Abstract
Introduction
rIX-FP is a coagulation factor IX (recombinant), albumin fusion protein with more than fivefold half-life prolongation over other standard factor IX (FIX) products available on the market.
Aim
This prospective phase II, open-label study evaluated the safety and efficacy of rIX-FP for the prevention of bleeding episodes during weekly prophylaxis and assessed the haemostatic efficacy for on-demand treatment of bleeding episodes in previously treated patients with haemophilia B.
Methods
The study consisted of a 10–14 day evaluation of rIX-FP pharmacokinetics (PK), and an 11 month safety and efficacy evaluation period with subjects receiving weekly prophylaxis treatment. Safety was evaluated by the occurrence of related adverse events, and immunogenic events, including development of inhibitors. Efficacy was evaluated by annualized spontaneous bleeding rate (AsBR), and the number of injections to achieve haemostasis.
Results
Seventeen subjects participated in the study, 13 received weekly prophylaxis and 4 received episodic treatment only. No inhibitors were detected in any subject. The mean and median AsBR were 1.25, and 1.13 respectively in the weekly prophylaxis arm. All bleeding episodes were treated with 1 or 2 injections of rIX-FP. Three prophylaxis subjects who were treated on demand prior to study entry had >85% reduction in AsBR compared to the bleeding rate prior to study entry.
Conclusion
This study demonstrated the efficacy for weekly routine prophylaxis of rIX-FP to prevent spontaneous bleeding episodes and for the treatment of bleeding episodes. In addition no safety issues were detected during the study and an improved PK profile was demonstrated.
Keywords: factor IX, haemophilia B, haemostasis, pharmacokinetics, prophylaxis
Introduction
Prophylaxis is currently considered as the optimal care for patients with severe haemophilia 1 since it reduces the incidence of all bleeding episodes and when initiated at a young age, also reduces the development of arthropathy 2–4. The half-life of standard FIX products is approximately 18 h, which requires 2 or 3 intravenous (IV) infusions per week to achieve effective bleeding prevention. This relatively high frequency of infusions makes it difficult to adhere to the prescribed treatment, especially for patients with poor venous access 5–7. Thus, a recombinant FIX product with an extended plasma half-life (t1/2) that would allow fewer injections to achieve and maintain haemostasis with either a prophylaxis regimen or on-demand therapy is highly desirable 8–11.
Coagulation factor IX (recombinant), albumin fusion protein (rIX-FP) is manufactured in Chinese Hamster Ovary (CHO) cells, by genetic fusion of human recombinant albumin to the C-terminus of rFIX via a cleavable linker. The short linker peptide, derived from an endogenous FIX sequence involved in FIX activation, enables in vivo cleavage of activated FIX from the albumin carrier moiety when required for coagulation 10,12,13.
In a previous phase I study, the pharmacokinetics (PK) of a single dose of rIX-FP were evaluated and showed favourable PK parameters compared to marketed products 14. rIX-FP has a 5.3-fold longer half-life, the sevenfold reduced clearance (CL), and the sevenfold greater AUC compared to previous FIX products, and a single dose of 50 IU kg−1 provided baseline-corrected trough levels of 13.4% and 7.4% FIX activity at 7 and 14 days respectively. Maintaining an adequate trough level is considered to be an important determinant of preventing break-through bleeding 15,16, though other PK parameters, including AUC and peak levels, may also play a role.
The present trial aimed to evaluate the efficacy of rIX-FP for the prevention of bleeding episodes during weekly prophylaxis and assess haemostatic efficacy for treatment of bleeding, in addition to assessing safety and PK of rIX-FP.
Materials and methods
Patients
Criteria for subject selection were based on the draft Guideline on the clinical investigation of recombinant and human plasma-derived FIX products 17. Patients were previously treated (≥150 exposure days [EDs] to FIX products) males with haemophilia B (FIX activity ≤ 2%) and aged 12–65 years. Patients with a history of neutralizing antibodies (inhibitors) to FIX, a CD4+ lymphocyte count <200 mm−3 (if HIV positive) or with a coagulation disorder other than haemophilia B were excluded from participation. Patients were recruited from two sites in two countries (Israel and Bulgaria). All patients (or the patient’s parents or legally acceptable representative) provided written informed consent prior to any trial-related activities. The study was approved by independent ethics committees, and was conducted in accordance with Good Clinical Practice (GCP) and the Declaration of Helsinki. The trial was registered at www.clinicaltrials.gov: NCT01361126.
Trial design
This trial was a prospective, open-label study to evaluate safety, PK and efficacy of rIX-FP, which is being developed for prophylaxis and treatment of bleeding episodes in patients with haemophilia B. The study consisted of a 10–14 day evaluation of rIX-FP PK, and a 3–5 month safety and efficacy evaluation period, which was extended up to 11 months to allow subjects to receive continuous treatment until enrolment in the subsequent study of rIX-FP (see Fig.1). Subjects receiving weekly prophylactic treatment were initially treated with 30 ± 5 IU kg−1. The dose could be adjusted based on bleeding phenotype, physical activity level and clinical outcome, while maintaining a 7-day treatment interval and individualized trough FIX activity level. No individual target trough FIX level was established a priori. All bleeding episodes which occurred during the treatment period of the study were treated with rIX-FP. For treatment of a bleeding episode, the dose was based upon subject’s PK profile, WFH guidelines and local standard of care, with a minimum dose of 25 IU kg−1 rIX-FP. All subjects self-administered rIX-FP treatment for both routine prophylaxis and treatment of bleeding episodes, and were recorded by subjects in an electronic diary.
Figure 1.
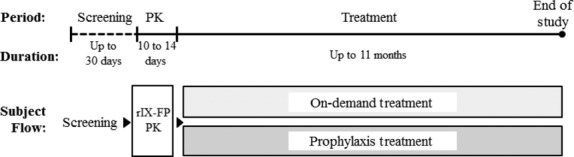
Study design. Schematic diagram of the trial design, including time periods, duration and subject flow. PK, pharmacokinetics.
The primary objective of the study was to evaluate the long-term safety of IV injections of rIX-FP. Safety was evaluated by nature and incidence of adverse events (AEs), changes in laboratory values and development of inhibitors against FIX or non-neutralizing antibodies against rIX-FP.
The secondary objectives of the study were to evaluate PK parameters following a single IV dose of 25 IU kg−1 rIX-FP, clinical response of weekly routine prophylaxis with rIX-FP with respect to the prevention of bleeding episodes and clinical response of bleeding episodes treated with rIX-FP.
The numbers of infusions to achieve haemostasis were tabulated to determine efficacy of rIX-FP for the treatment of bleeding episodes. Investigators also rated the efficacy of treatment of bleeding episodes on a prespecified 4-point scale, taking into account both number of infusions and subject-reported pain relief after treatment. Efficacy of routine prophylaxis was measured by the number of spontaneous bleeding episodes occurring during prophylaxis, displayed as an annualized bleeding rate.
Analytical methods
Factor IX activity was measured using a validated one-stage clotting method using Pathromtin SL (Siemens Healthcare Diagnostics, Marburg, Germany) as activator reagent, rIX-FP activity determination was performed using the Behring coagulation system. The results were interpreted using a reference curve, prepared from standard human plasma calibrated to the WHO standard for FIX, and the results are reported in per cent of norm or International Units.
Inhibitors were titrated by the Bethesda method according to the Nijmegen modification 18, a coagulation assay based on in vitro determination of activated partial thromboplastin time (aPTT) in human citrated plasma. A result ≥0.6 BU was defined as a positive result.
A tiered approach to immunogenicity testing for rIX-FP was employed. Antibodies to rIX-FP were tested in all patients before rIX-FP exposure and 4 weeks after exposure. A direct-binding ELISA assay detected antibodies against rIX-FP; if a positive signal was obtained, the plasma sample was retested in a separate direct-binding ELISA assay to confirm the specific antibody signal and to discriminate between antibodies against plasma-derived FIX, recombinant FIX (rFIX) and albumin.
The analyses of FIX activity, FIX antigen, inhibitors and antibodies against rIX-FP were performed in the central laboratory at CSL Behring, Marburg, Germany.
PK analysis and statistical methods
Pharmacokinetic samples were collected prior to dosing rIX-FP and at 30 min, 3, 24, 48, 72, 120, 168, 240 and 336 h after infusion. All PK parameters were calculated using actual collection times. PK analysis was performed by standard non-compartmental analysis using WinNonlin® Software (Pharsight: Cary, NC, USA). PK parameters included: area under the curve to last sample with quantifiable drug concentration (AUC0-t); area under the curve from time of dosing extrapolated to infinity, based on last observed FIX concentration (AUC0-inf); incremental recovery (IR0–30 min) according to the formula C30 min (IU dL−1)/Dose (IU kg−1); terminal half-life (t1/2); total body CL, normalized to body weight.
Safety endpoints were summarized using descriptive statistics, including all patients exposed to rIX-FP (safety population).
Drug product
rIX-FP is a single chain glycoprotein with a molecular weight of approximately 125 000 Da, synthesized in CHO cells. The manufacturing and formulation do not include addition of excipients from animal or human origin 12. rIX-FP was supplied as a lyophilized sterile formulation intended for IV injection in single-use vials of 500 and 1000 IU per vial, and was reconstituted with 2.5 mL sterile water for injection.
Results
Patient characteristics
Seventeen study subjects from haemophilia treatment centres in Israel and Bulgaria were screened and all were enrolled in the study. All subjects were Caucasian and non-Hispanic, and ages ranged from 13 to 46 years (mean 26 years). All 13 subjects enrolled in Israel received weekly prophylaxis treatment with rIX-FP for the duration of the study (range = 37–48 weeks), and all four subjects enrolled in Bulgaria received on-demand treatment (ODT) for bleeding episodes with rIX-FP for the duration of the study (range = 15–22 weeks). The subject disposition is outlined in Fig.2.
Figure 2.
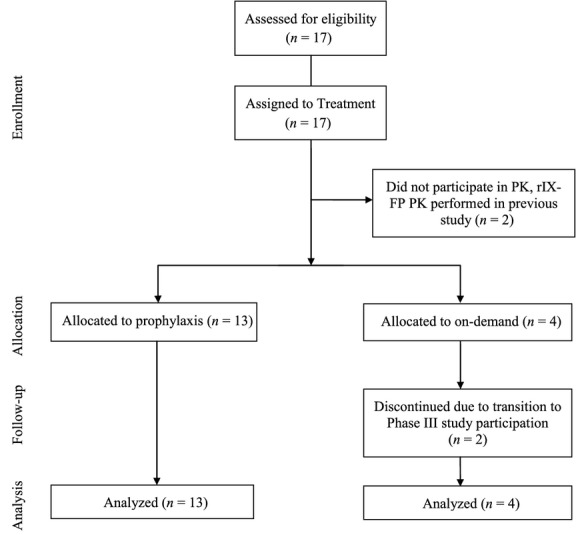
Disposition of patients. Schematic diagram showing the flow and disposition of patients in the trial.
Overall, prophylaxis subjects were younger than on-demand subjects, and included three subjects younger than 18 years. Prophylaxis subjects had fewer chronic hepatitis infections than on-demand subjects, and reported less joint damage (Table1).
Table 1.
Patient demographics and medical history
| Prophylaxis treatment N = 13 | On-demand treatment N = 4 | Total N = 17 | |
|---|---|---|---|
| Age, years, mean (min–max) | 23.2 (13–42) | 35.8 (27–46) | 26.1 (13–46) |
| <18 years, n (%) | 3 (23.1) | 0 | 3 (17.6) |
| Weight, kg, mean (min–max) | 64.1 (36.0–83.8) | 75.7 (62.4–93.0) | 66.8 (36.0, 93.0) |
| Race | |||
| White | 13 (100.0) | 4 (100.0) | 17 (100.0) |
| Previous exposure days to factor IX, mean (SD) | 861.9 (353.61) | 662.5 (131.50) | 815.0 (323.46) |
| Total bleeds 12 months prior to study entry, mean (SD) | 14.0 (17.97) | 27.0 (3.37) | 17.1 (16.63) |
| Spontaneous bleeds 12 months prior to study entry, mean (SD) | 9.2 (14.73) | 27.0 (3.37) | 13.4 (15.02) |
| Prior treatment | |||
| Prophylaxis, n (%) | 10 (76.9) | 0 | 10 (58.8) |
| On-demand, n (%) | 3 (23.1) | 4 (100.0) | 7 (41.2) |
| HIV, n (%) | 0 | 0 | 0 |
| HBV, n (%) | 0 | 1 (25.0) | 1 (5.9) |
| HCV, n (%) | 3 (23.1) | 2 (50.0) | 5 (29.4) |
| Haemophilic arthropathy, n (%) | 5 (38.5) | 4 (100.0) | 9 (52.9) |
| Synovitis, n (%) | 3 (23.1) | 0 | 3 (17.6) |
Min, minimum; max, maximum; n, number of patients.
All subjects had exposure to FIX products prior to study entry (range = 415–1450). In the prophylaxis treatment group, three subjects had been receiving ODT prior to study entry, and 10 subjects were receiving prophylaxis treatment with FIX products (2–3 times per week in 80% of the subjects). Those three subjects previously receiving ODT reported a much higher mean number of total bleeds in the 12 months prior to study entry than the prior prophylaxis subjects (43.3 vs. 5.2). Subjects in the on-demand group in the study had a mean of 27 total bleeds in the 12 months prior to study entry.
Pharmacokinetics
Pharmacokinetics of a single dose of 25 IU kg−1 rIX-FP were assessed at the beginning of the study in 15 subjects who had not previously received rIX-FP. The mean single-dose PK profile has been previously published 19, and the PK parameters were comparable to those previously reported from the phase I study 14. A dose of 25 IU kg−1 rIX-FP had a mean incremental recovery of 1.52 IU dL−1 per IU kg−1 and a mean half-life of 94.8 h. The mean baseline-uncorrected FIX activity at 7, 10 and 14 days were 5.6, 3.9 and 2.9 IU dL−1 following a single dose of 25 IU kg−1 rIX-FP.
Safety
rIX-FP was well tolerated in all subjects. The duration of treatment ranged from 259 to 335 days for prophylaxis subjects, and from 105 to 155 days for on-demand subjects, with subjects receiving prophylaxis participating in the study longer than on-demand subjects (mean = 315 vs. 131 days) due to the timing of enrolment into the subsequent study. There were a total of 718 EDs to rIX-FP, with a mean of 51.5 EDs to rIX-FP in the prophylaxis subjects and 12 EDs to rIX-FP in on-demand subjects. Nine prophylaxis subjects achieved at least 50 EDs during the study.
None of the subjects developed inhibitors to FIX or antibodies to rIX-FP following rIX-FP administration. There were no hypersensitivity reactions. There were no significant treatment-emergent findings in any safety-related parameters during the course of the study.
A total of 14 (82.4%) subjects reported 46 treatment-emergent AEs, none of which were considered related to rIX-FP by the investigator (Table2). All AEs were mild or moderate in severity. There were no serious AEs reported, and there were no withdrawals due to AEs. The most frequent classes of AEs were musculoskeletal disorders [7 (41.2%) subjects, 17 events] and injuries [seven (41.2%) subjects, nine events].
Table 2.
Overview of treatment emergent AEs
| rIX-FP, N (%) E | |||
|---|---|---|---|
| Prophylaxis | On demand | Total | |
| Number of subjects | 13 | 4 | 17 |
| AE leading to study withdrawal | 0 | 0 | 0 |
| Serious AEs (SAEs) | 0 | 0 | 0 |
| Any AEs | 13 (100.0) 45 | 1 (25.0) 1 | 14 (82.4) 46 |
| Severity of AEs | |||
| Mild | 13 (100.0) 42 | 1 (25.0) 1 | 14 (82.4) 43 |
| Moderate | 2 (15.4) 3 | 0 | 2 (11.8) 3 |
| Severe | 0 | 0 | 0 |
| AEs related to rIX-FP | 0 | 0 | 0 |
rIX-FP, coagulation factor IX (recombinant), albumin fusion protein; N, number of subjects with AEs; AE, adverse events.
Efficacy
Treatment of bleeds
Seven (53.8%) prophylaxis subjects and four (100%) on-demand subjects treated spontaneous bleeding episodes. During the study, a total of 85 bleeding episodes were treated with rIX-FP. All bleeding episodes were successfully treated with one (95.3%) or two (4.7%) doses of rIX-FP during the study (Fig.3a). All subjects were initially assigned a treatment dose of 30–35 IU kg−1 (mean of 33 IU kg−1) rIX-FP as prescribed by the protocol. The mean treatment dose for subjects in on-demand arm (all from Bulgaria) was 28 IU kg−1, while the mean treatment dose for subjects in the prophylaxis arm (all from Israel) was 62 IU kg−1. Nevertheless, bleeding episodes requiring a single dose of rIX-FP were similar between on-demand and prophylaxis subjects, 97.3% and 93.6% respectively. There were four bleeds requiring two doses of rIX-FP to achieve haemostasis. An additional five bleeds were treated with a single dose of rIX-FP, followed by the scheduled prophylaxis dose of rIX-FP within 30 h of the treatment dose.
Figure 3.
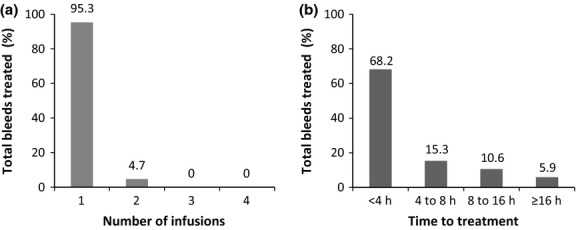
Treatment of Bleeds. (a) The number of coagulation factor IX (recombinant), albumin fusion protein (rIX-FP) infusions to achieve haemostasis for all treated bleeds. (b) The time between the start of a bleed to the first infusion of rIX-FP, for all treated bleeds.
The majority of infusions received a rating of excellent or good (96.5%) by the investigator in response to rIX-FP treatment. There were three bleeding episodes, the treatment of which received a moderate rating; these bleeds all resolved with one or two infusions, and the time to the first rIX-FP treatment was delayed more than 8 h after the start of the bleed. While subjects were encouraged to treat a bleed immediately, 16.4% of bleeds were treated more than 8 h after the start of the haemorrhage, and 5.9% more than 16 h after the start of the haemorrhage (Fig.3b).
Routine prophylaxis
All prophylaxis subjects were assigned a dose based on the individual subject’s PK, activity level and bleeding phenotype. The subjects maintained weekly routine prophylaxis with mean dose of 55 IU kg−1 rIX-FP, which was 63% of their previous weekly FIX consumption. Approximately, half of the prophylaxis subjects (46%, six subjects) did not have any spontaneous bleeding episodes for the 11-month duration of the study. Seven prophylaxis subjects reported 14 spontaneous bleeds during the prophylaxis portion of the study and two spontaneous bleeds occurred at the end of the PK period. Four of the seven subjects reporting spontaneous bleeds had a history of haemophilic arthropathy or synovitis in the joint; the remaining three subjects were all adolescents, two of whom were receiving only ODT prior to study entry.
Annualized bleeding rate
Overall, prophylaxis subjects reported fewer spontaneous bleeding episodes than the subjects receiving ODT only. On-demand subjects receiving rIX-FP for ODT (ODT-ODT) had a mean AsBR of 21.74 bleeds per year (Fig.4a), a 20% reduction compared to their historical AsBR. Prophylaxis subjects who received rIX-FP for prophylaxis (PT-PT) had a mean AsBR of 1.26 (Fig.4a). The prophylaxis and on-demand subjects who received rIX-FP during the study (PT-PT and ODT-ODT, respectively, Fig.4b) had a similar ABR for traumatic bleeding episodes. The mean total ABR (spontaneous and traumatic) during the study was 4.35 and 28.8 for prophylaxis and on-demand subjects, respectively.
Figure 4.
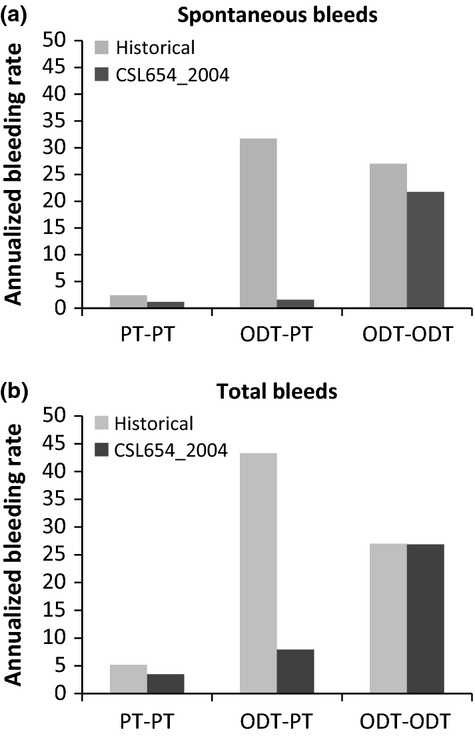
Annualized bleeding rate for prophylaxis with coagulation factor IX (recombinant), albumin fusion protein compared to historical bleeding rate. The mean annualized bleeding rate for spontaneous bleeds are shown in (a) and for all bleeds are shown in (b). The annualized bleeding rate for each patient was calculated as the number of bleeds during the time in the treatment period of the study in days, divided by 365.25. The historical bleeding rate for each patient was the number of bleeds in the 12 month period prior to study entry. PT, prophylaxis treatment; ODT, on-demand treatment.
In the prophylaxis treatment group, ten subjects received routine prophylaxis prior to study entry (PT-PT), and reported approximately 50% higher mean historical AsBR in the 12 months prior to study entry than with rIX-FP weekly prophylaxis (Fig.4a). Three subjects in the prophylaxis treatment group received ODT only prior to study entry (ODT-PT). These ODT-PT subjects reported a much higher historical mean AsBR of 31.7 in the 12 months prior to study entry than the PT–PT subjects (Fig.4a). After starting weekly prophylaxis with rIX-FP, ODT-PT subjects had a mean AsBR of 1.56 (Fig.4a) per year, a 95% reduction when compared to the historical data. The total ABR of the ODT-PT subjects was reduced by 83% to 7.3 bleeds per year with rIX-FP prophylaxis treatment (Fig.4b). The total ABR of the ODT-PT subjects after having received rIX-FP was higher than the overall prophylaxis treatment group (PT–PT, Fig.4b) due to a higher rate of traumatic bleeding episodes. However, traumatic bleeds were still reduced in the ODT-PT population by approximately 50% during prophylaxis treatment with rIX-FP, compared to the 12 months prior to study entry (5.8 vs. 11.3).
Discussion
Albumin fusion technology has been shown to be a very attractive technology to extend the half-life of coagulation factors, as human albumin is an abundant plasma protein and does not act as a trigger for the immune system. In the present study, there were no hypersensitivity reactions or development of inhibitors to FIX or antibodies to rIX-FP after over 700 repeated exposures to rIX-FP among 17 study subjects, nine of which achieved at least 50 EDs.
rIX-FP was very effective in the treatment of bleeding episodes, with haemostasis achieved after a single dose 95% of the time, and all bleeds effectively treated with one or two doses. This compares favourably to treatment with BeneFIX, which has been reported to effectively treat 81% of bleeds with a single dose in a clinical trial in previously treated patients 20. While there was a big difference in the rIX-FP dose used to treat bleeds from the two study centres, there is no apparent correlation between the dose of rIX-FP used and the number of treatments needed to achieve haemostasis. All of the prophylaxis subjects were assigned the same dose of rIX-FP for the treatment of bleeding episodes, as for weekly prophylaxis; this was done for patient convenience and to ensure patients utilized full vials, as decided by the treating physician. The difference in assigned rIX-FP doses for the treatment of bleeds may be the result of multiple factors, including the prophylaxis treatment status, the bleeding phenotype of the patient and local standard of care. Nevertheless, the study results indicated that the lower dose of rIX-FP was equally effective for the treatment of bleeding episodes. On-demand subjects also showed a 20% reduction in AsBR compared to their historical AsBR, suggesting that rIX-FP may prevent some spontaneous bleeding episodes in haemophilia B patients receiving ODT. Due to the small number of patients treated on demand in this study, additional data are needed from the phase III trial.
All prophylaxis subjects maintained weekly treatment interval for prophylaxis with rIX-FP, with excellent compliance, for 11 months during the study. The ABR of these subjects on weekly prophylaxis was less than their previous ABR when receiving prophylaxis 1, 2 or 3 times weekly with plasma-derived or recombinant FIX. Three subjects who were not receiving prophylaxis treatment prior to study entry had an 83% reduction in the total ABR compared to their reported ABR while on ODT. Remarkably, one of these three subjects had no spontaneous bleeding episodes during the study. While this is an extremely small sample size, switching from on demand to weekly prophylaxis treatment of rIX-FP dramatically reduced the bleeding rate for these subjects.
Eleven subjects in the prophylaxis arm and all four on-demand subjects continued their treatment with rIX-FP in the next phase of the clinical program of rIX-FP. The majority of prophylaxis subjects (10/11) switched to prophylaxis intervals of 10 and 14 days (five each) at a dose of 70–75 IU kg−1. All four on-demand subjects have switched to weekly prophylaxis at 36–38 IU kg−1 for over a year, and then switched to once every 14 days at 75 IU kg−1. This proof of concept study demonstrated that a less frequent prophylaxis treatment regimen is possible and effective with the extended half-life provided by rIX-FP. In addition, rIX-FP provided effective ODT for patients with this lifelong, debilitating bleeding disorder. The excellent PK, efficacy and safety profile may make rIX-FP an excellent choice for long-term once weekly to 14 days routine prophylaxis in patients with haemophilia B.
Acknowledgments
The authors thank the chairman of Data Review Committee, Dr Barbara Konkle, Puget Sound Blood Center, Seattle, USA, sub-investigators Drs J. Luboshitz and S. Lelazari, The Israeli National Hemophilia Center, Chaim Sheba Medical Center, Tel Hashomer, Israel, and study personnel in both study centres. Cindy Cochran, CSL Behring/US, provided the operational management; Stefanie Achenbach, CSL Behring/Germany, provided central laboratory testing support. This study would not have been possible without the support of the patients who participated. This study was sponsored by CSL Behring, Marburg, Germany.
Author contributions
U. Martinowitz and T. Lissitchkov performed research, collected data, interpreted data, wrote and revised the manuscript. E. Santagostino designed research, interpreted data and revised the manuscript. A. Lubetsky and G. Jotov performed research, collected data and revised the manuscript. T. Barazani- Brutman collected data. T. Moises provided analytical support. C. Voigt and I. Jacobs designed the research, analysed data, wrote and revised the manuscript.
Disclosures
U. Martinowitz received research support from CSL Behring to conduct the study, lecture fees and honoraria for consultancy from CSL Behring. T. Lissitchkov, A. Lubetsky, G. Jotov and T. Barazani- Brutman received research support from CSL Behring to conduct the study. E. Santagostino received fees as a speaker in meetings organized by CSL Behring and acted as a paid consultant for CSL Behring. T. Moises, C. Voigt and I. Jacobs were employed at CSL Behring.
References
- National Hemophilia Foundation. 2007. MASAC recommendation #179: concerning prophylaxis (regular administration of clotting factor concentrate to prevent bleeding). Available at http://www.hemophilia.org/NHFWeb/MainPgs/MainNHF.aspx?menuid=57&contentid=1007. Accessed February 25, 2015.
- Astermark J, Petrini P, Tengborn L, Schulman S, Ljung R, Berntorp E. Primary prophylaxis in severe haemophilia should be started at an early age but can be individualized. Br J Haematol. 1999;105:1109–13. doi: 10.1046/j.1365-2141.1999.01463.x. [DOI] [PubMed] [Google Scholar]
- Kreuz W, Escuriola-Ettingshausen C, Funk M, Schmidt H, Kornhuber B. When should prophylactic treatment in patients with haemophilia A and B start? The German experience. Haemophilia. 1998;4:413–7. doi: 10.1046/j.1365-2516.1998.440413.x. [DOI] [PubMed] [Google Scholar]
- Funk M, Schmidt H, Escuriola-Ettinghausen C, et al. Radiological and orthopedic score in pediatric hemophilic patients with early and late prophylaxis. Ann Hematol. 1998;77:171–4. doi: 10.1007/s002770050436. [DOI] [PubMed] [Google Scholar]
- De Moerloose P, Urbancik W, van den Berg HM, Richards M. A survey of adherence to haemophilia therapy in six European countries: results and recommendations. Haemophilia. 2008;14:931–8. doi: 10.1111/j.1365-2516.2008.01843.x. [DOI] [PubMed] [Google Scholar]
- Blanchette VS, Manco-Johnson M, Santagostino E, Ljung R. Optimizing factor prophylaxis for the haemophilia population: where do we stand? Haemophilia. 2004;10:97–104. doi: 10.1111/j.1365-2516.2004.00998.x. [DOI] [PubMed] [Google Scholar]
- Hacker MR, Geraghty S, Manco-Johnson M. Barriers to compliance with prophylaxis therapy in haemophilia. Haemophilia. 2001;7:392–6. doi: 10.1046/j.1365-2516.2001.00534.x. [DOI] [PubMed] [Google Scholar]
- Negrier C, Knobe K, Tiede A, Giangrande P, Moss J. Enhanced pharmacokinetic properties of a glycoPEGylated recombinant factor IX: a first human dose trial in patients with hemophilia B. Blood. 2012;118:2695–701. doi: 10.1182/blood-2011-02-335596. [DOI] [PubMed] [Google Scholar]
- Shapiro AD, Ragni MV, Valentino LA, et al. Recombinant factor IX-Fc fusion protein (rFIX-Fc) demonstrates safety and prolonged activity in a phase 1/2a study in hemophilia B patients. Blood. 2012;119:666–72. doi: 10.1182/blood-2011-07-367003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte S. Half-life extension through albumin fusion technologies. Thromb Res. 2009;124:S6–8. doi: 10.1016/S0049-3848(09)70157-4. [DOI] [PubMed] [Google Scholar]
- Knobe K, Berntorp E. New treatments in hemophilia: insights for the clinician. Ther Adv Hematol. 2012;3:165–75. doi: 10.1177/2040620712440007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzner HJ, Weimer T, Kronthaler U, Lang W, Schulte S. Genetic fusion to albumin improves the pharmacokinetic properties of Factor IX. Thromb Haemost. 2009;102:634–44. doi: 10.1160/TH09-04-0255. [DOI] [PubMed] [Google Scholar]
- Nolte MW, Nichols TC, Mueller-Cohrs J, et al. Improved kinetics of rIX-FP, a recombinant fusion protein linking factor IX with albumin, in cynomolgus monkeys and hemophilia B dogs. J Thromb Haemost. 2012;10:1591–9. doi: 10.1111/j.1538-7836.2012.04826.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santagostino E, Negrier C, Klamroth R, et al. Safety and pharmacokinetics of a novel recombinant fusion protein linking coagulation factor IX with albumin (rIX-FP) in hemophilia B patients. Blood. 2012;120:2405–11. doi: 10.1182/blood-2012-05-429688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins PW, Fischer K, Morfini M, Blanchette VS, Björkman S on behalf of International Prophylaxis Study Group (IPSG) Pharmacokinetics Expert Working Group. Implications of coagulation factor VIII and IX pharmacokinetics in the prophylactic treatment of haemophilia. Haemophilia. 2011;17:2–10. doi: 10.1111/j.1365-2516.2010.02370.x. [DOI] [PubMed] [Google Scholar]
- Ahnström J, Berntorp E, Lindvall K, Björkman S. A 6-year follow-up of dosing, coagulation factor levels and bleedings in relation to joint status in the prophylactic treatment of haemophilia. Haemophilia. 2004;10:689–97. doi: 10.1111/j.1365-2516.2004.01036.x. [DOI] [PubMed] [Google Scholar]
- European Medicines Agency. Committee for medicinal products for human use (CHMP), guideline in the clinical investigation of recombinant and human plasma-derived factor IX products. EMEA 2009CHMP/BPWP/144552/2009. Available at http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003634.pdf. Accessed February 25, 2015.
- Verbruggen B, Novakova I, Wessels H, Boezeman J, van den Berg M, Mauser-Bunschoten E. The Nijmegen modification of the Bethesda assay for factor VIII: C inhibitors: improved specificity and reliability. Thromb Haemost. 1995;73:247–51. [PubMed] [Google Scholar]
- Martinowitz U, Lubetsky A. Phase I/II, open-label, multicenter, safety, efficacy and PK study of a recombinant coagulation factor IX albumin fusion protein (rIX-FP) in subjects with hemophilia B. Thromb Res. 2013;131(Suppl. 2):S11–4. doi: 10.1016/S0049-3848(13)70152-X. [DOI] [PubMed] [Google Scholar]
- Benefix Package Insert. 2011. Available at http://labeling.pfizer.com/showlabeling.aspx?id=492. Accessed August 2, 2012.


