Abstract
Aims
The aim of this study was to assess how quickly and effectively duloxetine improves energy compared with placebo in patients with major depressive disorder (MDD).
Methods
Data from 10 randomised, double-blind, placebo-controlled clinical trials examining duloxetine (40–60 mg/day) vs. placebo in patients diagnosed with MDD were analysed. Change from baseline at Week 1 through Week 8 in Hamilton Depression Rating Scale (HAM-D) retardation subscale score (Item 1 – depressed mood, Item 7 – work and activities, Item 8 – retardation and Item 14 – genital symptoms) was assessed with mixed model repeated measures analysis. Positive predictive values and negative predictive values were calculated for predictor analysis.
Results
Patients treated with duloxetine (N = 1522) experienced statistically significantly (p ≤ 0.05) greater reductions in HAM-D retardation subscale scores vs. placebo (N = 1180) starting at Week 1 throughout Week 8 of treatment. Of the patients with early energy improvement (≥ 20% reduction in HAM-D retardation subscale scores) at Week 1, 48% achieved remission (HAM-D total score ≤ 7) at Week 8; 48% and 46% of patients who experienced early energy improvement at Weeks 2 and 4, respectively, achieved remission at Week 8.
Discussion
We demonstrated that treatment with duloxetine, quickly and with increasing magnitude over treatment time, improves low energy symptoms. As early as 1 week after starting treatment with duloxetine, improvement of low energy may serve as a predictor of remission at end-point.
Conclusions
Treatment with duloxetine improves energy in patients with MDD and early response in retardation may serve as a modest predictor of remission at end-point.
Clinical trials registration
ClinicalTrials.gov. Study Identifiers: NCT00036335; NCT00073411; NCT00406848 and NCT00536471. Studies HMAQa, HMAQb, HMATa, HMATb, HMBHa and HMBHb predate the registration requirement.
Data posting
ClinicalTrials.gov. Study Identifiers: NCT00406848; NCT00536471.
What’s known
Duloxetine improves depressive symptoms in patients with major depressive disorder (MDD).
What’s new
Duloxetine improves low energy symptoms in patients with MDD. Improvement of low energy as early as Week 1 of treatment in patients with MDD has modest predictive value for remission of depressive symptoms at Week 8.
Introduction
Symptoms associated with major depressive disorder (MDD) are very diverse, and include depressed mood, weight changes, insomnia or hypersomnia, psychomotor agitation, fatigue or loss of energy, feelings of worthlessness, diminished ability to concentrate and recurrent thoughts of death 1. Prevalence of fatigue and low energy is high among patients with MDD, and these symptoms are clinically relevant for patients seeking treatment for MDD 2,3. In a previous study, performed by general practitioners, fatigue was reported to be one of the most common symptoms (93.6%) in patients with major depression 4. In addition, a pan-European study found that the most common symptoms experienced by patients with depression were sleep problems (63%), tiredness (73%) and low mood (76%) 5. Fatigue often does not respond well to treatment with antidepressants, even in patients classified as treatment responders, and fatigue often persists as a symptom of depression between depressive episodes 6,7. Clinical studies assessing energy levels in patients with MDD commonly refer to low energy as tiredness, fatigue, loss of energy or reduced energy levels 3–5. A tool used in clinical studies to assess energy levels is the Hamilton Depression Rating Scale (HAM-D) retardation subscale 8 score (consisting of four HAM-D items: Item 1 – depressed mood; Item 7 – work and activities; Item 8 – retardation and Item 14 – genital symptoms; Items 1, 7 and 8 are also contained in the Maier subscale, which focuses on the core emotional symptoms of depression). Failure to treat residual symptoms of depression, such as fatigue and low energy, impedes the ability of patients to achieve remission 2.
Antidepressants that increase noradrenergic and dopaminergic effects have demonstrated superiority over serotonergic antidepressants in the treatment of depressive symptoms such as fatigue and loss of energy 9. Duloxetine is a potent inhibitor of serotonin (5-hydroxytryptamine) and norepinephrine reuptake, and is balanced in its affinity of binding to serotonin and norepinephrine transporter sites 10,11. Acute administration of duloxetine increases extracellular monoamine levels 12, thereby, enhancing monoaminergic tone. Duloxetine has demonstrated efficacy in the acute treatment of MDD in randomised, double-blind, placebo-controlled trials 13–15 and is known to improve retardation symptoms 16. However, the magnitude of improvement of low energy and retardation after treatment with duloxetine in patients with MDD has not been sufficiently assessed. In addition, while Katz et al. examined the predictive value of improvement of low energy after 2 weeks of treatment with duloxetine for the patients’ outcome at end-point 17, it is unknown if improvement of energy at earlier time points (e.g. 1 week of treatment) has predictive value for patient outcome. Finally, it is unknown if the effects of duloxetine differ between patients with high and low levels of retardation at baseline.
This study has two main objectives – to assess whether duloxetine is associated with faster and greater improvements of energy in patients with MDD compared with placebo, and to evaluate whether early improvement in energy could be an indicator for achieving response and/or remission, and, thus, impacts the clinical prognosis of MDD. An additional exploratory objective was to assess differences in the effects of duloxetine on retardation symptoms between patients with high levels of retardation at baseline, compared with patients with low levels of retardation at baseline.
Methods
We conducted a patient-level pooled analysis of data from 10 clinical trials comparing duloxetine 40–60 mg/day (doses approved in Japan) and placebo in adult patients with MDD to assess whether low energy in patients with MDD responds to treatment with duloxetine as assessed with the HAM-D retardation subscale score.
The protocols for the individual studies were reviewed and approved by the applicable organizational ethical review boards. The patients provided written informed consent before undergoing any study procedures, and the studies were conducted in accordance with the ethical principles of the Declaration of Helsinki, Good Clinical Practice and applicable laws and regulations.
Data sources
We conducted post hoc analyses of data from 10 randomised, double-blind, placebo-controlled clinical trials of duloxetine (HMAQa, HMAQb, HMATa, HMATb, HMBHa, HMBHb, HMCB, HMCR, HMFA, HMFS) 13,14,18–25 (Table1) in patients diagnosed with MDD by using the Diagnostic and Statistical Manual of Mental Disorders. The studies were included in an integrated database, which allowed patient-level analysis. Included in the analyses were all studies in the database that met the following criteria: patients diagnosed with MDD, acute placebo-controlled (with at least one duloxetine treatment arm receiving a dose approved in Japan, 40–60 mg/day) and depressive symptoms were assessed by using the HAM-D 26 so data from the HAM-D retardation subscale score were available. Only data from the acute treatment phases of the studies throughout Week 8 (up to Day 70) were included in the analyses. No studies with less than 6 weeks of study duration, and no maintenance treatment phases were included in the analyses; additionally, for the patient selection within the selected studies, data from patients who received duloxetine > 60 mg/day were excluded to align with the dose approved in Japan. Study protocols permitted minimum anxiolytic use by the patients; patients with psychosis, other psychiatric diseases and dementia were excluded from study participation.
Table 1.
Placebo-controlled studies of duloxetine in major depressive disorder included in the analyses
| Study acronym | Study location | Duration of acute phase (weeks) | Treatment and dose | Patient no. | Main inclusion criteria |
|---|---|---|---|---|---|
| HMAQa | USA | 10 | DLX 20–60 mg bid | DLX = 70 | Age: 18 through 65 years; |
| FLX 20 mg/day* | FLX = 33 | MDD (DSM-IV); current episode | |||
| PLB | PLB = 70 | duration ≥ 2 weeks; CGI-S ≥ 4; HAM-D17 total score ≥ 15 | |||
| HMAQb | USA | 10 | DLX 20–60 mg bid | DLX = 82 | Age: 18 through 65 years; |
| FLX 20 mg/day* | FLX = 37 | MDD (DSM-IV); current episode duration ≥ 2 weeks; | |||
| PLB | PLB = 75 | CGI-S ≥ 4; HAM-D17 total score ≥ 15 | |||
| HMATa | USA | 11 | DLX 20 mg bid | DLX = 175 | Age: ≥ 18 years; MDD (DSM-IV); HAM-D17 |
| DLX 40 mg bid | PRX = 89 | total score ≥ 15; CGI-S total score ≥ 4 | |||
| PRX 20 mg qd* | PLB = 90 | ||||
| PLB | |||||
| HMATb | USA | 11 | DLX 20 mg bid | DLX = 177 | Age: ≥ 18 years; MDD (DSM-IV); |
| DLX 40 mg bid | PRX = 87 | HAM-D17 total score ≥ 15; | |||
| PRX 20 mg qd* | PLB = 89 | CGI-S total score ≥ 4 | |||
| PLB | |||||
| HMBHa | USA | 11 | DLX 60 mg qd PLB | DLX = 123 | Age: ≥ 18 years; MDD (DSM-IV); |
| PLB = 122 | HAM-D total score ≥ 15; CGI-S ≥ 4 | ||||
| HMBHb | USA | 11 | DLX 60 mg qd PLB | DLX = 128 | Age: ≥ 18 years; MDD (DSM-IV); |
| PLB = 139 | HAM-D total score ≥ 15; CGI-S ≥ 4 | ||||
| HMCB | USA | 9 | DLX 60 mg qd PLB | DLX = 141 | Age: ≥ 18 years; MDD (DSM-IV); |
| PLB = 141 | HAM-D17 total score ≥ 15; CGI-S ≥ 4; BPI average pain (question 3) score ≥ 2 | ||||
| HMCR | USA | 8 | DLX 60 mg qd | DLX = 273 | Age: ≥ 18 years; MDD (DSM-IV): |
| ESC 10 mg qd* | ESC = 274 | CGI-S ≥ 4; MADRS total score ≥ 22 | |||
| PLB | PLB = 137 | ||||
| HMFA | USA | 12 | DLX 60 mg qd | DLX = 249 | Age: ≥ 65 years; MDD (DSM-IV-TR); |
| France | PLB | PLB = 121 | MMSE ≥ 20 | ||
| Mexico | |||||
| Puerto Rico | |||||
| HMFS | USA | 8 | DLX 60 mg qd PLB | DLX = 518 | Age: 18–65 years; MDD (DSM-IV-TR); MADRS ≥ 22; CGI-S ≥ 4 |
| Puerto Rico | PLB = 258 |
bid, twice daily administration; BPI, Brief Pain Inventory; CGI-S, Clinical Global Impression of Severity; DLX, duloxetine; DSM-IV, Diagnostic and Statistical Manual of Mental Disorders, 4th Edition; DSM-IV-TR, Diagnostic and Statistical Manual of Mental Disorders, 4th Edition, Text Revision; ESC, escitalopram; FLX, fluoxetine; HAM-D, Hamilton Depression Rating Scale; HAM-D17, 17-item Hamilton Depression Rating Scale; MADRS, Montgomery-Åsberg Depression Rating Scale; MDD, major depressive disorder; MMSE, Mini Mental Score Exam; PLB, placebo; PRX, paroxetine; qd, once daily administration; USA, United States of America.
Not used in the current analyses.
[Correction added on 21 July 2015, after first online publication: The duloxetine doses for HMAQa and HMAQb is previously wrong and has been changed to 20–60 mg bid].
Efficacy assessment
To evaluate how quickly changes in energy levels occur, change on the HAM-D retardation subscale 8 score (consisting of 4 HAM-D items: Item 1 – depressed mood; Item 7 – work and activities; Item 8 – retardation and Item 14 – genital symptoms) and change on individual items of the HAM-D retardation subscale were assessed at Week 1 (between Day 1 and Day 10), Week 2 (between Day 11 and Day 21), Week 4 (between Day 22 and Day 35), Week 6 (between Day 36 and Day 49) and Week 8 (between Day 50 and Day 70) after treatment initiation. Cleary and Guy established the retardation subscale of the HAM-D as a measure of loss of energy with factor analysis 8. Subsequently, Judge et al. successfully used the HAM-D retardation subscale as a measure of loss of energy 3.
To confirm if duloxetine’s effect on energy sustains over time, we examined the time to first onset of sustained improvement of energy using Kaplan–Meier analysis. Previous studies showed a 20% early improvement in depressive symptoms as clinically relevant and predictive of further improvement 17,27. Therefore, we chose 20% reduction in HAM-D retardation subscale scores as an indication of improvement in energy, and examined if duloxetine separates from placebo on this measure. First onset of sustained improvement of energy was defined as the first time point at which the HAM-D retardation subscale score was reduced by ≥ 20% and this reduction was maintained throughout Day 70 of treatment.
To investigate the clinical implications of this early improvement in energy on patients, we evaluated whether early improvement in energy (at Week 1, 2 or 4) could predict response/remission at Week 8 [missing data at Week 8 were imputed using last-observation-carried-forward (LOCF) analysis method]. Early improvement of energy was defined as a ≥ 20% decrease on the HAM-D retardation subscale according to Katz et al. 17, and, although they evaluated the predictive value of early improvement only at Week 2, we evaluated the predictive value of early improvement at Weeks 1, 2 and 4. For end-point assessment, response was defined as HAM-D total score ≥ 50% decrease from baseline, and remission was defined as HAM-D total score ≤ 7.
In addition, subgroup analyses were performed by using baseline HAM-D retardation subscale scores to categorise patients into two subgroups: patients with high levels of retardation at baseline (HAM-D retardation subscale score ≥ 8 at baseline) and patients with low levels of retardation at baseline (HAM-D retardation subscale score < 8 at baseline), as previously implemented by Judge et al. 3.
Statistical analyses
Based on intent-to-treat principles, all randomised patients assigned to duloxetine (40–60 mg/day) or placebo with baseline and at least one postbaseline HAM-D assessment were included in the analyses.
For treatment comparison, χ2 test, mixed model repeated measures (MMRM) or Cox proportional hazard model were used based on the variable type. The MMRM model included study, treatment, visit, baseline, treatment × visit and baseline × visit as fixed effects. The Cox proportional hazard model included study and treatment. A Kaplan–Meier survival curve was also created. All tests were conducted by using a significance level of 5%.
For the predictor analysis, positive predictive values (PPVs) and negative predictive values (NPVs) were calculated. The PPV expresses the proportion of patients who achieved response or remission among all patients who experienced early improvement of energy. The NPV expresses the proportion of patients who did not achieve response or remission among all patients who did not experience early improvement of energy.
For the subgroup analyses, analysis of covariance (ANCOVA) with treatment, level of baseline fatigue, their interaction, study and baseline were used. Interaction was evaluated by using a significance level of 10%.
All analyses were post hoc and no adjustments for multiplicity were made.
Results
Patient baseline characteristics
Of 2761 patients who were randomly assigned to treatment, 1555 patients received treatment with duloxetine, and 1206 patients received placebo. Most patients were female (64.3%) and Caucasian (76.3%). Patients had a mean [standard deviation (SD)] age of 46.2 (15.9) years and a mean (SD) HAM-D total score of 20.3 (5.19) at baseline. Gender and ethnicity distributions, age and baseline illness characteristics were similar between duloxetine and placebo groups (Table2).
Table 2.
Baseline demographics and illness characteristics
| Parameter | Duloxetine + Placebo (N = 2761) | Duloxetine (N = 1555) | Placebo (N = 1206) |
|---|---|---|---|
| Gender,n (%) | |||
| Male | 985 (35.7) | 559 (35.9) | 426 (35.3) |
| Female | 1776 (64.3) | 996 (64.1) | 780 (64.7) |
| Race/ethnic origin,n (%) | |||
| Caucasian | 2107 (76.3) | 1175 (75.6) | 932 (77.3) |
| African American | 287 (10.4) | 163 (10.5) | 124 (10.3) |
| Hispanic or Latino | 309 (11.2) | 183 (11.8) | 126 (10.4) |
| Other | 58 (2.1) | 34 (2.2) | 24 (2.0) |
| Age (years), mean (SD) | 46.2 (15.88) | 46.9 (16.12) | 45.3 (15.53) |
| HAM-D Scores, mean (SD) | |||
| HAM-D17 total | 20.3 (5.19) | 20.5 (5.25) | 20.2 (5.11) |
| HAM-D retardation subscale | 7.3 (1.97) | 7.3 (1.97) | 7.2 (1.96) |
| Item 1 – depressed mood | 2.6 (0.77) | 2.6 (0.74) | 2.6 (0.80) |
| Item 7 – work and activities | 2.6 (0.73) | 2.6 (0.72) | 2.6 (0.74) |
| Item 8 – retardation | 0.9 (0.76) | 0.9 (0.77) | 0.9 (0.75) |
| Item 14 – genital symptoms | 1.1 (0.84) | 1.1 (0.83) | 1.2 (0.84) |
| Current MDD episode,n (%) | |||
| First | 511 (18.5) | 284 (18.3) | 227 (18.8) |
| Other | 2015 (73.0) | 1206 (77.6) | 809 (67.1) |
| Missing | 235 (8.5) | 65 (4.2) | 170 (14.1) |
| Age at first episode (years), mean (SD) | 28.8 (14.2) | 29.1 (13.8) | 28.5 (14.6) |
HAM-D, Hamilton Depression Rating Scale; HAM-D17, 17-item Hamilton Depression Rating Scale; MDD, major depressive disorder; N, total number of patients; n, number of affected patients; SD, standard deviation.
Patient disposition
Fewer patients receiving treatment with duloxetine discontinued the studies early compared with placebo (duloxetine: 23.9% vs. placebo: 28.5%; P = 0.005). While early discontinuations due to lack of efficacy were more frequently observed in the placebo group compared with treatment with duloxetine (duloxetine: 3.2% vs. placebo: 9.9%; p < 0.0001), the opposite was true for early discontinuations because of adverse events (duloxetine: 8.1% vs. placebo: 4.6%; p = 0.003) (Table3).
Table 3.
Patient disposition
| Patient disposition | Duloxetine (N = 1555) | Placebo (N = 1206) | p-value |
|---|---|---|---|
| Early discontinuation, n (%) | 371 (23.9) | 344 (28.5) | 0.005 |
| Reasons for early discontinuations,n (%) | |||
| Adverse events | 126 (8.1) | 56 (4.6) | 0.003 |
| Patient decision | 82 (5.3) | 74 (6.1) | 0.196 |
| Lost to follow-up | 64 (4.1) | 59 (4.9) | 0.203 |
| Lack of efficacy | 50 (3.2) | 119 (9.9) | < 0.0001 |
| Protocol violation | 36 (2.3) | 22 (1.8) | 0.523 |
| Physician decision | 9 (0.6) | 9 (0.7) | 0.500 |
| Sponsor decision | 2 (0.1) | 3 (0.2) | 0.419 |
| Death | 1 (< 0.1) | 1 (< 0.1) | 0.822 |
| Other | 1 (< 0.1) | 1 (< 0.1) | 0.822 |
N, total number of patients; n, number of affected patients.
Changes in HAM-D retardation subscale scores and individual retardation subscale item scores
Patients treated with duloxetine experienced statistically significantly greater reductions in HAM-D retardation subscale scores compared with placebo beginning at Week 1 of treatment throughout Week 8 (Figure1). The observed differences in score changes between duloxetine and placebo groups became gradually bigger from Week 1 to Week 8 with increasing effect sizes (0.079–0.303).
Figure 1.
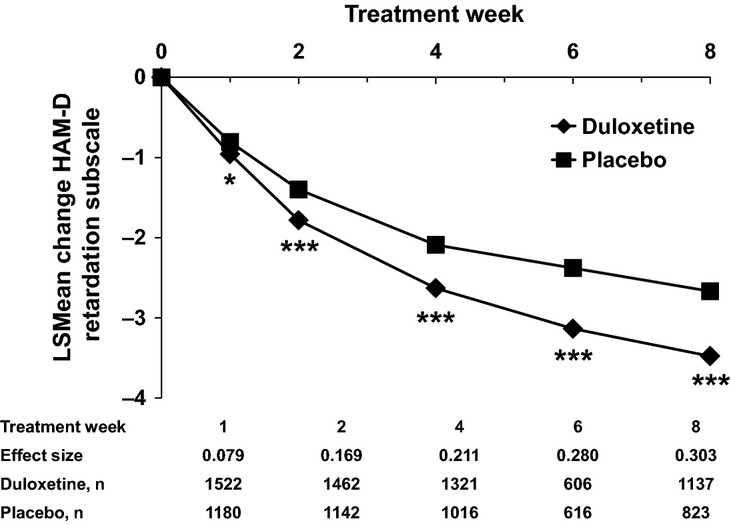
LSMean Changes of HAM-D Retardation Subscale Scores. The efficacy of duloxetine on HAM-D retardation subscale score was examined in comparison to placebo. The HAM-D retardation subscale consists of the following four items: Item 1 – depressed mood, Item 7 – work and activities, Item 8 – retardation, Item 14 – genital symptoms. These analyses were performed with MMRM. *p < 0.05, **p < 0.001, ***p < 0.0001. HAM-D, Hamilton Depression Rating Scale; LSMean, least squares mean; MMRM, mixed model repeated measures; n, number of patients
Among individual HAM-D items that are included in the retardation subscale, changes in the course of treatment and effect sizes differed. However, at Week 8, treatment with duloxetine was consistently associated with statistically significantly greater score reductions compared with placebo for all individual items (Figure2A–D).
Figure 2.
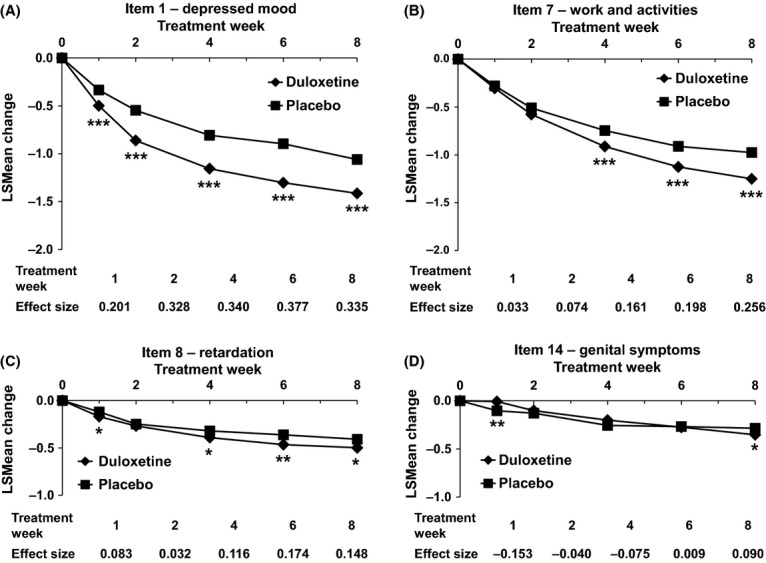
LSMean Score Changes of Individual Items of the HAM-D Retardation Subscale. LSMean changes of Item 1 – depressed mood (A), Item 7 – work and activities (B), Item 8 – retardation (C) and Item 14 – genital symptoms (D) are shown. *p < 0.05, **p < 0.001, ***p < 0.0001. MMRM analysis. Numbers of patients per treatment group and time point are identical to Figure1. HAM-D, Hamilton Depression Rating Scale; LSMean, least squares mean; MMRM, mixed model repeated measures
First onset of sustained 20% improvement of energy
The median [95% confidence interval (CI)] time to first onset of sustained improvement of energy was 28.0 days (26.0, 28.0) for patients receiving duloxetine and 42.0 days (36.0, 49.0) for patients receiving placebo. Treatment with duloxetine was associated with a significantly faster onset of efficacy compared with placebo with a hazard ratio (95% CI) of 1.4 (1.3, 1.5) (Figure3).
Figure 3.
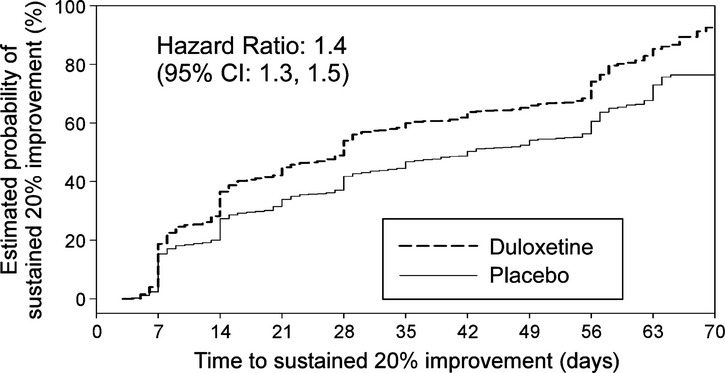
First onset of sustained improvement of energy. First onset of sustained improvement of energy was defined as the first time point when HAM-D retardation subscale score was reduced by ≥ 20% and the reduction was maintained throughout day 70 of treatment. The effect of duloxetine treatment on the first onset of sustained improvement of energy was compared with placebo by Cox proportional hazard model and Kaplan–Meier curve. CI, confidence interval; HAM-D, Hamilton Depression Rating Scale
Predictor analysis
Of the patients receiving treatment with duloxetine, 43.8% (681/1555) achieved response and 31.9% (496/1555) achieved remission at Week 8. To explore the predictive value of early improvement of energy for response and remission at Week 8, a predictor analysis was performed (Table4). Similar PPVs for early improvement of energy for response or remission at Week 8 were observed at Weeks 1, 2 and 4. Of the patients who experienced improvement of energy at Week 1 (≥ 20% reduction in HAM-D retardation subscale score), 48% achieved remission (HAM-D total score ≤ 7) and 60% achieved response (HAM-D total score ≥ 50% decrease from baseline) at Week 8. Early improvement of energy at Weeks 2 and 4 was associated with remission in 48% and 46% of patients and response in 63% and 62% of patients at Week 8. While PPVs for remission and response remained constant among Weeks 1, 2 and 4, NPVs increased over time (Table4).
Table 4.
Predictor analysis for response and/or remission by early improvement of retardation subscale scores
| End-point status | Predictive value | Early improvement at: | ||
|---|---|---|---|---|
| Week 1 | Week 2 | Week 4 | ||
| Response | PPV (n/N) | 60% (329/548) | 63% (468/747) | 62% (525/850) |
| NPV (n/N) | 63% (564/900) | 70% (421/605) | 77% (279/361) | |
| Remission | PPV (n/N) | 48% (261/548) | 48% (356/747) | 46% (394/850) |
| NPV (n/N) | 75% (678/900) | 80% (485/605) | 86% (310/361) | |
Early improvement: ≥ 20% reduction in HAM-D retardation subscale scores at Week 1, 2 or 4. Response: HAM-D total score ≥ 50% decrease from baseline at Week 8 (LOCF). Remission: HAM-D total score ≤ 7 at Week 8 (LOCF). HAM-D, Hamilton Depression Rating Scale; LOCF, last-observation-carried-forward; N, total number of patients; n, number of affected patients; NPV, negative predictive value; PPV, positive predictive value.
HAM-D retardation subscale score and 17-item HAM-D total score changes in patients with high vs. low retardation
To evaluate the effect of baseline energy levels on patient outcome at Week 8, patients were grouped by baseline retardation subscale scores in two subgroups: patients with high retardation (HAM-D retardation subscale score ≥ 8 at baseline) and patients with low retardation (HAM-D retardation subscale score < 8 at baseline). A significant interaction (significance level: 0.1) was observed between treatment and baseline retardation levels for baseline through week 8 change on the HAM-D retardation subscale score (Figure4A). When comparing mean change from baseline in HAM-D retardation subscale scores between the two subgroups in patients receiving placebo, patients with high retardation seemed to show smaller overall decreases compared with patients with low retardation. When comparing mean change from baseline between the two subgroups in patients receiving duloxetine, patients with high or low retardation experienced similar score changes from baseline through Week 8 on the retardation subscale [LSMean (standard error) changes: high retardation – duloxetine = −3.1 (0.1); placebo = −2.1 (0.1); low retardation – duloxetine = −3.0 (0.1); placebo = −2.4 (0.1)] (Figure4A). When examining mean changes from baseline within both retardation subgroups, patients treated with duloxetine showed statistically significantly greater decreases compared with placebo (Figure4A). Similar trends were observed for changes on the HAM-D total score from baseline to week 8 (Figure4B).
Figure 4.
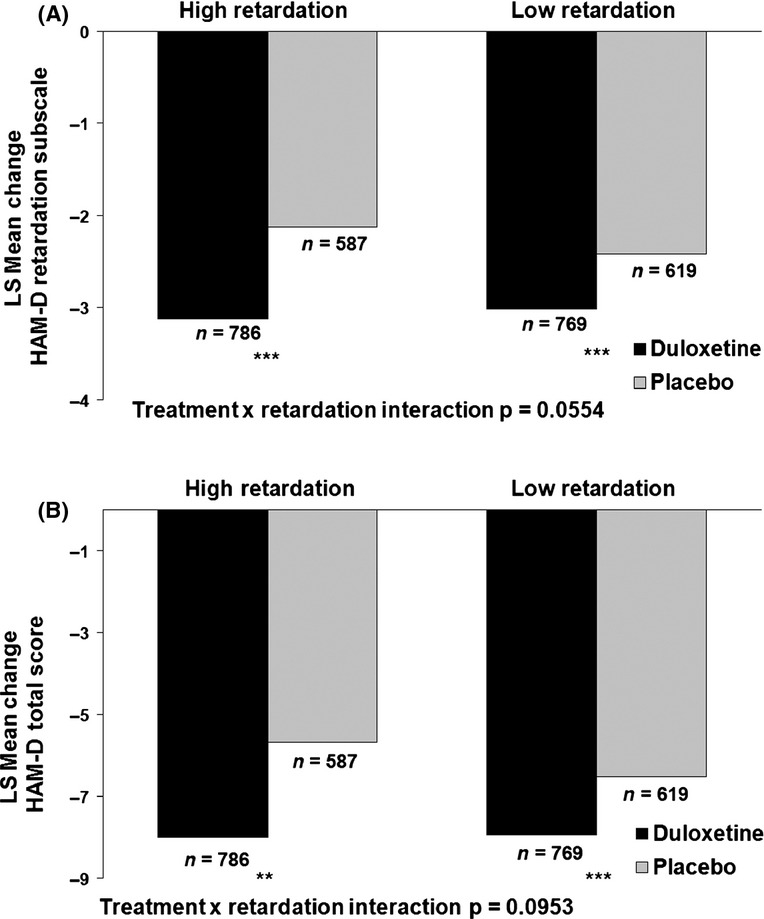
HAM-D retardation subscale and total score changes in high and low retardation patients. (A) Baseline to week 8 HAM-D retardation subscale score changes – last-observation-carried-forward analysis. (B) Baseline to week 8 HAM-D total score changes – last-observation-carried-forward analysis. The effect of baseline energy levels on week 8 (LOCF) HAM-D retardation subscale score changes (A) and HAM-D total score changes (B) were analysed. Patients were grouped into high retardation (HAM-D retardation subscale score ≥ 8 at baseline) and low retardation (HAM-D retardation subscale score < 8 at baseline) subgroups. These analyses were performed by ANCOVA. **p < 0.01, ***p < 0.001. ANCOVA, analysis of covariance; HAM-D, Hamilton Depression Rating Scale; LSMean, least squares mean; n, number of patients
Discussion
The analyses presented here, based on a big sample size drawing data from 10 clinical trials, provide evidence that duloxetine improves low energy symptoms (as assessed with the HAM-D retardation subscale score) quicker and to a greater degree than placebo in patients with MDD. This observation is consistent with Shelton et al., who demonstrated that duloxetine improves scores on the HAM-D retardation subscale starting after 1–2 weeks of treatment 16. This study similarly observed improvement of HAM-D retardation subscale scores after 1 week of treatment with duloxetine and efficacy was maintained throughout Week 8, with increasing magnitude over time. In addition, the results of the Kaplan–Meier analysis confirmed that the initial energy improvement, which is clinically relevant, sustains over time.
Early improvement in energy levels that was observed in this study re-emphasises the role of noradrenergic action in the treatment of MDD. Previously, the noradrenergic neurotransmitter system has been reported to be primarily associated with arousal and activity 28, and Katz et al. demonstrated early improvement in psychomotor retardation after treatment with the selective norepinephrine reuptake inhibitor (SNRI) desipramine 17. Singh et al. demonstrated greater reduction in retardation symptoms after treatment with venflafaxine, a SNRI, compared with treatment with escitalopram, a selective serotonin reuptake inhibitor (SSRI) 29. A pooled analysis by Papakostas et al. indicated that patients with MDD displaying prominent symptoms of fatigue/sleepiness may benefit more from treatment with buporprion, a norepinephrine-dopamine reuptake inhibitor, compared with treatment with SSRI 30. Here, we demonstrate that the serotonin and norepinephrine reuptake inhibitor, duloxetine, improves low energy starting at Week 1 throughout Week 8, reinforcing and confirming the earlier observations.
At Week 8, the effect size for improvement on the HAM-D retardation subscale was 0.3 when comparing duloxetine with placebo, consistent with previous observations by Shelton et al. 16. Among the four items constituting the HAM-D retardation subscale, changes in response to treatment were more pronounced for Items 1 and 7, while Items 8 and 14 presented smaller changes.
The predictor analysis demonstrates the clinical meaning of early improvement. In the current analyses, patients had a 32% likelihood of achieving remission at the initiation of treatment with duloxetine; however, if early improvement after 1 week was observed, the patients’ likelihood of achieving remission increased to 48%, and the likelihood was similar after 2 and 4 weeks. Similar to our observations, Katz et al. reported that a 20% decrease in the HAM-D retardation subscale score after 2 weeks of treatment is a good predictor for patient outcome (PPV = 55.4% and NPV = 80.4% for achieving sustained remission) 17. Our data further demonstrate that the predictive value after 1 week is similar to that after 2 weeks. Thus, improvement of energy after 1 week may be a modest but substantially better predictor than baseline for remission at end-point.
A significant interaction between the level of baseline fatigue and treatment was observed for the improvement of low energy and overall depressive symptoms. Treatment effect is defined as the difference between response to placebo and response to active treatment, therefore, the observed interaction could indicate duloxetine has a greater treatment effect in patients with high retardation compared with patients with low retardation at baseline. As statistically significant differences were observed between patients treated with duloxetine vs. patients receiving placebo in both retardation subgroups, we can reasonably state that duloxetine provides a certain level of clinical benefits for patients, regardless of baseline retardation level. However, the observed significant interaction may indicate that patients with high retardation at baseline may benefit more from duloxetine treatment than patients with low retardation at baseline when compared with placebo treatment. This may be explained by the potential difference in placebo response for both groups; patients with high retardation may respond less to placebo than patients with low retardation at baseline 31.
The interpretation of our results is limited by several factors. For the evaluation of low energy, the HAM-D retardation subscale was used, but this scale might not completely reflect changes in energy in patients with MDD, and not all items included in the HAM-D retardation subscale are directly related to energy levels (e.g. Item 14 – genital symptoms). Considering that all items contained in the HAM-D retardation subscale, with the exception of Item 14, are also contained in the Maier subscale, similar results might be observed with the Maier subscale. However, analyses involving the Maier subscale were out-of-scope for the current analyses. Furthermore, HAM-D retardation subscale score changes contribute to HAM-D total score changes; consequently, changes in HAM-D retardation subscale scores are correlated with changes in HAM-D total scores. However, while the items contained in the retardation subscale contribute to the HAM-D total score, those items are not solely driving change in the total score and contribute to remission in conjunction with other HAM-D items. Comparisons of the impact of HAM-D retardation subscale score changes vs. changes in other HAM-D subscales on HAM-D total score changes were not the focus of the analyses presented here. Differences in the time points and frequency of HAM-D assessments in the included studies made it difficult to evaluate all patients in the same manner at each of the included time points (e.g. Weeks 1, 2, 4 and 6). The generalisability of our results to a clinical practice setting may be impacted by the inclusion and exclusion criteria that are inherent to clinical trials. Since this was a post hoc analysis and no adjustments for multiplicity were made, the powering of the study should be considered when evaluating the outcomes of the variables. The dose of duloxetine used in the current analyses was limited to maximally 60 mg/day to not exceed doses approved in Japan. However, duloxetine 60 mg/day has been confirmed as effective in the treatment of MDD and is commonly used globally 32,33. Finally, comparisons among different HAM-D subscales and their predictive values for overall patient outcome were out-of-scope for the current analyses. Future studies are warranted to address this question.
In conclusion, we demonstrated that treatment with duloxetine quickly and with increasing magnitude over treatment time improves low energy symptoms, compared with placebo in patients with MDD. As early as 1 week after starting treatment with duloxetine, improvement of low energy can serve as a modest predictor of remission at end-point. In addition, duloxetine demonstrated a greater treatment effect in patients with high retardation vs. low retardation at baseline.
Author contributions
Drs Harada, Kato, Fujikoshi, Wohlreich, Tokuoka and Ms. Berggren contributed to the conception of the study, interpretation of the data, drafting and critical revision of the manuscript and provided final approval of the manuscript before submission. In addition, Dr Fujikoshi and Ms. Berggren performed the data analysis.
Acknowledgments
This manuscript was sponsored by Eli Lilly Japan K.K. The authors thank Dr Ralf Jaeger, full-time employee of Accovion, for statistical analysis support. In addition, the authors thank Ms. Aki Yoshikawa, full-time employee of Eli Lilly Japan K.K., for writing and logistical support and Dr Alexandra Heinloth and Ms. Angela Lorio, both full-time employees of inVentiv Health Clinical, LLC, for writing and editorial support. Eli Lilly Japan K.K. contracted Accovion for statistical support and inVentiv Health Clinical for writing and editorial support.
References
- Kennedy SH. Core symptoms of major depressive disorder: relevance to diagnosis and treatment. Dialogues Clin Neurosci. 2008;10:271–7. doi: 10.31887/DCNS.2008.10.3/shkennedy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joliat MJ, Brown EB, Miner CM. Changes in energy after switching from daily citalopram, paroxetine, or sertraline to once-weekly fluoxetine. J Clin Psychopharmacol. 2004;24:464–7. doi: 10.1097/01.jcp.0000132345.35713.16. [DOI] [PubMed] [Google Scholar]
- Judge R, Plewes JM, Kumar V, Koke SC, Kopp JB. Changes in energy during treatment of depression: an analysis of fluoxetine in double-blind, placebo-controlled trials. J Clin Psychopharmacol. 2000;20:666–72. doi: 10.1097/00004714-200012000-00013. [DOI] [PubMed] [Google Scholar]
- Maurice-Tison S, Verdoux H, Gay B, Perez P, Salamon R, Bourgeois ML. How to improve recognition and diagnosis of depressive syndromes using international diagnostic criteria. Br J Gen Pract. 1998;48:1245–6. [PMC free article] [PubMed] [Google Scholar]
- Tylee A, Gastpar M, Lepine JP, Mendlewicz J. DEPRES II (Depression Research in European Society II): a patient survey of the symptoms, disability and current management of depression in the community. DEPRES Steering Committee. Int Clin Psychopharmacol. 1999;14:139–51. doi: 10.1097/00004850-199905002-00001. [DOI] [PubMed] [Google Scholar]
- Conradi HJ, Ormel J, de Jonge P. Presence of individual (residual) symptoms during depressive episodes and periods of remission: a 3-year prospective study. Psychol Med. 2011;41:1165–74. doi: 10.1017/S0033291710001911. [DOI] [PubMed] [Google Scholar]
- McClintock SM, Husain MM, Wisniewski SR, et al. Residual symptoms in depressed outpatients who respond by 50% but do not remit to antidepressant medication. J Clin Psychopharmacol. 2011;31:180–6. doi: 10.1097/JCP.0b013e31820ebd2c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleary M, Guy W. Factor analysis of the Hamilton Depression Scale. Drugs Exp Clin Res. 1975;1:115–20. [Google Scholar]
- Nutt D, Demyttenaere K, Janka Z, et al. The other face of depression, reduced positive affect: the role of catecholamines in causation and cure. J Psychopharmacol. 2007;21:461–71. doi: 10.1177/0269881106069938. [DOI] [PubMed] [Google Scholar]
- Bymaster FP, Beedle EE, Findlay J, et al. Duloxetine (Cymbalta), a dual inhibitor of serotonin and norepinephrine reuptake. Bioorg Med Chem Lett. 2003;13:4477–80. doi: 10.1016/j.bmcl.2003.08.079. [DOI] [PubMed] [Google Scholar]
- Wong DT, Bymaster FP. Dual serotonin and noradrenaline uptake inhibitor class of antidepressants potential for greater efficacy or just hype? Prog Drug Res. 2002;58:169–222. doi: 10.1007/978-3-0348-8183-8_5. [DOI] [PubMed] [Google Scholar]
- Karpa KD, Cavanaugh JE, Lakoski JM. Duloxetine pharmacology: profile of a dual monoamine modulator. CNS Drug Rev. 2002;8:361–76. doi: 10.1111/j.1527-3458.2002.tb00234.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detke MJ, Lu Y, Goldstein DJ, Hayes JR, Demitrack MA. Duloxetine, 60 mg once daily, for major depressive disorder: a randomized double-blind placebo-controlled trial. J Clin Psychiatry. 2002;63:308–15. doi: 10.4088/jcp.v63n0407. [DOI] [PubMed] [Google Scholar]
- Goldstein DJ, Mallinckrodt C, Lu Y, Demitrack MA. Duloxetine in the treatment of major depressive disorder: a double-blind clinical trial. J Clin Psychiatry. 2002;63:225–31. doi: 10.4088/jcp.v63n0309. [DOI] [PubMed] [Google Scholar]
- Nemeroff CB, Schatzberg AF, Goldstein DJ, et al. Duloxetine for the treatment of major depressive disorder. Psychopharmacol Bull. 2002;36:106–32. [PubMed] [Google Scholar]
- Shelton RC, Prakash A, Mallinckrodt CH, et al. Patterns of depressive symptom response in duloxetine-treated outpatients with mild, moderate or more severe depression. Int J Clin Pract. 2007;61:1337–48. doi: 10.1111/j.1742-1241.2007.01444.x. [DOI] [PubMed] [Google Scholar]
- Katz MM, Meyers AL, Prakash A, Gaynor PJ, Houston JP. Early symptom change prediction of remission in depression treatment. Psychopharmacol Bull. 2009;42:94–107. [PubMed] [Google Scholar]
- Brannan SK, Mallinckrodt CH, Brown EB, Wohlreich MM, Watkin JG, Schatzberg AF. Duloxetine 60 mg once-daily in the treatment of painful physical symptoms in patients with major depressive disorder. J Psychiatr Res. 2005;39:43–53. doi: 10.1016/j.jpsychires.2004.04.011. [DOI] [PubMed] [Google Scholar]
- Detke MJ, Lu Y, Goldstein DJ, McNamara RK, Demitrack MA. Duloxetine 60 mg once daily dosing versus placebo in the acute treatment of major depression. J Psychiatr Res. 2002;36:383–90. doi: 10.1016/s0022-3956(02)00060-2. [DOI] [PubMed] [Google Scholar]
- Goldstein DJ, Lu Y, Detke MJ, Wiltse C, Mallinckrodt C, Demitrack MA. Duloxetine in the treatment of depression: a double-blind placebo-controlled comparison with paroxetine. J Clin Psychopharmacol. 2004;24:389–99. doi: 10.1097/01.jcp.0000132448.65972.d9. [DOI] [PubMed] [Google Scholar]
- Nierenberg AA, Greist JH, Mallinckrodt CH, et al. Duloxetine versus escitalopram and placebo in the treatment of patients with major depressive disorder: onset of antidepressant action, a non-inferiority study. Curr Med Res Opin. 2007;23:401–16. doi: 10.1185/030079906X167453. [DOI] [PubMed] [Google Scholar]
- Oakes TM, Myers AL, Marangell LB, et al. Assessment of depressive symptoms and functional outcomes in patients with major depressive disorder treated with duloxetine versus placebo: primary outcomes from two trials conducted under the same protocol. Hum Psychopharmacol. 2012;27:47–56. doi: 10.1002/hup.1262. [DOI] [PubMed] [Google Scholar]
- Perahia DG, Wang F, Mallinckrodt CH, Walker DJ, Detke MJ. Duloxetine in the treatment of major depressive disorder: a placebo- and paroxetine-controlled trial. Eur Psychiatry. 2006;21:367–78. doi: 10.1016/j.eurpsy.2006.03.004. [DOI] [PubMed] [Google Scholar]
- Pigott TA, Prakash A, Arnold LM, Aaronson ST, Mallinckrodt CH, Wohlreich MM. Duloxetine versus escitalopram and placebo: an 8-month, double-blind trial in patients with major depressive disorder. Curr Med Res Opin. 2007;23:1303–18. doi: 10.1185/030079907X188107. [DOI] [PubMed] [Google Scholar]
- Robinson M, Oakes TM, Raskin J, Liu P, Shoemaker S, Nelson JC. Acute and long-term treatment of late-life major depressive disorder: duloxetine versus placebo. Am J Geriatr Psychiatry. 2014;22:34–45. doi: 10.1016/j.jagp.2013.01.019. [DOI] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stassen HH, Delini-Stula A, Angst J. Time course of improve ment under antidepressant treatment: a survival-analytical approach. Eur Neuropsychopharmacol. 1993;3:127–35. doi: 10.1016/0924-977x(93)90264-m. [DOI] [PubMed] [Google Scholar]
- Morilak DA, Frazer A. Antidepressants and brain monoaminergic systems: a dimensional approach to understanding their behavioural effects in depression and anxiety disorders. Int J Neuropsychopharmacol. 2004;7:193–218. doi: 10.1017/S1461145704004080. [DOI] [PubMed] [Google Scholar]
- Singh AB, Bousman CA, Ng CH, Byron K, Berk M. Psychomotor depressive symptoms may differentially respond to venlafaxine. Int Clin Psychopharmacol. 2013;28:121–6. doi: 10.1097/YIC.0b013e32835f1b9f. [DOI] [PubMed] [Google Scholar]
- Papakostas GI, Nutt DJ, Hallett LA, Tucker VL, Krishen A, Fava M. Resolution of sleepiness and fatigue in major depressive disorder: a comparison of bupropion and the selective serotonin reuptake inhibitors. Biol Psychiatry. 2006;60:1350–5. doi: 10.1016/j.biopsych.2006.06.015. [DOI] [PubMed] [Google Scholar]
- Bialik RJ, Ravindran AV, Bakish D, Lapierre YD. A comparison of placebo responders and nonresponders in subgroups of depressive disorder. J Psychiatry Neurosci. 1995;20:265–70. [PMC free article] [PubMed] [Google Scholar]
- Ball SG, Desaiah D, Zhang Q, Thase ME, Perahia DG. Efficacy and safety of duloxetine 60 mg once daily in major depressive disorder: a review with expert commentary. Drugs Context. 2013;3:212–45. doi: 10.7573/dic.212245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowen PJ, Ogilvie AD, Gama J. Efficacy, safety and tolerability of duloxetine 60 mg once daily in major depression. Curr Med Res Opin. 2005;21:345–56. doi: 10.1185/030079905X30680. [DOI] [PubMed] [Google Scholar]


