea was as described previously (Yang et al., 2010). Transgenic lines were identified by Hygromycin B selection and by PCR amplification of a junction fragment consisting of vector and MSH1 gene sequence. The primers used for MSH1-RNAi line confirmation are listed in Supplementary Table S1 at JXB online.
Transcriptome analysis in CMS and revertant lines of B. juncea
Total RNAs isolated from floral buds of CMS and REV19 lines of B. juncea were used for global transcript analysis by RNA-seq. The protocols for library construction and sequencing were the standard procedures provided by Illumina (NEBNextUltraTM RNA library Prep kit, Illumina, USA). The sequencing was performed using the Illumina HiSeq™ 2500 System according to the manufacturer’s protocol (50bp single read module). An average of 8.5 G clean reads for each library was used for differential gene expression analysis. For each sequenced library, the read counts were adjusted using the edger program package through one scaling normalized factor. Differential expression analysis was performed using the DEGseq R package. P values were adjusted using the Benjamini & Hochberg method. Corrected P-value of 0.005 and log2 (fold change) of 1 were set as the threshold for significantly differential expression. Gene Ontology (GO) enrichment analysis of differentially expressed genes was implemented by GOseq R package, in which gene length bias was corrected. GO terms with corrected P-value <0.05 were considered significantly enriched by differentially expressed genes. Clusters of orthologous groups (COG) analysis was used as an online service (www.ncbi.nlm.nih.gov/COG/).
Differentially expressed genes by RNA-seq were annotated based on whole genome sequence information. Then we selected 15 annotated anther development-associated genes to represent candidate genes involved in Arabidopsis anther development (Chang et al., 2011a). Quantitative (q)PCR was used to study expression patterns of these selected anther development-associated genes. The primers for these anther-related genes are listed in Supplementary Table S1 at JXB online.
Results
Identification of fertility reversion in B. juncea
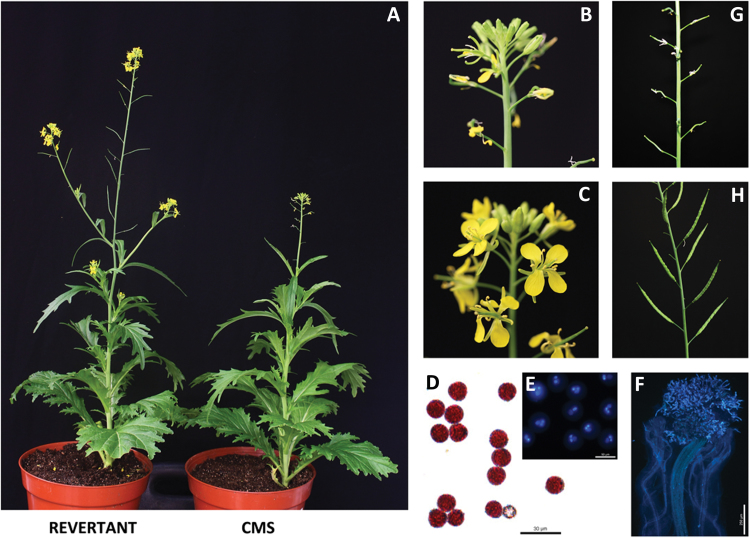
Fifty-three seeds from self-pollination were collected from 39 CMS B. juncea (T84-66A) plants, of which two seeds gave rise to male fertile plants, designated revertants REV19 and REV21, and the remaining were male sterile plants (see Supplementary Table S2 and Supplementary Fig. S1 at JXB online). REV19 displayed earlier flowering than the CMS isoline (Fig. 1A), with full flower structure and normal stamens (Fig. 1B, C). Pollen from REV19 appeared normal based on Alexander staining (Fig. 1D), DAPI staining (Fig. 1E), and in situ germination on stigmas (Fig. 1F). Consequently, seed set was fully recovered in REV19 compared with the CMS line (Fig. 1G, H). REV19 progeny showed full fertility in three consecutive self-crossed generations, but could not restore fertility to the CMS line in crossing as a pollen parent, indicating that the reversion represents a cytoplasmic event.
Fig. 1.
Phenotypes of cytoplasmic male sterile and revertant lines of B. juncea. (A) CMS and REV19 plants. (B) CMS inflorescence. (C) REV19 inflorescence. (D) Alexander staining of pollen from REV19. (E) DAPI staining of pollen from REV19. (F) In situ germination on stigma of pollen from REV19. (G) Silique from CMS line by self-crossing. (H) Silique of REV19 by self-crossing. (This figure is available in color at JXB online).
Mitochondrial genome rearrangement and SSS of ORF220 in CMS and REV lines
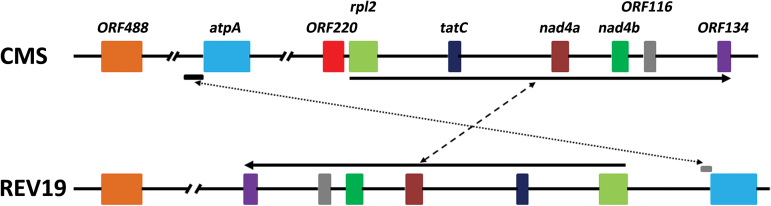
We compared mitochondrial DNA in CMS and REV19 using assembled mt genome scaffolds, confirming that REV19 is not a fertile maintainer line contaminant (see Supplementary Fig. S2 at JXB online). We previously identified the CMS-associated ORF220 from CMS B. juncea (Yang et al., 2010). We compared mitochondrial DNA intervals encompassing CMS-associated ORF220 and flanking regions in the two lines, and observed two genome rearrangements around ORF220—a genomic insertion of atpA and a reverse complement sequence composed of several mitochondrial genes (Fig. 2). Results indicated that ORF220 and its flanking regions undergo extensive genomic rearrangement between CMS and REV19 (Supplementary Fig. S3). We also observed several additional mitochondrial rearrangements in other regions of the mitochondrial genomes between CMS and REV19 (Supplementary data).
Fig. 2.
Schematic diagram of ORF220 and its flanking regions. Dashed lines show rearrangement events. Solid lines represent recombination direction. (This figure is available in color at JXB online).
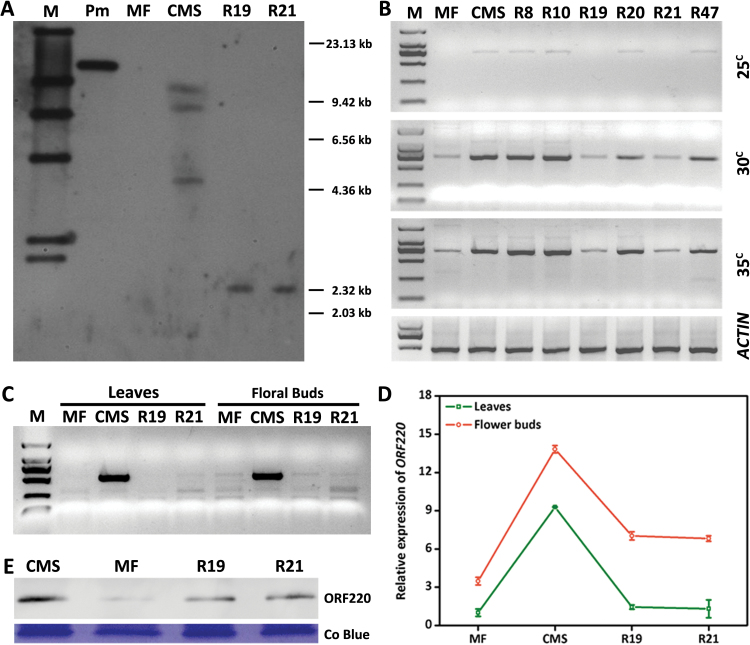
Different configurations of CMS-associated ORF220 were found in the CMS, REV19, REV21, and fertility maintainer (MF) lines, indicated by DNA gel blotting (Fig. 3A). We checked ORF220 copy number by PCR-based amplification, showing evidence of SSS in the various lines (Fig. 3B). Expression of ORF220 was significantly increased in the CMS line and decreased in REV19 based on RT-PCR and qRT-PCR (Fig. 3C, D), as well as protein gel blotting (Fig. 3E). The apparent correspondence of ORF220 copy number with gene expression levels in the male sterile and revertant lines suggests that SSS of ORF220 is associated with fertility reversion in CMS B. juncea.
Fig. 3.
SSS and expression of ORF220. (A) DNA gel blot probed with ORF220 following HindIII digestion. Pm (plasmid). (B) SSS analysis of ORF220. (C) Steady state transcript levels of ORF220 by semi-RT-PCR and qRT-PCR (D). (E) Protein levels of ORF220 by protein gel blot analysis. (This figure is available in color at JXB online).
Mitochondrially targeted ORF220 causes male sterility in Arabidopsis
To further test the association of ORF220 with male sterility, we developed Arabidopsis lines containing ORF220 gene constructions with and without a mitochondrial targeting presequence and under control of the CaMV 35S (constitutive) and AP3 (flower-specific) promoters (see Supplementary Fig. S4 at JXB online). Plants containing the construct with the 35S promoter, with and without presequence, showed evidence of slightly reduced growth (Fig. 4A). In total, 17 plants that were transformed with the mitochondrially targeting construct were male sterile and plants transformed with the construct lacking presequence showed no evidence of sterility when expressed under control of the 35S promoter (Supplementary Table S3; Fig. 4B–E). Moreover, in constructs containing the AP3 promoter, 28 plants containing the construct with mitochondrial presequence were male sterile and one plant with the construct lacking presequence showed male sterility (Supplementary Table S3; Fig. 4G–J). These results are consistent with our hypothesis that mitochondrially localized ORF220 causes male sterility.
Fig. 4.
Mitochondrially targeted expression of ORF220 in Arabidopsis thaliana. (A) Transgenic plants with or without mitochondrial (mtT) presequence under control of the CaMV 35S promoter. (B) WT flower. (C) 35S::ORF220 flower. (D) 35S::mtT::ORF220 flower. (E) Silique from WT, 35S::ORF220, and 35S::mtT::ORF220 plant top-down. (F) Transgenic plants with or without mitochondrial presequence under the AP3 promoter. (G) WT flower. (H) AP3::ORF220 flower. (I) AP3:mtT::ORF220 flower. (J) Silique from WT, AP3::ORF220, and AP3::mtT::ORF220 plant top-down. (This figure is available in color at JXB online).
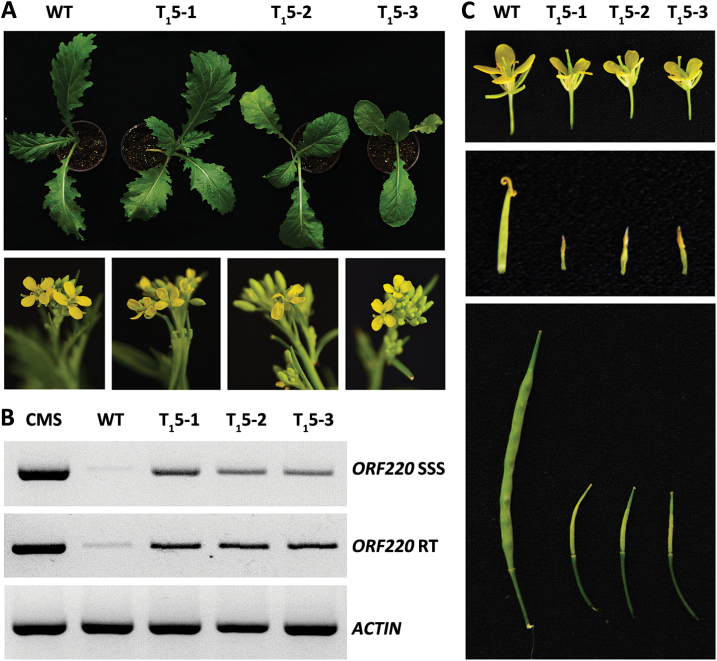
Phenotypes and ORF220 SSS in MSH1-RNAi lines
Two MSH1 genes were isolated from the B. juncea genome with high amino acid sequence similarity to their ortholog in Arabidopsis (see Supplementary Fig. S5 at JXB online). We developed four independent MSH1-RNAi lines of B. juncea with confirmed suppression of MSH1 expression, where two of the lines showed male sterility in the T1 generation. Varied leaf shape and normal flowering were also observed in the MSH1-RNAi lines (Fig. 5A). ORF220 copy number assays showed evidence of SSS following MSH1 suppression (Fig. 5B), and transcript levels of ORF220 were correspondingly increased in MSH1-RNAi lines (Fig. 5B). Comparison of three other mitochondrial genes in these lines indicated no evidence of gene alteration or copy number shifting (Supplementary Fig. S6). B. juncea MSH1-RNAi lines produced small flowers (Fig. 5C), and stamen development was severely affected, such that anthers were not observed (Fig. 5C). The three MSH1-RNAi lines produced no seed by self-pollination (Fig. 5C), although seed set occurred with pollen from the WT. These results indicate that MSH1 suppression can lead to SSS of ORF220 and male sterility in B. juncea.
Fig. 5.
MSH1-RNAi construction in B. juncea and SSS of ORF220. (A) Seedling and inflorescence of WT and MSH1-RNAi lines. (B) SSS and transcript levels of ORF220 in WT and MSH1-RNAi lines. (C) Flowers, stamens, and siliques of self-crossing from WT and MSH1-RNAi lines. (This figure is available in color at JXB online).
Anther development-associated gene expression
We employed RNA-seq to identify global transcriptional differences between CMS and REV19 lines of B. juncea, and to investigate the nature of mitochondrial retrograde regulation associated with fertility reversion. In total, we found 4880 differentially expressed genes between CMS and REV19 lines (see Supplementary Table S4; Supplementary Fig. S7). The identified genes were involved in metabolic processes, response to stimulus, biological regulation, developmental processes, reproduction, and reproductive processes by GO analysis (Supplementary Fig. S8). By COG analysis, the differentially expressed genes involved functions in replication, recombination and repair, energy production and conversion, carbohydrate transport and metabolism, cell cycle control, cell division, chromosome partitioning, and signal transduction (Supplementary Fig. S9).
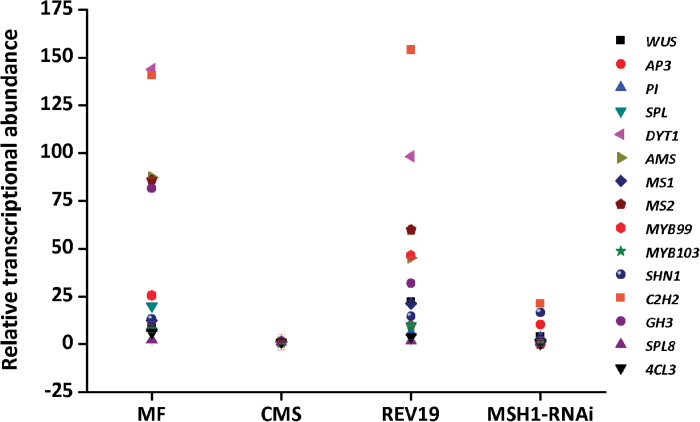
We selected 15 anther development-associated genes (Table 1) to investigate transcription patterns among MF, CMS, REV19, and MSH1-RNAi lines of B. juncea. Expression of these anther development genes is up-regulated in REV19 compared with CMS lines, accompanied by reversion from male-sterile to fertile. Moreover, these genes are down-regulated in the MSH1-RNAi line compared with WT, with transition from male fertile to sterile (Fig. 6). For example, at early-stage initiation of anther development, the expression of WUS and several MADS-box genes, including AP3, AG, and PI, increased in REV19 relative to CMS, and showed decrease in the MSH1-RNAi line relative to WT (Fig. 6). During anther morphogenesis, the key regulatory gene for microsporogenesis SPOROCYTELESS (SPL) was restored to normal transcript levels in REV19, and decreased in MSH1-RNAi compared with WT (Fig. 6). We also found that expression of DYT1, AMS, MS1, MS2, MYB99, and MYB103 were correspondingly increased in REV19 compared with CMS, and decreased in the MSH1-RNAi line compared with WT at late-stage tapetum function and pollen development (Fig. 6). These results indicate that male-sterility induction by MSH1 suppression and fertility reversion—via SSS—are accompanied by corresponding changes in anther-associated gene expression, implying a relationship between mitochondrial genome behavior and anther development programs.
Table 1.
Transcriptional analysis of anther development-associated genes by RNA-seq
| Gene ID | CMS reads | REV19 reads | Log2 FC (CMS/REV19) | Ortholog in Arabidopsis | Annotation |
|---|---|---|---|---|---|
| Bju009726 | 4 | 42 | –3.325892244 | WUS | WUSCHEL, homeobox gene controlling the stem cell |
| Bju047574 | 108 | 321 | –1.881915185 | AP3 | APETELA3, floral homeotic gene encoding a MADS domain transcription factor |
| Bju012907 | 138 | 308 | –1.472805418 | PI | PISTILLATA, floral homeotic gene encoding a MADS domain transcription factor |
| Bju010658 | 1 | 35 | –4.246938286 | SPL | SPOROCYTELESS, initiation of micro- and megagametogenesis |
| Bju083268 | 22 | 855 | –5.518191891 | DYT1 | DYSFUNCTIONAL TAPETUM 1 |
| Bju076135 | 12 | 634 | –5.892604764 | AMS | ABORTED MICROSPORES |
| Bju004296 | 0.01 | 34 | –4.992803589 | MS1 | MALE STERILITY 1 |
| Bju014803 | 43 | 1911 | –5.752855182 | MS2 | MALE STERILITY 2 |
| Bju003047 | 1 | 178 | –6.557555136 | MYB99 | MYB transcription factor |
| Bju027475 | 0.01 | 21 | –4.325437835 | MYB103 | MYB transcription factor |
| Bju072885 | 2 | 47 | –4.154850764 | At1g02040 | zinc finger (C2H2 type) family protein |
| Bju024267 | 56 | 169 | –1.891531152 | 4CL3 | pollen exine formation |
| Bju028126 | 32 | 2446 | –4.325437835 | AT5G13380 | Auxin-responsive GH3 family protein, pollen exine formation |
| Bju002493 | 54 | 186 | –2.080138807 | SHN1/WIN1 | ERF/AP2 transcription factor |
| Bju038196 | 109 | 26 | 1.702239773 | SPL8 | SQUAMOSA PROMOTER BINDING PROTEIN-LIKE 8 |
Fig. 6.
Transcriptional analysis of anther development-associated genes in fertile maintainer, CMS, REV19, and MSH1-RNAi lines of B. juncea. (This figure is available in color at JXB online).
Discussion
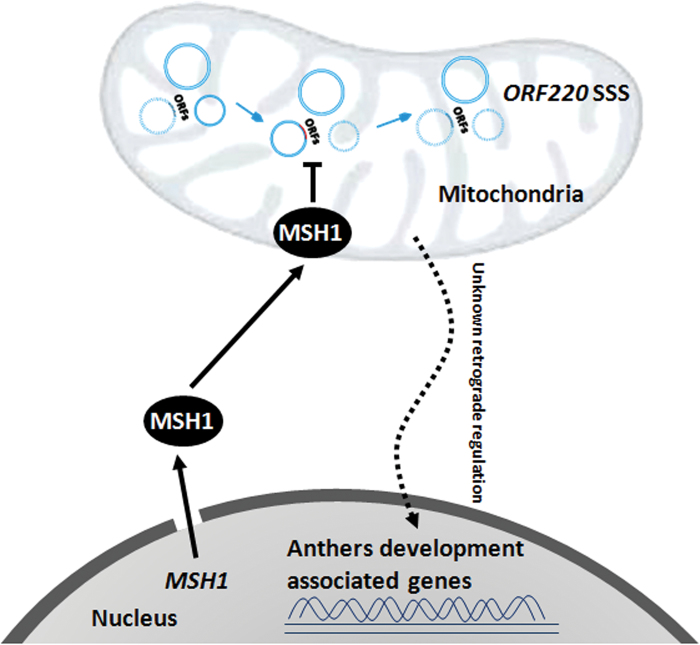
CMS and fertility restoration are valuable components of hybrid breeding systems in crops, deriving from competitive interactions between mitochondrial and nuclear genomes (Ma, 2013; Chen and Liu, 2014). Spontaneous fertility reversion sporadically occurs in some CMS systems, providing insight into the relationship of mitochondrial SSS and plant reproductive behavior (Escote et al., 1985; Rottmann et al., 1987; Janska et al., 1998; Feng et al., 2009). These spontaneous fertility reversion events are influenced in frequency by nuclear background (Mackenzie et al., 1988; Small et al., 1988), and can be problematic to commercial interests for CMS implementation. In most fertility reversion cases previously reported, the nuclear genes involved in triggering mitochondrial genome rearrangement are largely unknown. In the case of MSH1, previous evidence suggests that the loss of MSH1 function creates conditions conducive to mitochondrial asymmetric DNA exchange (Davila et al., 2011). We propose that the spontaneous SSS of ORF220 for fertility reversion in B. juncea is associated with processes controlled, at least in part, by MSH1 (Fig. 7).
Fig. 7.
A working model for MSH1 mediating ORF220 SSS and causing male sterility. (This figure is available in color at JXB online).
SSS of ORF220 is associated with spontaneous fertility reversion
Mitochondrial DNA rearrangements are often observed in some CMS systems upon reversion to fertility (Fauron et al., 1987; Smith et al., 1987; Escotecarlson et al., 1988; Mackenzie et al., 1988; Bellaoui et al., 1998; Janska et al., 1998), with dramatic reduction in relative copy number of the CMS sequence in each case. Here, we demonstrated that the SSS of CMS-associated ORF220 occurs in association with fertility reversion in CMS B. juncea. To confirm that ORF220 is sufficient to condition the CMS phenotype, ORF220 was mitochondrially targeted, and the transgenic plants displayed male sterility in both Arabidopsis and B. juncea (Yang et al., 2010). The amenability of this system to both transgenic induction and to fertility reversion provides a valuable opportunity for more detailed investigations of factors influencing nuclear–mitochondrial stability.
Depressed expression of MSH1 caused SSS of ORF220 and male sterility
It is not clear the extent to which MSH1 variation might have influenced spontaneous CMS reversion in natural systems. In the case of CMS common bean, SSS of the CMS-associated pvs-orf239 was associated with changes in a single nuclear gene that, at that time, was designated Fr (Mackenzie and Chase, 1990). It has not been determined whether Fr might represent MSH1 or a gene modulating MSH1.
Plant mitochondrial genome stability is controlled by nuclear recombination surveillance mechanisms that include at least three nuclear genes, MSH1, RecA3, and OSB1 (Zaegel et al., 2006; Shedge et al., 2007; Arrieta-Montiel et al., 2009). Of these three genes, disruption of MSH1 can also influence male sterility (Sandhu et al., 2007). Here, we present evidence that further substantiates this causality by SSS of CMS-associated ORF220 when MSH1 is suppressed. Pollen fertility was significantly reduced in MSH1-RNAi lines of B. juncea. However, the MSH1-associated SSS process observed in Arabidopsis Col-0 does not result in male sterility, indicating that not all SSS events necessarily give rise to a CMS phenotype (Abdelnoor et al., 2003). This response in Arabidopsis may be due to the lack of a CMS-associated mitochondrial sequence in the Col-0 ecotype (Gobron et al., 2013). Furthermore, only partial male sterility was observed in MSH1-RNAi lines of B. juncea in this study, and in MSH1-RNAi lines of tomato and tobacco plants previously (Sandhu et al., 2007). SSS events are bidirectional and dynamic, so that only a fraction of CMS-associated genes might achieve a threshold to cause male sterility.
This fertility reversion mechanism is distinguished from fertility recovery by nuclear fertility restoration. CMS-associated gene expression has been observed to be modulated by nuclear restorer genes, which also sometimes affect mitochondrial metabolism to confer biochemical activities that facilitate pollen development (Liu et al., 2001; Hanson and Bentolila, 2004; Chase, 2007; Ma, 2013; Chen and Liu, 2014). In nature, it is reasonable to assume that both restorer and reversion mechanisms are operational, with restorer systems providing a means of recovering self-fertility under conditions when CMS plants are located within cross-compatible populations, and reversion providing a means of self-pollination when CMS plants are in reproductive isolation.
Fertility reversion is associated with anther development-associated gene expression
Early anther development includes stamen identity determination, lobed anther structure morphogenesis, anther cell layer specification, and early microspore development processes. Molecular genetic studies have uncovered crucial molecules and transcription factors that function in determining anther cell types and in controlling gene expression regulatory networks for anther development (Chang et al., 2011a; Pearce et al., 2015). We observed that the reproductive dynamics created by manipulating mitochondrial genome behavior in B. juncea includes altered expression of several anther development genes in CMS, REV19, WT, and MSH1-RNAi lines. Increased expression of WUSCHEL (WUS), APETELA3 (AP3), and PISTILLATA (PI) occurred with recovery of floral structure development in the REV19 line. Correspondingly, reduced expression of these genes may cause the abnormal adhesive structure of petal and stamen observed in MSH1-RNAi lines. SPOROCYTELESS (SPL)—essential for the formation of reproductive cells and microsporogenesis—was altered in expression, suggesting its role in specifying the reproductive cell fate in these lines (Liu et al., 2009). We observed increased expression of SPL in REV19 and decreased expression in MSH1-RNAi lines, suggesting the action of SPL in fertility conversion. These results indicate that male sterility and fertility reversion, caused by the SSS of ORF220 and mediated by MSH1, involve differential regulation of anther development networks.
A distinctive characteristic of plant mitochondrial genomes is their recombinational versatility. The SSS activity of mitochondrial genomes likely serves as an important mechanism for maintaining appropriate function while retaining mitochondrial adaptive genetic diversity (Small et al., 1987). Our findings here suggest that it is feasible to directly manipulate the MSH1-mediated sterility–fertility reversion mechanism in crops, a promising first step toward enhancing breeding potential by creating CMS or controlling fertility reversion behavior.
Supplementary data
Supplementary data are available at JXB online.
Supplementary Fig. S1. Candidate revertant lines of B. juncea.
Supplementary Fig. S2. Comparison of mitochondrial DNA from CMS and revertant lines of B. juncea.
Supplementary Fig. S3. Mitochondrial genome rearrangement of the atpA gene.
Supplementary Fig. S4. Schematic diagram of ORF220 gene construction.
Supplementary Fig. S5. MSH1 from B. juncea and comparison with its ortholog in Arabidopsis thaliana.
Supplementary Fig. S6. SSS of mitochondrial genes in MSH1-RNAi lines relative to WT.
Supplementary Fig. S7. Genes differentially expressed between CMS and REV19 lines of B. juncea by RNA-seq.
Supplementary Fig. S8. Gene Ontology enrichment analysis of differentially expressed genes.
Supplementary Fig. S9. COG analysis of differentially expressed genes.
Supplementary Table S1. Candidate revertant events from CMS B. juncea.
Supplementary Table S2. Fertility of ORF220 expression in Arabidopsis.
Supplementary Table S3. Primers used in this study.
Supplementary Table S4. Genes differentially expressed between CMS and REV19 by RNA-seq.
Supplementary data. Assembled mitochondrial genomic scaffolds of CMS and REV19 lines.
Acknowledgements
This work is supported by grants from the National Natural Science Foundation of China (31372063).
References
- Abdelnoor RV, Yule R, Elo A, Christensen AC, Meyer-Gauen G, Mackenzie SA. 2003. Substoichiometric shifting in the plant mitochondrial genome is influenced by a gene homologous to MutS. Proceedings of the National Academy of Sciences of the United States of America 100, 5968–5973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrieta-Montiel M, Lyznik A, Woloszynska M, Janska H, Tohme J, Mackenzie S. 2001. Tracing evolutionary and developmental implications of mitochondrial stoichiometric shifting in the common bean. Genetics 158, 851–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrieta-Montiel M, Mackenzie SA. 2010. Plant mitochondrial genomes and recombination. In: Kempken F, ed. Advances in plant biology: plant mitochondria , Springer: New York, pp. 65–84. [Google Scholar]
- Arrieta-Montiel MP, Shedge V, Davila J, Christensen AC, Mackenzie SA. 2009. Diversity of the Arabidopsis Mitochondrial Genome Occurs via Nuclear-Controlled Recombination Activity. Genetics 183, 1261–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellaoui M, Martin-Canadell A, Pelletier G, Budar F. 1998. Low-copy-number molecules are produced by recombination, actively maintained and can be amplified in the mitochondrial genome of Brassicaceae: relationship to reversion of the male sterile phenotype in some cybrids. Molecular and General Genetics 257, 177–185. [DOI] [PubMed] [Google Scholar]
- Chahal A, Sidhu HS, Wolyn DJ. 1998. A fertile revertant from petaloid cytoplasmic male-sterile carrot has a rearranged mitochondrial genome. Theoretical and Applied Genetics 97, 450–455. [Google Scholar]
- Chang F, Wang YX, Wang SS, Ma H. 2011a. Molecular control of microsporogenesis in Arabidopsis. Current Opinion in Plant Biology 14, 66–73. [DOI] [PubMed] [Google Scholar]
- Chang SX, Yang TT, Du TQ, Huang YJ, Chen JM, Yan JY, He JB, Guan RZ. 2011b. Mitochondrial genome sequencing helps show the evolutionary mechanism of mitochondrial genome formation in Brassica. BMC Genomics 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase CD. 2007. Cytoplasmic male sterility: a window to the world of plant mitochondrial-nuclear interactions. Trends in Genetics 23, 81–90. [DOI] [PubMed] [Google Scholar]
- Chen JM, Guan RZ, Chang SX, Du TQ, Zhang HS, Xing H. 2011. Substoichiometrically different mitotypes coexist in mitochondrial genomes of Brassica napus L. Plos One 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen LT, Liu YG. 2014. Male Sterility and Fertility Restoration in Crops. Annual Review of Plant Biology , 65, 579–606. [DOI] [PubMed] [Google Scholar]
- Clark E, Gafni Y, Izhar S. 1988. Loss of Cms-specific mitochondrial-dna arrangement in fertile segregants of petunia hybrids. Plant Molecular Biology 11, 249–253. [DOI] [PubMed] [Google Scholar]
- Clough SJ, Bent AF. 1998. Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant Journal 16, 735–743. [DOI] [PubMed] [Google Scholar]
- Curtis MD, Grossniklaus U. 2003. A gateway cloning vector set for high-throughput functional analysis of genes in planta. Plant Physiology 133, 462–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davila JI, Arrieta-Montiel MP, Wamboldt Y, Cao J, Hagmann J, Shedge V, Xu YZ, Weigel D, Mackenzie SA. 2011. Double-strand break repair processes drive evolution of the mitochondrial genome in Arabidopsis. BMC Biology 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escote LJ, Gabaylaughnan SJ, Laughnan JR. 1985. Cytoplasmic reversion to fertility in Cms-S maize need not involve loss of linear mitochondrial plasmids. Plasmid 14, 264–267. [DOI] [PubMed] [Google Scholar]
- Escotecarlson LJ, Gabaylaughnan S, Laughnan JR. 1988. Reorganization of mitochondrial genomes of cytoplasmic revertants in Cms-S inbred line Wf9 in Maize. Theoretical and Applied Genetics 75, 659–667. [Google Scholar]
- Fauron CM, Havlik M, Brettell RI. 1990. The mitochondrial genome organization of a maize fertile cmsT revertant line is generated through recombination between two sets of repeats. Genetics 124, 423–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauron CMR, Abbott AG, Brettell RIS, Gesteland RF. 1987. Maize Mitochondrial-DNA rearrangements between the normal type, the Texas male sterile cytoplasm, and a fertile revertant Cms-T regenerated plant. Current Genetics 11, 339–346. [Google Scholar]
- Feng X, Kaur AP, Mackenzie SA, Dweikat IM. 2009. Substoichiometric shifting in the fertility reversion of cytoplasmic male sterile pearl millet. Theoretical and Applied Genetics 118, 1361–1370. [DOI] [PubMed] [Google Scholar]
- Gobron N, Waszczak C, Simon M, Hiard S, Boivin S, Charif D, Ducamp A, Wenes E, Budar F. 2013. A cryptic cytoplasmic male sterility unveils a possible gynodioecious past for Arabidopsis thaliana. Plos One 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson MR, Bentolila S. 2004. Interactions of mitochondrial and nuclear genes that affect male gametophyte development. Plant Cell 16, S154–S169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He SC, Abad AR, Gelvin SB, Mackenzie SA. 1996. A cytoplasmic male sterility-associated mitochondrial protein causes pollen disruption in transgenic tobacco. Proceedings of the National Academy of Sciences of the United States of America 93, 11763–11768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janska H, Sarria R, Woloszynska M, Arrieta-Montiel M, Mackenzie SA. 1998. Stoichiometric shifts in the common bean mitochondrial genome leading to male sterility and spontaneous reversion to fertility. Plant Cell 10, 1163–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerschen A, Napoli CA, Jorgensen RA, Muller AE. 2004. Effectiveness of RNA interference in transgenic plants. FEBS Letters 566, 223–228. [DOI] [PubMed] [Google Scholar]
- Liu F, Cui XQ, Horner HT, Weiner H, Schnable PS. 2001. Mitochondrial aldehyde dehydrogenase activity is required for male fertility in maize. Plant Cell 13, 1063–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu XD, Huang J, Parameswaran S, Ito T, Seubert B, Auer M, Rymaszewski A, Jia G, Owen HA, Zhao DZ. 2009. The SPOROCYTELESS/NOZZLE Gene Is Involved in Controlling Stamen Identity in Arabidopsis. Plant Physiology 151, 1401–1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo DP, Xu H, Liu ZL, et al. 2013. A detrimental mitochondrial-nuclear interaction causes cytoplasmic male sterility in rice. Nature Genetics 45, 573–577. [DOI] [PubMed] [Google Scholar]
- Ma H. 2013. A battle between genomes in plant male fertility. Nature Genetics 45, 472–473. [DOI] [PubMed] [Google Scholar]
- Mackenzie SA, Chase CD. 1990. Fertility restoration is associated with loss of a portion of the mitochondrial genome in cytoplasmic male-sterile common bean. Plant Cell 2, 905–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenzie SA, Pring DR, Bassett MJ, Chase CD. 1988. Mitochondrial-DNA rearrangement associated with fertility restoration and cytoplasmic reversion to fertility in cytoplasmic male sterile Phaseolus-Vulgaris L. Proceedings of the National Academy of Sciences of the United States of America 85, 2714–2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marechal A, Brisson N. 2010. Recombination and the maintenance of plant organelle genome stability. New Phytologist 186, 299–317. [DOI] [PubMed] [Google Scholar]
- Pearce S, Ferguson A, King J, Wilson ZA. 2015. FlowerNet: a gene expression correlation network for anther and pollen development. Plant Physiology 167, 1717–1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottmann WH, Brears T, Hodge TP, Lonsdale DM. 1987. A mitochondrial gene is lost via homologous recombination during reversion of CMS T maize to fertility. EMBO Journal 6, 1541–1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandhu APS, Abdelnoor RV, Mackenzie SA. 2007. Transgenic induction of mitochondrial rearrangements for cytoplasmic male sterility in crop plants. Proceedings of the National Academy of Sciences of the United States of America 104, 1766–1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz-Linneweber C, Small I. 2008. Pentatricopeptide repeat proteins: a socket set for organelle gene expression. Trends in Plant Science 13, 663–670. [DOI] [PubMed] [Google Scholar]
- Shedge V, Arrieta-Montiel M, Christensen AC, Mackenzie SA. 2007. Plant mitochondrial recombination surveillance requires unusual RecA and MutS homologs. Plant Cell 19, 1251–1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shedge V, Davila J, Arrieta-Montiel MP, Mohammed S, Mackenzie SA. 2010. Extensive rearrangement of the Arabidopsis mitochondrial genome elicits cellular conditions for thermotolerance. Plant Physiology 152, 1960–1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Y, Cai Q, Gao M, Wang X. 1996. Isolation and genetic characterization of a fertility-restoring revertant induced from cytoplasmic male sterile rice. Euphytica 90, 17–23. [Google Scholar]
- Small I, Suffolk R, Leaver CJ. 1989. Evolution of plant mitochondrial genomes via substoichiometric intermediates. Cell 58, 69–76. [DOI] [PubMed] [Google Scholar]
- Small ID, Isaac PG, Leaver CJ. 1987. Stoichiometric differences in DNA-molecules containing the atpa gene suggest mechanisms for the generation of mitochondrial genome diversity in maize. EMBO Journal 6, 865–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small ID, Earle ED, Escotecarlson LJ, Gabaylaughnan S, Laughnan JR, Leaver CJ. 1988. A comparison of cytoplasmic revertants to fertility from different Cms-S maize sources. Theoretical and Applied Genetics 76, 609–618. [DOI] [PubMed] [Google Scholar]
- Smith RL, Chowdhury MKU, Pring DR. 1987. Mitochondrial-DNA rearrangements in pennisetum associated with reversion from cytoplasmic male-sterility to fertility. Plant Molecular Biology 9, 277–286. [DOI] [PubMed] [Google Scholar]
- Woodson JD, Chory J. 2008. Coordination of gene expression between organellar and nuclear genomes. Nature Reviews Genetics 9, 383–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu YZ, Arrieta-Montiel MP, Virdi KS, et al. 2011. MutS HOMOLOG1 is a nucleoid protein that alters mitochondrial and plastid properties and plant response to high light. Plant Cell 23, 3428–3441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang JH, Liu XY, Yang XD, Zhang MF. 2010. Mitochondrially-targeted expression of a cytoplasmic male sterility-associated orf220 gene causes male sterility in Brassica juncea. BMC Plant Biology 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaegel V, Guermann B, Le Ret M, Andres C, Meyer D, Erhardt M, Canaday J, Gualberto JM, Imbault P. 2006. The plant-specific ssDNA binding protein OSB1 is involved in the stoichiometric transmission of mitochondrial DNA in Arabidopsis. Plant Cell 18, 3548–3563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerbino DR, Birney E. 2008. Velvet: Algorithms for de novo short read assembly using de Bruijn graphs. Genome Research 18, 821–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.