Abstract
Objective
The objective of this study was to compare the efficacy, tolerability, and safety of AVP-825, an investigational bi-directional breath-powered intranasal delivery system containing low-dose (22 mg) sumatriptan powder, vs 100 mg oral sumatriptan for acute treatment of migraine in a double-dummy, randomized comparative efficacy clinical trial allowing treatment across multiple migraine attacks.
Background
In phases 2 and 3, randomized, placebo-controlled trials, AVP-825 provided early and sustained relief of moderate or severe migraine headache in adults, with a low incidence of triptan-related adverse effects.
Methods
This was a randomized, active-comparator, double-dummy, cross-over, multi-attack study (COMPASS; NCT01667679) with two ≤12-week double-blind periods. Subjects experiencing 2-8 migraines/month in the past year were randomized 1:1 using computer-generated sequences to AVP-825 plus oral placebo tablet or an identical placebo delivery system plus 100 mg oral sumatriptan tablet for the first period; patients switched treatment for the second period in this controlled comparative design. Subjects treated ≤5 qualifying migraines per period within 1 hour of onset, even if pain was mild. The primary end-point was the mean value of the summed pain intensity differences through 30 minutes post-dose (SPID-30) using Headache Severity scores. Secondary outcomes included pain relief, pain freedom, pain reduction, consistency of response across multiple migraines, migraine-associated symptoms, and atypical sensations. Safety was also assessed.
Results
A total of 275 adults were randomized, 174 (63.3%) completed the study (ie, completed the second treatment period), and 185 (67.3%) treated at least one migraine in both periods (1531 migraines assessed). There was significantly greater reduction in migraine pain intensity with AVP-825 vs oral sumatriptan in the first 30 minutes post-dose (least squares mean SPID-30 = 10.80 vs 7.41, adjusted mean difference 3.39 [95% confidence interval 1.76, 5.01]; P < .001). At each time point measured between 15 and 90 minutes, significantly greater rates of pain relief and pain freedom occurred with AVP-825 treatment compared with oral sumatriptan. At 2 hours, rates of pain relief and pain freedom became comparable; rates of sustained pain relief and sustained pain freedom from 2 to 48 hours remained comparable. Nasal discomfort and abnormal taste were more common with AVP-825 vs oral sumatriptan (16% vs 1% and 26% vs 4%, respectively), but ∼90% were mild, leading to only one discontinuation. Atypical sensation rates were significantly lower with AVP-825 than with conventional higher dose 100 mg oral sumatriptan.
Conclusions
AVP-825 (containing 22 mg sumatriptan nasal powder) provided statistically significantly greater reduction of migraine pain intensity over the first 30 minutes following treatment, and greater rates of pain relief and pain freedom within 15 minutes, compared with 100 mg oral sumatriptan. Sustained pain relief and pain freedom through 24 and 48 hours was achieved in a similar percentage of attacks for both treatments, despite substantially lower total systemic drug exposure with AVP-825. Treatment was well tolerated, with statistically significantly fewer atypical sensations with AVP-825.
Keywords: migraine, AVP-825, sumatriptan, comparative trial, intranasal, OptiNose
Head-to-head studies provide data on relative risks and benefits of drugs, allowing an evidence-based rationale for making treatment decisions when choosing among therapeutic alternatives. For this reason, comparative studies are increasingly required by the US Affordable Care Act, the US Patient-Centered Outcomes Research Institute, and payers and health-care providers. Yet, studies directly comparing migraine medications have rarely been conducted, despite the fact that migraine is prevalent, disabling, and exacts a substantial impact on patients, payers, and society.1
Sumatriptan is the most widely prescribed triptan for migraine and is available in different formulations in different countries, including oral, subcutaneous injection, liquid nasal spray, and suppository.2,3 Of formulations approved in the US, oral sumatriptan is preferred by patients and by far the most commonly prescribed,2,3 but has been associated with variable absorption and delayed onset of efficacy, because of slowed gastric emptying during migraine and in migraineurs generally, as well as with triptan-related adverse effects such as tingling, and chest, jaw, or neck tightness (ie, atypical sensations).2 Despite the well-established efficacy of triptans for acute treatment of migraine and the availability of multiple formulations, many patients remain dissatisfied with existing triptan options for a number of reasons, including slow onset of efficacy with oral treatment.4
AVP-825, a novel intranasal delivery system containing low-dose sumatriptan powder (22 mg), is under investigation for the acute treatment of migraine.5,6 AVP-825 delivers sumatriptan more effectively than traditional liquid nasal spray to the upper posterior segments of the nasal passages using a breath-powered bi-directional intranasal delivery system that redirects patients’ exhaled air containing sumatriptan powder into the nostril, widens nasal passages, naturally seals the soft palate (reducing drug deposition in the throat and mouth), and delivers drug beyond the narrow nasal valve7 (Fig. 1). Delivery of drug with this novel method may confer several advantages, including drug distribution across a significantly wider area of absorbent mucosa in the upper posterior nasal cavity compared with traditional liquid nasal spray,5 which is thought to explain the proven faster absorption of sumatriptan powder delivered with this technology relative to traditional liquid nasal spray and oral tablets,8 and to create potential for reduced drug swallowing and reduced first-pass hepatic metabolism. The low dose (∼15-16 mg of drug is delivered into the nose9) is associated with significantly lower systemic exposure vs oral tablet (100 mg) or subcutaneous injection (6 mg), which may lead to improved tolerability.8 Previous phases 2 and 3 placebo-controlled AVP-825 trials in acute treatment of migraine showed early onset of efficacy (significantly higher rates of pain relief as early as 30 minutes post-dose in the phase 3 trial) accompanied by sustained headache relief through 48 hours and a low level of triptan-related adverse effects.9,10
Figure 1.
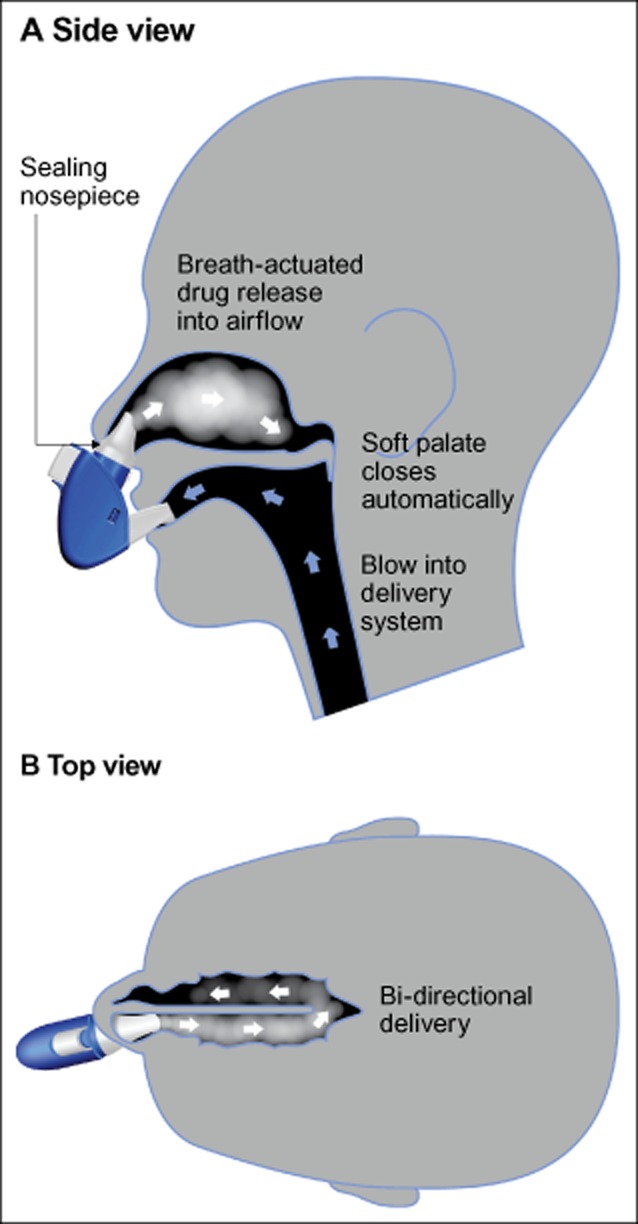
AVP-825: illustration of breath-powered delivery of sumatriptan powder. AVP-825 delivers low-dose sumatriptan powder to the upper posterior nasal regions beyond the narrow nasal valve, an area of richly vascular mucosa conducive to rapid drug absorption into the systemic circulation.
This study (COMPASS) compared the efficacy and safety of AVP-825 to the most widely prescribed migraine treatment, oral sumatriptan at 100 mg. COMPASS was designed to avoid limitations of other comparative migraine studies,11–21 and to minimize bias favoring AVP-825 by optimizing dosing conditions for oral sumatriptan. In order to maximize the opportunity for clinical response with oral sumatriptan, participants were required to begin treatment early (within 1 hour of migraine onset), even if pain was mild, and to use the most efficacious dose of oral sumatriptan (100 mg) in an early intervention paradigm.22
The objective of this study was to compare the efficacy, tolerability, and safety of AVP-825, an investigational bi-directional breath-powered intranasal delivery system containing low-dose (22 mg) sumatriptan powder, vs 100 mg oral sumatriptan for acute treatment of migraine in a double-dummy, randomized comparative efficacy clinical trial allowing treatment across multiple migraine attacks.
Methods
Standard Protocol Approvals, Registrations, and Patient Consents
COMPASS (NCT01667679, clinicaltrials.gov) was conducted in accordance with the Declaration of Helsinki, all relevant US federal regulations, and in compliance with the International Conference on Harmonization guideline for Good Clinical Practice. The protocol, informed consent forms, and other study-related documents were approved by an Institutional Review Board/Ethics Committee at each center. Written informed consent was obtained from each subject prior to protocol-related activities.
Study Design
COMPASS was a randomized, double-blind, double-dummy, active-comparator cross-over study (August 2012–March 2014) conducted at 13 outpatient centers throughout the US. Both cross-over and parallel group trial designs are recommended by International Headache Society guidelines for controlled trials of drugs in migraine.23 This study consisted of two double-blind periods, each lasting ≤12 weeks, in which subjects treated ≤5 qualifying migraines with study medication (bi-directional breath-powered intranasal delivery system plus oral tablet). Eligible subjects were randomly assigned (unstratified blocked randomization sequences generated using SAS® Version 9.2, SAS Institute Inc., Cary, NC, USA, or higher) by interactive web-based response system. Subject randomization was done 1:1 to one of two treatment sequences that in each period consisted of an intranasal delivery system (active or placebo) and a tablet (active or placebo). Specifically, the treatment sequences were as follows: (1) AVP-825 (containing 22 mg sumatriptan nasal powder) plus placebo tablet (Ranbaxy Pharmaceuticals, Inc., Jacksonville, FL, USA) in treatment period 1 followed by an identical placebo delivery system (containing lactose powder) plus 100 mg sumatriptan tablet (Ranbaxy Pharmaceuticals, Inc.) in treatment period 2 or 2) identical placebo delivery system plus 100 mg sumatriptan tablet (treatment period 1) followed by AVP-825 plus oral placebo tablet (treatment period 2). After treating the 5th qualifying migraine or upon reaching 12 weeks in treatment period 1, whichever was first, the subject then crossed over to the alternate treatment regimen (treatment period 2) and continued with that until the 5th qualifying migraine was treated or upon reaching 12 weeks in treatment period 2, whichever was first. Subjects, investigators, sponsor, and staff remained blinded during the study. Randomization codes were maintained within the interactive web-based response system.
Qualifying migraines met the International Headache Society criteria with at least mild (grade 1) intensity. Subjects were to administer treatment within 1 hour of onset of a qualifying migraine. For each administration, subjects were to use the intranasal delivery system first followed by an oral tablet. The intranasal dose was administered using a novel bi-directional breath-powered intranasal delivery system using 2 nosepieces (1 application into each nostril), starting with the nostril on the side of the headache. At dispensing, subjects were trained to use the new intranasal delivery system, but subsequently treated headaches without medical supervision or oversight. Subjects recorded outcomes in an electronic diary.
A second dose of study drug was allowed after all diary assessments were completed for the 120 minute time point, and up to 24 hours after the first dose if there was no relief or the headache worsened/recurred. Rescue medication was permitted if there was no relief or headache worsened/recurred at 120 minutes after the second dose. Subjects were withdrawn from the study for failure to treat any migraines in the 12 weeks of either treatment period 1 or 2.
Subjects
Adults (18-65 years) with a diagnosis of episodic migraine with or without aura (according to The International Classification of Headache Disorders, 2nd Edition, 1st revision, May 2005) for at least 1 year prior to screening were recruited from the outpatient centers of investigators where they were receiving care, and in some instances via advertising or referral from other clinics. Eligible subjects experienced 2-8 migraines/month in the 12 months before screening. Subjects had verified airflow through both nostrils, the ability to close the soft palate (eg, inflate a balloon), and the ability to use the bi-directional breath-powered intranasal delivery system correctly.
Subjects were excluded if they could not distinguish migraine from other headaches or if they had: headache of any kind ≥15 days/month, hemiplegic or basilar-type migraine, a history or symptoms/signs of ischemic cardiac, cerebrovascular, or peripheral vascular syndromes, uncontrolled hypertension, severe hepatic impairment, or a history of epilepsy. Subjects were excluded if they had systemic disease or a neurologic or psychiatric condition, which in the opinion of the investigator would contraindicate participation. Subjects with a history of nonresponse to sumatriptan or ≥2 other triptans, current use of medication for migraine prophylaxis not at a stable dose for 30 days before screening, chronic opioid therapy (>3 consecutive days in the 30 days before screening), known nasal obstruction, or current uncontrolled nasopharyngeal illness were excluded, as were subjects who previously participated in the phase 3 AVP-825 TARGET9 trial.
Outcome Measures
Efficacy
The mean value of the SPID-30, defined as the sum of pain intensity differences from baseline through 30 minutes post-dose, was the primary end-point, with a higher number indicating greater reduction in pain intensity. Headache Severity score on a standard ordinal 4-point scale (pain intensity of 0 = none, 1 = mild, 2 = moderate, 3 = severe) was recorded by subjects just before the intake of study medication (baseline) and at 10, 15, 30, 45, 60, 90, and 120 minutes post-dose. The differences in pain intensity values from baseline to 10, 15, and 30 minutes post-dose were used to calculate the SPID-30. The null hypothesis asserted that there is no difference between treatments in the SPID-30.
Pain intensity differences and summed pain intensity differences have been widely used in pain studies and in some migraine studies.15,23–25 In this study, the SPID-30 was determined to be a preferred primary measure of early treatment effect for statistic purposes and to more directly test the study objective/hypothesis vs an earlier designated primary end-point (proportion of attacks with pain reduction, defined as a decrease in Headache Severity score of ≥1 point, at a single 30 minutes time point). SPID-30 was formally specified as the primary end-point after enrollment was completed, but before database lock and unblinding of study data. However, the proportion of attacks with pain reduction at 30 minutes (and at other time points) was also evaluated and included as a secondary end-point, and data for both outcomes are reported in full.
All secondary efficacy end-points were considered exploratory. Key secondary efficacy end-points were evaluated at 10, 15, 30, 45, 60, 90, and 120 minutes post-dose and included pain relief (reduction of Headache Severity score from moderate or severe to none or mild) and pain freedom (reduction of Headache Severity score from mild, moderate, or severe to none). Other secondary efficacy end-points included sustained pain relief (pain relief reported at 120 minutes followed by no worsening of pain or second dose of study medication or rescue medication through 24 and 48 hours after initial dose), sustained pain freedom (no pain at 120 minutes with no recurrence of headache, use of a second dose of study medication or rescue medication taken through 24 and 48 hours after initial dose), pain freedom for headaches treated when the baseline intensity was mild vs moderate/severe, presence of migraine-associated symptoms (nausea, photophobia, phonophobia, or vomiting), subject self-assessment of meaningful pain relief, pain reduction, clinical disability (performance of daily activities of 0 = no disability, 1 = mildly impaired, 2 = moderately impaired, 3 = severely impaired, bed rest may be necessary), and consistency of pain relief across multiple migraines (for the first two, ≥two of the first three, or three of the first three migraines in each period). Additional secondary efficacy end-points were evaluated and will be presented in a subsequent article.
Safety assessments included treatment-emergent adverse events (TEAEs), laboratory measures (hematology, serum chemistry, and urinalysis), and electrocardiograms. The pre-specified TEAE of atypical sensations (symptoms in the chest, arms, hands or feet of warm/hot sensation, burning sensation, feeling of chest heaviness, pressure, feeling of tightness, numbness, or feeling strange) was evaluated at 120 minutes post-dose.
Data Analysis
The sample size was based on proportion of attacks with pain reduction and assumed average 30 minutes pain-reduction rates of 38% (AVP-825) vs 25.1% (oral sumatriptan) based on a previous trial.26 With a 2-sided α = 0.05 and a correlation of 0.20, the power was 80% for 3 migraines and 85% for 4 migraines per subject (since not all subjects would treat 5 migraines). A protocol revision updated the assumed withdrawal rate to 30% (vs 10%) and allowed a larger number of subjects in order to maintain the planned statistical power. Approximately 138 subjects were to be enrolled per sequence group (approximately 276 total). Tables and listings were produced using SAS® Version 9.2 or higher.
Efficacy analyses used the full analysis set (FAS), which included all subjects who received ≥1 dose of AVP-825 and ≥1 dose of oral sumatriptan, and recorded ≥1 post-treatment assessment in both cross-over treatment periods. The FAS definition was pre-specified in the study protocol. The Safety Set included all subjects who received ≥1 dose of AVP-825 or oral sumatriptan.
The primary efficacy end-point (mean value of the SPID-30 over all headaches during a period) was analyzed using an analysis of covariance model with fixed effects for treatment, period, and treatment sequence, and with subject as a random effect. Consistency of pain relief across migraines was evaluated using McNemar’s exact test. Other secondary efficacy end-points and the percentage of migraine attacks with TEAEs of atypical sensations were analyzed using generalized estimating equations models with treatment, sequence, and period as fixed effects and a compound symmetry correlation structure within subject. For efficacy analyses, missing values were replaced by carrying forward the preceding value (last observation carried forward). Statistical significance was accepted for P < .05 for the primary efficacy end-point; all other efficacy end-points were exploratory. Other safety variables were analyzed descriptively.
Results
Subject Disposition and Study Populations
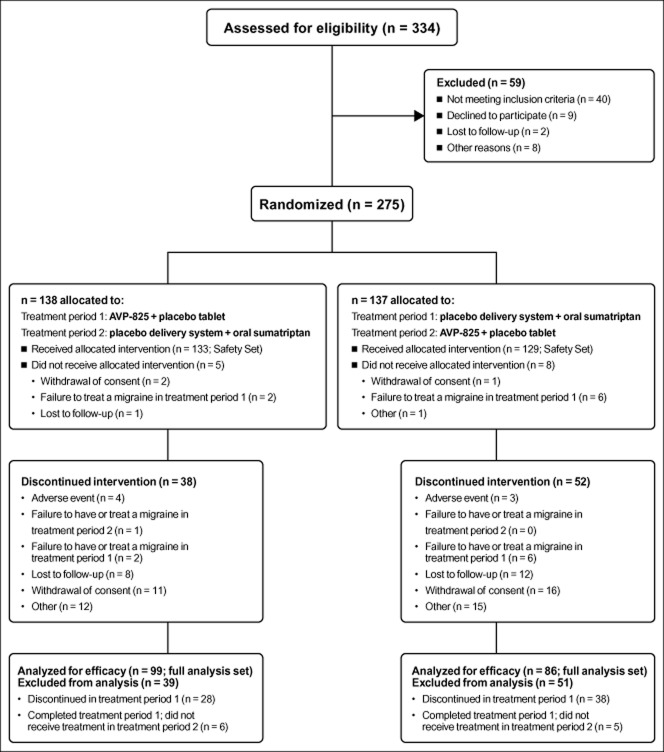
A total of 275 subjects were randomized, and 262 (95.3%) used at least 1 dose of study medication and comprised the Safety Set (Fig. 2). A total of 185 (67.3%) subjects contributed efficacy data (ie, treated at least 1 migraine in each cross-over treatment period) and comprised the FAS, in which 1531 migraines were treated over the entire study. Most subjects in the FAS treated 2-5 migraines in a treatment period (95% AVP-825, 92% oral sumatriptan), and a majority treated 5 migraines (60% AVP-825, 61% oral sumatriptan). The median number of migraines treated per subject was 5 (1-6). Completion rates for treatment period 1 (ie, subjects who treated ≥1 qualifying migraine in treatment period 1 and entered treatment period 2) were 79.7% (AVP-825) and 69.3% (oral sumatriptan); of these subjects, 85.5% (AVP-825) and 84.2% (oral sumatriptan) completed treatment period 2 (ie, subjects who treated ≥1 qualifying headache and either had 5 qualifying headaches or completed the full 12 weeks in treatment period 2). Overall, 63.3% of randomized participants completed the study (subjects who completed treatment period 2 completed the study). Demographic and baseline characteristics were comparable for the two treatment sequences (Table 1).
Figure 2.
Subject flow diagram. The full analysis set (FAS) included all subjects who received ≥1 dose of AVP-825 and ≥1 dose of oral sumatriptan, and recorded ≥1 post-treatment assessment for each treatment. Subjects completed treatment period 1 if they treated ≥1 qualifying migraine in treatment period 1 and went into treatment period 2. Subjects completed treatment period 2 if they treated 5 qualifying migraines or ≥1 qualifying migraine and completed the full 12 weeks in treatment period 2. Subjects were considered study completers if they completed treatment period 2.
Table 1.
Subject Demographics and Baseline Characteristics
| Treatment period 1 | Treatment period 1 | ||
|---|---|---|---|
| AVP-825 + placebo tablet | Placebo delivery system +100 mg oral sumatriptan tablet | ||
| Treatment period 2 | Treatment period 2 | ||
| Placebo delivery system +100 mg oral sumatriptan tablet | AVP-825 + placebo tablet | ||
| Total | |||
| Safety Set, n | 133 | 129 | 262 |
| Age (years), mean (SD) | 39.5 (12.6) | 40.7 (11.9) | 40.1 (12.2) |
| Female, n (%) | 107 (80.5) | 115 (89.1) | 222 (84.7) |
| Migraine attacks per month (past 12 months), mean (SD) | 4.8 (2.0) | 4.9 (1.9) | 4.9 (1.9) |
| Full Analysis Set, n | 99 | 86 | 185 |
| Migraine attacks per month (past 12 months), mean (SD) | 5.0 (2.0) | 4.8 (1.9) | 4.9 (1.9) |
| Has monthly migraine ≥moderate severity (past 12 months), n (%) | 99 (100) | 86 (100) | 185 (100) |
| Historical pain intensity of treated migraine headaches, n (%) | |||
| None | 6 (6.1) | 6 (7.0) | 12 (6.5) |
| Mild | 40 (40.4) | 35 (40.7) | 75 (40.5) |
| Moderate | 38 (38.4) | 40 (46.5) | 78 (42.2) |
| Severe | 14 (14.1) | 5 (5.8) | 19 (10.3) |
| Migraine type (past 12 months), n (%) | |||
| Aura only | 0 | 1 (1.2) | 1 (0.5) |
| With aura | 29 (29.3) | 27 (31.4) | 56 (30.3) |
| Without aura | 79 (79.8) | 69 (80.2) | 148 (80.0) |
| Presence of (past 6 months),*n (%) | |||
| Vomiting | 37 (37.4) | 27 (31.4) | 64 (34.6) |
| Nausea | |||
| None | 15 (15.2) | 12 (14.0) | 27 (14.6) |
| Mild | 32 (32.3) | 34 (39.5) | 66 (35.7) |
| Moderate | 40 (40.4) | 29 (33.7) | 69 (37.3) |
| Severe | 12 (12.1) | 11 (12.8) | 23 (12.4) |
| Photophobia | |||
| None | 1 (1.0) | 2 (2.3) | 3 (1.6) |
| Mild | 14 (14.1) | 13 (15.1) | 27 (14.6) |
| Moderate | 40 (40.4) | 37 (43.0) | 77 (41.6) |
| Severe | 44 (44.4) | 34 (39.5) | 78 (42.2) |
| Phonophobia | |||
| None | 7 (7.1) | 4 (4.7) | 11 (5.9) |
| Mild | 19 (19.2) | 17 (19.8) | 36 (19.5) |
| Moderate | 42 (42.4) | 37 (43.0) | 79 (42.7) |
| Severe | 31 (31.3) | 28 (32.6) | 59 (31.9) |
| No prior use of intranasal medication, n (%) | 96 (97.0) | 84 (97.7) | 180 (97.3) |
Subjects may have had more than one of the listed symptoms.
SD = standard deviation.
Efficacy
The primary end-point showed greater reduction in migraine pain intensity with AVP-825 vs oral sumatriptan that was statistically significant over the first 30 minutes post-dose (least squares mean SPID-30 = 10.80 vs 7.41, adjusted mean difference 3.39 [95% confidence interval 1.76, 5.01]; P < .001). Statistically significant differences in rates of pain relief (Fig. 3A) and pain freedom (Fig. 3B) favored AVP-825 vs oral sumatriptan at every time point from 15 to 90 minutes post-dose. Pain relief and pain freedom at 120 minutes, as well as sustained pain relief and pain freedom through 24 and 48 hours, were achieved in a similar percentage of attacks for both treatments (Fig. 3). Differences in pain reduction (the earlier designated primary end-point) rates were also statistically significant in favor of AVP-825 vs oral sumatriptan from 15 to 60 minutes post-dose (Table 2).
Figure 3.
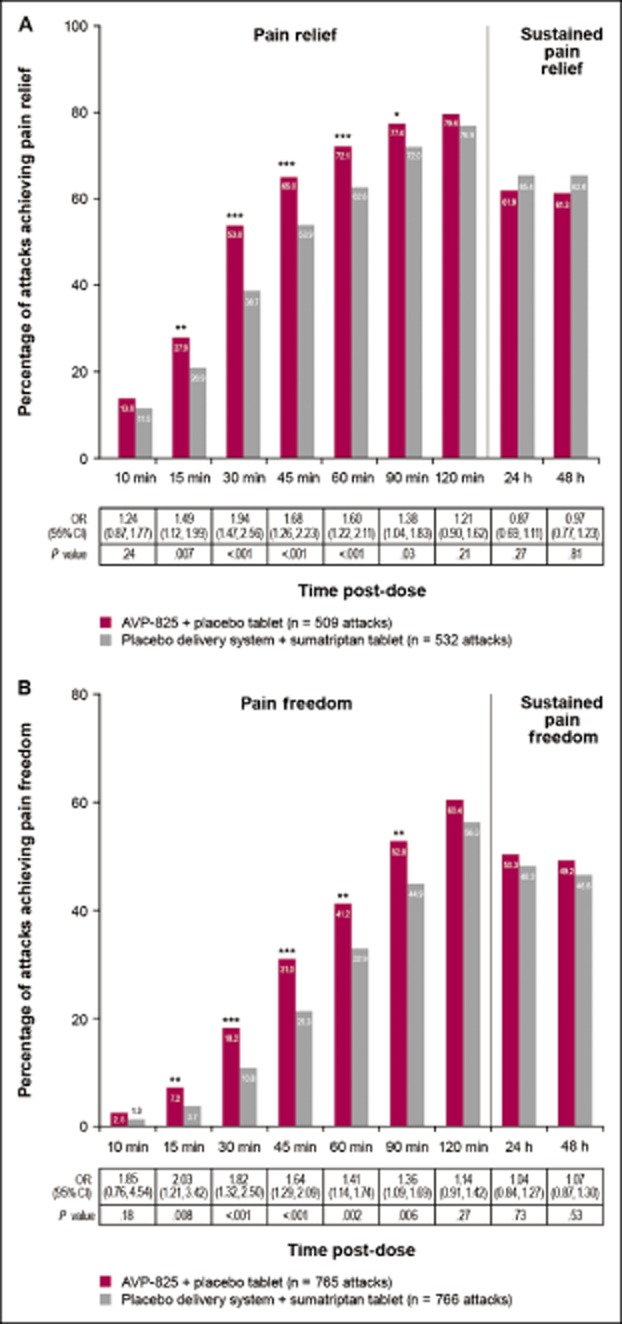
Percentage of migraine attacks achieving (A) pain relief and sustained pain relief and (B) pain freedom and sustained pain freedom (full analysis set). For pain relief, percentages are based on the total number of attacks treated when pain was moderate or severe. Pain relief = pain level reduced to none or mild. Sustained pain relief = pain level reduced to none or mild with no worsening of headache, second dose of study drug, or use of rescue medication over 24 hours or 48 hours after the attack. Pain freedom = pain level reduced to none (grade 0). Sustained pain freedom = grade 0 from 120 minutes to over 24 hours, and 48 hours after the initial dose with no recurrence of headache, or rescue medication/second dose up to 24 and 48 hours. Odds ratios (OR) are based on the fitted generalized estimating equations models.*P < .05, **P < .01, ***P < .001.
Table 2.
Other Secondary Efficacy Outcomes at Each Post-Dose Time Point
| Pre-dose | 10 minutes | 15 minutes | 30 minutes | 45 minutes | 1 hour | 1.5 hours | 2 hours | |
|---|---|---|---|---|---|---|---|---|
| Pain reduction, percentage of attacks | ||||||||
| AVP-825 (n = 765 attacks) | 11.5 | 26.4 | 49.0 | 60.7 | 67.2 | 74.6 | 78.0 | |
| Oral sumatriptan (n = 766 attacks) | 10.2 | 19.6 | 35.2 | 49.9 | 59.8 | 69.8 | 75.2 | |
| OR (95% CI) | 1.16 (0.85, 1.60) | 1.51 (1.20, 1.90) | 1.79 (1.45, 2.21) | 1.56 (1.24, 1.96) | 1.37 (1.10, 1.71) | 1.26 (0.99, 1.61) | 1.15 (0.89, 1.49) | |
| P value | .35 | <.001 | <.001 | <.001 | .006 | .07 | .29 | |
| Pain freedom, percentage of mild attacks | ||||||||
| AVP-825 (n = 256 attacks) | 4.7 | 14.1 | 29.3 | 43.8 | 51.6 | 63.7 | 69.9 | |
| Oral sumatriptan (n = 234 attacks) | 3.4 | 9.4 | 17.9 | 29.9 | 41.5 | 57.3 | 66.2 | |
| OR (95% CI) | 1.45 (0.51, 4.09) | 1.59 (0.94, 2.69) | 1.87 (1.33, 2.64) | 1.70 (1.18, 2.46) | 1.45 (1.00, 2.11) | 1.35 (0.90, 2.04) | 1.13 (0.74, 1.70) | |
| P value | .48 | .08 | <.001 | .005 | .05 | .15 | .58 | |
| Pain freedom, percentage of moderate/severe attacks | ||||||||
| AVP-825 (n = 509 attacks) | 1.4 | 3.7 | 12.6 | 24.6 | 36.0 | 47.3 | 55.6 | |
| Oral sumatriptan (n = 532 attacks) | 0.4 | 1.1 | 7.7 | 17.5 | 29.1 | 39.5 | 51.9 | |
| OR (95% CI) | 3.65 (0.62, 21.38) | 3.83 (1.12, 13.11) | 1.76 (1.05, 2.95) | 1.58 (1.14, 2.18) | 1.37 (1.05, 1.81) | 1.40 (1.07, 1.84) | 1.16 (0.88, 1.53) | |
| P value | .15 | .03 | .03 | .006 | .02 | .02 | .30 | |
| Nausea, percentage of attacks | ||||||||
| AVP-825 (n = 765 attacks) | 33.5 | 30.1 | 26.1 | 20.9 | 17.5 | 14.1 | 11.1 | 10.6 |
| Oral sumatriptan (n = 766 attacks) | 33.8 | 30.0 | 27.2 | 25.3 | 22.6 | 19.2 | 15.1 | 11.9 |
| OR (95% CI) | 1.01 (0.85, 1.20) | 0.99 (0.73, 1.36) | 0.89 (0.69, 1.15) | 0.73 (0.56, 0.96) | 0.68 (0.51, 0.91) | 0.66 (0.49, 0.89) | 0.67 (0.47, 0.94) | 0.85 (0.60, 1.20) |
| P value | .92 | .97 | .37 | .02 | .009 | .006 | .02 | .35 |
| Photophobia, percentage of attacks | ||||||||
| AVP-825 (n = 765 attacks) | 74.2 | 68.8 | 63.9 | 52.0 | 45.0 | 37.3 | 32.2 | 25.4 |
| Oral sumatriptan (n = 766 attacks) | 78.6 | 74.9 | 72.8 | 64.0 | 56.7 | 46.5 | 37.2 | 30.3 |
| OR (95% CI) | 0.82 (0.67, 1.01) | 0.66 (0.48, 0.90) | 0.55 (0.41, 0.74) | 0.58 (0.45, 0.74) | 0.64 (0.51, 0.80) | 0.74 (0.60, 0.91) | 0.86 (0.67, 1.09) | 0.84 (0.65, 1.08) |
| P value | .06 | .01 | <.001 | <.001 | <.001 | .005 | .21 | .16 |
| Phonophobia, percentage of attacks | ||||||||
| AVP-825 (n = 765 attacks) | 60.5 | 57.0 | 53.1 | 44.1 | 35.8 | 29.8 | 25.9 | 21.3 |
| Oral sumatriptan (n = 766 attacks) | 63.4 | 61.2 | 59.0 | 51.3 | 44.3 | 37.7 | 30.8 | 25.1 |
| OR (95% CI) | 0.91 (0.77, 1.07) | 0.72 (0.52, 1.00) | 0.65 (0.51, 0.84) | 0.68 (0.55, 0.84) | 0.67 (0.54, 0.82) | 0.69 (0.55, 0.87) | 0.82 (0.63, 1.06) | 0.85 (0.65, 1.12) |
| P value | .25 | .05 | <.001 | <.001 | <.001 | .002 | .12 | .25 |
| Vomiting, percentage of attacks | ||||||||
| AVP-825 (n = 765 attacks) | 3.3 | 3.5 | 1.6 | 1.7 | 1.2 | 1.4 | 1.2 | 1.0 |
| Oral sumatriptan (n = 766 attacks) | 2.6 | 2.2 | 1.2 | 1.3 | 1.3 | 1.3 | 0.9 | 1.6 |
| OR (95% CI) | 1.47 (0.89, 2.43) | 1.38 (0.69, 2.78) | 2.14 (0.49, 9.31) | 1.37 (0.50, 3.77) | 0.94 (0.35, 2.50) | 1.10 (0.38, 3.20) | 0.84 (0.20, 3.50) | 0.46 (0.12, 1.77) |
| P value | .13 | .36 | .31 | .54 | .90 | .86 | .81 | .26 |
| Meaningful pain relief, percentage of attacks | ||||||||
| AVP-825 (n = 765 attacks) | 14.0 | 25.1 | 47.8 | 61.6 | 69.5 | 77.8 | 82.2 | |
| Oral sumatriptan (n = 766 attacks) | 7.4 | 16.2 | 34.7 | 49.3 | 60.7 | 72.7 | 79.5 | |
| OR (95% CI) | 2.13 (1.45, 3.11) | 1.74 (1.35, 2.25) | 1.75 (1.40, 2.20) | 1.65 (1.32, 2.06) | 1.49 (1.17, 1.90) | 1.29 (1.00, 1.66) | 1.15 (0.88, 1.50) | |
| P value | <.001 | <.001 | <.001 | <.001 | .001 | .05 | .31 | |
| Clinical Disability Scale, least squares mean change from pre-dose (SE) | ||||||||
| AVP-825 (n = 185 patients) | −0.08 (0.017) | −0.18 (0.025) | −0.42 (0.038) | −0.60 (0.046) | −0.73 (0.049) | −0.83 (0.052) | −0.92 (0.054) | |
| Oral sumatriptan (n = 185 patients) | −0.03 (0.017) | −0.09 (0.025) | −0.26 (0.038) | −0.43 (0.046) | −0.56 (0.049) | −0.72 (0.052) | −0.85 (0.054) | |
| Adjusted mean difference (95% CI) | −0.04 (−0.08, −0.01) | −0.09 (−0.14, −0.04) | −0.16 (−0.23, −0.09) | −0.17 (−0.25, −0.08) | −0.16 (−0.26, −0.07) | −0.11 (−0.21, −0.02) | −0.07 (−0.17, 0.03) | |
| P value | .02 | <.001 | <.001 | <.001 | <.001 | .02 | .17 | |
OR are based on the fitted generalized estimating equations models.
CI = confidence interval; OR = odds ratio; SE = standard error.
For attacks treated when pain was mild (32% of attacks in the FAS), pain freedom rates were significantly greater with AVP-825 vs oral sumatriptan at 30 and 45 minutes post-dose (Table 2; P < .001 and P = .005, respectively). For attacks treated when pain was moderate or severe (68% of attacks in the FAS), differences in pain freedom rates were statistically significant in favor of AVP-825 vs oral sumatriptan at all time points from 15 to 90 minutes post-dose (Table 2). For subjects who treated multiple migraine attacks in both treatment periods, a significantly greater proportion had consistency of pain relief at 30 minutes across multiple migraines with AVP-825 vs oral sumatriptan (Table 3). For example, in patients treating ≥2 attacks in each period (n = 165 patients), AVP-825 was superior to 100 mg oral sumatriptan for the rate of pain relief (37.6% vs 18.8%, P < .0001) at 30 minutes post-dose for the first 2 attacks treated (Table 3). For patients treating ≥3 attacks in each period (n = 140 patients), AVP-825 was superior to oral sumatriptan at 30 minutes post-dose for pain relief in ≥2 of the first 3 migraine attacks treated (51.4% vs 33.6%, P = .0002) and in 3 of the first 3 migraine attacks treated (27.1% vs 12.1%, P = .0002; Table 3). At no time point and for no efficacy end-point was the statistical comparison in favor of oral sumatriptan.
Table 3.
Consistency of Pain Relief Across Multiple Migraines
| 30 minutes | 1 hour | 2 hours | |
|---|---|---|---|
| Pain relief for the first 2 migraines in each treatment period, percentage of subjects who treated ≥2 attacks in both periods | |||
| AVP-825 (n = 165) | 37.6 | 52.1 | 64.2 |
| Oral sumatriptan (n = 165) | 18.8 | 45.5 | 62.4 |
| P value | <.001 | .20 | .80 |
| Pain relief for ≥2 of the first 3 migraines in each treatment period, percentage of subjects who treated ≥3 attacks in both periods | |||
| AVP-825 (n = 140) | 51.4 | 71.4 | 83.6 |
| Oral sumatriptan (n = 140) | 33.6 | 64.3 | 81.4 |
| P value | <.001 | .17 | .68 |
| Pain relief for 3 of the first 3 migraines in each treatment period, percentage of subjects who treated ≥3 attacks in both periods | |||
| AVP-825 (n = 140) | 27.1 | 41.4 | 57.9 |
| Oral sumatriptan (n = 140) | 12.1 | 40.7 | 57.1 |
| P value | <.001 | 1.0 | 1.0 |
Pain relief is defined as reduction of Headache Severity score from moderate or severe to none or mild.
P values are based on McNemar’s test.
Statistically significant reductions in migraine-associated symptom rates were observed with AVP-825 vs oral sumatriptan (Table 2). The percentage of migraine attacks with nausea was significantly lower with AVP-825 vs oral sumatriptan at all time points from 30 to 90 minutes post-dose. Photophobia and phonophobia rates were significantly lower with AVP-825 as early as 10 and 15 minutes, respectively, through 60 minutes post-dose. The baseline rate of vomiting was low (<4%), and there were no statistically significant between-group differences for vomiting rates post-dose.
For subject-assessed meaningful pain relief, statistically significant differences favoring treatment with AVP-825 were seen from 10 to 60 minutes post-dose (Table 2). In addition, Clinical Disability Scale scores improved at successive time points, with statistically significant differences favoring AVP-825 from 10 to 90 minutes post-dose (Table 2).
Safety and Tolerability
No serious TEAEs occurred, and <2% of subjects experienced a TEAE leading to discontinuation with either treatment (Table 4). TEAEs were experienced by 53.9% of subjects with AVP-825 and 32% of subjects with oral sumatriptan and most were mild in severity (Table 4). Local administration-site TEAEs such as nasal discomfort and abnormal product taste were more common with AVP-825 but were deemed mild in nearly 90% of cases (Table 4) and led to only one discontinuation. There were no clinically relevant changes in laboratory values. One subject had a clinically significant electrocardiogram abnormality of t-wave inversion at the end-of-study visit (oral sumatriptan period), with no clinically significant abnormality at the follow-up visit approximately 4 months later.
Table 4.
Summary of Treatment-Emergent Adverse Events
| AVP-825 + placebo tablet | Placebo delivery system +100 mg oral sumatriptan tablet | |
|---|---|---|
| n = 219 | n = 228 | |
| n (%) | n (%) | |
| TEAEs | 118 (53.9) | 73 (32.0) |
| Mild | 96 (43.8) | 52 (22.8) |
| Moderate | 17 (7.8) | 14 (6.1) |
| Severe | 5 (2.3) | 7 (3.1) |
| Serious TEAEs | 0 | 0 |
| TEAEs leading to discontinuation | 4 (1.8) | 3 (1.3) |
| Most common TEAEs (>5% in either treatment group) | ||
| Abnormal product taste | 57 (26.0) | 9 (3.9) |
| Mild | 53 | 9 |
| Moderate | 3 | 0 |
| Severe | 1 | 0 |
| Nasal discomfort | 34 (15.5) | 3 (1.3) |
| Mild | 30 | 3 |
| Moderate | 4 | 0 |
| Severe | 0 | 0 |
TEAEs were coded using MedDRA (version 14.1; Copyright © 2011 International Federation of Pharmaceutical Manufacturers and Associations. All Rights Reserved.) preferred terms. A subject with more than one event with the same preferred term was counted once for that term.
TEAEs = treatment-emergent adverse events.
For each attack, subjects were asked to report if they experienced atypical sensations, described as symptoms in the chest, arms, hands, or feet: warm/hot sensation, burning sensation, feeling of heaviness, pressure, feeling of tightness, numbness, or feeling strange often associated with triptan use. Treatment-emergent atypical sensations occurring within 120 minutes after administration of the study drug were significantly lower with AVP-825 compared with oral sumatriptan (2% of 512 attacks vs 5% of 512 attacks, odds ratio 0.40 [95% confidence interval 0.19, 0.85]; P = .02).
Discussion
The COMPASS study compares the efficacy and safety of AVP-825 (containing 22 mg sumatriptan nasal powder) vs the most efficacious dose (100 mg) of oral sumatriptan for the acute treatment of migraine, providing the opportunity to comparatively assess an investigational product with lower drug exposure against a well-established standard of care. Prior to COMPASS, one comparative efficacy trial of intranasal sumatriptan (traditional liquid nasal spray) vs oral sumatriptan (100 mg) had been described briefly27 and was a precedent for the COMPASS study design. Several COMPASS design aspects are notable when interpreting the findings. As AVP-825 has a faster absorption profile than oral sumatriptan,8 COMPASS was designed to reduce bias against oral sumatriptan by selection of the most efficacious oral sumatriptan dose (100 mg) for early migraine treatment,22 use of a dummy delivery system in the oral sumatriptan group to account for any potential benefits associated with the delivery system alone, and the protocol-specified requirement for early treatment (within 1 hour of migraine onset, even if pain was mild). The cross-over design minimized subject variation associated with parallel treatment arms, although it should be noted that as with any cross-over design, patients have an opportunity to compare treatment experiences, which may potentially impact blinding. While the evaluation of multiple migraine attacks per subject could have increased the rate of adverse events and discontinuations because of the longer treatment duration of a multi-attack vs a single attack study, it also increased the data available for analysis relative to other migraine studies (>1500 migraines assessed in COMPASS), as well as provided safety and efficacy information with repeated use, as might occur in routine clinical practice. The fact that most subjects (>90% for AVP-825 and oral sumatriptan) treated multiple migraines in COMPASS suggests that the subjects had confidence in both treatments and tolerated them well.
The low dose of sumatriptan delivered with AVP-825 (∼15-16 mg9) showed significantly greater early efficacy on the primary end-point (SPID-30) compared with 100 mg oral sumatriptan. Rates of early pain relief and pain freedom also significantly favored AVP-825 over oral sumatriptan, starting at 15 minutes and at every time point through 90 minutes post-dose. Multiple other efficacy measures, including pain reduction (original primary end-point) also showed significant early benefit of AVP-825 over oral sumatriptan. The clinical significance of the difference was supported by corresponding statistically significant early benefits of AVP-825 over oral sumatriptan on subject-rated meaningful pain relief and on clinical disability. Consistent with early relief of migraine pain, significantly greater reductions in migraine-associated symptoms, particularly phonophobia and photophobia, were observed with AVP-825 vs oral sumatriptan. Additionally, AVP-825 resulted in a more reliable (consistent) early response across multiple migraine attacks than 100 mg oral sumatriptan. At no time point was oral sumatriptan statistically superior to AVP-825 for an efficacy measure. As is typical, there was no adjustment for multiple comparisons for secondary outcomes, so the secondary outcome analyses should not be considered “hypothesis testing.” However, the earlier onset of efficacy observed with AVP-825 is remarkably consistent across primary and all secondary efficacy measurements for pain and associated symptoms in this study and is consistent with results from previous placebo-controlled phase 2 and 3 AVP-825 trials.9,10 The early migraine efficacy observed with AVP-825 at a lower dose than the 100 mg oral sumatriptan may reflect quick systemic absorption of sumatriptan powder when delivered by the bi-directional breath-powered delivery system to the highly absorptive upper posterior surfaces of the nasal cavity and to cranial nerve structures potentially relevant for migraine therapy.5,8 Other factors potentially contributing to early onset of effect with this unique intranasal form of delivery at the lower dose include possible serotonergic action at nerve endings in the nasal cavity5,9,28 or direct delivery to the brain (via olfactory or trigeminal nerves), but no direct evidence for these mechanisms has been established.7,29
The results of COMPASS indicate that the main difference in efficacy between AVP-825 and 100 mg oral sumatriptan occurs early, within 2 hours post-dose, likely due in large part to faster sumatriptan absorption with AVP-825.8 The fact that the effect of AVP-825 and 100 mg oral sumatriptan was not significantly different on any measure at 2 hours post-dose nor on measures of sustained efficacy (pain relief and pain freedom at 24 or 48 hours post-dose) indicates that sustained pain response is not sacrificed in exchange for the earlier onset of efficacy with AVP-825 or lower total systemic exposure. The clinical relevance of the early onset of efficacy is that it corresponds to a timeframe when patients feel improvement in treatment is needed. Multiple studies have reported that early efficacy onset and complete pain relief are important determinants of patient satisfaction with migraine therapy.30,31
Acknowledging that drug performance in different clinical trials may reflect specific trial conditions, relatively high response rates with 100 mg oral sumatriptan were observed in COMPASS (eg, 1-hour pain relief rate after oral sumatriptan was 63% for COMPASS vs 29% in a meta-analysis of previous oral sumatriptan trials3). Expectation of receiving an effective treatment in both study periods may have played a role. Additionally, use of non-oral routes of administration, devices, or surgical procedures in clinical trials has been associated with increased placebo response in multiple other settings, including pain trials.32–34 In addition, the bi-directional breath-powered intranasal delivery system and its unique aerodynamic characteristics may contribute to efficacy through neurochemical effects of CO2 delivery and/or removal of nitric oxide at trigeminal nerve endings, although this has not been established.5,6
Post-treatment pain freedom was evaluated as a function of headache severity at baseline time of treatment (mild vs moderate/severe). Pain freedom rates were generally higher for headaches treated when mild than those treated when moderate/severe, regardless of treatment. This is consistent with studies that have supported the concept of a “treat while mild” paradigm to achieve optimal patient outcomes.3 AVP-825 treatment of mild attacks yielded significantly higher pain freedom rates than oral sumatriptan at 30 and 45 minutes post-dose. Pain freedom rates also were significantly higher for AVP-825 compared with oral sumatriptan at early time points in the moderate/severe subgroup, indicating faster AVP-825 efficacy even when treatment was not delivered when migraine pain was mild.
As noted, AVP-825 containing 22 mg sumatriptan is associated with a much lower overall sumatriptan exposure8 (proportional to the ∼15-16 mg sumatriptan delivered9) than 100 mg oral sumatriptan, which may underlie favorable tolerability. In COMPASS, TEAEs observed with AVP-825 consisted predominantly of mild administration site effects, and the overall tolerability profile was similar to that observed in the previous phase 3 trial.9 The classification of abnormal product taste as “mild” by ∼90% of those who experienced this TEAE is important because general clinical consensus is that bad taste is a frequent reason for lack of use of traditional liquid nasal sumatriptan preparations. Even with the majority of patients treating multiple attacks in this study, both treatments were generally well tolerated, with only 7 patients discontinuing because of TEAEs (4 in an AVP-825 period, 3 in an oral sumatriptan period).
It has been demonstrated that the incidence of specific triptan-related adverse events is significantly higher and more accurate when the patient is queried rather than when the information is obtained by spontaneous report.35 In COMPASS, patients were specifically questioned regarding treatment-emergent atypical sensations, and the incidence of these adverse events was significantly lower with AVP-825 than oral sumatriptan, indicating an important clinical difference between the treatments.
Conclusion
COMPASS is a rigorously designed comparative efficacy study, which demonstrates that the investigational AVP-825 bi-directional breath-powered intranasal delivery system provides an earlier reduction of migraine pain intensity, which was statistically and clinically significant, and higher rates of pain relief and pain freedom within 30 minutes, without loss of sustained efficacy, than the most effective dose of oral sumatriptan (100 mg) despite substantially lower drug exposure. In addition, AVP-825 conferred statistically significantly fewer triptan-related adverse effects than 100 mg oral sumatriptan. As oral sumatriptan is the most commonly utilized triptan for the acute treatment of migraine, results of this trial may challenge the current migraine treatment paradigm.
Acknowledgments
Jim McGinley, PhD (McGinley Statistical Consulting), provided feedback on the statistical analysis plan and data analyses of the study. Merrilee Johnstone, PhD, and Jennifer Hepker, PhD (Prescott Medical Communications Group, Chicago, IL), Scott Siegert, PharmD (Avanir Pharmaceuticals, Inc., Aliso Viejo, CA), and Ken Shulman, DO (Avanir Pharmaceuticals, Inc., Aliso Viejo, CA), provided technical editing and copyediting of the article.
Glossary
- FAS
full analysis set
- SPID-30
summed pain intensity differences through 30 minutes
- TEAE
treatment-emergent adverse event
Statement of Authorship
Category 1
-
(a) Conception and Design
Stewart J. Tepper; Roger K. Cady; Stephen Silberstein; John Messina; Ramy A. Mahmoud; Per G. Djupesland; Paul Shin; Joao Siffert
-
(b) Acquisition of Data
Stewart J. Tepper; Roger K. Cady; Stephen Silberstein
-
(c) Analysis and Interpretation of Data
Stewart J. Tepper; Roger K. Cady; Stephen Silberstein; John Messina; Ramy A. Mahmoud; Per G. Djupesland; Paul Shin; Joao Siffert
Category 2
-
(a) Drafting the Manuscript
Stewart J. Tepper; Roger K. Cady; Stephen Silberstein; John Messina; Ramy A. Mahmoud; Per G. Djupesland; Paul Shin; Joao Siffert
-
(b) Revising It for Intellectual Content
Stewart J. Tepper; Roger K. Cady; Stephen Silberstein; John Messina; Ramy A. Mahmoud; Per G. Djupesland; Paul Shin; Joao Siffert
Category 3
-
(a) Final Approval of the Completed Manuscript
Stewart J. Tepper; Roger K. Cady; Stephen Silberstein; John Messina; Ramy A. Mahmoud; Per G. Djupesland; Paul Shin; Joao Siffert
References
- Buse DC, Loder EW, Gorman JA, et al. Sex differences in the prevalence, symptoms, and associated features of migraine, probable migraine and other severe headache: Results of the American Migraine Prevalence and Prevention (AMPP) Study. Headache. 2013;53:1278–1299. doi: 10.1111/head.12150. [DOI] [PubMed] [Google Scholar]
- Johnston MM, Rapoport AM. Triptans for the management of migraine. Drugs. 2010;70:1505–1518. doi: 10.2165/11537990-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Derry CJ, Derry S, Moore RA. Sumatriptan (all routes of administration) for acute migraine attacks in adults – Overview of Cochrane reviews. Cochrane Database Syst Rev. 2014;(5) doi: 10.1002/14651858.CD009108.pub2. CD009108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigal M, Rapoport A, Aurora S, Sheftell F, Tepper S, Dahlof C. Satisfaction with current migraine therapy: Experience from 3 centers in US and Sweden. Headache. 2007;47:475–479. doi: 10.1111/j.1526-4610.2007.00752.x. [DOI] [PubMed] [Google Scholar]
- Djupesland PG, Messina JC, Mahmoud RA. Breath powered nasal delivery: A new route to rapid headache relief. Headache. 2013;53(Suppl. 2):72–84. doi: 10.1111/head.12186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tepper SJ. Clinical implications for breath-powered powder sumatriptan intranasal treatment. Headache. 2013;53:1341–1349. doi: 10.1111/head.12166. [DOI] [PubMed] [Google Scholar]
- Djupesland PG. Nasal drug delivery devices: Characteristics and performance in a clinical perspective – A review. Drug Deliv Transl Res. 2013;3:42–62. doi: 10.1007/s13346-012-0108-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obaidi M, Offman E, Messina J, Carothers J, Djupesland PG, Mahmoud RA. Improved pharmacokinetics of sumatriptan with breath powered nasal delivery of sumatriptan powder. Headache. 2013;53:1323–1333. doi: 10.1111/head.12167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cady RK, McAllister PJ, Spierings ELH, et al. A randomized, double-blind, placebo-controlled study of breath powered nasal delivery of sumatriptan powder (AVP-825) in the treatment of acute migraine (the TARGET study) Headache. 2015;55:88–100. doi: 10.1111/head.12472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djupesland PG, Docekal P Czech Migraine Investigators Group. Intranasal sumatriptan powder delivered by a novel breath-actuated bi-directional device for the acute treatment of migraine: A randomised, placebo-controlled study. Cephalalgia. 2010;30:933–942. doi: 10.1177/0333102409359314. [DOI] [PubMed] [Google Scholar]
- Diener HC, Jansen JP, Reches A, et al. Efficacy, tolerability and safety of oral eletriptan and ergotamine plus caffeine (Cafergot) in the acute treatment of migraine: A multicentre, randomised, double-blind, placebo-controlled comparison. Eur Neurol. 2002;47:99–107. doi: 10.1159/000047960. [DOI] [PubMed] [Google Scholar]
- Dowson AJ, Massiou H, Lainez JM, Cabarrocas X. Almotriptan is an effective and well-tolerated treatment for migraine pain: Results of a randomized, double-blind, placebo-controlled clinical trial. Cephalalgia. 2002;22:453–461. doi: 10.1046/j.1468-2982.2002.00394.x. [DOI] [PubMed] [Google Scholar]
- Geraud G, Olesen J, Pfaffenrath V, et al. Comparison of the efficacy of zolmitriptan and sumatriptan: Issues in migraine trial design. Cephalalgia. 2000;20:30–38. doi: 10.1046/j.1468-2982.2000.00004.x. [DOI] [PubMed] [Google Scholar]
- Goadsby PJ, Ferrari MD, Olesen J, et al. Eletriptan in acute migraine: A double-blind, placebo-controlled comparison to sumatriptan. Eletriptan Steering Committee. Neurology. 2000;54:156–163. doi: 10.1212/wnl.54.1.156. [DOI] [PubMed] [Google Scholar]
- Goldstein J, Ryan R, Jiang K, et al. Crossover comparison of rizatriptan 5 mg and 10 mg vs sumatriptan 25 mg and 50 mg in migraine. Rizatriptan Protocol 046 Study Group. Headache. 1998;38:737–747. doi: 10.1046/j.1526-4610.1998.3810737.x. [DOI] [PubMed] [Google Scholar]
- Pascual J, Vega P, Diener HC, Allen C, Vrijens F, Patel K. Comparison of rizatriptan 10 mg vs zolmitriptan 2.5 mg in the acute treatment of migraine. Rizatriptan-Zolmitriptan Study Group. Cephalalgia. 2000;20:455–461. doi: 10.1046/j.1468-2982.2000.00069.x. [DOI] [PubMed] [Google Scholar]
- Sandrini G, Farkkila M, Burgess G, Forster E, Haughie S, Eletriptan Steering C. Eletriptan vs sumatriptan: A double-blind, placebo-controlled, multiple migraine attack study. Neurology. 2002;59:1210–1217. doi: 10.1212/wnl.59.8.1210. [DOI] [PubMed] [Google Scholar]
- Bomhof M, Paz J, Legg N, Allen C, Vandormael K, Patel K. Comparison of rizatriptan 10 mg vs naratriptan 2.5 mg in migraine. Eur Neurol. 1999;42:173–179. doi: 10.1159/000008094. [DOI] [PubMed] [Google Scholar]
- Tfelt-Hansen P, Teall J, Rodriguez F, et al. Oral rizatriptan vs oral sumatriptan: A direct comparative study in the acute treatment of migraine. Rizatriptan 030 Study Group. Headache. 1998;38:748–755. doi: 10.1046/j.1526-4610.1998.3810748.x. [DOI] [PubMed] [Google Scholar]
- Tfelt-Hansen P, Henry P, Mulder LJ, Scheldewaert RG, Schoenen J, Chazot G. The effectiveness of combined oral lysine acetylsalicylate and metoclopramide compared with oral sumatriptan for migraine. Lancet. 1995;346:923–926. doi: 10.1016/s0140-6736(95)91554-0. [DOI] [PubMed] [Google Scholar]
- Tfelt-Hansen P, Ryan RE., Jr Oral therapy for migraine: Comparisons between rizatriptan and sumatriptan. A review of four randomized, double-blind clinical trials. Neurology. 2000;55:S19–S24. [PubMed] [Google Scholar]
- Winner P, Landy S, Richardson M, Ames M. Early intervention in migraine with sumatriptan tablets 50 mg vs 100 mg: A pooled analysis of data from six clinical trials. Clin Ther. 2005;27:1785–1794. doi: 10.1016/j.clinthera.2005.11.009. [DOI] [PubMed] [Google Scholar]
- Tfelt-Hansen P, Pascual J, Ramadan N, et al. Guidelines for controlled trials of drugs in migraine: Third edition. A guide for investigators. Cephalalgia. 2012;32:6–38. doi: 10.1177/0333102411417901. [DOI] [PubMed] [Google Scholar]
- Lipton RB, Stewart WF, Ryan RE, Jr, Saper J, Silberstein S, Sheftell F. Efficacy and safety of acetaminophen, aspirin, and caffeine in alleviating migraine headache pain: Three double-blind, randomized, placebo-controlled trials. Arch Neurol. 1998;55:210–217. doi: 10.1001/archneur.55.2.210. [DOI] [PubMed] [Google Scholar]
- Tfelt-Hansen P, McCarroll K, Lines C. Sum of pain intensity differences (SPID) in migraine trials. A comment based on four rizatriptan trials. Cephalalgia. 2002;22:664–666. doi: 10.1046/j.1468-2982.2002.00402.x. [DOI] [PubMed] [Google Scholar]
- Charlesworth BR, Dowson AJ, Purdy A, Becker WJ, Boes-Hansen S, Farkkila M. Speed of onset and efficacy of zolmitriptan nasal spray in the acute treatment of migraine: A randomised, double-blind, placebo-controlled, dose-ranging study vs zolmitriptan tablet. CNS Drugs. 2003;17:653–667. doi: 10.2165/00023210-200317090-00005. [DOI] [PubMed] [Google Scholar]
- Dahlof C. Sumatriptan nasal spray in the acute treatment of migraine: A review of clinical studies. Cephalalgia. 1999;19:769–778. doi: 10.1046/j.1468-2982.1999.1909769.x. [DOI] [PubMed] [Google Scholar]
- Ivanusic JJ, Kwok MM, Ahn AH, Jennings EA. 5-HT(1D) receptor immunoreactivity in the sphenopalatine ganglion: Implications for the efficacy of triptans in the treatment of autonomic signs associated with cluster headache. Headache. 2011;51:392–402. doi: 10.1111/j.1526-4610.2011.01843.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djupesland PG, Messina JC, Mahmoud RA. The nasal approach to delivering treatment for brain diseases: An anatomic, physiologic, and delivery technology overview. Ther Deliv. 2014;5:709–733. doi: 10.4155/tde.14.41. [DOI] [PubMed] [Google Scholar]
- Davies GM, Santanello N, Lipton R. Determinants of patient satisfaction with migraine therapy. Cephalalgia. 2000;20:554–560. doi: 10.1046/j.1468-2982.2000.00082.x. [DOI] [PubMed] [Google Scholar]
- Smelt AF, Louter MA, Kies DA, et al. What do patients consider to be the most important outcomes for effectiveness studies on migraine treatment? Results of a Delphi study. PLoS One. 2014;9:e98933. doi: 10.1371/journal.pone.0098933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redberg RF. Sham controls in medical device trials. N Engl J Med. 2014;371:892–893. doi: 10.1056/NEJMp1406388. [DOI] [PubMed] [Google Scholar]
- Moseley JB, O’Malley K, Petersen NJ, et al. A controlled trial of arthroscopic surgery for osteoarthritis of the knee. N Engl J Med. 2002;347:81–88. doi: 10.1056/NEJMoa013259. [DOI] [PubMed] [Google Scholar]
- Oken BS. Placebo effects: Clinical aspects and neurobiology. Brain. 2008;131:2812–2823. doi: 10.1093/brain/awn116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feleppa M, Apice G, D’Alessio A, Fucci S, Bigal ME. Tolerability of acute migraine medications: Influence of methods of assessment and relationship with headache attributes. Cephalalgia. 2008;28:1012–1016. doi: 10.1111/j.1468-2982.2008.01643.x. [DOI] [PubMed] [Google Scholar]