Abstract:
Abstract: Autologous platelet gel (APG) was developed in the early 1990s as a byproduct of platelet-rich plasma sequestration during cardiac surgery. Although APG has been approved for postoperative healing, there have been no published studies that evaluate the effects of APG on sternal healing postcardiopulmonary bypass. The purpose for this study was to examine the effects of APG on postoperative sternal wound healing evidenced by subjective reports of chest and leg pain, the amount of measurable bruising incurred, and platelet indices both preoperatively and postoperatively.
Keywords: autologous platelet gel (APG), platelet-rich plasma (PRP), platelet-poor plasma (PPP), growth factors
Autologous platelet gel (APG) is a byproduct of platelet-rich plasma (PRP) sequestration (1,2). APG stimulates regional osteogenesis (or bone growth) within bones, which promotes soft tissue repair and regeneration. Clinical studies have demonstrated the benefits of APG application during cosmetic surgeries by enhancing soft tissue healing (3–5). Other studies also have demonstrated benefits in oral and maxillofacial surgery as APG-enhanced bone grafts (6–8). PRP has three to five times the native concentration of platelets (7). When PRP is combined with thrombin and calcium, the clotting cascade is activated, converting fibrinogen to fibrin with subsequent platelet degranulation. Substances released from the degranulated platelets include serotonin, catecholamines, adenosine diphosphatase, fibrinogen, fibronectin, adenosine triphosphatase, factor V, Von Willebrand factor VIII, thromboxane A2, and calcium. Platelets then become trapped in a fibrin matrix/mesh that produces a stable clot from specific receptors for fibrin, collagen, and adhesive glycoproteins. This fibrin matrix/mesh is similar to a native fibrin clot that also allows normal cellular infiltration of monocytes, fibroblasts, and other cells important in wound healing. A viscous coagulum is formed rapidly with resulting hemostasis. In addition, platelets also release a number of platelet-derived growth factors that enhance wound healing by autocrine (having an effect on its own cell membrane) and paracrine (having an effect on another cell membrane) mechanisms that include platelet-derived growth factor, transforming growth factor-beta, platelet-derived endothelial cell growth factor, plateletderived angiogenesis factor, and insulin-like growth factor (9).
MATERIALS AND METHODS
This study was a prospective randomized study that examined the effects of APG on postoperative sternal wound healing, evidenced by subjective reports of pain, the amount of measurable bruising incurred, and platelet indices both preoperatively and postoperatively.
After approval from the Wichita Medical Research & Education Foundation Institutional Review Board was obtained, 30 patients who underwent cardiopulmonary bypass with a sternal incision were recruited to participate in this study. All subjects were provided detailed information about the study, and informed consent was obtained. Subjects were not paid to participate. Signed informed consents will be kept in a locked drawer in the principle investigator’s (PI) office. No subjects refused participation. Subjects from both genders were recruited, and additional inclusion criteria were that their age required that subjects had to be 30–85 years of age and scheduled for coronary artery bypass surgery through a mediastinal incision.
Patients were assigned randomly to either the treatment or control group in succession (without replacement) by drawing either “treatment” or “control” from prewritten pieces of paper in an envelope to prevent against bias (10). Demographic variables such as age, history of smoking, and diabetes were collected from the patient’s chart by the PI.
Subjective patient reports of postoperative pain were measured from 0 to 10, with “0” representing “no pain” and “10” representing the worse possible pain. Subjects were instructed how to rate their pain by the PI. Postoperative wound infections, swelling, bruising, and postoperative blood loss were measured on the morning of postoperative days 1 and 3 in the intensive care init and approximately 30 days postoperatively during the subject’s visit with their physician. A certified clinical technician, intensive care registered nurses, one nurse from the postoperative floor, and an office nurse agreed to collect data for the specific study end points. Data collectors were blinded to the experimental and control conditions. All data were given to the PI, who entered the data onto password-protected computer files.
The investigators for this study were not compensated. Funding was received from the Jesse P. Willis Endowed Fund for Heart Research through the Wichita Medical Research and Education Foundation, Wichita, Kansas. Helena Laboratory Platelet Works donated the test tubes for the platelet aggregation tests for this study. Fresenius Medical Care Extracorporeal Alliance agreed to fund the costs relating to the equipment and labor necessary to produce the APG. Medtronic provided the Magellan Autologous Platelet Separator Disposables Kit, Magellan Spray Tip, MST 700, and the Magellan Ratio Dispenser Kit, MRD500.
Wound photographs were taken to compare and visualize the differences between the experimental and control group healing and to access if possible post-operative infection (Table 1). A ruler (millimeter) was placed next to the wound to measure length and width of redness and/or swelling. Nurses were educated to take the pictures at the appropriate distance with the included ruler, identification card, and camera.
Table 1.
Criteria for defining a surgical-site infection (SSI).*
Superficial Incisional SSI Infection occurs within 30 days after the operation and infection involves only skin or subcutaneous tissue of the incision and at least one of the following:
Do not report the following conditions as SSI:
Note: Specific criteria are used for identifying infected episiotomy and circumcision sites and burn wounds. Deep-Incisional SSI Infection occurs within 30 days after the operation if no implant† is left in place or within 1 year if implant is in place and the infection appears to be related to the operation and infection involves deep soft tissues (e.g., fascial and muscle layers) of the incision and at least one of the following:
Notes:
|
Mangram AJ, Horan TC, Pearson ML, Silver LC, Jarvis WR (11).
National Nosocomial Infection Surveillance definition: a nonhuman-derived implantable foreign body (e.g., prosthetic heart valve, nonhuman vascular graft, mechanical heart, or hip prosthesis) that is permanently placed in a patient during surgery.
In addition, all blood work (platelet indices, hemoglobin, and hematocrits) were performed at the hospital facility. All blood work gathered was the standard of care except for the postoperative platelet indices obtained from the experimental group. The platelet counts and platelet aggregation tests were measured using a 60-mL syringe that contained 52 mL of the patient’s blood and 8 mL of anticoagulant citrate dextrose (ACD). The final product platelet count was taken from the platelet-rich plasma (PRP) syringe that was handed off from the surgical field. The blood was transferred into plastic redtopped tubes because glass would activate the platelets and decreasing the total platelet count. Laboratory personnel first diluted the blood sample at a rate of 4:1 with an isotonic solution, which prevents clumping to provide a more accurate platelet count. The platelet count was displayed on an automatic cell counter, and laboratory personnel then multiplied their results by 4. The platelet concentrate was gently agitated and kept at room temperature to maintain platelet viability (12).
The Medtronic Magellan™ Autologous Platelet Separator System and the Medtronic Magellan™ Autologous Platelet Separator Disposables Kit (Minneapolis, MN) were donated for this study. All manufacturers’ guidelines for use were followed. Platelet-poor plasma (PPP) was sprayed on subcuticular tissue before the closure of the chest incision and before the closure of the leg incision after the saphenous vein harvest. Approximately 20 mL of PPP was applied to the subcuticular tissue using the Magellan Spray Tip, MST 700, and the Magellan Ratio Dispenser Kit, MRD500. Approximately 16 mL of PRP was applied to the sternum before sternal wiring using the Magellan Cannula Tip, MCT600, and to make a “caulking bead” on the sternum after sternal wires were tightened (13).
Data were analyzed using t tests and repeated measures analysis of variance (14) when appropriate using SPSS 11.5 (SPSS Inc., Chicago, IL). Probability was set at p < .05. Effect sizes were calculated to determine the strength of relationships (15).
ANALYSIS AND DISCUSSION
Descriptive statistics were calculated to provide information about the subjects. Eight of 30 subjects were women. Eleven subjects were diabetic, and seven subjects were smokers. Overall, the control group was slightly older (M = 68.47 years, SD = 14.59) than the experimental group (M = 61.87 years, SD = 9.47), and had slightly higher baseline platelet counts (M = 208.80k, SD = 34.32) than the experimental group (M = 192.33k, SD = 62.81).
Statistical analyses for the outcome variables of interest (chest and leg pain, chest and leg bruising, and platelet indices) revealed mixed results. Overall, the experimental group reported less chest and leg pain than the control group during all measurement times (Table 2).
Table 2.
Chest and leg pain means and standard deviations between groups on postoperative days 1, 3, and the office visit (OV).
| Variable (pain scale 0–10) | Control Group (n = 15) Mean (SD) | Experimental Group (n = 15) Mean (SD) |
|---|---|---|
| Chest pain day 1 | 4.47 (2.06) | 1.47 (0.83) |
| Chest pain day 3 | 4.53 (2.33) | 1.40 (0.75) |
| Chest pain office visit | 2.27 (1.33) | 0.53 (0.64) |
| Leg pain day 1 | 3.06 (1.62) | 1.33 (0.72) |
| Leg pain day 3 | 2.80 (2.27) | 1.46 (0.74) |
| Leg pain office visit | 2.33 (1.72) | 0.53 (0.64) |
A one-way repeated measures analysis of variance was calculated to examine differences in perceived chest and leg pain within and between groups (14) over time. The amount of perceived chest pain was significantly less over time in the experimental group,
which might possibly reflect a treatment effect; however, as the natural course of healing occurs one would assume that all pain would decrease over time. The effect size was calculated and revealed a moderately large effect for the treatment group over time, η2 = .56.
Results from another one-way repeated measures analysis of variance was calculated to examine differences in perceived leg pain over time. This analysis revealed nonsignificant multivariate results but significant univariate results,
with a moderate effect size, η2 = .39. Although the total sample size was too small to support a difference between groups, a moderate effect size may indicate a treatment effect.
There was a large amount of variance in recording the amount of bruising per subject, which made meaningful statistical analysis problematic, because some subjects bruised more than others. One suggestion to handle extreme scores was to eliminate them and re-run the analysis. Because our study began with a small sample size, this would further compromise our results. We present four pictures to illustrate two cases. The subject from the control group was one of a few who developed excessive bruising, whereas the subject represented from the experimental group had little bruising. These pictures in no way reflect upon the judgment of our results. They are for illustrative purposes only.
Platelet indices were analyzed using a t test to evaluate whether baseline mean ADP platelet counts differed from post ADP platelet counts in the experimental group (Table 3).
Table 3.
Platelet means and standard deviations from the experimental group.
| n = 15 | Mean (SD) |
|---|---|
| Baseline platelet count (mm3) | 200.57 k (51.64) |
| Baseline ADP platelet (mm3) | 122.53 k (73.85) |
| Baseline aggregation % | 38.73 (18.10) |
| PRP platelet count (mm3) | 1010.87 (685.33) |
| PRP ADP platelet count (mm3) | 47.07 (22.11) |
| PRP aggregation % | 532.53 (461.48) |
| Platelet yield from Magellan | 5.07 (1.81) |
Figure 1.

Postoperative day 3 leg incision, experimental group.
Figure 2.
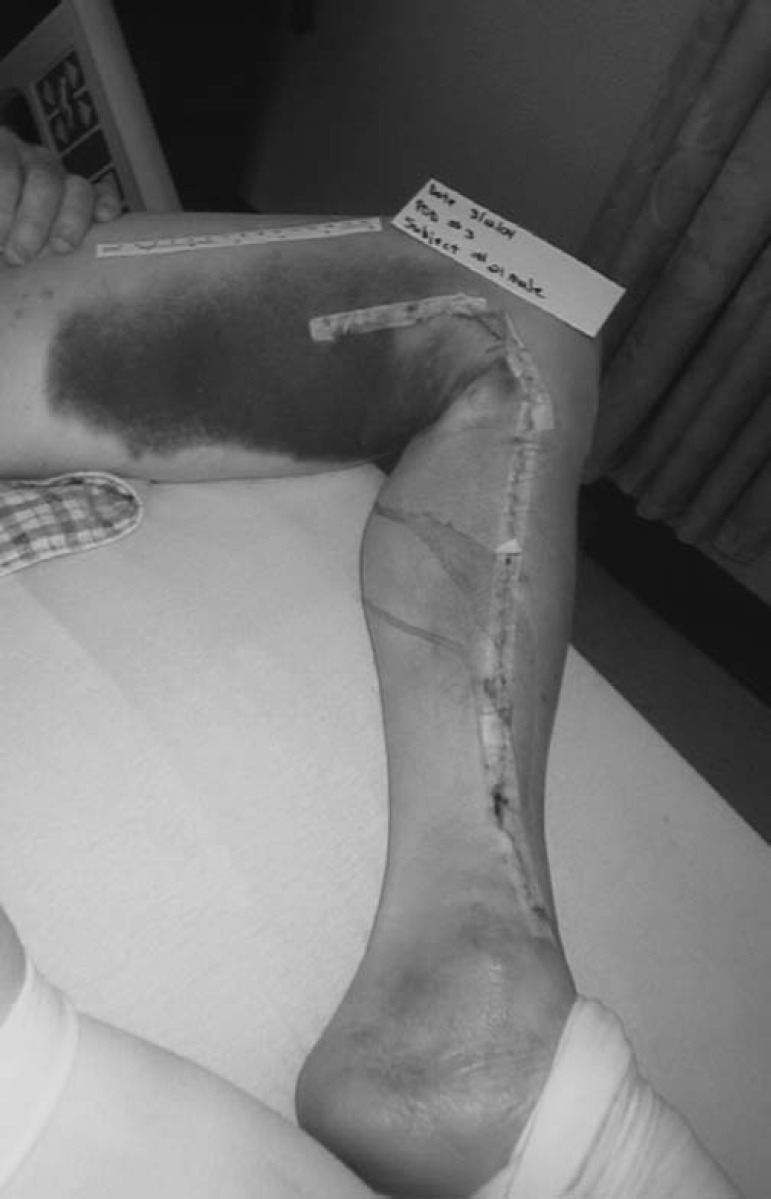
Postoperative day 3 leg incision, control group.
Figure 3.
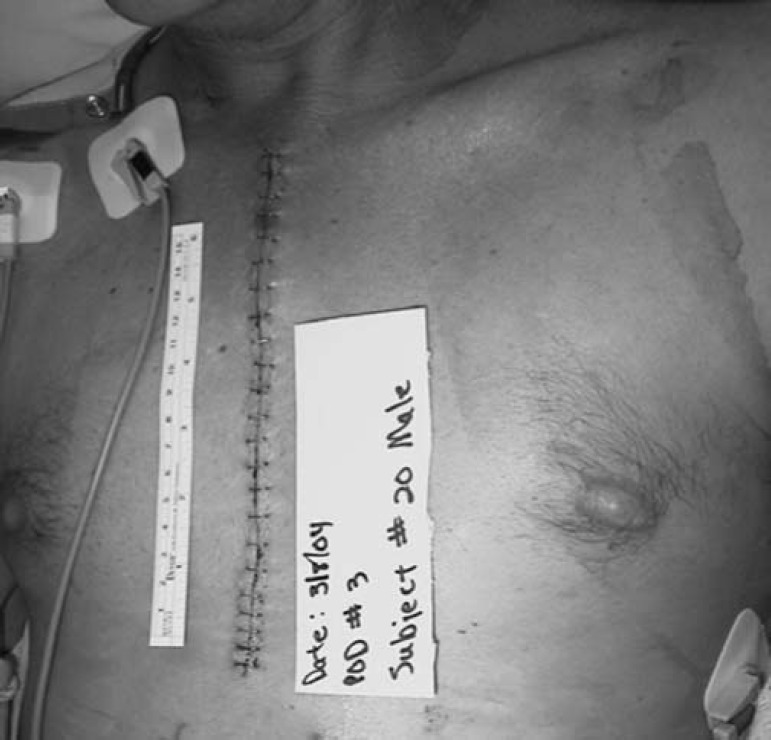
Postoperative day 3 chest incision, experimental group.
Figure 4.
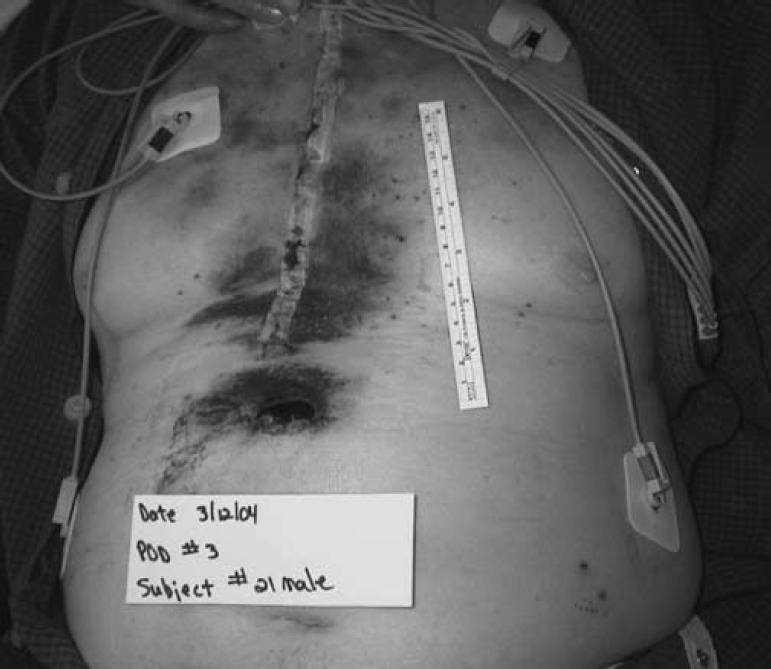
Postoperative day 3 chest incision, control group.
Statistical results indicated that the mean post ADP platelet count was significantly higher (M = 532.53, SD = 119.15) than the mean baseline ADP platelet count (M = 122.53, SD = 73.85),
CONCLUSIONS
In this pilot study, we found support that chest and leg pain were decreased in the experimental group, and we observed that the experimental group had less bruising, although not statistically substantiated. There were several limitations that challenged our statistical conclusions, such as a small sample size for between group analyses; however, our sample size was adequate for within group analyses (14). In addition, the control group platelet indices were not measured postoperatively, thereby preventing a group comparison and we question whether there could be a difference detected between groups. All data collected from one institution limit generalizability.
One cannot ignore the moderate effect sizes obtained through data analyses; however, power needs to be increased to determine if a treatment effect with Autologous Platelet Gel does affect patient healing and recovery post cardiopulmonary bypass surgery. Our results warrant further investigations including long-term/longitudinal follow-up.
ACKNOWLEDGMENTS
We acknowledge the support and input from Timothy Dickinson, MS, Director, Research and Development, FMC EA; data collection by Patricia Rymer, CCT, FMC EA, Wesley Medical Center; Tanya Winter, RN, BSN, Wichita Surgical Specialists; Nancy McClure, RN, Wichita Surgical Specialists; and Cindy Hagerty, RN, BSN, RN, Mid America Surgical Specialists.
REFERENCES
- 1.Oz MC, Jeevanandam V, Smith CR, Williams MR, Kaynar AM, Frank RA, Mosca R, Reiss RF, Rose EA.. Autologous fibrin glue from intraoperatively collected platelet-rich plasma. Ann Thorac Surg. 1992;53:530–1. [DOI] [PubMed] [Google Scholar]
- 2.Tawes RL, Sydorak GR, DuVall TB.. Autologous fibrin glue: the last step in operative hemostasis. Am J Surg. 1994;168:120–2. [DOI] [PubMed] [Google Scholar]
- 3.Man D, Plosker H, Winland-Brown JE, Saltz R.. The use of autologous platelet-rich plasma (platelet gel) and autologous platelet-poor plasma (fibrin glue) in cosmetic surgery. Plast Reconstr Surg. 2001;107:238–9. [DOI] [PubMed] [Google Scholar]
- 4.Powell DM, Chang E, Farriow EH.. Recovery from deep-plane rhytidectomy following unilateral wound treatment with autologous platelet gel: a pilot study. Arch Facial Plast Surg. 2001;3:245–50. [DOI] [PubMed] [Google Scholar]
- 5.Bhanot S, Alex JC.. Current applications of platelet gels in facial plastic surgery. Facial Plast Surg. 2002;18:27–33. [DOI] [PubMed] [Google Scholar]
- 6.Robiony M, Polini F, Costa F, Politi M.. Osteogenesis distraction and platelet-rich plasma for bone restoration of the severe atrophic mandible: Prelimary results. J Oral Maxillofac Surg. 2002;60:630–5. [DOI] [PubMed] [Google Scholar]
- 7.Marx R, Carlson E, Eichstaedt R, Schimmele S, Strauss J, Goergeff K.. Platelet-derived growth factor enhancement for bone grafts. Oral Radiol Endod. 1998;85:638–46. [DOI] [PubMed] [Google Scholar]
- 8.Kim SG, Chung CH, Kim YK, Park JC, Li SC.. Use of particulate dentin-plaster of Paris combination with/without platelet-rich plasma in the treatment of bone defects around implants. Int J Oral Maxillofac Implants. 2002;107:238–9. [PubMed] [Google Scholar]
- 9.Petrungara PS.. The use of platelet rich plasma with growth factors (autologous platelet gel) to enhance hard and soft tissue healing and maturation in the reconstruction of the maxillary pneumatized sinus. Contemp Periodontics Implantol. 2001;1:2. [Google Scholar]
- 10.Rosenthal R, Rosnow RL.. Essentials of Behavioral Research. Boston: McGraw-Hill; 1991. [Google Scholar]
- 11.Mangram AJ, Horan TC, Pearson ML, Silver LC, Jarvis WR.. Guideline for prevention of surgical site infection, 1999. Hospital Infection Control Practices Advisory Committee. Infect Control Hosp Epidemiol. 1999;20:250–78; quiz 279–80. [DOI] [PubMed] [Google Scholar]
- 12.Fresenius Medical Care Extracorporeal Autologous Platelet Gel Training Module. 2003;41. [Google Scholar]
- 13.Medtronic Magellan Autologous Platelet Separator System Instructions for Use.
- 14.Green SB, Salkind NJ, Akey TM.. Using SPSS for Windows. Upper Saddle River, NJ: Prentice Hall; 2000. [Google Scholar]
- 15.Cohen J.. A power primer. Psychol Bull. 1992;112:155–9. [DOI] [PubMed] [Google Scholar]


