Abstract:
Pediatric patients undergoing surgical correction of congenital heart diseases using cardiopulmonary bypass (CPB) are subjected to hypothermia. Core temperature is cooled down to 26–28°C during CPB. Postoperative hypothermia in these patients remains a source of long-intensive care unit (ICU) stay. Therefore, this study was performed to build a rewarming strategy aiming to improve the cardiac performance, minimize the early after-drop in both core and foot temperatures, and to achieve early achievement of homeostasis. Thirty pediatric patients of acyanotic congenital heart diseases were randomly allocated into one of three equal groups of 10. Group I was kept at 3°C between nasopharyngeal and heater-cooler unit water temperatures during rewarming whereas group II and group III were kept at 5°C and 7°C, respectively. The following parameters were measured: 1) cardiac performance (cardiac index and peak velocity); 2) cumulative amrinone consumption, blood lactate levels, and total body oxygen consumption; 3) intraoperative and postoperative peak and trough core and foot temperatures; and 4) time to extubation and ICU stay. Group I patients showed statistically significant increase in cardiac index and peak velocity compared with groups II and III, at p < 0.05 and p < 0.025, respectively. Statistically, the consumption of amrinone was significantly decreased in group I compared with groups II and III, with p < 0.005 and p < 0.0005, respectively, at 6 hours postoperatively. Group I showed an insignificant increase in blood lactate level, where groups II and III showed significant increases compared with controls (p < 0.001 at 6 hours postoperatively). Intraoperatively, both trough core and peak foot temperatures of group I patients statistically were significantly higher than in group III patients at p < 0.0005 and p < 0.05, respectively. The same applies in the ICU as regards to the time to core temperature (p < 0.005) and the rate of foot warming (p < 0.01). It was found that a difference of 3°C (group I) between nasopharyngeal and heater-cooler unit water temperatures during rewarming demonstrated the best outcome compared with 5°C and 7°C differences (groups II and III, respectively). This outcome was obvious in the following parameters: 1) the best cardiac performance (cardiac index and peak velocity); 2) the lowest values of cumulative amrinone consumption and blood lactate level; 3) the least after-drop in both core and foot temperatures; and 4) achievement of early homeostasis, shortest ICU stays, and conservation of the ICU resources.
Keywords: cardiopulmonary bypass, slow rewarming, amrinone, blood lactate
The ability to establish and maintain a core body temperature is one of many physiologic adaptations that must occur for humans to survive. The significance of thermal regulation for neonates was appreciated as early as the twentieth century, when Budin noted a significant difference in neonatal mortality rates among infants with different body temperatures (1). Thermoregulatory vasoconstriction is comparably impaired in infants and children given halothane or isofurane (2). Four mechanisms exist by which heat is lost in pediatrics, namely, conduction, radiation, convection, and evaporation, whereas only two mechanisms for heat production exist, namely nonshivering thermogenesis and shivering thermogenesis.
Nonshivering thermoregulatory defense fails to increase the metabolic rate in infants who are anaesthetized with propofol (3). Hypothermia remains a common problem because of its deleterious hemodynamic, hemostatic, immune, and metabolic effects (4). Patients undergoing cardiac surgery often are cooled to nasopharyngeal temperatures of 26–28°C and, in pediatric patients, this temperature may even decrease should conditions necessitate deep or profound hypothermia with circulatory arrest. Although the nasopharyngeal temperature is restored to prebypass levels at the end of cardiopulmonary bypass (CPB), a considerable mass of peripheral tissues remain at subnormal temperatures subsequent to the redistribution of heat from the core to the periphery, which causes a decrease in nasopharyngeal temperature ranging from 1 to 3°C, termed “after-drop” (5). This post-bypass hypothermia can be ameliorated in adults by vasodilation with nitroprusside or prolonged rewarming (6). Therefore, CPB is associated with large thermal perturbations, both deliberate and unintentional, that have significant clinical consequences (7). The cardiac index and peak velocity are important hemodynamic parameters used to determine the cardiac performance and are measured using Doppler ultrasound device.
Amrinone is a bipyridine derivative. It acts as a selective phosphodiesterase III inhibitor and is a noncatecholamine and nonglycoside that exerts a competitive inhibitory action on an isoenzyme fraction of PDE, leading to an increased intracellular concentration of cyclic adenosine monophosphate in the myocardium and vascular smooth muscle, which results in the stimulation of protein kinases; therefore, phosphorylating substances are responsible for the inward movement of calcium ions (8). As a result, amrinone produces a dose-dependent positive inotropic and vasodilator effects, manifesting as increased cardiac output and decreased left ventricular end-diastolic pressure. Amrinone has a wide therapeutic index of approximately 100:1 (9) and provides a higher cardiac output and more favorable oxygen dynamic than the combination of dopamine and nitroglycerin (10).
Lactate is a marker of anaerobic metabolism, and tissue oxygen-deficient lactic acidosis indicates an imbalance between the metabolic needs of the tissue and their oxygen supply. Measuring arterial lactate concentration is a prompt, easy, and relatively noninvasive way to estimate tissue oxygen metabolism (11). Elevated serum lactate levels have been correlated with rates of mortality (12). Studies that investigate the role of serum lactate in clinical entities such as shock, extracorporeal life support, myocardial infarction, and trauma (13,14) have shown a strong correlation between high blood lactate levels and an increased risk of morbidity and mortality. An absolute lactate level or range that correlates with morbidity or mortality has not been defined, although the peak lactate level may indicate low cardiac output or reduced tissue oxygen extraction. The change in lactate level over time may be an acceptable marker of the response to therapeutic interventions and subsequent outcome (15).
The aim of this study was to evaluate three different rewarming strategies in pediatric patients with congenital acyanotic heart diseases who were subjected to total surgical correction using CPB and the impact of these strategies on the total inotropic support, blood lactate values, and the after-drop in body temperatures in the immediate first 6 hours postoperatively.
MATERIALS AND METHODS
This study was conducted at Abul-Reish Pediatric University Hospital at the Cardiothoracic Theatre and intensive care unit (ICU) after the approval of the department of Anesthesia, Faculty of Medicine Cairo University and the local Ethics and Research Committee. This study lasted 18 months (January 2002 to June 2003). Informed consent was obtained from either the parents or guardians of each pediatric patient that was included in this study. This study entailed 30 pediatric patients with congenital acyanotic heart diseases who were subjected to surgical cardiac correction. Patients were randomly and equally separated into three groups of 10 patients each. The CPB prime was warmed up to patient core temperature to minimize the temperature shift at the start of CPB. The core temperature was monitored using a nasopharyngeal temperature probe (Space Labs Medical, Inc. Monitor, Redmond, WA; model no. 90385; serial no. 385-217779) inserted to a distance of 2–3 cm in the nasopharynx.
The cooling phase was started gradually with temperature differences not more than 7°C between nasopharyngeal and the heater-cooler unit water. The cooling time count was initiated at the start of cooling using a heater-cooler unit 3–5 minutes after beginning of CPB and ended when core temperature reached 26–28°C. Group I was subjected to only a 3°C temperature difference between the nasopharyngeal and both the heater-cooler unit water temperatures and the thermo-blanket during the rewarming phase of CPB; group II was subjected to 5°C temperature differences and group III to 7°C temperature differences. This difference was achieved by gradually increasing the heater-cooler unit water temperature as guided by nasopharyngeal temperature. CPB was terminated when the nasopharyngeal temperature reached 37.5°C, and the thermo-blanket was kept at temperature of 38°C until the end of operation. The rewarming time count ended with the termination of CPB. The blood (in form of washed red blood cells) added to CPB circuit to achieve the hemoglobin not less than 10 g/dL. The operating room temperature was elevated gradually during rewarming to 30°C by the end of operation.
Included in this study were patients of both sexes who were within the age range of 6 months to 3 years and who were at least in the 50th percentile of the ideal growth curve. Patients with evidence of hepatic or renal impairment either clinically or biochemically, history of bronchial asthma or chest infection, patients on anti-failure medications, patients with gastrointestinal disturbances, leukocytosis or fever, were excluded. For their anesthetic management, all patients received a 1 M premedicant in the form of ketamine 2 mg/kg + midazolam 0.2 mg/kg + atropine 0.02 mg/kg 15 minutes before admission to the operating theater and thus resulted in a calm infant with no secretions or saliva. In the operating theater, ECC lead II, pulse oximeter, and a noninvasive blood pressure monitor were applied. A thermo-blanket was used for warming and set at 38°C.
A peripheral intravenous (IV) line was inserted and cephotaxime 50 mg/kg was given slowly. This infusion was followed by fentanyl 3 μg/kg and ketamine 1 mg/kg IV. Endotracheal intubation was facilitated with pancuronium 0.l mg/kg. Ventilation then was adjusted to maintain both normocarbia and normoxia as justified by serial blood gases analysis. Invasive blood pressure monitoring was achieved via a 22-G cannula that was inserted into the radial artery of the nondominant hand. Central venous cannulation was achieved by placing double lumen catheters 4- or 5-Fr size, using the Seldinger’s technique, into the right internal jugular vein. Temperature was continuously monitored both centrally (nasopharyngeally) and peripherally (big toe of left foot), and urine output was monitored via a urinary catheter attached to the urine bag. An initial activated clotting time (ACT) value was taken as baseline.
Anesthesia was maintained using O2, isoflurane, and fentanyl to ensure adequate levels of both hypnosis and analgesia. Extra muscle relaxation was provided with pancuronium in boluses of 0.03 mg/kg IV. Heparin in a dose of 400 IU/kg IV was given and followed in 8 minutes by ACT estimation. Blood gases were taken as frequently as the clinical situation necessitated. A hollow fiber oxygenator (CAPIOX SX10R hollow fiber oxygenator, no. 050-125-000; TERUMO Corp., Tokyo, Japan) was used as a part of the CPB set at a nonpulsatile flow of 100–150 mL/kg/min (Roller pump, 10S1316; Stöckert S.N., GMBH, Germany). The heater-cooler unit used also was from Stöckert. Cold potassium rich-cardioplegia (30 mEq/L) with blood-crystalloid 1:2 and NaHCO3 was used in this study; the dose was 20 mL/kg, which was followed by 10 mL/kg at 20- to 30-minute intervals. At the end of CPB, a reversal of heparin’s effects was achieved with protamine sulfate in a dose of 1 mg to each 100 IU heparin given. Inotropic support (amrinone) was initiated at core temperature of 35°C and, before discontinuation of the CPB circuit, all patients received a slow IV bolus of 0.75 mg/kg; then, IV infusion was set and the dosage was manipulated to the hemodynamic response. Calculation of amrinone cumulative doses (per kilogram of body weight) started at the end of CPB. The amrinone cumulative doses represent the support better than the actual dose at a given time point.
Blood lactate was measured using the STAT PROFILE PLUS 9 ANALYZER (Nova Biomedical Corp., Waltham, MA) with a measurement range of 0.3–20.0 mmol/L and with an error of ±0.03%. The sample volume required is 200 μL.
At 6 hours postoperatively, cardiac index and peak velocity were measured using a transesophageal Doppler ultrasound device (CARDIO Q monitor, Model no. 9051-6901; Deltex TM Medical Limited, Chichester, UK). The principle states “the perceived frequency of sound or light waves emitted by or reflected from a moving object alters proportionately to the relative velocity between the object and the receiver” (16). With CARDIO Q monitor, a stationary esophageal probe directs a continuous 4-MHz beam of ultrasound waves towards the blood flowing in the descending aorta. The shift in the frequency of the reflected ultrasound waves caused by the moving blood cells is translated by the CARDIO Q monitor into a real time display of the velocity of the blood against time. Analysis of this waveform allows information on a range of cardiac parameter to be derived (17).
Also at 6 hours postoperatively, the total body oxygen consumption was calculated according the following equation:
where Qt is cardiac output, CaO2 arterial is the O2 content, and CvO2 is the venous O2 content. O2 content is summarized in two ways: 1) the physical form: α × PaO2, where α is the solubility coefficient for O2 (0.003 mL/dL/mmHg) and Pa O2 is the O2 partial pressure; and 2) the chemical form: Hb × SO2 × 1.31 mL O2, where Hb is the patient hemoglobin, SO2 is the O2 saturation, and 1.31 mL is the O2 carried in 1 g of hemoglobin at 100% SO2. The significance of total body oxygen consumption differences between the three groups calculated compared with each other.
The extubation was done when patients achieved the accepted parameters according to our standard ICU protocol.
The following variables were measured or calculated in this study:
Patients’ characteristics (Table 1).
Cardiac index and peak velocity at 6 hours postoperatively (Table 2).
Cumulative amrinone consumption (Figure 1).
Blood lactate levels (Figure 2).
Total body oxygen consumption at 6 hours postoperatively.
Intraopertive and postoperative data and temperature measurements (Tables 3 and 4; Figures 3 and 4).
Table 1.
Patients’ characteristics.
| Group I | Group II | Group III | |
|---|---|---|---|
| Sex (M/F) | 6/4 | 5/5 | 4/6 |
| Age (months) | 16.9 ± 5.53 | 14.6 ± 6.5 | 15.4 ± 7.63 |
| Weight (kg) | 10.2 ± 1.55 | 9.8 ± 2.1 | 10.1 ± 3 |
| Height (m) | 0.75 ± 0.05 | 0.73 ± 0.08 | 0.74 ± 0.09 |
| BSA (m2) | 0.5 ± 0.06 | 0.48 ± 0.08 | 0.49 ± 0.1 |
| BMI (kg/m2) | 13.65 ± 1.66 | 13.04 ± 1.79 | 13.42 ± 2.48 |
| Type of surgery (n): | |||
| ASD | 2 | 3 | 2 |
| VSD | 5 | 4 | 6 |
| VSD with PS | 3 | 3 | 2 |
BSA, body surface area; BMI, body mass index; ASD, atrial septal defect; VSD, ventricular septal defect; PS, pulmonary stenosis.
Table 2.
Cardiac index and peak velocity at 6 hours postoperation.
| Group I | Group II | Group III | |
|---|---|---|---|
| Cardiac index (l/min/m2) | 3.16 ± 0.28 | 2.87 ± 0.32* | 2.8 ± 0.34† |
| Peak velocity (cm/sec) | 105 ± 6.9 | 97.2 ± 10* | 94.7 ± 12† |
As compared with Group I: *p < 0.05; †p < 0.025.
Figure 1.
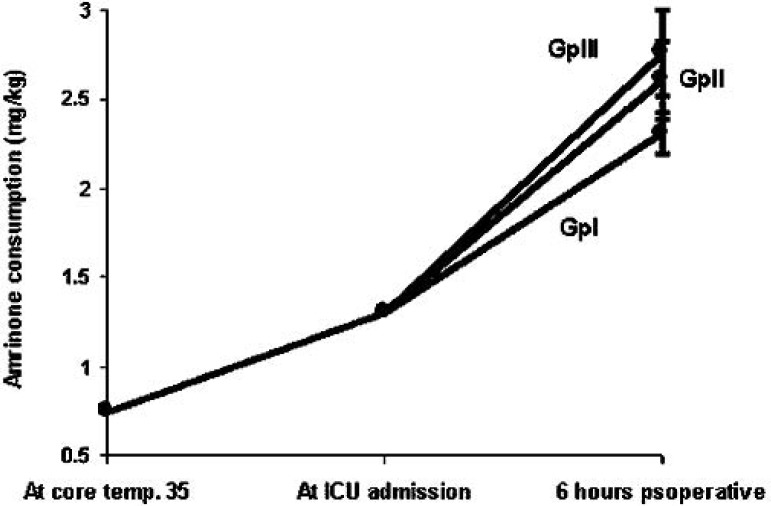
Cumulative amrinone consumption in groups I, II, and III.
Figure 2.
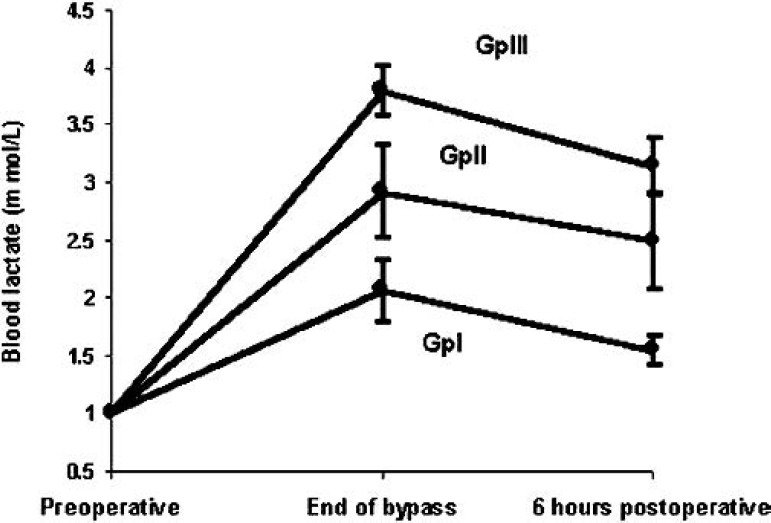
Blood lactate levels in groups I, II, and III.
Table 3.
Intraoperative data and temperature measurements.
| Group I | Group II | Group III | |
|---|---|---|---|
| CPB time (min) | 126.8 ± 7.9 | 110.8 ± 8.28† | 98.5 ± 9.18†b |
| Aortic cross clamp time (min) | 38.1 ± 8.31 | 42.2 ± 7.96 | 40.8 ± 9.63 |
| Cold core temperature (°C) | 26.3 ± 2.46 | 25.8 ± 3.13 | 26.1 ± 2.78 |
| Cooling time (min) | 51.3 ± 4.99 | 50.3 ± 6.13 | 53.1 ± 7.39 |
| Rewarming time (min) | 75.5 ± 4.33 | 60.5 ± 4.95† | 45.4 ± 3.72†c |
| Rate of core rewarming (°C/min) | 0.196 ± 0.016 | 0.248 ± 0.023† | 0.34 ± 0.027†c |
| Core temperature (°C): | |||
| Peak | 37.6 ± 0.53 | 37.3 ± 0.68 | 37.5 ± 0.62 |
| Trough | 36.3 ± 0.64 | 36.0 ± 0.51 | 35.6 ± 0.48†a |
| Foot temperature (°C): | |||
| Peak | 31.3 ± 1.92 | 30.8 ± 1.74 | 29.7 ± 2.06* |
| Trough | 29.6 ± 2.01 | 29.2 ± 1.97 | 28.1 ± 2.14 |
CPB, cardiopulmonary bypass. As compared to group I: *p < 0.05, †p < 0.0005; As compared Group III to Group II: ap < 0.05: bp < 0.005; cp < 0.0005.
Table 4.
Postoperative data and temperature measurements.
| Group I | Group II | Group III | |
|---|---|---|---|
| Time to core temperature 37°C (°C/h) | 2.2 ± 1.25 | 3.1 ± 1.37 | 4.6 ± 1.98§a |
| Rate of core warming (°C/h) | 0.46 ± 0.18 | 0.42 ± 0.16 | 0.44 ± 0.19 |
| Rate of foot warming (°C/h) | 0.38 ± 0.09 | 0.31 ± 0.07* | 0.28 ± 0.08‡ |
| Time to extubation (h) | 4.6 ± 2.8 | 6.8 ± 2.61* | 7.4 ± 3.12† |
| ICU stay (h) | 28.4 ± 12.14 | 35.1 ± 11.36 | 42.8 ± 14.68† |
ICU, intensive care unit; As compared to Group 1: *p < 0.05, †p < 0.025, ‡p < 0.01, §p < 0.005; As compared Group III to Group II: ap < 0.05.
Figure 3.

Core temperature in typical patients in groups I, II, and III from the end of CPB (0 minutes) to 6 hours postoperatively.
Figure 4.

Foot temperature in typical patients in groups I, II, and III from the end of CPB (0 minutes) to 6 hours postoperatively.
All variables are expressed as mean (SD). One-way and two-way analysis of variance were used as appropriate to detect statistical differences. A p < 0.05 was considered significant.
RESULTS
There were no statistically significant differences in sex, age, weight, height, body surface area, or in the body mass index in the patients (Table 1). Regarding the cardiac index and peak velocity, at 6 hours postoperatively there was a statistically significant increase in group I compared with groups II and III at p < 0.05 and p < 0.025, respectively (Table 2). Cumulative amrinone consumption (mg/kg) showed no statistical significance upon arrival at the ICU among groups. However, at 6 hours postoperatively, group I consumed 2.31 ± 0.11 mg/kg versus 2.61 ± 0.22 mg/kg for group II at p < 0.005 versus 2.76 ± 0.24 mg/kg for group III at p < 0.0005. No statistically significant differences existed between groups II and III (Figure 1).
Regarding blood lactate (mmol/L), there were no statistically significant differences in preoperative control values in between groups. At the end of CPB blood lactate was 2.065 ± 0.26, 2.92 ± 0.40, and 3.8 ± 0.22 for groups I, II, and III, respectively, with significant increase at p < 0.001 compared to controls. At 6 hours postoperatively, blood lactate was 1.55 ± 0.12, 2.5 ± 0.41, and 3.15 ± 0.25 for groups I, II, and, III, respectively, with significant increase in groups II and III at p < 0.001 compared with controls but with a statistically insignificant increase in group I compared with controls. Among groups, p < 0.0001 existed in between group I and groups II and III, but there was no statistically significant differences between groups II and III at both the end of CPB and at 6 hours postoperatively (Figure 2).
Total body oxygen consumption calculations showed an insignificant increase in group I (6.31 ± 0.37 mL/kg) compared with groups II and III (5.95 ± 0.5 and 5.8 ± 0.52 mL/kg, respectively). Regarding time of CPB, there was a significant increase in group I compared with groups II and III at p < 0.0005 and in group II compared with group III at p < 0.005. Regarding the rewarming time and rate of core rewarming, there was a significant increase in group I compared with groups II and III at p < 0.0005 and in group II compared with group III at p < 0.0005. However, there were no statistically significant differences among the groups regarding aortic cross clamp time, cold core temperature, or the cooling time (Table 3).
In the operating theater, there were no statistically significant differences among groups as regards to the peak core temperature or the trough foot temperature, whereas statistically significant differences existed in the trough core temperature between groups I and III at p < 0.0005 and in between group II and III at p < 0.05; regarding peak foot temperature, statistically significant differences existed only between groups I and III at p < 0.05 (Table 3).
In the ICU, the time to core temperature (37°C) was the highest in group III compared with groups I and II, at p < 0.005 at p < 0.05, respectively. The rate of foot warming (°C/h) was significantly increased in group I compared with groups II and III at p < 0.05 and p < 0.01.There was no statistically significant differences in between groups regarding the rate of core warming (°C/h) (Table 4). Typical core and foot temperatures in different groups of patients were shown in Figures 3 and 4.
Regarding the time to extubation, group I patients showed a statistically significant shorter time compared with groups II and III, at p < 0.05 and p < 0.025, respectively. In addition, group I showed statistically a significantly shorter ICU stay compared with group III at p < 0.025 (Table 4). There were no statistical significant differences between groups regarding oher clinical data observed in ICU, such as blood loss, total IV fluids, and the occurrence of postoperative complications. The hemodynamics, arterial blood gases, kidney functions, urine output, liver functions, hemoglobin, hematocrit, blood glucose, and blood chemistry were kept at or near-normal values throughout the study period.
DISCUSSION
Hypothermic CPB for the surgical correction of congenital heart diseases is routine in our institution. Rewarming at the end of CPB is of utmost importance to maintain homeostasis. This study dealt with three different rewarming techniques that aimed reaching the best outcome regarding the cardiac performance in terms of cardiac index and peak velocity, total inotropic support consumption, blood lactate, and temperature after-changes in both the operating theater and the 1CU.
This study showed that slow rewarming resulted in good peripheral tissue perfusion in group I compared with groups II and III and resulted in better cardiac performance. This improvement was shown by better cardiac index and peak velocity in group I compared with the other groups.
The cumulative amrinone consumption concept was used because this presents better the support than the actual dose at a given time point, which can change rapidly. Statistically significant differences among the groups existed only at 6 hours postoperatively. The hemodynamic effects of amrinone in pediatric patients during CPB and postoperatively were studied; amrinone proved useful in producing enough of a vasodilating effect without major side effects (18). This was also proven by a more recent study comparing the efficacy and safety of amrinone versus a combination of dopamine and nitroglycerin in neonates after reconstructive surgery for transposition of great arteries. The authors showed that amrinone provides a higher cardiac output and more favorable oxygen dynamics than a combination of dopamine and nitroglycerin (10). The pharmacokinetics of amrinone showed a slower elimination rate in neonates than in infants, mainly because of the immature renal function in neonates (19); however, our patient population was older and none failed to show any renal dysfunction throughout the study period.
Blood lactate measurements are being used clinically as an indicator of circulatory impairment and the overall state of oxygenation of patients in critical care (15). In this study, only group I patients had normal blood lactate levels at both end of CPB and at 6 hours postoperatively, whereas group II and group III patients had mild-tomoderate lactataemia throughout the study period. Munoz et al. (20) evaluated the change in lactate level during CPB and the possible predictive value in identifying patients at high risk of morbidity and mortality after surgery for congenital heart disease. They found that the largest increment in lactate level occurred during CPB, which decreased between the postbypass period and on admission to the 1CU. These highest values were in patients who had circulatory arrest at all time points. They concluded that hyperlactataemia during CPB or persisting in the postoperative ICU is an early indicator of postoperative morbidity and mortality, with an optimal sensitivity of 82% and specificity of 80% (20).
A more recent study by Shime et al. (11) investigated the perioperative blood lactate levels in pediatric heart surgery on a large series of 112 patients aged 5 days to 17 years with different cardiac pathology to predict the severity of an outcome. They found significantly and sustained increases in lactate at 16 hours postoperatively (first day) in neonates subjected to the greatest stresses of profound hypothermia and longer CPB in patients with longer ICU stays and in those who died later. They came to the conclusion that, hyperlactataemia >2.2 mmol/L during the first day predicted death with a sensitivity of 82% and a specificity of 72%. The measurement of early postoperative lactate levels, reflecting postoperative ability to eliminate intraoperative hyperlactaemia, is a better way of assessing the severity of a pediatric patient’s condition after cardiac surgery. The study conducted by Li et al. (21) showed no significant differences in oxygen consumption, oxygen delivery, and oxygen extraction between the group with lactate levels between 2 and 3 mmol/L and the group with normal blood lactate levels at the end of surgery and two hours then after. They concluded that mild lactataemia is common and does not appear to reflect oxygen delivery or oxygen consumption or a more complicated recovery.
An important entity in this study was the evaluation of both core and foot temperatures and the after-drop at these sites in both the operating theater and the ICU by using three rewarming strategies as mentioned in the methodology. To our best knowledge, this study is the first to investigate the best rewarming strategy. The best results were shown in group I patients because they had the least after-drop in both core and foot temperature, the lowest values in blood lactate, better cardiac index and peak velocity, and the least cumulative amrinone support throughout the study period. Early homeostasis was shown in group I patients, as evidenced by earlier time to extubation and consequently ICU stay. In a study on adult patients submitted to coronary revascularization surgery, only the rate of foot warming under enoximone showed better results than in the control group, and the mean time to core temperature (37°C) was 4.4 hours (22). A more recent study on adults investigating the determinants of core temperature at the time of admission to the ICU aftercardiac surgery found that the rewarming endpoint at the time of separation from CPB was the best and only significant predictor of core temperature (p = 0.004) and that weight, height, body habits, and nitroprusside administration had no statistical significance. Yet, a warm ambient temperature may be beneficial after separation from CPB (23).
A former comparative study was undertaken to evaluate the differences in body temperatures after CPB in pediatric versus adult patients. The authors studied the after-drop in both the nasopharyngeal and the rectal temperatures 1 hour after termination of CPB. They found that the after-drop occurred to a lesser degree in the pediatric patients, which was attributed to the more-efficient supplying of external heat in pediatric patients, in whom there is a larger body surface area to weight ratio. However, the rewarming technique was identical in both groups of patients (24).
This study involved 30 pediatric patients undergoing surgical corrections of homogenous distribution. Our aim in the future is to investigate the rewarming rate in different heart pathologies, in neonates and younger infants, and in those with failure to thrive, in addition to the possible influence of the rewarming rate on different anesthetic techniques as well as on neurocognitive outcome.
REFERENCES
- 1.Bisonnette B, Davis PJ.. Thermal regulation-physiology and perioperative management in infants and children. In: Ed Motoyama EK, Avis PJ eds. Smith’s Anesthesia for Infants and Children. St. Louis: Mosby-Year Book; 1996;139–158. [Google Scholar]
- 2.Bissonnette B, Sessler DI.. Thermoregulatory thresholds for vasoconstriction in pediatric patients anesthetized with halothane or isoflurane and caudal bupivacaine. Anesthesiology. 1992;76:387. [DOI] [PubMed] [Google Scholar]
- 3.Plattner O, Semsroth M, Sessler DI, Papousek A, Klasen C, Wagner O.. Lack of nonshivering thermogenesis in infants anesthetized with fentanyl and propofol. Anesthesiology. 1997;86:772–7. [DOI] [PubMed] [Google Scholar]
- 4.Spaniol SE, Bond EF, Brengelmann GL, Savage M, Pozos RS.. Shivering following cardiac surgery: predictive factors, consequences and characteristics. Am J Crit Care. 1994;3:356–67. [PubMed] [Google Scholar]
- 5.Ramsay JG, Ralley FE, Whalley DG, Delli Colli P, Wynands JE.. Site of temperature monitoring and prediction of after drop after open heart surgery. Can Anaesth Soc J. 1985;32:607–12. [DOI] [PubMed] [Google Scholar]
- 6.Joachimsson PO, Nystrom SO, Tyden H.. Postoperative ventilatory and circulatory effects of heating after aortocoronary bypass surgery. Extended rewanning during cardiopulmonary bypass and postoperative radiant heat supply. Acta Anaesthesiol Scand. 1987;31:543–9. [DOI] [PubMed] [Google Scholar]
- 7.Rajek A, Lenhardt R, Sessler DI, et al. Tissue heat content and distribution during and after cardiopulmonary bypass at 31°C and 27°C. Anesthesiology. 1998;88:1511–8. [DOI] [PubMed] [Google Scholar]
- 8.Skoyles JR, Sherry KM.. Pharmacology, mechanisms of action and uses of selective phosphodiestrase inhibitors. Br J Anaesth. 1992;68:293–302. [DOI] [PubMed] [Google Scholar]
- 9.Stoelting RK.. Renin, plasma kinins and serotonin. In: Pharmacology & Physiology in Anesthetic Practice. 3rd ed. New York: Lippincott-Raven; 1999:398–408. [Google Scholar]
- 10.Laitinen P, Happonen JM, Sairanen H, Peltola K, Rautiainen P.. Amrinone versus dopamine and nitroglycerin in neonates after arterial switch operation for transposition of the great arteries. J Cardiothorac Vasc Anesth. 1999;13:186–90. [DOI] [PubMed] [Google Scholar]
- 11.Shime N, Kageyama K, Ashida H, Ueda M, Kitamura Y, Tanaka Y.. Periopcralivc assessment of blood lactate levels in pediatric heart surgery. Masui. 2001;50:752–7. [PubMed] [Google Scholar]
- 12.Astiz ME, Rackow EC.. Assessing perfusion failure during circulatory shock. Crit Care Clin. 1993;9:299–312. [PubMed] [Google Scholar]
- 13.Cowan BN, Burns HJ, Boyle P, Ledingham IM.. The relative prognostic value of lactate and haemodynamic measurements in early shock. Anaesthesia. 1984;39:750–5. [DOI] [PubMed] [Google Scholar]
- 14.Grayck EN, Meliones JN, Kern FH, Hansell DR, Ungerleider RM, Greeley WJ.. Elevated serum lactate correlates with intracranial hemorrhage in neonates treated with extracorporeal life support. Pediatrics. 1995;96:914–7. [PubMed] [Google Scholar]
- 15.Toffaletti J.. Elevations in blood lactate: overview of use in critical care. Scand J Clin Lab Invest. 1996;224:10–7. [DOI] [PubMed] [Google Scholar]
- 16.Gan TJ, Horacek A, Maroof M.. Intraoperative volume expansion guided by esophageal Doppler improved postoperative outcome and shorten hospital stay. Anesth Analg. 1999;88:S1–424, S179.10083853 [Google Scholar]
- 17.Tibby SM, Hatherill M, Durward A, Murdoch IA.. Are transoesophageal Doppler parameters a reliable guide to paediatric haemodynamic status and fluid management? Intensive Care Med. 2001;27:201–5. [DOI] [PubMed] [Google Scholar]
- 18.Imai M, Yamaguchi M, Ohashi H, et al. Hemodynamic effects of amrinone in children during cardiopulmonary bypass and postoperative 12 hours. Kyobu Geka. 1997;50:1018–21. [PubMed] [Google Scholar]
- 19.Laitinen P, Ahonen J, Olkkola KT, Peltola K, Rautiainen P, Rasanen J.. Pharmacokinetics of amrinone in neonates and infants. J Cardiothorac Vasc Anesth. 2000;14:378–82. [DOI] [PubMed] [Google Scholar]
- 20.Munoz R, Laussen PC, Palacio G, Zienko L, Piercey G, Wessel DL.. Changes in whole blood lactate levels during cardiopulmonary bypass for surgery for congenital cardiac disease: an early indicator of morbidity and mortality. J Thorac Cardiovasc Surg. 2000;119:155–62. [DOI] [PubMed] [Google Scholar]
- 21.Li J, Schulze-Neick I, Lincoln C, et al. Oxygen consumption after cardiopulmonary bypass surgery in children: determinants and implications. J Thorac Cardiovasc Surg. 2000;119:525–33. [DOI] [PubMed] [Google Scholar]
- 22.Duthie DJ, Woolman PS, Doyle AR.. Enoximone and warming after hypothermic cardiopulmonary bypass. Br J Anaesth. 1995;75:43–6. [DOI] [PubMed] [Google Scholar]
- 23.El-Rahmany HK, Frank SM, Vannier CA, Schneider G, Okasha AS, Bulcao CF.. Determinants of core temperature at the time of admission to intensive care following cardiac surgery. J Clin Anesth. 2000;12:177–83. [DOI] [PubMed] [Google Scholar]
- 24.Huang FY, Wang MJ, Huang HH.. Differences in temperature changes between pediatric and adult patients after cardiopulmonary bypass. J Cardioth Vase Anesth. 1993;7:66–8. [DOI] [PubMed] [Google Scholar]


