Abstract
Purpose
Innate immune signaling elicited by polyinosinic-polycytidylic acid (poly I:C) induces IL-7 production and early inflammatory responses in the salivary gland and accelerates the development of Sjögren's syndrome (SS)-like sialadenitis. Whether poly I:C can induce similar responses in the lacrimal gland (LAC) has not been characterized. In this study, we examined the early responses and pathologic changes of the LAC tissue in response to poly I:C treatment.
Methods
Poly I:C or recombinant human IL-7 was injected intraperitoneally into C57BL/6 mice, and the LAC was harvested at different time points. Expression of chemokines and cytokines in the LAC was measured by RT-PCR, immunofluorescence staining, and immunohistochemistry. Leukocytic infiltration and caspase-3 activation were analyzed by hematoxylin and eosin staining and immunohistochemistry. Serum antinuclear antibody levels were also determined. Tear secretion was measured by phenol red cotton threads.
Results
Administration of poly I:C induced IL-7 gene expression and protein production in the LAC. Poly I:C also induced the expression of CXCR3 ligands, monocyte chemoattractant protein-1, IL-23p19, and TNF-α in the LAC in an IL-7–dependent fashion. Similarly to poly I:C, administration of exogenous IL-7 also up-regulated these proinflammatory mediators. Furthermore, repeated administration of poly I:C to C57BL/6 mice over an 8-day period caused leukocytic infiltration and caspase-3 activation in the LAC, antinuclear antibody production, and impaired tear secretion.
Conclusions
Poly I:C induces IL-7 production, early inflammatory responses, and characteristic pathologies of SS-like dacryoadenitis in non–autoimmune-prone C57BL/6 mice. These findings provide new evidence that viral infection-elicited innate immune signaling may be one of the early triggers of SS-like dacryoadenitis.
Keywords: lacrimal gland, Sjogren's syndrome, poly I:C, interleukin-7, innate immune response
The innate immune system provides crucial early protection against pathogen infections and also critically influences the subsequent adaptive immune responses. Activation of the Toll-like receptors (TLRs) expressed on host cells by pathogen-associated molecular patterns expressed on microbes leads to activation, maturation, and production of inflammatory mediators and initiates the host responses to pathogens.1–3 In addition to protective host responses, TLR-mediated early inflammatory responses can also develop into inflammatory diseases or autoimmune diseases that cause damages to host tissues.3 Activation of TLR3 by viral double-stranded RNA and its synthetic analog polyinosinic-polycytidylic acid (poly I:C) can trigger inflammatory diseases of lung, kidney, and liver.4–7
Viral infection-induced TLR signaling is implicated as a possible trigger for the development of Sjögren's syndrome (SS), an autoimmune disease that primarily affects the lacrimal and salivary gland.8–11 We and others have previously shown that poly I:C administration facilitates the development and onset of SS-like sialadenitis in mouse models of SS.11 In particular, we demonstrate that poly I:C induces IL-7 production and IL-7–dependent inflammatory responses in salivary gland tissues in both normal C57BL/6 mice and a mouse model of SS to promote SS-like salivary gland inflammation.12 Poly I:C induces a T helper 1 (Th1)-skewing inflammatory response in the salivary gland as indicated by the production of IL-12p40, IFN-γ, and CXCR3 ligands.12 Moreover, we have reported that endogenous and exogenous IL-7 enhances Th1 responses and plays an essential role in the development and onset of SS-like exocrinopathy of salivary glands.13 These findings provide additional evidence for the pathologic consequences of excessive IL-7 production induced by innate immune signaling14 that can facilitate the pathogenesis of development and onset of multiple autoimmune disorders, mainly by enhancing Th1 and T cytotoxic 1 (Tc1) immune responses in the target tissues.15–18
The lacrimal gland (LAC) is a primary target organ affected by SS. The autoimmune response and the subsequent pathologic changes in this gland lead to impaired tear production and dry eye symptoms, inflicting significant discomfort and pain to the patients and increasing the risks of corneal ulceration and infection. Current treatment for SS is mostly palliative, with no cure or effective biological treatment available. It is therefore crucial to identify the triggers and define the pathogenic mechanisms in the development and persistence of LAC pathologies in SS, in order to develop targeted and effective therapeutic strategies. In comparison to the salivary gland, the effect of innate immune signaling, such as that induced by poly I:C, on the LAC tissues in either normal C57BL/6 mice or SS disease models is largely unknown. Whether poly I:C elicits similar or distinct inflammatory response in the LAC compared to the salivary gland has remained uncharacterized. In this study, we examined the effect of poly I:C administration on IL-7 production and inflammatory responses in the LAC.
Methods
Mice
C57BL/6 mice were purchased from the Jackson Laboratory (Bar Harbor, ME, USA) and kept under pathogen-free conditions. All the animal experiments were conducted with adherence to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research and with approval from the Institutional Animal Care and Use Committee at the Forsyth Institute.
In Vivo Administration of Poly I:C and IL-7
Female C57BL/6 mice received intraperitoneal (IP) injection of 100 μg poly I:C (Sigma-Aldrich Corp., St. Louis, MO, USA) and were euthanized 6 hours later to harvest the extraorbital LAC for further analysis. In some experiments, female C57BL/6 mice received IP injection of 100 μg control IgG (2A3; BioLegend), anti–IL-7Rα (A7R34; BioXcell, West Lebanon, NH, USA), anti-IFNAR1 (MAR1-5A3; BioLegend, San Diego, CA, USA), or anti–IFN-γ (XMG1.2; BioLegend) 2 hours before receiving poly I:C injection. Mice were then euthanized, and the LAC was harvested 6 or 24 hours after poly I:C injection. For repeated administration of poly I:C, 100 μg poly I:C was injected to C57BL/6 mice every 2–3 days, and mice were euthanized 8 or 16 days after the initial injection to harvest the LAC for analysis. For IL-7 administration, female C57BL/6 mice were IP injected with 20 μg recombinant human (rh) IL-7 (kindly provided by Biological Resources Branch, National Cancer Institute, Bethesda, MD, USA) and euthanized 24 hours later to collect organs for further analysis. The endotoxin level of the rh IL-7 was below 1 × 10−4 EU/μg protein.
Histologic Analysis, Immunofluorescence, and Immunohistochemical Staining
Tissue samples were fixed in 4% paraformaldehyde, embedded in paraffin, and sectioned to 5 μm thickness. Sections were deparaffinized, rehydrated, stained with hematoxylin and eosin, and examined for leukocyte infiltration under a light microscope. For immunofluorescence staining, the rehydrated sections were subjected to antigen unmasking process and incubated with phycoerythrin-conjugated anti-mouse CXCL9 (MIG-2F5.5; Biolegend), goat anti-mouse CXCL10, or goat anti-mouse IL-7 (Santa Cruz Biotechnology, Santa Cruz, CA, USA), followed by Alexa Fluor647-conjugated anti-goat IgG. The stained samples were examined with a Leica laser scanning confocal microscope (Zeiss, Oberkochen, Germany). Images were average projections of three optical sections and processed with the Leica confocal software. For immunohistochemical staining, the rehydrated sections were incubated with goat anti-mouse IL-23p19 antibody (Santa Cruz Biotechnology) at 4°C overnight, followed by biotinylated anti-goat IgG using the VECTASTAIN Elite ABC Kit (Vector Labs, Burlingame, CA, USA) according to the manufacturer's instructions.
Measurement of Tear Flow Rate
Mice were anesthetized with a mixture of ketamine hydrochloride and xylazine hydrochloride and then received IP injection of a mixture of isoproterenol and pilocarpine. One minute after the injection, a Zone-Quick phenol red cotton thread (FCI Ophthalmics, Pembroke, MA, USA) was gently placed at the ocular surface of the lateral canthus and held in place for precisely 60 seconds. The thread was then removed from the eye, and the length of the red-stained part was measured with the scale provided along with the threads.
Detection of Activated Caspase-3
Detection of active caspase-3 in the LAC sections was performed using the SignalStain Apoptosis (Cleaved Caspase-3) IHC Detection Kit (Cell Signaling Technology, Danvers, MA, USA) according to the manufacturer's manual.
Detection of Serum Antinuclear Antibodies
Antinuclear antibodies (ANAs) in the mouse serum were detected using HEp-2 human epithelial cell substrate slides (INOVA Diagnostics, San Diego, CA, USA) according to the manufacturer's protocol. After staining, the samples were examined under a wide-field fluorescence microscope (Zeiss) at a magnification of 400×. Images presented were processed using Zeiss software (ZEN blue edition).
Real-Time RT-PCR
The LAC was cut into small fragments and ground into single cells between two frosted glass slides. The cells were then filtered through a 200-μm nylon mesh to generate a single cell suspension. RNA extraction, real-time RT-PCR, and primer sequences have been described previously.12
Statistical Analysis
Statistical significance was determined by Student's t-test (two-tailed, two-sample equal variance). P ≤ 0.05 was considered statistically significant.
Results
Induction of IL-7 Expression in the LAC by Poly I:C
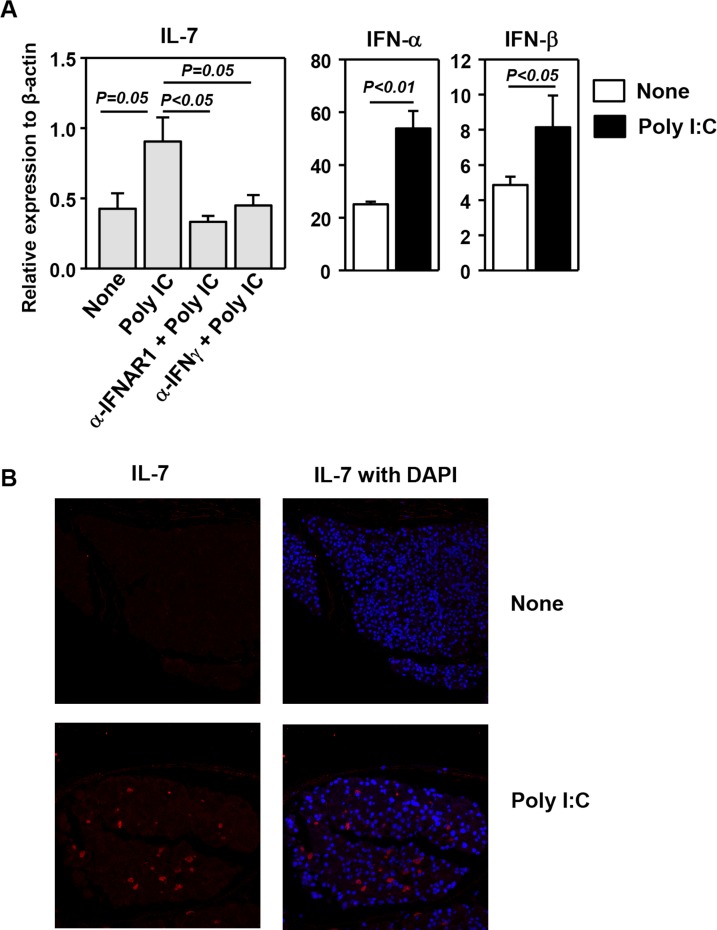
We and others have previously shown that in vivo administration of poly I:C induces IL-7 production in the salivary gland, lung, and liver. To determine whether poly I:C has a similar effect on the LAC tissues, we injected 100 μg poly I:C IP into C57BL/6 mice and analyzed the amount of IL-7 mRNA 6 hours later. Real-time RT-PCR showed that poly I:C markedly up-regulated IL-7 mRNA levels in the LAC. We previously reported that both type 1 IFNs and IFN-γ are required for the optimal induction of IL-7 by poly I:C in the salivary gland. To assess whether similar mechanisms are operating in the LAC, we injected neutralizing monoclonal anti-IFNAR1 or anti–IFN-γ antibody to C57BL/6 mice before administration of poly I:C and found that the induction of IL-7 expression by poly I:C was completely abrogated by either anti-IFNAR1 or anti–IFN-γ treatment (Fig. 1A). Consistent with a requirement for IFNs in IL-7 induction, poly I:C administration up-regulated the gene expression of IFN-α and IFN-β (Fig. 1A). Immunofluorescence staining showed an increase in IL-7 protein levels in the LAC upon poly I:C treatment (Fig. 1B). Hence, innate immune signaling elicited by poly I:C results in increased IL-7 mRNA and protein levels in the LAC tissue.
Figure 1.
Induction of IL-7 expression in the LAC by poly I:C. (A) Real-time PCR analysis of mRNA levels of IL-7 and IFNs in the LAC from C57BL/6 mice 6 hours after injection of poly I:C and indicated neutralizing antibodies. The results are presented relative to that of β-actin (n = 4). (B) Immunofluorescence staining of IL-7 in the LAC sections from C57BL/6 mice 24 hours after poly I:C injection. Data are representative of analyses of 10 mice (2–3 mice per experiment for a total of four independent experiments).
Induction of CXCR3 Ligands in the LAC by Poly I:C and IL-7
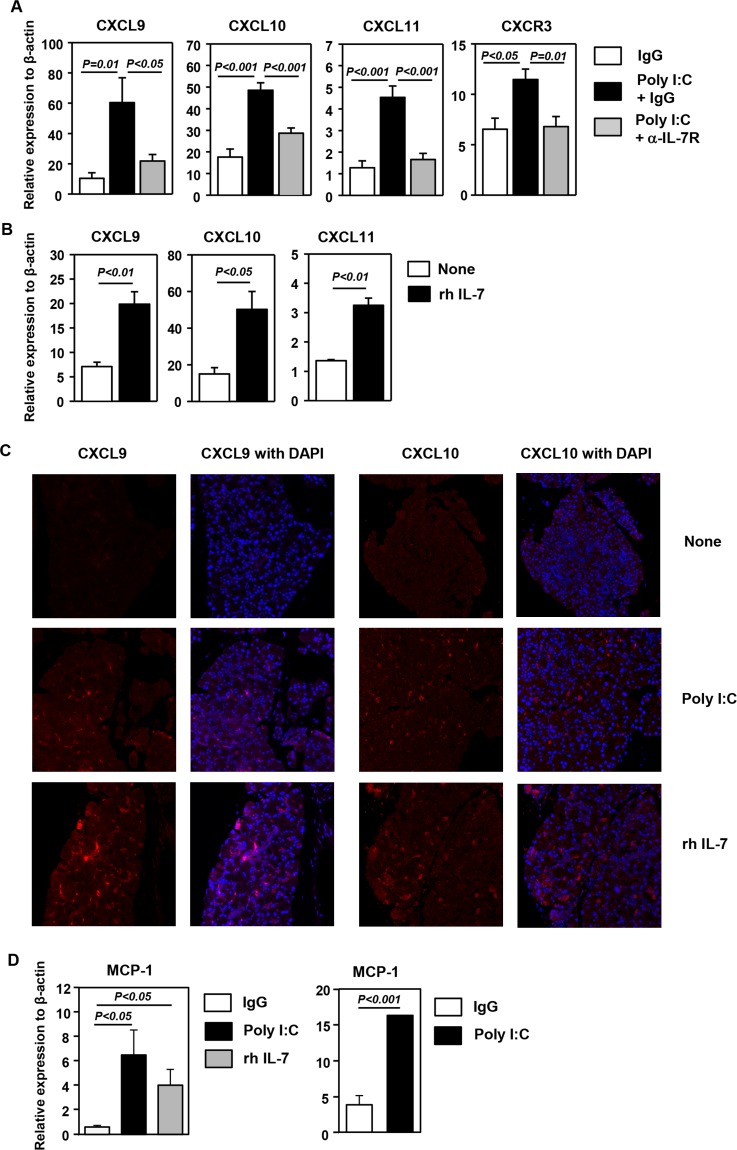
We have previously shown that poly I:C can induce the expression of several CXCR3 ligands, which are chemokines that preferentially attract CXCR3-expressing Th1, Tc1, and NK cells, to the salivary gland and lung in an IL-7–dependent manner. To determine whether similar events are induced by poly I:C in the LAC, we pretreated C57BL/6 mice with an anti–IL-7Rα antibody, which competitively inhibits IL-7 binding to IL-7Rα,12,13 or its isotype control IgG before IP administration of poly I:C. Poly I:C treatment induced a considerable increase in the expression of all three CXCR3 ligands, CXCL9, 10, and 11, in the LAC, and this increase was dramatically reduced by anti–IL-7Rα treatment (Fig. 2A). Accordingly, mRNA levels of CXCR3, which is expressed predominantly by IFN-γ–producing Th1, Tc1, and NK cells, were also increased in the LAC by poly I:C treatment, and this increase was almost completely abrogated by IL-7Rα blockade (Fig. 2A). We next assessed whether administration of exogenous IL-7 can exert similar effects as poly I:C to induce these chemokines in the LAC. Administration of rh IL-7 to C57BL/6 mice led to a considerable increase in mRNA levels of all three CXCR3 ligands in the LAC 1 day after the injection (Fig. 2B). Consistent with the changes in mRNA levels, immunofluorescence staining showed that both poly I:C and IL-7 treatment increased CXCL9 and 10 protein expression in the LAC (Fig. 2C). Hence, poly I:C up-regulates the expression of CXCR3 ligands in the LAC in an IL-7–dependent fashion. Moreover, IL-7 treatment has a similar promoting effect on the expression of these chemokines as poly I:C.
Figure 2.
Induction of CXCR3 ligands in the LAC by poly I:C and IL-7 treatment. (A) Real-time PCR analysis of mRNA levels of CXCR3 and its ligands in the LAC from C57BL/6 mice 24 hours after injection of poly I:C together with a blocking anti–IL-7Rα antibody or its isotype control IgG. The results are presented relative to that of β-actin (n = 6). (B) Real-time PCR analysis of mRNA levels of CXCR3 and its ligands in the LAC from C57BL/6 mice 24 hours after rh IL-7 injection, presented relative to that of β-actin (n = 3). Data are representative of four independent experiments. (C) Immunofluorescence staining of CXCL9 in the LAC sections from the mice treated with poly I:C described above. Data are representative of analyses of 10 mice each group (2–3 mice per experiment for a total of four independent experiments). (D) Expression level of MCP-1 in the LAC from C57BL/6 mice 24 hours after injection of poly I:C or rh IL-7 (left) or from mice 6 hours after injection of poly I:C (right), presented relative to that of β-actin (n = 4). Data are representative of three independent experiments.
We previously reported that both poly I:C and IL-7 treatment can up-regulate the expression of monocyte chemoattractant protein-1 (MCP-1), which induces tissue migration of monocytes/macrophages and NK cells, in the lung tissue.4,19,20 We thus examined whether poly I:C and IL-7 can also induce the same event in the LAC. Indeed, administration of either poly I:C or rh IL-7 led to a significant elevation in MCP-1 mRNA levels in the LAC 24 hours after injection (Fig. 2D). Moreover, a significant increase in MCP-1 transcripts could also be detected as early as 6 hours after poly I:C treatment (Fig. 2D). Hence, poly I:C and IL-7 can up-regulate MCP-1 expression in the LAC, which may in turn facilitate the recruitment of innate immune cells, especially NK cells and macrophages.
Induction of Proinflammatory Cytokines in the LAC by Poly I:C and IL-7 Administration
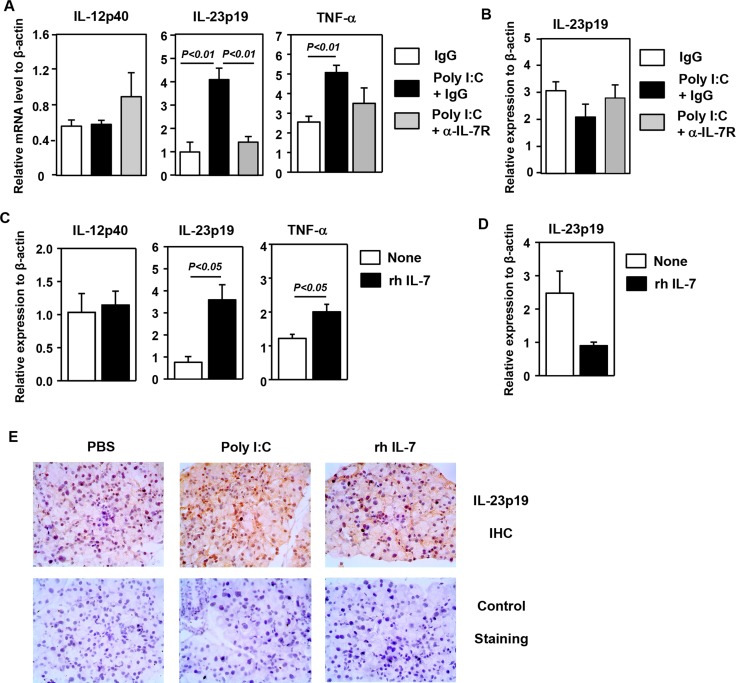
In addition to CXCR3 ligands, expression of several proinflammatory cytokines, including IL-23p19 and TNF-α, was also up-regulated by poly I:C, and this up-regulation was almost completely abolished by anti–IL-7Rα treatment (Fig. 3A). The expression of IL-12p40 and IL-12p35 in the LAC was not induced by poly I:C treatment (Fig. 3A and data not shown). In contrast to the LAC, expression of IL-23p19 was not up-regulated in the submandibular salivary gland upon poly I:C treatment (Fig. 3B). Administration of rh IL-7 up-regulated expression of IL-23p19 and TNF-α in the LAC without affecting that of IL-12p40 and IL-12p35 (Fig. 3C and data not shown). In contrast to the LAC, expression of IL-23p19 was not up-regulated in the submandibular gland by IL-7 administration (Fig. 3D). In accordance with the changes in IL-23p19 gene expression, immunohistochemical staining showed that poly I:C and IL-7 treatment increased IL-23p19 protein levels in the LAC (Fig. 3E). In conclusion, poly I:C up-regulates the expression of IL-23p19 and TNF-α in the LAC in an IL-7–dependent fashion. Moreover, IL-7 treatment has a similar inducing effect on the expression of these proinflammatory cytokines as poly I:C.
Figure 3.
Induction of proinflammatory cytokines in the LAC by poly I:C and IL-7 treatment. (A) The mRNA levels of proinflammatory cytokines in the LAC from mice receiving poly I:C as described in Figure 2 (n = 4). Data are representative of four independent experiments. (B) The IL-23p19 gene expression in the submandibular gland from mice treated with poly I:C (n = 6). (C) The mRNA levels of cytokines in the LAC in mice injected with rh IL-7 (n = 3). Data are representative of four independent experiments. (D) The IL-23p19 gene expression in the submandibular gland in mice treated with rh IL-7 (n = 3). (E) Immunohistochemical staining of IL-23p19 in the LAC sections from mice treated with poly I:C or rh IL-7. Negative control staining with only the secondary antibody is also shown (lower panels). Data are representative of three independent samples.
Induction of SS-Like Pathologic Changes in the LAC by Repeated Administration of Poly I:C
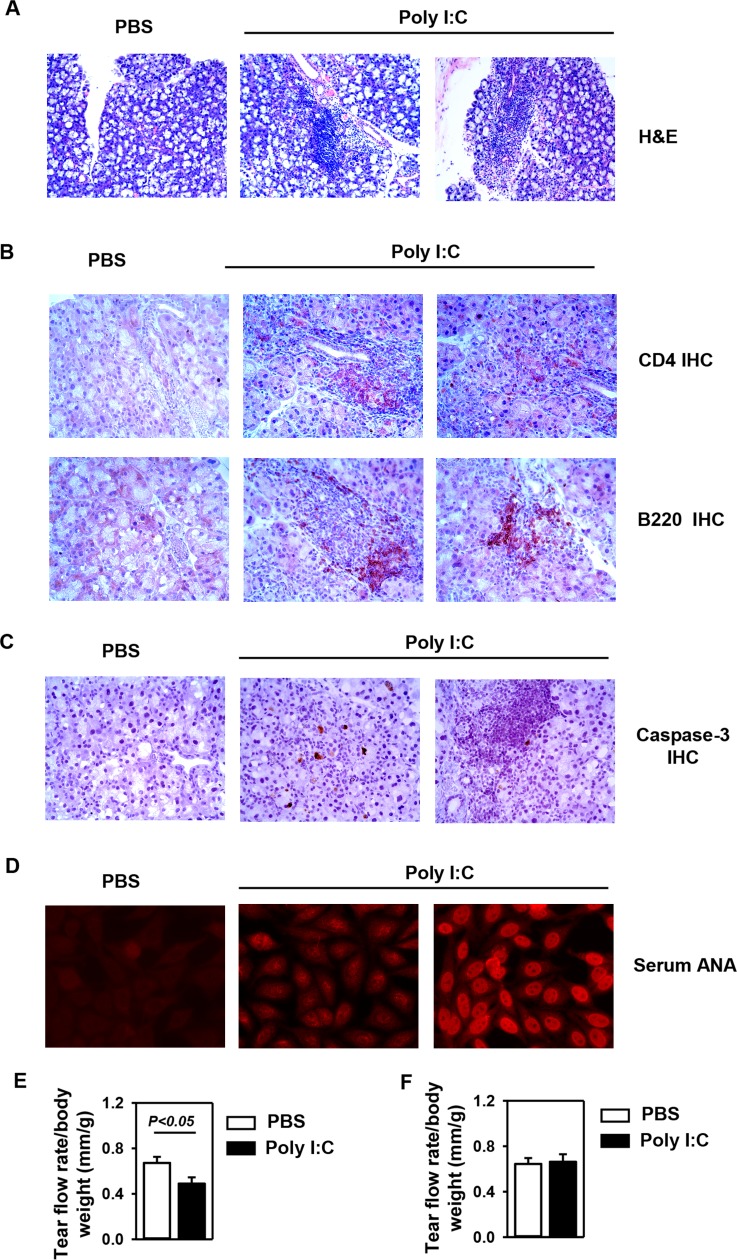
Chronic viral infection has been implicated as a trigger or contributing factor for SS and a number of other inflammatory diseases. Previous reports have shown that repeated poly I:C administration, mimicking persistent viral infection, can accelerate SS-like sialadenitis and cause salivary gland dysfunction in mouse models of SS.10,12 Repeated poly I:C has also been shown to induce lung inflammation and exacerbates neurodegenerative disease in mice.5,21 Therefore, having demonstrated that poly I:C can rapidly induce the expression of multiple chemokines and proinflammatory cytokines in the LAC, we next assessed whether repeated poly I:C treatment over time can lead to the development and onset of SS-like dacryoadenitis. We administered 100 μg poly I:C to C57BL/6 mice every 2–3 days for a total of three times and harvested the LAC 8 days after the initial injection. Hematoxylin and eosin staining showed that poly I:C treatment induced the appearance of leukocytic foci in the LAC in 55% of the mice (Fig. 4A). Analysis of the composition of the foci by immunohistochemical staining for CD4 and B220 showed the presence of CD4 T cells and B cells, which is characteristic of SS-like disease (Fig. 4B). Moreover, having shown that IFN-γ and TNF-α are elevated in the LAC upon poly I:C treatment, we next assessed whether poly I:C treatment can induce apoptosis of the LAC tissues, as these cytokines are known to induce apoptosis of salivary gland epithelial cells. Immunohistochemical staining showed that in contrast to PBS-treated control mice, mice treated with poly I:C contained considerable amount of cells positive for cleaved, active caspase-3 in LAC tissues, indicating the onset of apoptosis process and tissue damage (Fig. 4C). Additionally, analysis for the presence of serum ANA, another characteristic parameter of SS-like disease, showed that serum ANA production was also induced to various degrees by poly I:C treatment (Fig. 4D). In comparison, a single poly I:C injection caused the appearance of leukocytic foci in only 20% of the mice and did not induce ANA production 1 day after the injection, and no pathologies were observed 8 days after the single injection as well (data not shown). These results indicated that repeated poly I:C treatment is required for the induction of the pathologies of SS-like dacryoadenitis. Finally, repeated poly I:C treatment in an 8-day period led to a significant reduction in the tear flow rate, indicating an impaired secretory function (Fig. 4E). To determine whether the SS-like pathologies induced by repeated poly I:C treatment can persist without continuous poly I:C treatment, we followed the mice for an additional 8 days after the initial three injections of poly I:C. The results showed that the tear flow rate, tissue inflammation, and ANA all returned back to levels comparable to the control group (Fig. 4F and data not shown), indicating that the pathologies of SS-like dacryoadenitis subsided and disappeared over time after the poly I:C treatment stopped. Hence, repeated poly I:C administration over a period of 8 days is sufficient to cause characteristic pathologic and functional changes of SS-like dacryoadenitis in the non–autoimmune-prone C57BL/6 mice, and continuous poly I:C stimulation may be needed to sustain these disease pathologies.
Figure 4.
Induction of SS-like dacryoadenitis by repeated administration of poly I:C. The C57BL/6 mice received poly I:C or control PBS injection every 2–3 days and were euthanized 8 days after the initial injection. (A) Hematoxylin and eosin staining of the LAC sections. One PBS-treated and two independent poly I:C-treated samples are shown. (B) Immunohistochemical staining of CD4 and B220 in the LAC sections. (C) Immunohistochemical staining of cleaved, activated caspase-3 in LAC sections. (D) Measurement of serum ANA levels. One PBS-treated and two independent poly I:C-treated samples are shown. All the data are representative of analyses of 11 PBS-treated mice and 12 poly I:C-treated mice. (E) Tear flow rate, represented by the length of the red area of the phenol red cotton thread, normalized to body weight (n = 14). (F) C57BL/6 mice received poly I:C or PBS injection every 2–3 days and were euthanized 16 days after the initial injection. Tear flow rate relative to body weight is shown (n = 10).
Discussion
The present study demonstrates that innate immune signaling elicited by poly I:C can rapidly induces IL-7 production and IL-7–dependent early inflammatory responses in the LAC of normal non–autoimmune-prone C57BL/6 mice in vivo. Moreover, IL-7 is sufficient to induce most of the inflammatory responses that poly I:C does. The response of LAC to poly I:C and IL-7 bears many resemblance to that of the salivary gland but differs in the induction of IL-23p19. Finally, repeated poly I:C treatment over a period of 8 days is sufficient to cause characteristic pathologies of SS-like dacryoadenitis, including impaired tear secretion, in C57BL/6 mice.
Interleukin-7 is elevated in a variety of autoimmune and inflammatory diseases and plays a critical pathogenic role in these diseases,15–17,22–25 including SS as we have recently reported.13 Moreover, we have shown that poly I:C treatment can induce IL-7 production and IL-7–dependent early inflammatory responses in the salivary gland, and accelerate the development of SS-like sialadenitis.12 Here we provide evidence that poly I:C can similarly induce IL-7 production and IL-7–dependent early inflammatory response in the LAC. In addition, we show that administration of exogenous IL-7 is sufficient to replicate most of the effect of poly I:C on the LAC tissue. The chemokines and cytokines that are up-regulated by poly I:C and IL-7 in both the LAC and salivary gland include MCP-1, CXCR3 ligands, type 1 IFNs, IFN-γ, and TNF-α.
The main difference between the early response in the LAC and the salivary gland to poly I:C and IL-7 is the up-regulation of IL-23p19 in the former but not in the latter. This difference could be attributed to different properties of LAC versus salivary gland epithelial cells or to the different cellular types in the two glands that respond to poly I:C and IL-7. For instance, we have previously shown that macrophages are the main sources of CXCL9 in the lung in response to poly I:C and IL-7 treatment,4 but are not the main sources of CXCL9 in the salivary gland upon the same stimulations. The precise cell types and populations in the LAC that respond to poly I:C to produce IL-7, type 1 IFNs, IL-23, and CXCL9-11 and mediate the inflammatory response require further characterization, especially by using in vitro stimulation and culture systems of the purified cell populations. It has been reported that poly I:C can induce the production of these cytokines and chemokines from dendritic cells, monocytes, macrophages, and NK cells,26–30 as well as in salivary gland epithelial cells.31,32 In addition, we previously reported that poly I:C treatment induces IL-7 production from a human salivary gland epithelial cell line (HSG) in vitro,12 suggesting that poly I:C can directly act on exocrine gland epithelial cells to induce IL-7 expression. Therefore, we hypothesize that lacrimal gland epithelial cells and innate immune cells are the primary cell types that rapidly and directly respond to poly I:C to produce IL-7, proinflammatory cytokines, and chemokines and elicit the early inflammatory response. As a result of the early inflammatory response, T and B cells are subsequently recruited to the LAC and activated to cause or promote tissue damage, autoantibody production, and secretory dysfunction. These hypotheses will be tested in our future studies.
Importantly, repeated poly I:C injection over an 8-day period causes the appearance of leukocytic foci that contain T and B cells in the LAC of more than half of the mice. The up-regulation of CXCR3 ligands likely contributes to the migration of T cells, especially CXCR3+ Th1 and Tc1 cells, to the LAC. In addition, CXCR3 ligands, such as CXCL9 and 10, are implicated in the migration of B cells and plasma cells to local tissues.33,34 Additional chemokines may also be induced by poly I:C treatment and play a role in the recruitment of these cells, which will be investigated in the future. Poly I:C has been shown to induce apoptosis of primary salivary gland cells from SS patients.35,36 Here we demonstrate that poly I:C may have similar apoptosis-inducing effect on the LAC cells as it promotes the activation of caspase-3 in these cells, and the underlying mechanisms require further characterization. Interestingly, elevation in serum ANA levels, a characteristic serologic change in SS and several other rheumatic diseases, is also induced by repeated poly I:C treatment within 8 days after the initial treatment. Importantly, we show that the secretory function of the LAC is impaired by repeated poly I:C treatment. Therefore, activation of innate signaling by poly I:C is capable of initiating not only the early innate inflammatory response but also adaptive immune response and major characteristic pathologies of SS-like dacryoadenitis in the LAC in non–autoimmune-prone C57BL/6 mice.
Consistent with previous reports that repeated poly I:C administration accelerates SS-like sialadenitis in mouse models of this disease, induces lung inflammation, and exacerbates neurodegeneration in mice,5,10,12,21 we show that repeated poly I:C administration can also cause SS-like dacryoadenitis in non–autoimmune-prone C57BL/6 mice and that a single poly I:C injection is not able to do so. Repeated poly I:C stimulation mimics persistent viral infection, which is implicated as one of the environmental triggers of SS and a number of other inflammatory diseases. Moreover, consistent with a previous report that the salivary gland regains its normal secretory function after the cessation of poly I:C treatment,10 we also found that the pathologies of SS-like dacryoadenitis cannot be sustained for long without continuous poly I:C treatment in C57BL/6 mice, suggesting that chronic or repeated viral infection might be needed for the long-term persistence of SS, which is a feature of this disease. Future studies will examine the effect of poly I:C on the development of autoimmune dacryoadenitis in combination with other environmental or genetic factors. In addition, whether local application of poly I:C to the lacrimal gland or the eye can also cause SS-like dacryoadenitis, in the absence of a systemic immune response, will be tested.
Poly I:C can activate several downstream signaling pathways, including the TLR3, melanoma differentiation-associated gene 5, and RNA-activated protein kinase (PKR) pathways.37–40 Induction of B-cell activating factor in human salivary gland cells is completely PKR dependent and partially TLR3 dependent.40 Currently we do not know which signaling pathway mediates poly I:C-induction of IL-7 expression in the LAC or salivary gland. We hypothesize that similar pathways are involved in these two types of glands, and our future studies will address this possibility.
In the salivary gland, poly I:C-induced IL-7 production is critically dependent on type 1 IFNs and IFN-γ. Moreover, treatment with a combination of IFN-α and IFN-γ can effectively induce IL-7 production from HSG cells in vitro. Similarly, the IL-7–inducing effect of poly I:C in the LAC is also dependent on type 1 IFNs and IFN-γ. Whether these cytokines are sufficient to induce IL-7 production in the LAC and salivary gland in vivo and the downstream molecular pathways involved in these processes are questions that await further investigation.
Conclusions
Poly I:C induces excessive IL-7 production and early inflammatory responses in the LAC and subsequently causes signature pathologic changes of SS-like dacryoadenitis in non–autoimmune-prone C57BL/6 mice. These findings, for the first time, define the effect of virus analog poly I:C on the LAC tissues and provide evidence that innate immune signaling, such as that elicited by viral infections, can initiate and promote the development of SS-like dacryoadenitis. Hence, targeting virus-induced innate signaling and its downstream inflammatory mediators may be a potentially effective strategy to treat SS.
Acknowledgments
The authors thank Ekta S. Patel, PhD, for technical help in some experiments.
Supported by grants from the National Institutes of Health (P30DE020751, R01 DE023838).
Disclosure: J. Zhou, None; J.-O. Jin, None; J. Du, None; Q. Yu, None
References
- 1. Kawai T,, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010; 11: 373–384. [DOI] [PubMed] [Google Scholar]
- 2. Arpaia N,, Barton GM. Toll-like receptors: key players in antiviral immunity. Curr Opin Virol. 2011; 1: 447–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Marshak-Rothstein A. Toll-like receptors in systemic autoimmune disease. Nat Rev Immunol. 2006; 6: 823–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jin JO,, Yu Q. Systemic administration of TLR3 agonist induces IL-7 expression and IL-7-dependent CXCR3 ligand production in the lung. J Leukoc Biol. 2013;93:413. – 425. [DOI] [PMC free article] [PubMed]
- 5. Stowell NC,, Seideman J,, Raymond HA,, et al. Long-term activation of TLR3 by poly(I:C) induces inflammation and impairs lung function in mice. Respir Res. 2009; 10: 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Patole PS,, Grone HJ,, Segerer S,, et al. Viral double-stranded RNA aggravates lupus nephritis through Toll-like receptor 3 on glomerular mesangial cells and antigen-presenting cells. J Am Soc Nephrol. 2005; 16: 1326–1338. [DOI] [PubMed] [Google Scholar]
- 7. Lang KS,, Georgiev P,, Recher M,, et al. Immunoprivileged status of the liver is controlled by Toll-like receptor 3 signaling. J Clin Invest. 2006; 116: 2456–2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Voulgarelis M,, Tzioufas AG. Pathogenetic mechanisms in the initiation and perpetuation of Sjogren's syndrome. Nat Rev Rheumatol. 2010; 6: 529–537. [DOI] [PubMed] [Google Scholar]
- 9. Mariette X,, Gottenberg JE. Pathogenesis of Sjogren's syndrome and therapeutic consequences. Curr Opin Rheumatol. 2010; 22: 471–477. [DOI] [PubMed] [Google Scholar]
- 10. Deshmukh US,, Nandula SR,, Thimmalapura PR,, Scindia YM,, Bagavant H. Activation of innate immune responses through Toll-like receptor 3 causes a rapid loss of salivary gland function. J Oral Pathol Med. 2009; 38: 42–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nandula SR,, Scindia YM,, Dey P,, Bagavant H,, Deshmukh US. Activation of innate immunity accelerates sialoadenitis in a mouse model for Sjogren's syndrome-like disease. Oral Dis. 2011; 17: 801–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jin JO,, Shinohara Y,, Yu Q. Innate immune signaling induces interleukin-7 production from salivary gland cells and accelerates the development of primary Sjogren's syndrome in a mouse model. PLoS One. 2013; 8: e77605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jin JO,, Kawai T,, Cha S,, Yu Q. Interleukin-7 enhances Th1 response to promote the development of Sjogren's syndrome-like autoimmune exocrinopathy. Arthritis Rheum. 2013; 65: 2132–2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sawa Y,, Arima Y,, Ogura H,, et al. Hepatic interleukin-7 expression regulates T cell responses. Immunity. 2009; 30: 447–457. [DOI] [PubMed] [Google Scholar]
- 15. Lee LF,, Axtell R,, Tu GH,, et al. IL-7 promotes T(H)1 development and serum IL-7 predicts clinical response to interferon-beta in multiple sclerosis. Sci Transl Med. 2011; 3:93ra68. [DOI] [PMC free article] [PubMed]
- 16. Hartgring SA,, Willis CR,, Alcorn D,, et al. Blockade of the interleukin-7 receptor inhibits collagen-induced arthritis and is associated with reduction of T cell activity and proinflammatory mediators. Arthritis Rheum. 2010; 62: 2716–2725. [DOI] [PubMed] [Google Scholar]
- 17. Totsuka T,, Kanai T,, Nemoto Y,, et al. IL-7 Is essential for the development and the persistence of chronic colitis. J Immunol. 2007; 178: 4737–4748. [DOI] [PubMed] [Google Scholar]
- 18. Pellegrini M,, Calzascia T,, Toe JG,, et al. IL-7 engages multiple mechanisms to overcome chronic viral infection and limit organ pathology. Cell. 2011; 144: 601–613. [DOI] [PubMed] [Google Scholar]
- 19. Deshmane SL,, Kremlev S,, Amini S,, Sawaya BE. Monocyte chemoattractant protein-1 (MCP-1): an overview. J Interferon Cytokine Res. 2009; 29: 313–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bracke KR,, Demedts IK,, Joos GF,, Brusselle GG. CC-chemokine receptors in chronic obstructive pulmonary disease. Inflamm Allergy Drug Targets. 2007; 6: 75–79. [DOI] [PubMed] [Google Scholar]
- 21. Field R,, Campion S,, Warren C,, Murray C,, Cunningham C. Systemic challenge with the TLR3 agonist poly I:C induces amplified IFNalpha/beta and IL-1beta responses in the diseased brain and exacerbates chronic neurodegeneration. Brain Behav Immunity. 2010; 24: 996–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Shinohara T,, Nemoto Y,, Kanai T,, et al. Upregulated IL-7 receptor alpha expression on colitogenic memory CD4+ T cells may participate in the development and persistence of chronic colitis. J Immunol. 2011; 186: 2623–2632. [DOI] [PubMed] [Google Scholar]
- 23. Yamazaki M,, Yajima T,, Tanabe M,, et al. Mucosal T cells expressing high levels of IL-7 receptor are potential targets for treatment of chronic colitis. J Immunol. 2003; 171: 1556–1563. [DOI] [PubMed] [Google Scholar]
- 24. Hartgring SA,, Bijlsma JW,, Lafeber FP,, van Roon JA. Interleukin-7 induced immunopathology in arthritis. Ann Rheum Dis. 2006; 65(suppl 3):iii69 – iii74. [DOI] [PMC free article] [PubMed]
- 25. Lee LF,, Logronio K,, Tu GH,, et al. Anti-IL-7 receptor-alpha reverses established type 1 diabetes in nonobese diabetic mice by modulating effector T-cell function. Proc Natl Acad Sci U S A. 2012; 109: 12674–12679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kumar H,, Koyama S,, Ishii KJ,, Kawai T,, Akira S. Cutting edge: cooperation of IPS-1- and TRIF-dependent pathways in poly IC-enhanced antibody production and cytotoxic T cell responses. J Immunol. 2008; 180: 683–687. [DOI] [PubMed] [Google Scholar]
- 27. Longhi MP,, Trumpfheller C,, Idoyaga J,, et al. Dendritic cells require a systemic type I interferon response to mature and induce CD4+ Th1 immunity with poly IC as adjuvant. J Exper Med. 2009; 206: 1589–1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lundberg AM,, Drexler SK,, Monaco C,, et al. Key differences in TLR3/poly I:C signaling and cytokine induction by human primary cells: a phenomenon absent from murine cell systems. Blood. 2007; 110: 3245–3252. [DOI] [PubMed] [Google Scholar]
- 29. Miyake T,, Kumagai Y,, Kato H,, et al. Poly I:C-induced activation of NK cells by CD8 alpha+ dendritic cells via the IPS-1 and TRIF-dependent pathways. J Immunol. 2009; 183: 2522–2528. [DOI] [PubMed] [Google Scholar]
- 30. Garrett S,, Fitzgerald MC,, Sullivan KE. LPS, and poly I:C induce chromatin modifications at a novel upstream region of the IL-23 p19 promoter. Inflammation. 2008; 31: 235–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Li J,, Jeong MY,, Bae JH,, et al. Toll-like receptor3-mediated induction of chemokines in salivary epithelial cells. Korean J Physiol Pharmacol. 2010; 14: 235–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ittah M,, Miceli-Richard C,, Gottenberg JE,, et al. Viruses induce high expression of BAFF by salivary gland epithelial cells through TLR- and type-I IFN-dependent and -independent pathways. Eur J Immunol. 2008; 38: 1058–1064. [DOI] [PubMed] [Google Scholar]
- 33. Nicholas MW,, Dooley MA,, Hogan SL,, et al. A novel subset of memory B cells is enriched in autoreactivity and correlates with adverse outcomes in SLE. Clin Immunol. 2008; 126: 189–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Meinl E,, Krumbholz M,, Hohlfeld R. B lineage cells in the inflammatory central nervous system environment: migration, maintenance, local antibody production, and therapeutic modulation. Ann Neurol. 2006; 59: 880–892. [DOI] [PubMed] [Google Scholar]
- 35. Nakamura H,, Horai Y,, Suzuki T,, et al. TLR3-mediated apoptosis and activation of phosphorylated Akt in the salivary gland epithelial cells of primary Sjogren's syndrome patients. Rheumatol Int. 2013; 33: 441–450. [DOI] [PubMed] [Google Scholar]
- 36. Manoussakis MN,, Spachidou MP,, Maratheftis CI. Salivary epithelial cells from Sjogren's syndrome patients are highly sensitive to anoikis induced by TLR-3 ligation. J Autoimmunity. 2010; 35: 212–218. [DOI] [PubMed] [Google Scholar]
- 37. Baccala R,, Hoebe K,, Kono DH,, Beutler B,, Theofilopoulos AN. TLR-dependent and TLR-independent pathways of type I interferon induction in systemic autoimmunity. Nature Med. 2007; 13: 543–551. [DOI] [PubMed] [Google Scholar]
- 38. Kato H,, Takeuchi O,, Sato S,, et al. Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature. 2006; 441: 101–105. [DOI] [PubMed] [Google Scholar]
- 39. Wang Q,, Miller DJ,, Bowman ER,, et al. MDA5 and TLR3 initiate pro-inflammatory signaling pathways leading to rhinovirus-induced airways inflammation and hyperresponsiveness. PLoS Pathog. 2011; 7: e1002070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ittah M,, Miceli-Richard C,, Gottenberg JE,, Sellam J,, Lepajolec C,, Mariette X. B-cell-activating factor expressions in salivary epithelial cells after dsRNA virus infection depends on RNA-activated protein kinase activation. Eur J Immunol. 2009; 39: 1271–1279. [DOI] [PubMed] [Google Scholar]