Abstract
Background
The islet size distribution in a preparation may contribute to islet transplant outcomes. Larger islets may exhibit poorer therapeutic value and this may be because of oxygen diffusion limitations that worsen in proportion to islet size.
Methods
To test this hypothesis, we studied the impact of islet size index (ISI) and other islet product characteristics on outcomes following islet autotransplant (IAT) in recipients receiving a marginal islet dose (2000–4999 islet equivalents (IEs) per kg body weight from 1/1/2009–6/11/2012 at the University of Minnesota (n=58). ISI was defined as the number of IE divided by the number of islet particles (IPs) in a preparation; an ISI <1 indicates a mean islet diameter that is <150-μm. The primary post-IAT outcome was 6-month insulin use status.
Results
Logistic regression analysis indicated that IPs/kg (p=0.001), IEs/kg (p=0.019), total IPs transplanted (p=0.040) and ISI (p=0.074) were most strongly correlated with the primary outcome. The ISI (mean ± standard error) was lower for recipients achieving insulin independence at 6-months (0.71±0.05) versus those partially (0.83±0.05) or completely (1.00±0.07) insulin-dependent. The combination of islet dose (expressed as units IPs/kg) and ISI exhibited a sensitivity of 75% and specificity of 74% in predicting insulin independence in this population of patients.
Conclusion
IAT recipients of a marginal islet mass were more likely to achieve insulin independence when transplanted with a greater number of smaller islets.
Keywords: islet size index, islet autotransplant, total pancreatectomy, insulin independence
Introduction
Islet size distribution may contribute to outcomes following islet transplantation. Prior to revascularization, islets are non-perfused cell aggregates that rely on diffusion to obtain oxygen and nutrients. Diffusion limitations worsen in proportion to the islet radius, and thus β-cells located near the center of larger islets have the poorest access to the substrates required for insulin secretion (1). This may explain prior findings that larger islets secrete less insulin than smaller islets (2–4), and that smaller islets have greater therapeutic value with intraportal islet allotransplant (3).
Total pancreatectomy (TP) with islet autotransplant (IAT) has become a reasonable therapeutic option for patients suffering from the intractable pain of chronic pancreatitis. Following TP, the pancreas can be processed to retrieve the tissue needed for IAT. TP-IAT enables patients to remove the source of their pain while alleviating the brittle diabetes that would normally accompany removal of the pancreas. When compared with islet allografts, islet autografts have fewer confounding factors that influence graft survival; islet allografts are subjected to longer cold ischemia times, immune rejection and immunosuppressant toxicity (5), all which are believed to contribute to posttransplant islet loss and all of which islet autografts do not experience. This is in part why significantly more islets are required to achieve insulin independence following islet allotransplant (5, 6). Hence, IAT may be a better model for the study of factors like islet size distribution and viability that may influence outcomes in either auto- or allotransplant. Study of these factors via IAT may help interpret outcomes following islet allotransplant.
In this study, we examined the effect of the islet size index (ISI) and other islet product characteristics on outcomes following IAT in recipients of marginal islet doses. ISI was defined as the number of islet equivalents (IEs) divided by the number of islet particles (IPs) in a preparation; an ISI below 1 indicates a mean islet diameter in a preparation that is lower than 150-μm. We hypothesized that the ISI would be predictive of insulin use status post-IAT, and that islet preparations with lower ISI would be associated with lower post-IAT insulin requirements than those with higher ISI.
Results
Patient demographic data are stratified into three cohorts based on insulin use status at 6 months post-IAT (Table 1). Of all patients in this study, 8 (13.8%) were insulin-independent at 6 months post-IAT, whereas 19 (32.8%) were partially insulin-dependent and 31 (53.4%) were completely insulin-dependent. There was improved long-term blood glucose control in recipients with successful IAT versus recipients who required a basal-bolus insulin regimen; there were differences in hemoglobin A1C (HbA1C) between those with insulin independence (p=0.03) or partial insulin dependence (p=0.01) and those with complete insulin dependence, but no differences between those with insulin independence and those with partial insulin dependence (p=0.6). There were no overall differences between cohorts in the age, gender, body mass index (BMI), etiology of pancreatitis, degree of pancreatic fibrosis or duration of pancreatitis. There was an overall difference between cohorts in pre-IAT fasting blood glucose (FBG) (p=0.04), but this did not trend with the primary outcome.
Table 1.
Selected patient characteristics as stratified by primary post-IAT outcome
| Insulin use status at 6 mos. |
||||
|---|---|---|---|---|
| Characteristic | Independence | Partial dependence | Complete dependence | One-way ANOVA or Chi-square testa |
|
| ||||
| Mean (± SE) or n (% of cohort) | p-value | |||
| Patient | 8 (13.8) | 19 (32.8) | 31 (53.4) | - |
| Age (years) | 29.9 (± 4.4) | 37.9 (± 3.1) | 36.6 (± 2.3) | 0.34 |
| Gender | 0.24 | |||
| Female | 7 (87.5) | 17 (89.5) | 22 (71.0) | - |
| BMI | 22.7 (± 2.0) | 25.3 (± 1.3) | 26.5 (± 1.2) | 0.32 |
| Pre-IAT FBG | 90.8 (± 2.9) | 87.4 (± 1.8) | 94.5 (± 1.9) | 0.04* |
| HbA1Cb | 5.8 (± 0.2) | 5.9 (± 0.1) | 6.8 (± 0.3) | 0.01* |
| Etiology of pancreatitis | 0.26 | |||
| Idiopathic | 4 (50.0) | 8 (42.1) | 9 (29.0) | - |
| Pancreas divisum | 1 (12.5) | 5 (26.3) | 7 (22.6) | - |
| Sphincter of Oddi dysfunction | 0 (0) | 5 (26.3) | 4 (12.9) | - |
| Familial/hereditary | 3 (37.5) | 1 (5.3) | 4 (12.9) | - |
| Cystic fibrosis | 0 (0) | 0 (0) | 3 (9.7) | - |
| Alcoholism | 0 (0) | 0 (0) | 2 (6.5) | - |
| Biliary obstruction | 0 (0) | 0 (0) | 2 (6.5) | - |
| Degree of fibrosisc | 5.4 (± 0.7) | 4.9 (± 0.4) | 5.4 (± 0.4) | 0.67 |
| Duration of pancreatitis (years) | 7.6 (± 2.2) | 5.3 (± 0.9) | 8.9 (± 1.5) | 0.34 |
Abbreviations: ANOVA, analysis of variance; HbA1C, hemoglobin A1C; IAT, islet autotransplant; IE, islet equivalent; IP, islet particle; kg, kilogram (recipient body weight); SE, standard error (of the mean); TV, tissue volume;
One-way ANOVA was used for comparisons of continuous variables, whereas the Chi-square test was used for categorical variables;
HbA1C data were available for 7, 19 and 26 patients with insulin independence, partial dependence and complete dependence, respectively;
Based on a relative histologic scoring system, with 10 representing the most severe fibrosis;
p<0.05.
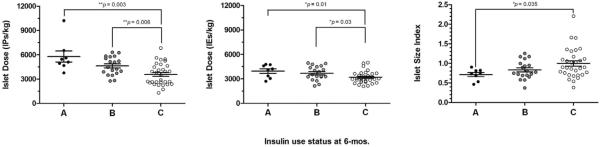
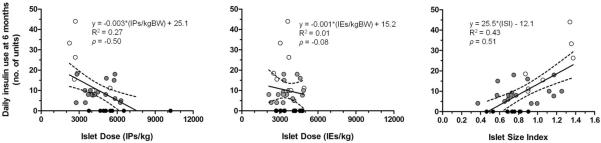
There were no overall differences between cohorts in the total number of IEs transplanted or the total tissue volume (TV) transplanted. However, there were overall differences between cohorts in the islet dose, both IPs/kg (p=0.001) and IEs/kg (p=0.02), the total IPs transplanted (p=0.04), ISI (p=0.07) and proportion of islets >200 μm in diameter (p=0.09). Notably, the ISI was lower for recipients achieving insulin independence at 6 months (0.71±0.05) versus those partially (0.83±0.05) or completely (1.00±0.07) insulin-dependent. Similar trends were observed for other strongly associated parameters (Table 2). There were differences in IPs/kg (p=0.003), IEs/kg (p=0.01) and ISI (p=0.04) when comparing patients achieving insulin independence versus those who were completely insulin-dependent (Figure 1). Lower insulin requirements at 6 months post-IAT were correlated with higher IPs/kg (ρ=−0.50) and a lower ISI (ρ=0.51) (Figure 2). In contrast, IEs/kg exhibited poor correlation with insulin requirements at 6 months post-IAT (ρ=−0.08). When all variables were included in a multivariate logistic regression analysis, only pre-IAT FBG (odds ratio [OR]=0.55, p=0.058) and IPs/kg (OR=2.22, p=0.0005) were significantly associated with insulin independence at 6 months.
Table 2.
Islet product characteristics stratified by primary post-IAT outcome
| Insulin use status at 6 mos. |
||||
|---|---|---|---|---|
| Characteristic | Independence | Partial dependence | Complete dependence | One-way ANOVA or Kruskal-Wallis testa |
|
| ||||
| Mean (± SE) | p-value | |||
| Islet size index | 0.71 (± 0.05) | 0.83 (± 0.05) | 1.00 (± 0.07) | 0.07 |
| Proportion >200 μm (%) | 6.6 (± 1.5) | 8.4 (± 0.8) | 10.3 (± 0.9) | 0.09 |
| IPs/kg | 5,785 (± 692) | 4,624 (± 259) | 2,569 (± 241) | 0.001 ** |
| IEs/kg | 3,947 (± 284) | 3,688 (± 195) | 3,183 (± 133) | 0.02* |
| IPs transplanted | 343,588 (± 45,590) | 315,445 (± 25,316) | 253,804 (± 17,015) | 0.04* |
| IEs transplanted | 239,531 (± 30,306) | 260,876 (± 24,356) | 236,724 (± 14,586) | 0.68 |
| TV transplanted (mL) | 11.1 (± 1.8) | 10.4 (± 1.1) | 8.7 (± 0.9) | 0.31 |
Abbreviations: ANOVA, analysis of variance; IAT, islet autotransplant; IE, islet equivalent; IP, islet particle; kg, kilogram (recipient body weight); SE, standard error (of the mean); TV, tissue volume;
One-way ANOVA was used for comparisons in which the sample data were normally distributed (as confirmed by the D'Agostino-Pearson omnibus normality test), and the Kruskal-Wallis test was used when the sample data were not normally distributed;
p<0.05;
p<0.01.
Figure 1.
Vertical scatter plots illustrating the islet dose (left, in units of number of islet particles [IPs] per kilogram [kg] recipient body weight; middle, in units of number of islet equivalents [IE] per kg) and islet size index (right) for each patient as stratified by insulin use status at 6 months posttransplant. The `A' refers to insulin independence (solid circles), the `B' refers to partial insulin dependence (grey circles), and the `C' refers to complete insulin dependence (unfilled circles), as defined in the Methods section. The horizontal lines and error bars represent the mean values and the standard error from the mean. The Kruskal-Wallis test indicates significant overall differences in the IPs/kg (p=0.001), IEs/kg (p=0.02) and near differences in the islet size index (p=0.07) with respect to the primary categorical outcome. Individual cohorts were directly compared using either the non-paired t test or the Mann-Whitney test (depending on normality of data), and the significant p-values are shown.
Figure 2.
Daily insulin use at 6 months posttransplant versus the islet dose (left, in units of number of islet particles [IPs] per kilogram [kg] recipient body weight; middle, in units of number of islet equivalents [IE] per kg) and islet size index (right, ISI) for each patient, with bivariate linear regression analysis illustrating clear associations for both islet product parameters. The dotted lines indicate the bounds of the 95% confidence interval associated with the linear fit. Correlation analysis yield Spearman's rank correlation coefficients (ρ) that are also shown; IPs/kg (ρ=−0.50) and ISI (ρ=0.51) had good correlation with daily insulin use at 6 months, whereas IEs/kg did not (ρ=−0.08). Solid circles represent patients achieving insulin independence, grey circles represent patients with partial insulin dependence, and unfilled circles represent patients with complete insulin dependence.
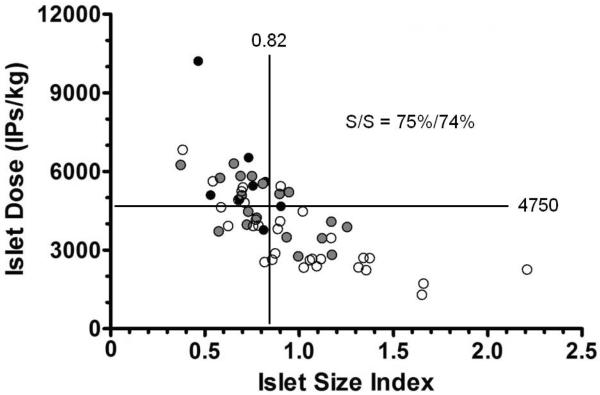
When a threshold islet dose of ≥4750 IPs/kg was tested for its utility in predicting post-IAT insulin independence (vs. insulin dependence [either partial or complete]), it was found to have a sensitivity of 75% and specificity of 68%. When a threshold ISI of ≤0.82 was tested for its predictive utility, it was found to have a sensitivity of 88% and specificity of 54%, indicating that ISI is associated with a lower false negative rate than IPs/kg. The combination of IPs/kg and ISI has a sensitivity of 75% and specificity of 74% for predicting post-IAT insulin independence, improving the false positive rate by 6% when compared to using IPs/kg alone (Figure 3).
Figure 3.
Plot depicting the islet dose (in units of number of islet particles (IPs) per kilogram [kg] recipient body weight) versus islet size index (ISI) for each islet product preparation. Analysis with a threshold islet dose of ≥4750 IPs/kg and a threshold ISI of ≤0.82 indicates that the combination of the two islet product parameters is associated with a sensitivity of 75% and specificity of 74% for predicting insulin independence in this population of patients. Solid circles represent patients achieving insulin independence, grey circles represent patients with partial insulin dependence, and unfilled circles represent patients with complete insulin dependence.
Discussion
When indicating the amount of transplantable tissue in an islet preparation, it is conventional to quantify based on IEs with little regard to the unadjusted number of IPs or distribution in islet size. In this study, we observed that the IPs/kg and the ISI provided additional utility for predicting metabolic outcomes in TP-IAT recipients receiving a marginal islet dose. Both higher IPs/kg and a lower ISI (smaller islets) were associated with a greater likelihood of achieving improved blood glucose control. The islet product parameter that was most strongly associated with insulin independence was islet dose quantified as the number of IPs/kg, indicating that IPs/kg was an even better predictor for insulin independence than IEs/kg. This may reflect the increased susceptibility of the metric of IEs/kg to counter-specific error. Combining ISI with IPs/kg improved the specificity for predicting post-IAT insulin independence. This is important since it is apparent that ISI provides additional information about the islet preparation that is lost when converting IPs to IEs.
We speculate that the differences in graft function and survival between smaller and larger islets is due in part to oxygen and nutrient diffusion limitations, particularly in the early posttransplant period (<10–14 days) during which islet revascularization has not yet occurred (7, 8). This concept could be important over an even longer time frame (9–11). Whatever the time frame, there is evidence suggesting that >50% of islets may be lost in the early posttransplant period (8, 12). Islet doses that are currently transplanted should theoretically be adequate to reverse diabetes in every case since experience with surgical pancreatectomy has indicated that the native pancreas has considerable insulin-secreting reserve, requiring in some cases the removal of >90% of the pancreas to cause overt diabetes (13). If a normal human adult pancreas contains 1 million islets (14), then 100 thousand transplanted islets (or ~1,400 IEs/kg in a 70-kg recipient) should be adequate to prevent diabetes assuming all islets survive and engraft. However, our experience with clinical islet transplantation has shown that the transplanted islet mass far exceeds what would appear to be theoretically necessary, with >5,000 IEs/kg likely needed to achieve insulin independence in IAT (5, 6, 15), and >10,000–13,000 IEs/kg in islet allotransplant (16–19), even with the most potent induction immunotherapy currently-available (20). Islets may be lost due to factors unique to the clinical transplant site (the liver), which is the same site used in both islet auto- and allotransplant.
One of the possible causes for islet loss may be inadequate oxygenation. Of all causes of islet loss following intraportal islet transplant, oxygenation is often overlooked due to the assumption that direct access to the blood stream should be enough to provide the necessary oxygen supply. However, there have been few attempts to confirm if this is true. There have been two studies that directly (21) or indirectly (11) measured the oxygen partial pressure (pO2) near or within intraportally transplanted islets, and both found that the pO2 may be very low (5-10 mm Hg).
We can calculate the pO2 drop (ΔpO2) from the surface to the center of an islet to better appreciate the approximate oxygenation requirements for an islet of a particular size. This can be done by using the Thiele modulus, which is a ratio of the rates of oxygen consumption and diffusion, and can be rearranged to calculate the ΔpO2 for a spherical islet with equal access to the same oxygen supply across its entire surface (best-case scenario):
| [1] |
where OCR is the oxygen consumption rate (mol/cm3/sec), R is the islet radius (cm), and (αD) is the oxygen permeability (mol/cm/mm Hg/sec) in islet tissue. Using actual OCR data normalized to DNA content (OCR/DNA) from the islet preparations of this study (n=25), we can estimate the ΔpO2 in islets of different sizes. The mean OCR/DNA (119.4 nmol/min/mg DNA) can be converted to a mean volumetric OCR (1.2·10−8 mol/cm3/sec) by assuming 1 human IE contains 10.4 ng DNA, which has been validated experimentally (22). Using Equation 1 and our actual data, we estimate the expected ΔpO2 to be about 1, 9 and 33 mm Hg for islet diameters of 50, 150 and 300 µm. Furthermore, oxygenation would worsen in the setting of thrombosis, inflammation or islet clumping. If the studies of Carlsson et al. (21) and Olsson et al. (11) correctly measured the pO2 available to islets in the liver, then all but the smallest islets (<100-μm diameter) would have adequate oxygenation. It is important to note that the required oxygen supply for healthy islet function is higher than the required oxygen supply for islet survival; insulin secretion is significantly affected at local pO2 values of 5-10 mm Hg (1) whereas islet cell survival can occur above the lower critical pO2 value of ~0.1 mm Hg (23).
Some of our study results are corroborated by MacGregor et al. (2), Lehmann et al. (3), and Fujita et al. (4), who each studied the impact of islet size on islet function and survival. MacGregor et al. reported that smaller rat islets were more viable and secreted more insulin in vitro as compared to larger rat islets (2). Furthermore, the authors reported that 1000 IEs transplanted under the kidney capsule in diabetic nude mice ameliorated hyperglycemia in 80% receiving smaller islets versus none receiving larger islets. Lehmann et al. reported similar findings except with human islets (3). The authors presented histological data indicating that the greatest number of pyknotic nuclei were found in the core of larger islets and under hypoxic (versus normoxic) culture conditions. They also reported that IPs/kg and ISI correlated strongly with stimulated C-peptide measurements in recipients of an islet allotransplant (n=7). Most recently, Fujita et al. reported that the amount of insulin secreted per IE was significantly lower for larger human islets as compared to smaller human islets, showing an almost stepwise decrease in insulin secretion with incrementally larger islet fractions (4). These and our studies provide evidence that islet size distribution matters and that smaller islets may be better able to reverse diabetes following intraportal islet transplantation, and it may be because a smaller fraction of the intraislet β-cell mass is exposed to oxygen-limited conditions.
There are several limitations to this study. Firstly, our study population included few patients achieving insulin independence at 6 months post-IAT and may limit the strength of the conclusions. Secondly, though there were not many differences in the demographic profiles between cohorts, this was a retrospective study comparing transplanted preparations with lower versus higher ISI. It may not be possible to conduct a prospective randomized trial since islet processing involves the recipient's own pancreas, and the yield, quality and size of these islets may not be significantly modifiable. We are not advocating that the pancreas be overdigested to produce smaller islets, since the islet yield and quality may be adversely affected, which has been shown with islet allotransplant (24). Thirdly, the mechanism by which smaller islets achieve better glycemic control is not fully known. We did not have pre- or post-IAT histopathological data showing a larger proportion of β-cells in smaller islets or larger regions of cell death in larger islets. It will be important to better elucidate the involved mechanism(s) with future work.
In conclusion, islet size may be an important determinant of post-IAT outcomes, particularly in recipients receiving marginal islet doses. Our preliminary data suggest that post-IAT function as measured by insulin independence and insulin requirements at 6 months is superior in patients receiving islet preparations with more IPs/kg and smaller ISI. Further analysis with larger numbers of recipients must be done to evaluate whether the islet dose in combination with the ISI can enable improved prediction of post-IAT outcomes. Future study will also include examining the predictive utility of ISI on outcomes in the more confounded case of islet allotransplant.
Materials and Methods
Study Design
Data from all patients who underwent TP-IAT and received a marginal islet dose (2,000–4,999 IEs per kg body weight) from 1/1/2009–6/11/2012 at the University of Minnesota were included in the analysis (n=58). Exclusion criteria included pre-existing diabetes mellitus or impaired glucose tolerance, or prior partial pancreatectomy with IAT. Post-IAT, insulin was weaned slowly to maintain fasting blood glucose of 80–125 mg/dL, post-prandial blood glucose of <150–180 mg/dL, and HbA1C of <6.5%. Each patient was stratified into 1 of 3 cohorts depending on their insulin use status at 6 months (primary post-IAT outcome); insulin independence was defined as no documented use of insulin (n=8), partial insulin dependence was defined as the use of a daily long-acting insulin analog with rare use of rapid-acting insulin (<1 unit per day)(n=19), and complete insulin dependence was defined as using multiple daily injections of long-acting and/or rapid-acting insulin and/or analogs (n=31). When insulin use status was between strata, the patient was stratified into the cohort reflecting worse islet graft function. Where sufficient data was available (in the form of patient glucose logs or self-report), the mean total daily insulin requirements were calculated at 6 months post-IAT.
We examined the impact of several islet product characteristics on the primary post-IAT outcome, including: ISI, total IPs transplanted, IPs transplanted per kg, total IEs transplanted, IEs transplanted per kg, proportion of islets >200-μm in diameter and total TV transplanted.
Total pancreatectomy and islet isolation, purification and transplantation
TP-IAT was performed as described previously (15). Briefly, TP was done to preserve the blood supply to the pancreas until immediately before resection, which minimized the warm ischemia time to the pancreas. Following TP, the pancreas was surface-cooled and taken to the islet isolation laboratory. The basic method for islet isolation and purification has been described previously (15). Briefly, each pancreas was distended by an intraductal infusion of collagenase solution and then digested at 37°C using a Ricordi chamber (25), which has been designed to mechanically disrupt the gland during enzymatic digestion. After digestion, the islet preparation may have been additionally purified by density gradient centrifugation (26). The decision to purify the islet preparation was based on the post-digest TV, with >0.25 cm3/kg generally serving as an indication for purification. Aliquots of the islet product were then manually counted and used to estimate the total number of IPs in the preparation. Based on stratifying each IP into an approximate size range during the manual count, the total number of IPs in the preparation was converted to a total number of IEs by assuming that a perfectly spherical 150-μm diameter islet represented the volume of 1 IE (22). ISI (a dimensionless quantity) was also calculated by dividing the total number of IEs in a preparation by the total number of IPs. Some of the preparations (n=25) were also evaluated for their fractional viability by directly measuring OCR/DNA (27–29). The typical islet product preparation time ranged from 3.5–6.5 hours. Islet products were suspended in CMRL culture media (Mediatech, Inc., Manassas, VA) containing 2.5% m/v human serum albumin and taken to the operating room. Heparin (70 mg/kg) was administered systemically and the islet product was directly infused under gravity into the portal vein over a 15–60 minute period. Half of the heparin dose was mixed in with the islet preparation. During the infusion, the portal pressures were continuously monitored and if they exceeded 25 cm H20 the remainder of the islet preparation was infused into the peritoneal cavity. Patients were given low-molecular weight heparin for the duration of their hospitalization. Partial thromboplastin time was not routinely monitored. Aspirin was given for a platelet count >800,000/microliter. Doppler ultrasound was routinely ordered at 5–7 days and 2 weeks postoperatively in all patients to assess for portal venous thrombosis, hematoma and intraperitoneal bleeding. All patients were placed on an insulin drip in the immediate post-operative period, which was then transitioned to subcutaneous insulin injections at approximately 1 week post-operatively. This was done to minimize metabolic stress on the islets while bridging to engraftment. Target blood glucose levels have been summarized elsewhere (30).
Statistical analysis
Univariate analysis for overall comparisons between study cohorts was performed by oneway analysis of variance (ANOVA) for continuous variables or the Chi-square test for categorical variables. The distribution of data was tested for normality using the D'Agostino-Pearson omnibus normality test. For data not normally distributed, the Kruskal-Wallis test was substituted for the one-way ANOVA. Direct comparison between study cohorts was performed using the unpaired t test (for normally distributed data) or the Mann-Whitney test (for non-normally distributed data). Bivariate linear regression analysis was used to quantify the degree of correlation between the daily insulin usage at 6 months post-IAT and islet product parameters, and included correlation analysis to compute the Spearman's rank correlation coefficient (denoted by ρ). Sensitivity and specificity analysis was performed for IPs/kg and ISI. A multivariate analysis was done with all parameters being screened for inclusion in the final model using bivariate associations and stepwise logistic regression; the parameters considered in this model included ISI, IEs/kg, IPs/kg, transplanted tissue volume, and selected pre-IAT patient characteristics (BMI, FBG, degree of pancreatic fibrosis, and duration of pancreatitis). Data are presented as mean ± standard error (SE). Statistical significance corresponded to p-values <0.05 using a 95% confidence interval. All statistical analyses were performed using either SAS Version 9.2 (SAS Institute, Inc., Cary, NC) or Graphpad Prism Version 5.03 (Graphpad Software, Inc., La Jolla, CA).
Acknowledgements
The authors would like to thank Hang McLaughlin for her assistance in the preparation of this manuscript. Dr. Melena Bellin is supported by a career development award from NIDDK (K23DK084315-01A1).
Abbreviations
- ANOVA
analysis of variance
- BMI
body mass index
- DNA
deoxyribonucleic acid
- FBG
fasting blood glucose
- HbA1C
hemoglobin A1C
- IAT
islet autotransplantation
- IE
islet equivalent
- IP
islet particle
- ISI
islet size index
- OCR
oxygen consumption rate
- OCR/DNA
OCR normalized to DNA content (measure of fractional viability)
- OR
odds ratio
- pO2
oxygen partial pressure
- SE
standard error
- TP
total pancreatectomy
- TV
tissue volume
Footnotes
Authorship Contributions: TMS: Participated in research design, writing of the paper, performance of the research and data analysis
JJW: Participated in research design, writing of the paper, performance of the research and data analysis
DMR: Participated in research design, writing of the paper, performance of the research and data analysis
ANB: Participated in research design and writing of the paper
DERS: Participated in performance of the research
GJB: Participated in writing of the paper and performance of the research
TBD: Participated in writing of the paper and performance of the research
TLP: Participated in performance of the research
SMV: Participated in performance of the research
BJH: Participated in performance of the research
KKP: Participated in research design, writing of the paper, performance of the research and data analysis
MDB: Participated in research design, writing of the paper, performance of the research and data analysis
Disclosure: The authors have no conflicts of interest to disclose.
References
- 1.Dionne KE, Colton CK, Yarmush ML. Effect of hypoxia on insulin secretion by isolated rat and canine islets of Langerhans. Diabetes. 1993;42(1):12. doi: 10.2337/diab.42.1.12. [DOI] [PubMed] [Google Scholar]
- 2.MacGregor RR, Williams SJ, Tong PY, Kover K, Moore WV, Stehno-Bittel L. Small rat islets are superior to large islets in in vitro function and in transplantation outcomes. Am J Physiol Endocrinol Metab. 2006;290(5):E771. doi: 10.1152/ajpendo.00097.2005. [DOI] [PubMed] [Google Scholar]
- 3.Lehmann R, Zuellig RA, Kugelmeier P, et al. Superiority of small islets in human islet transplantation. Diabetes. 2007;56(3):594. doi: 10.2337/db06-0779. [DOI] [PubMed] [Google Scholar]
- 4.Fujita Y, Takita M, Shimoda M, et al. Large human islets secrete less insulin per islet equivalent than smaller islets in vitro. Islets. 2011;3(1):1. doi: 10.4161/isl.3.1.14131. [DOI] [PubMed] [Google Scholar]
- 5.Sutherland DE, Gruessner AC, Carlson AM, et al. Islet autotransplant outcomes after total pancreatectomy: a contrast to islet allograft outcomes. Transplantation. 2008;86(12):1799. doi: 10.1097/TP.0b013e31819143ec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bellin MD, Sutherland DE, Beilman GJ, et al. Similar islet function in islet allotransplant and autotransplant recipients, despite lower islet mass in autotransplants. Transplantation. 2011;91(3):367. doi: 10.1097/TP.0b013e318203fd09. [DOI] [PubMed] [Google Scholar]
- 7.Menger MD, Jaeger S, Walter P, Feifel G, Hammersen F, Messmer K. Angiogenesis and hemodynamics of microvasculature of transplanted islets of Langerhans. Diabetes. 1989;38(Suppl 1):199. doi: 10.2337/diab.38.1.s199. [DOI] [PubMed] [Google Scholar]
- 8.Eriksson O, Eich T, Sundin A, et al. Positron emission tomography in clinical islet transplantation. Am J Transplant. 2009;9(12):2816. doi: 10.1111/j.1600-6143.2009.02844.x. [DOI] [PubMed] [Google Scholar]
- 9.Carlsson PO, Palm F, Mattsson G. Low revascularization of experimentally transplanted human pancreatic islets. J Clin Endocrinol Metab. 2002;87(12):5418. doi: 10.1210/jc.2002-020728. [DOI] [PubMed] [Google Scholar]
- 10.Mattsson G, Jansson L, Carlsson PO. Decreased vascular density in mouse pancreatic islets after transplantation. Diabetes. 2002;51(5):1362. doi: 10.2337/diabetes.51.5.1362. [DOI] [PubMed] [Google Scholar]
- 11.Olsson R, Olerud J, Pettersson U, Carlsson PO. Increased numbers of low-oxygenated pancreatic islets after intraportal islet transplantation. Diabetes. 2011;60(9):2350. doi: 10.2337/db09-0490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davalli AM, Ogawa Y, Ricordi C, Scharp DW, Bonner-Weir S, Weir GC. A selective decrease in the beta cell mass of human islets transplanted into diabetic nude mice. Transplantation. 1995;59(6):817. [PubMed] [Google Scholar]
- 13.Weir GC, Bonner-Weir S, Leahy JL. Islet mass and function in diabetes and transplantation. Diabetes. 1990;39(4):401. doi: 10.2337/diab.39.4.401. [DOI] [PubMed] [Google Scholar]
- 14.Korc M, et al. The Pancreas. Raven Press; New York: 1993. Normal function of endocrine pancreas; p. 751. [Google Scholar]
- 15.Sutherland DE, Radosevich DM, Bellin MD, et al. Total pancreatectomy and islet autotransplantation for chronic pancreatitis. J Am Coll Surg. 2012;214(4):409. doi: 10.1016/j.jamcollsurg.2011.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shapiro AM, Lakey JR, Ryan EA, et al. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med. 2000;343(4):230. doi: 10.1056/NEJM200007273430401. [DOI] [PubMed] [Google Scholar]
- 17.Ryan EA, Lakey JR, Rajotte RV, et al. Clinical outcomes and insulin secretion after islet transplantation with the Edmonton protocol. Diabetes. 2001;50(4):710. doi: 10.2337/diabetes.50.4.710. [DOI] [PubMed] [Google Scholar]
- 18.Ryan EA, Paty BW, Senior PA, et al. Five-year follow-up after clinical islet transplantation. Diabetes. 2005;54(7):2060. doi: 10.2337/diabetes.54.7.2060. [DOI] [PubMed] [Google Scholar]
- 19.Markmann JF, Deng S, Huang X, et al. Insulin independence following isolated islet transplantation and single islet infusions. Ann Surg. 2003;237(6):741. doi: 10.1097/01.SLA.0000072110.93780.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bellin MD, Barton FB, Heitman A, et al. Potent induction immunotherapy promotes long-term insulin independence after islet transplantation in type 1 diabetes. Am J Transplant. 2012;12(6):1576. doi: 10.1111/j.1600-6143.2011.03977.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carlsson PO, Palm F, Andersson A, Liss P. Markedly decreased oxygen tension in transplanted rat pancreatic islets irrespective of the implantation site. Diabetes. 2001;50(3):489. doi: 10.2337/diabetes.50.3.489. [DOI] [PubMed] [Google Scholar]
- 22.Colton CK, Papas KK, Pisania A, et al. Characterization of islet preparations. In: Halberstadt C, Emerich DF, editors. Cellular Transplantation: From Laboratory to Clinic. Academic Press; Waltham, MA: 2007. p. 85. [Google Scholar]
- 23.Avgoustiniatos ES, Colton CK. Effect of external oxygen mass transfer resistances on viability of immunoisolated tissue. Ann N Y Acad Sci. 1997;831:145. doi: 10.1111/j.1749-6632.1997.tb52192.x. [DOI] [PubMed] [Google Scholar]
- 24.Nano R, Clissi B, Melzi R, et al. Islet isolation for allotransplantation: variables associated with successful islet yield and graft function. Diabetologia. 2005;48(5):906. doi: 10.1007/s00125-005-1725-3. [DOI] [PubMed] [Google Scholar]
- 25.Ricordi C, Lacy PE, Scharp DW. Automated islet isolation from human pancreas. Diabetes. 1989;38(Suppl 1):140. doi: 10.2337/diab.38.1.s140. [DOI] [PubMed] [Google Scholar]
- 26.Anazawa T, Matsumoto S, Yonekawa Y, et al. Prediction of pancreatic tissue densities by an analytical test gradient system before purification maximizes human islet recovery for islet autotransplantation/allotransplantation. Transplantation. 2011;91(5):508. doi: 10.1097/TP.0b013e3182066ecb. [DOI] [PubMed] [Google Scholar]
- 27.Papas KK, Pisania A, Wu H, Weir GC, Colton CK. A stirred microchamber for oxygen consumption rate measurements with pancreatic islets. Biotechnol Bioeng. 2007;98(5):1071. doi: 10.1002/bit.21486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Papas KK, Colton CK, Nelson RA, et al. Human islet oxygen consumption rate and DNA measurements predict diabetes reversal in nude mice. Am J Transplant. 2007;7(3):707. doi: 10.1111/j.1600-6143.2006.01655.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Papas KK, Colton CK, Qipo A, et al. Prediction of marginal mass required for successful islet transplantation. J Invest Surg. 2010;23(1):28. doi: 10.3109/08941930903410825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bellin MD, Balamurugan AN, Pruett TL, Sutherland DE. No islets left behind: islet autotransplantation for surgery-induced diabetes. Curr Diab Rep. 2012;12(5):580. doi: 10.1007/s11892-012-0296-1. [DOI] [PMC free article] [PubMed] [Google Scholar]