Abstract
Clinical observations and studies on different animal models of acquired epilepsy consistently demonstrate that blood-brain barrier (BBB) leakage can be an important risk factor for developing recurrent seizures. However, the involved signaling pathways remain largely unclear. Given the important role of thrombin and its major receptor in the brain, protease-activated receptor 1 (PAR1), in the pathophysiology of neurological injury, we hypothesized that PAR1 may contribute to status epilepticus (SE)-induced epileptogenesis and that its inhibition shortly after SE will have neuroprotective and antiepileptogenic effects. Adult rats subjected to lithium-pilocarpine SE were administrated SCH79797 (a PAR1 selective antagonist) after SE termination. Thrombin and PAR1 levels and neuronal cell survival were evaluated 48 hr following SE. The effect of PAR1 inhibition on animal survival, interictal spikes (IIS) and electrographic seizures during the first two weeks after SE and behavioral seizures during the chronic period were evaluated. SE resulted in a high mortality rate and incidence of IIS and seizures in the surviving animals. There was a marked increase in thrombin, decrease in PAR1 immunoreactivity and hippocampal cell loss in the SE-treated rats. Inhibition of PAR1 following SE resulted in a decrease in mortality and morbidity, increase in neuronal cell survival in the hippocampus and suppression of IIS, electrographic and behavioral seizures following SE.
These data suggest that the PAR1 signaling pathway contributes to epileptogenesis following SE. Because breakdown of the BBB occurs frequently in brain injuries, PAR1 inhibition may have beneficial effects in a variety of acquired injuries leading to epilepsy.
Keywords: Thrombin, protease-activated receptor, hippocampus, pilocarpine, epileptogenesis, status epilepticus
INTRODUCTION
Impairment of blood–brain barrier (BBB) integrity is commonly observed in conjunction with traumatic brain injury, stroke, tumors and infections; conditions which can lead to seizures and the development of epilepsy (Albayrak et al., 1997; Chodobski et al., 2011; Latour et al., 2004; On et al., 2013; Stolp and Dziegielewska, 2009; Vezzani and Friedman, 2011). Short- and long-lasting increases of BBB permeability during seizures and status epilepticus (SE) have been demonstrated in different animal models of chronic epilepsy (Friedman, 2011) and in patients with epilepsy (Mihály and Bozóky, 1984; Oby and Janigro, 2006). Moreover, BBB opening or intracerebral injection of blood components may directly evoke seizures and lead to the generation of an epileptic focus (Lee et al., 1997; van Vliet et al., 2007). However, the specific pathways activated as a consequence of BBB disruption participating in the development of chronic epilepsy remains unclear.
There is now substantial evidence that besides its key role in coagulation, serum- derived protein thrombin participates in many mechanisms important for normal brain functioning and during pathological conditions involving abnormal neuronal synchronization, neurodegeneration and inflammation (Luo et al., 2007; Turgeon et al., 2000). Among the possible blood components involved in seizure generation during BBB opening (albumin, iron, thrombin) only thrombin has been shown to produce early-onset seizures (Lee et al., 1997; Tomkins et al., 2007; Willmore et al., 1978). Depending on the concentration, the effect of thrombin in the CNS might be protective or deleterious (Xi et al., 2003). At low concentrations, thrombin rescues neurons from death after brain insults (Jiang et al., 2002). In contrast, the alteration of BBB integrity during pathological conditions may lead to dramatic increases of thrombin levels in the CNS (Woitzik et al., 2011). Thrombin, through the activation of specific protease-activated receptors (PAR) expressed by neurons and glial cells, is implicated in the exacerbation of brain damage, seizures and induction of inflammation and neurogenesis, all processes that frequently occur during epileptogenesis (Tanuja Rohatgi et al., 2004). Finally, deficiency of PAR1, a major thrombin receptor in the brain, protects against neuronal damage and neurologic deficits in different models of experimental brain insults thus indicating a significant role of PAR1 signaling in the development of neurodegenerative disorders (Chen et al., 2012; Junge et al., 2003; Manaenko et al., 2013; Olson et al., 2004; Wang et al., 2012).
Here we tested the hypothesis that PAR1 contributes to SE-induced neuronal damage and epileptogenesis. Using the lithium-pilocarpine model of SE, which was shown to be associated with BBB dysfunction (Ndode-Ekane et al., 2010), we have found a marked increase in the thrombin level and decrease in PAR1 immunoreactivity at 48 hr after SE. Repetitive injection of the PAR1 antagonist after SE results in a decrease in animal mortality rate, improvement of functional recovery, decrease of SE-induced cell loss and suppression of epileptiform activity and seizures following SE. Our data indicates that PAR1-mediated signaling is involved in epileptogenesis induced by SE and that PAR1 could be a potential novel molecular target for antiepileptic drug therapy.
MATERIAL AND METHODS
Animals
All experimental procedures were performed in accordance with the guidelines set by the National Institute of Health for the humane treatment of laboratory animals and approved by the Animal Care Committees of Bogomoletz Institute of Physiology and the University of Vermont College of Medicine. Eighty adult (P50–70) male Wistar rats were used throughout all aspects of histological (n = 12), behavioral (n = 40) and electrophysiological (n = 28) studies.
Lithium-pilocarpine status epilepticus model
In order to decrease animal mortality, SE initiation was performed by repeated administration of low doses of pilocarpine as described previously (Glien et al., 2001). Rats were injected intraperitoneally (ip) with lithium chloride (127 mg/kg, 1 ml/kg) 19–20 hr before administration of pilocarpine. Pilocarpine was injected (10 mg/kg, ip) at 30 min intervals until SE was induced. The total dose of pilocarpine administration ranged from 10–50 mg/kg. SE was considered to start when the rat developed stage V seizures according to Racine’s scale (Racine, 1972). Only 2 of 76 rats did not develop stage V seizures. These animals were excluded from further analysis. The SE onset time ranged from 26–150 min (average onset time was 78.8 ± 4.5 min). Seizures were terminated with sevoflurane at 90–120 min after SE onset for histological and behavioral studies (SE was terminated before 120 min if the rat developed wild running and severe myoclonic jerks with falling, typically a sign of impending death if the SE continues). For the electrophysiological studies of animals which underwent intrahippocampal electrode implantation, seizures were terminated at 1 hr after SE onset (Salami et al., 2014). This shorter duration of SE for the electrophysiological studies was based on previous studies (Chauvière et al., 2012; Salami et al., 2014) showing a duration of SE of 60 min or less is sufficient to study the dynamics of the interictal spikes and spontaneous seizures in pilocarpine-induced epileptogenesis. After SE, rats were injected with 5% dextrose in lactate Ringer’s solution three times a day for about 3 days and fed with milk (chocolate flavor) and moistened rodent chow three times a day until they were able to eat independently. The food and water intake (determined from the weight of the uneaten chow and leftover water in each cage) and rats’ weight were examined daily by a technician for 11 days after SE.
Drug administration
The PAR1 specific blocker, SCH79797, or appropriate volume of vehicle was ip injected 20–30 min after SE termination and thereafter injections were repeated once a day for 10 consecutive days. SCH79797 was injected at a concentration of 25 μg/kg. This concentration was shown to be optimal for the reduction of myocardial necrosis observed during experimental ischemia and for the decrease of brain water content and amelioration of neurological deficits following surgical brain injury (Manaenko et al., 2013; Strande et al., 2007).
Preparation of sections for light and confocal microscopy
Eight rats with SE (SE+vehicle group: n = 4, and SE+SCH group: n = 4) and age-matched control rats (n = 4) that received all injections, except for pilocarpine which was substituted by saline, were used in the histological studies. Animals with SE received two injections (at 20–30 min after SE termination and 24 hr latter) of SCH79797 or vehicle before sacrifice at 48 hr following SE. For deep anesthesia rats were given ip 70 mg/kg ketamine (Pfizer, Finland) and 2.5 mg/kg xylazine (Bayer, Germany). Anesthetized rats were transcardially perfused with 20 mL phosphate-buffered saline (PBS; 0.1 M, pH 7.4) with heparin (15 units/ml) followed by 50 mL of 4% paraformaldehyde (PFA, Sigma, USA) solution in PBS. Hippocampi were removed, post-fixed for 24 hr at 4°C in 4% PFA and thoroughly washed in 0.1 M PBS. Transversal sections 30–50 μm thick from each rat were cut with a step 250 μm starting at 1200–1500 μm from the dorsal end of hippocampus using vibratome (Leica Biosystems, USA). The sections were kept in 0.1 M PBS with 0.02% sodium azide 4–8 weeks at 4°C prior to immunohistochemistry or Nissl staining with thionin.
Histological analysis
The sections were washed in 0.1 M phosphate buffer, cryoprotected in a mixture of 20% DMSO, 2% glycerol in PBS for 60 min and permeabilized by freezing/thawing three times at −20°C. Sections were then blocked with 0.5% normal goat serum and 1% albumin bovine serum in 0.1 M PBS (blocking solution) for 1 hr at room temperature, and incubated overnight at 4°C in primary antibodies: anti-Thrombin HC polyclonal goat antibody (1:100; sc-23335, Santa Cruz Biotechnology, USA); anti-Thrombin R (PAR) polyclonal rabbit antibody (1:100; sc-5605, Santa Cruz Biotechnology, USA); anti-NeuN monoclonal antibody, clone A60 (1:1000; MAB377, Millipore, USA); anti-Glial Fibrillary Acidic Protein (GFAP) monoclonal antibody, clone GA5 (1:1000; MAB360, Millipore, USA) in blocking solution. Sections were thoroughly rinsed in 0.1 M PBS and incubated for 90 min at room temperature in 0.1 M PBS containing 1% albumin bovine serum and appropriate secondary antibodies (all from Invitrogen, USA): Alexa 568-conjugated donkey anti-goat antibody (1:800; A-11057); Alexa 647-conjugated donkey anti-rabbit antibody (1:800; A-31573); Alexa 488-conjugated donkey anti-mouse antibody (1:800; A-21202). Cell nuclei were stained with DAPI (4′,6′-diamidino-2-phenylindole, 100 nM). Sections were finally rinsed three times in 0.1 M PBS and then mounted onto glass microscope slides in Dako fluorescent mounting medium (Dako, Denmark) for confocal microscopy. The immunofluorescent images were captured using a confocal microscope FluoView™ FV1000 (Olympus America Inc., Center Valley, PA) and histological measures were performed using ImageJ software (National Institutes of Health, USA). Immunohistochemical analysis was performed in hippocampal CA1 pyramidal region and adjacent zones (no more than 200 μm from the pyramidal CA1 area layer.) The area of analysis is shown on Figure 1A. Images were taken at a resolution of 1024×1024 and have similar brightness and contrast (oil objective - 60×). Data are presented as immunopositive area of fluorescence (i.e. Thrombin+, PAR+) per mm2 (a.u.).
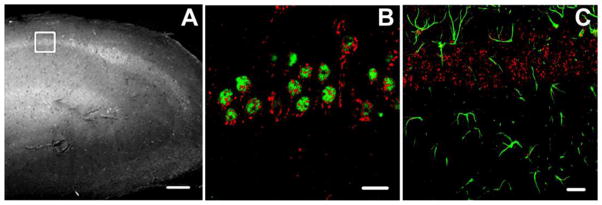
Figure 1.
Localization of PAR1 in the CA1 region of the hippocampus. (A) The region of interest for the histological analysis is marked by a white square on the phase contrast image (scale bar 500 μm). (B) Confocal image of hippocampal cells double stained for anti-PAR1 polyclonal antibody (red) and anti-NeuN monoclonal antibody (green) in the control group revealed that PAR1 immunoreactivity is predominant in neurons. (C) Immunohistochemistry revealed a weak PAR1 immunoreactivity in cells expressing specific astrocyte marker GFAP (green). Scale bars for B and C = 50 μm.
In our experiments we observed a loss of NeuN immunoreactivity in SE group (data not shown). Recent studies suggest that, although NeuN is consistently expressed by hippocampal neurons, different brain insults could impair NeuN expression, so the loss of NeuN immunoreactivity does not necessarily reflect the amount of cell loss (McPhail et al., 2004; Unal-Cevik et al., 2004). Therefore, in our study SE-induced neuronal damage was estimated using thionin staining. The sections were washed in 0.1 M phosphate buffer, mounted on gelatin-coated slides, defatted and hydrated through a graded series of ethanol (50%, 70%, 96% at 2 min), air-dried at 37°C and stained 30 sec in a 0.02% thionin (T-409, Fisher Scientific, Waltham, MA,) in 70% ethanol. Then, sections were washed for 1 min using 70% and 96% ethanol, air-dried at 37°C and cover-slipped with Pertex Mounting Media (Leica Biosystems, USA). The images were captured at a resolution of 1744×1308 using confocal microscope FluoView™ FV1000 (Olympus Inc., USA) and phase-contrast objective (40×). Only intact cells of CA1 area identified by morphological features were taken into account, condensed and damaged cells were excluded. Data are presented as the number of cell per mm2.
Electrophysiological analysis
For electrophysiological studies, animals underwent surgery with intrahippocampal implantation of the recording electrode into the CA1 region of the dorsal part of hippocampus. Briefly, animals were deeply anesthetized using 4% sevoflurane (for the initial induction) in an O2 carrier and then placed in the stereotaxic frame with a custom made anesthesia mask. Sevoflurane was reduced to 1.5–2% and ip injections of ketamine (4 mg/kg every 30 min) were made to provide adequate surgical anesthesia. Lidocaine (5mg/kg) was administrated subcutaneously prior the surgery and at the end of the surgery in order to provide additional local post-surgical analgesia. A bipolar wire electrode (50 μm in diameter; California Fine Wire, Grover Beach, CA) was introduced through a burr hole in the skull into the right dorsal hippocampus, using the following coordinates: anterior/posterior (AP) −3.7 mm; medial/lateral (ML) ±2.5 mm; dorsal/ventral (DV) −2.0 mm. A ground electrode was implanted into the cerebellum. Two additional microscrews were driven into the frontal bone to provide better fixation to the skull. Connector for the electrodes was located on the surface of the skull and was cemented to the skull using fast drying dental acrylic. After surgery, animals were allowed to recover for about 7–10 days before SE induction. During recovery, animals were housed individually and maintained under 12 hr light/dark-cycle with free access to food and water.
Intrahippocampal EEG recordings were performed using a differential amplifier (A-M Systems, Carlsborg, WA) (bandpass 1 Hz–10 kHz). Signals were digitized by analogue-to-digital converter (National Instruments Corp., Austin, TX) and collected using WinWCP software (J. Dempster, University of Strathclyde, Glasgow, UK). The EEG recordings were performed for two days before SE induction and then daily for 14 days (60 min per day). Interictal spikes (IIS) were defined as brief (80–200 ms) high-amplitude sharply contoured waveforms that were distinct from background patterns in the EEG that occurred in isolation on a background of otherwise normal activity. Electrographic seizures (ES) consisted of rhythmic spikes with a frequency of 1 Hz or more lasting at least 10 sec. No epileptiform activity was seen in recordings performed before SE induction. To estimate number of spontaneous behavioral seizures at chronic stage, 3–3.5 months after SE rats were video monitored for 12 hr/day for one week. Only stage IV–V seizures (rearing with forelimb clonus or rearing and falling with forelimb clonus) were counted (Racine, 1972).
Statistical analysis
Analysis of histological, electrophysiological and video data was performed by investigators “blinded” to treatment conditions. IIS and ES were detected using Clampfit (Molecular Devices, Sunnyvale, CA, USA) and Mini Analysis (Synaptosoft, Decatur, GA, USA) software. Data analysis was performed using GraphPad Prism (GraphPad Software, San Diego, CA, USA) and Origin (OriginLab Corporation, Northampton, MA, USA) software. Video recordings were visually analyzed for detection of spontaneous behavioral seizures. Duration and frequency (per week) of the seizures was obtained for each rat, averaged per group and compared. Animal mortality rate and recovery from weight loss after SE was obtained from animals which did not undergo surgery. Only animals which survived the first week after SE were used for the electrophysiological studies. The effect of PAR1 inhibition on IIS occurrence was analyzed using the repeated measures ANOVA test. The IIS count did not include the IIS presented during ES. Survival rate and probability of observing spontaneous seizures were analyzed using Fisher’s exact test. For statistical analysis of histological data we used one-way ANOVA followed by a post-hoc Tukey’s multiple comparisons test. The remainder of the statistical comparisons was made by applying two-tailed Student’s t-test. In the electrophysiological studies “n” represents the number of animals and in the histological studies “n” is the total number of sections analyzed for each experimental group. For the histological analysis of PAR1 immunoreactivity and thionin staining we used 5 sections from 4 animals in each experimental group (total n = 20). For the assay of thrombin immunoreactivity we additionally used 2–3 sections per animal in each experimental group (total n = 30). All data are shown as mean ± SEM and the difference is considered to be significant at p < 0.05.
RESULTS
Localization of PAR1 in hippocampal CA1 region
It was shown previously that within the hippocampal formation, PARs are predominantly localized in the pyramidal cell layers (Striggow et al., 2001). Although the localization of PAR1 has been shown for neurons and glia in different brain regions (Bourgognon et al., 2013; Han et al., 2011; Junge et al., 2003; Striggow et al., 2001), the information about cell specific localization of PAR1 in the CA1 region of hippocampus is scarce. The aim of this set of experiment was to determine the phenotype of PAR1-positive cells in the area of our analysis (Fig. 1A) in control conditions. To elucidate the phenotype of PAR1-positive cells we performed multi-labeled immunohistochemistry using neuron-specific (anti-NeuN) or astrocyte-specific (anti-GFAP) antibodies together with an anti-PAR1 antibody. As shown in Figure 1B anti-PAR1 antibody staining was mainly co-localized with NeuN, indicative of the neuronal phenotype of PAR1-positive cells (n = 10). PAR1-GFAP double-staining showed a weak co-localization of these two proteins (n = 10, Fig.1C). Thus, in the pyramidal CA1 region of the hippocampus, PAR1 is predominantly expressed by neurons.
Effect of PAR1 inhibition on thrombin and PAR1 immunoreactivity and cell number in CA1 region of hippocampus following SE
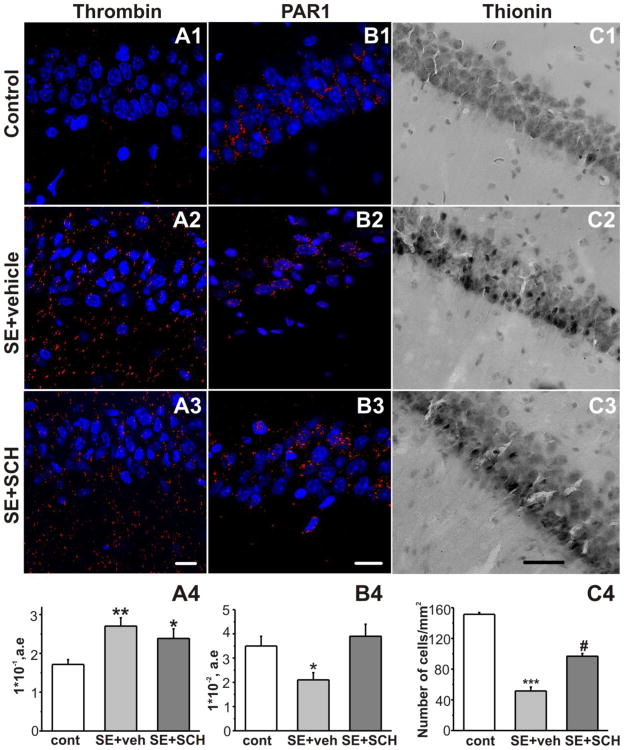
Weak thrombin immunoreactivity was observed in the CA1 region in the control group (Fig. 2A1). It was reported, that maximal BBB damage in the CA1 region is observed 2 days after pilocarpine induced-SE (Ndode-Ekane et al., 2010). In our study the thrombin level was significantly increased in the area of interest (Fig. 1A) at 48 hr after SE termination (0.27 ± 0.02 [n = 30] compared to controls 0.17 ± 0.01 [n = 30], p = 0.002, Fig. 2A1,A2,A4). Injection of PAR1 inhibitor SCH79797 (25 μg/kg) following SE termination did not alter the SE-induced increase in the thrombin level (at 48 hr after SE: SE+SCH group: 0.24 ± 0.02 [n = 30] compared to the SE+vehicle group: 0.27 ± 0.02 [n = 30], p = 0.5, Fig. 2A2–4).
Figure 2.
Effect of PAR1 inhibition on thrombin and PAR1 immunostaining and thionin staining in the CA1 region of the hippocampus following SE. Staining of hippocampal sections with DAPI (blue), anti-thrombin polyclonal antibody (A, red, scale bar 50 μm), anti-PAR1 polyclonal antibody (B, red, scale bar 50 μm) and thionin staining (C, scale bar 200 μm) in the control group (1), 48 hr after SE in the vehicle-treated (2) and the SCH-treated (3) groups. CA1 nuclei were visualized with DAPI staining. (A4) SE-induced increase in thrombin immunoreactivity was not affected by ip injection of the PAR1 inhibitor (averaged data from the CA1 area). (B4) PAR1 inhibition restored the SE-induced decrease of PAR1 immunoreactivity in the CA1 area. (C4) Thionin staining revealed significant cell loss in the CA1 pyramidal layer 48 hr following SE. Treatment with SCH 79797 resulted in the decrease of SE-induced cell loss. ***p<0.001, **p<0.01, *p<0.05 compared to control; #p<0.01 compared to SE+vehicle group.
We next examined the effect of SE on the pattern of PAR1 expression in the CA1 region of the hippocampus. Analysis revealed a significant loss of PAR1 immunoreactivity at 48 hr after SE (2.1 ± 0.3 [n = 20] compared to controls 3.5 ± 0.4 [n = 20], p = 0.03, Fig. 2B1,B2, B4). Injection of the PAR1 antagonist SCH79797 at 1 and 24 hr following SE termination prevented the decrease in PAR1 antigen signal (at 48 hr after SE: SE+SCH: 3.9 ± 0.5 [n = 20] compare to controls, p = 0.8, Fig. 2B3, B4).
Significant neuronal cell loss in the CA1 pyramidal region was seen in the SE+vehicle treated group at 48 hr after SE using thionin staining (51.5 ± 5.2 [n = 20] compared to controls 151.4 ± 2.3 [n = 20], data are presented as the number of cells per mm2, p < 0.0001, Fig. 2C1,C2,C4). In the SE+SCH group the number of cells was significantly greater than in the SE+vehicle group (at 48 hr 96.8 ± 3.5 [n = 20], p < 0.0001, Fig. 2C2–C4).
Effect of PAR1 inhibition on animal mortality rate and recovery from weight loss after SE
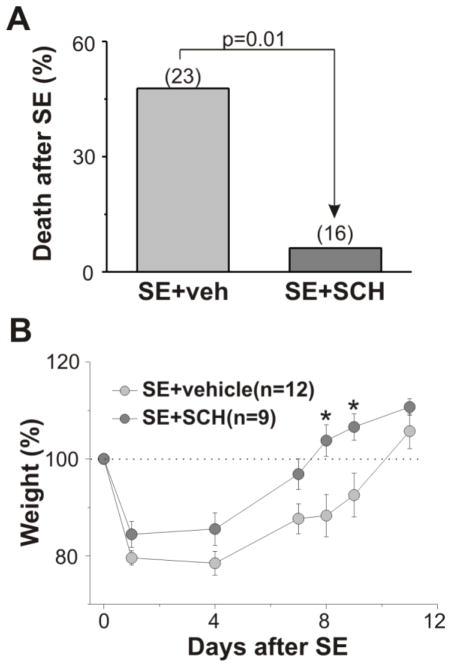
Although we used repeated administration of low doses of pilocarpine to induce SE, the mortality rate following SE was quite high. All animals that survived the initial SE were treated with the PAR1 inhibitor or vehicle. In the SE+vehicle group, 11 of 23 animals (47.8%) died within first two weeks after SE (average 5.4 ± 0.7 day, range 2–10 day), while in the SE+SCH group only 1 of 16 animals died after SE (6.3%, 7th day after SE). Thus, administration of the PAR1 antagonist shortly after SE significantly decreased mortality (p = 0.01, Fig 3A). On the third day after SE, 12 of 15 animals (80%) in the SE+SCH group and only 6 of 11 animals (55.5%) from the SE+vehicle group were able to drink and eat by their own and show a normal behavior. In addition, there was a significant increase in recovery from the weight loss in animals following SCH injection (Fig. 3B).
Figure 3.
Effect of PAR1 inhibition on survival rate and recovery from weight loss after SE. (A) Injection of PAR1 antagonist resulted in a substantial decrease in the delayed mortality rate following SE. (B) Effect of PAR1 antagonist on rat weights following SE. Number of animals used for analysis is provided in the parentheses. *p<0.05.
Effects of selective PAR1 antagonist on interictal spikes and spontaneous seizures after SE
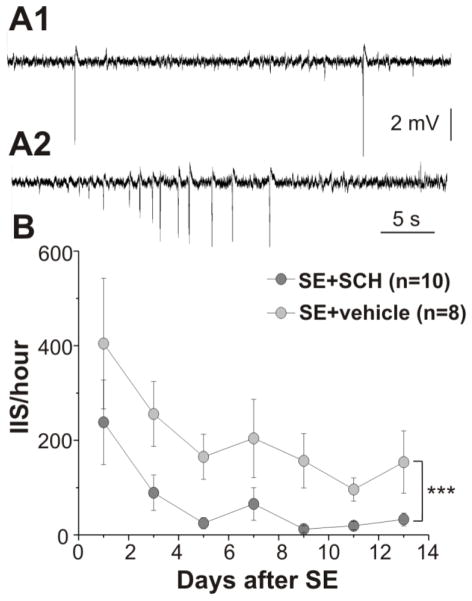
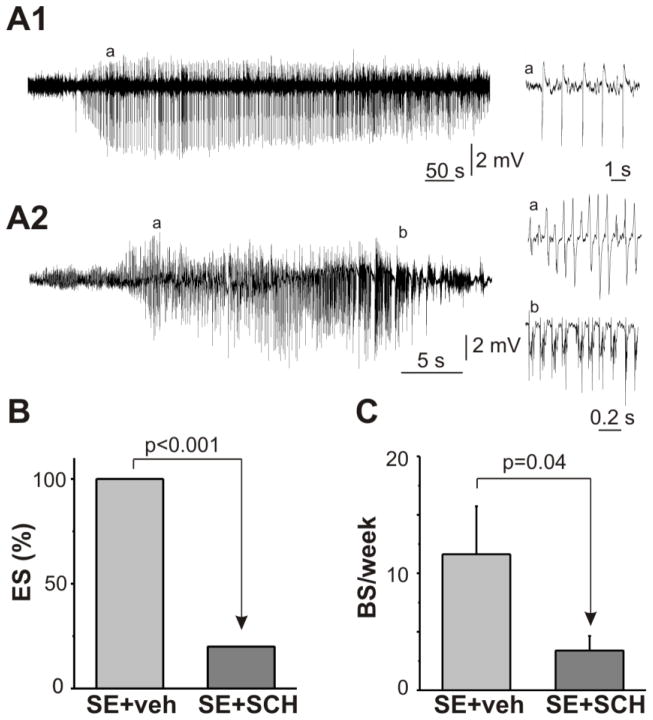
Other authors have found that electrographic seizure and IIS in the hippocampal formation are present in animals during the latent period after SE (Chauvière et al., 2012; Isaev et al., 2011; Mazzuferi et al., 2012; Salami et al., 2014; Staley et al., 2011). Furthermore, the presence of IIS over the first several days after SE before the occurrence of the first spontaneous seizure is associated with the development of chronic epilepsy (Chauvière et al., 2012; Salami et al., 2014). In the next set of experiments, we therefore estimated the appearance of IIS and electrographic spontaneous seizures in the CA1 pyramidal region during the first two weeks after SE using intrahippocampal EEG recordings in SE+vehicle and SE+SCH groups. The first day after SE, IIS were recorded in all rats (n = 8) in the SE+vehicle and in 6 out of 10 rats in the SE+SCH group. IIS occurred irregularly or in clusters of various duration (Fig. 4A1,2). The day-by-day analysis shows that the IIS frequency gradually decreased during the first week after SE in both groups (1st and 7th day after SE; SE+vehicle: from 404.6 ± 138.4 to 204 ± 82.7 events/hr [n = 8] and SE+SCH: from 238.0 ± 89.1 to 65.3 ± 34.7 events/hr [n = 10]) and thereafter remained stable for the rest of the recording period. Figure 4B shows that treatment with the PAR1 antagonist results in significant suppression of IIS over the first two weeks after SE (p = 0.003). We did not observe behavioral seizures during EEG recordings in any of the groups.
Figure 4.
Injection of the PAR1 antagonist produced a significant suppression of IIS in the CA1 region of hippocampus during the first two weeks after SE. Extracellular recording of field potentials from the CA1 region of the dorsal part of the hippocampus in rats experienced SE. (A1) IIS recorded on the second day after SE in a SE-vehicle treated rat. (A2) Example of IIS cluster recorded on the 7th day after SE in the same rat. (B) Cumulative data from IIS recordings collected over the first two weeks following SE. Repetitive injection of PAR1 antagonist after SE decreased the probability of IIS recording. Number of animals used for analysis shown in parentheses. ***p<0.005
Two types of electrographic seizures (ES) were observed during the recording period: prolonged low-frequency oscillations (Type I, maximal frequency 1–3 Hz, Fig. 5A1) lasted hundreds of seconds and short high-frequency (Type II, maximal frequency more than 10 Hz) oscillations consisted tonic and clonic activity (Fig. 5A2). Both types of ES could be observed separately or together during the one hour period of the EEG recording. During the entire recording period Type I ES was observed in all rats in the SE+vehicle group (onset 3.9 ± 1.2 day, 2.0 ± 0.7 event per rat, duration 459.8 ± 69.7 s, 16 events) and only in 2 out of 10 rats in SE+SCH group (onset Day 4 and Day 7 after SE, duration 608.5 ± 234.8 s, 4 events). Type II ES was observed only in one rat in SE+vehicle group beginning from Day 8 after SE (4 events, duration 32.5 ± 4.8 s, maximal frequency 18.0 ± 1.5 Hz). None of rats in the SE-SCH group had Type II seizures during the entire recording period. Figure 5B demonstrates that PAR1 inhibition resulted in a significant suppression in the occurrence of ES recorded from CA1 region during the first two weeks after SE.
Figure 5.
Effect of the PAR1 antagonist on spontaneous seizures after SE. Extracellular recording of field potentials from the CA1 region of the dorsal part of the hippocampus during the first two weeks after SE. (A1) Prolonged low-frequency oscillations recorded on the 8th day after SE in the SE+SCH treated rat. (A2) Electrographic seizures (ES) recorded on the 9th day after SE in the SE+vehicle treated rat. Tonic (a) and clonic (b) activity shown on an expended time scale (right). (B) PAR1 inhibition decreased the probability of ES recording during the first two weeks after SE. (C) Summary plot shows the effect of PAR1 inhibition on the occurrence of behavioral seizures (BS) during the chronic period after pilocarpine-induced SE. Mean ± SE.
All 8 rats in the SE+vehicle group and 6 out of 10 rats in the SE+SCH group had behavioral seizures (BS) during one week of video monitoring 3–3.5 months following the SE (p = 0.09). BS was observed only in animals that have a history of IIS or ES during post-SE EEG monitoring. PAR1 inhibition reduced the likelihood of observing BS during the chronic period after SE (SE+vehicle [n = 8] vs SE+SCH group [n = 10], p = 0.04, Fig. 5C), without alteration of BS duration (SE+vehicle 38.9 ± 2.8 s vs SE+SCH 42.5 ± 2.3 s, p = 0.4).
DISCUSSION
The main findings of our study is that SE produces a significant increase in thrombin and decrease in PAR1 immunoreactivity in the CA1 region of hippocampus and the administration of the PAR1 antagonist, SCH79797, shortly after SE termination decreases animal mortality, reduces SE-induced cell loss and suppresses the epileptiform activity and behavioral seizures.
Effect of PAR1 inhibition on thrombin and PAR1 immunoreactivity after SE
A significant increase in the thrombin level in the CA1 region of hippocampus was observed 48 hr after SE. Both short-term and long-term increases in the thrombin level and PAR1 activation have been observed following different brain insults such as ischemia, neuronal brain injury, inflammation, conditions which can compromise the development of chronic epilepsy(Chapman, 2006; Chen et al., 2012; Manaenko et al., 2013; Rohatgi et al., 2004; Striggow et al., 2001; Wang et al., 2012). One common feature of these conditions is a disturbance of BBB integrity, which even without detectable hemorrhage allows large molecule weight proteins such as albumin or globulins to enter the brain. Robust BBB disruption was observed during the first few days following pilocarpine-induced SE with significant increase of IgG extravasation observed for at least 60 days following SE (Ndode-Ekane et al., 2010). This data along with animal studies, where SE was induced by injection of kainic acid or electrical stimulation, and clinical studies in individuals with epilepsy, indicates that BBB leakage is a common pathology observed following SE (van Vliet et al. 2007; Oby and Janigro 2006; Gulati et al. 1987). Another source of thrombin increase following brain injury can be up-regulation of thrombin expression from the brain-derived prothrombin (Dihanich et al., 1991; Riek-Burchardt et al., 2002; Striggow et al., 2001). Further analyses of SE-induced alteration of the thrombin level in conjunction with changes of the BBB integrity need to clarify the source of the increased thrombin level after SE.
Increase in the thrombin level in the CA1 region of hippocampus following SE correlated with the decrease in PAR1 immunoreactivity in the pyramidal CA1 region. As was shown previously, upon activation, PAR1 is rapidly internalized into the cell and degraded (Macfarlane et al., 2001). We hypothesize that the increase in the level of thrombin after SE could activate PAR1, which will result in the decrease of PAR1 immunoreactivity. Inhibition of PAR1 by antagonist prevented the receptor activation and internalization in the presence of thrombin (Figure 2A3–B3). However there are several other explanations for the reduction of PAR1 immunoreactivity in the SE+vehicle group, such as SE-induced loss of PAR1 expression, SE-induced cell destruction and decrease of CA1 cell numbers (Fig. 2C). Future investigations are needed to clarify the mechanism responsible for the decrease of PAR1 levels following SE.
Effect of PAR1 inhibition on cell loss after SE
The role of thrombin as an important modulator of cell viability is well established in vivo and in vitro for central and peripheral neuronal cells (Donovan et al. 1997; Thirumangalakudi et al., 2009; Choi et al. 2003; de Castro Ribeiro et al. 2006; Fujimoto et al. 2007; Smirnova et al. 1998). It was suggested that in low concentrations thrombin can initiate neuroprotection, but a large dose of thrombin causes neurodegeneration (Striggow et al., 2000). However, several studies indicate that in the conditions of stress thrombin may cause cell death even in low concentrations (Choi et al., 2005; Weinstein et al., 1998). Most effects of thrombin in neuronal tissue are mediated by proteolytic activation of the major thrombin receptor in the brain, PAR1, and previous studies in models of surgical brain injury and ischemia indicate that lack of PAR1 or pharmacological blockade of PAR1 confer significant neuroprotection (Chen et al., 2012; Junge et al., 2003; Manaenko et al., 2013; Wang et al., 2012). To our knowledge, the present study is the first to demonstrate that inhibition of PAR1 results in a significant decrease of SE-induced cell loss.
Low mortality rate after PAR1 inhibition
In our study the majority of animals survived the first 24 hr after SE termination. A substantial delayed mortality (rate 47.8%) was observed over first two weeks following SE, which corresponds to the data reported for humans (DeLorenzo et al., 2009) and mice (Jiang et al., 2013; Levin et al., 2012) experiencing SE. Injection of PAR1 inhibitor significantly increased the survival rate (93.8%). Similar results were obtained in recent studies where conditional neuronal deletion of the cyclooxygenase-2 (COX-2) gene, inhibition of prostaglandin receptor EP2 and the use of drugs displaying anti-inflammatory effects result in decreases in the delayed mortality after SE, implicating the inflammatory processes in delayed mortality after SE (Jiang et al., 2013; Levin et al., 2012; Marchi et al., 2011; Sierra-Marcos et al., 2014). PAR1 is strongly involved in inflammatory signaling and thrombin has been shown to induce COX-2 expression and prostaglandin E2 release via PAR1 activation (Lo et al., 2009; Syeda et al., 2006). The effect of PAR1 inhibition on both morbidity and delayed mortality rate after SE may be at least be partly explained by inhibition of pro-inflammatory pathways.
Effect of PAR1 inhibition on the development of spontaneous seizures after SE
Although the period between SE and the appearance of the first spontaneous behavioral seizure is termed the “latent” or “silent” period, recent studies using continuous EEG monitoring show that IIS and ES can be readily detected during this period and that the occurrence of IIS shortly after SE can be a predictive marker of spontaneous seizures (Chauvière et al., 2012; Salami et al., 2014; White et al., 2010). Our aim was to determine whether the electrophysiological features of the neuronal network activity are influenced by PAR1 inhibition. Both IIS and ES were detected shortly after SE in all animals in the SE+vehicle group. In the SE+SCH group, a striking decrease in the probability to record IIS and ES was observed during the first two weeks after SE. The occurrence of electrographic epileptiform activity was strongly correlated with the development of spontaneous behavioral seizures in both SE+vehicle and SE+SCH groups. In the SE-SCH group we observed a substantial decrease in the occurrence of behavioral seizures in the chronic period after SE compared to the SE+vehicle group. These data indicate that PAR1 significantly contributes to the epileptogenesis in the lithium-pilocarpine model of TLE. Although our study is similar to most antiepileptic drug trials in patients where EEGs are obtained sporadically and seizure counts are obtained by seizure diaries, behavioral monitoring of seizures during the chronic period almost certainly underestimates the number of seizures, particularly if the animal is having non-convulsive seizures. Before concluding that inhibition of PAR1 prevents or reduces epileptogenesis, additional studies using continuous EEG and video monitoring as well as replicating our results with a different animal model of TLE will be necessary (Galanopoulou et al., 2014).
Possible mechanisms
There are various ways PAR1 activation can participate in epileptogenesis. An influx of thrombin and other PAR1 activator plasmin into the intracerebral environment as a consequence of BBB opening during SE may cause acute and local damage, but may also switch on the mechanisms related to underlying brain damage, metabolic disturbances, astrocytic dysfunction, inflammation, long-term changes in BBB integrity and neuronal network activity. As we have shown, PAR1 activation may amplify and even induce seizures by an increase of membrane depolarization through the activation of persistent sodium currents via the PKC depending pathway (Isaeva et al., 2012). Through activation of PAR1 thrombin can trigger the generation of epileptic seizures by reducing the inhibitory and increasing the excitatory tone in hippocampal neurons (Maggio et al., 2013). PAR1 activation during transient focal ischemia exacerbates neuronal damage through the enhancement of NMDA receptor signaling (Hamill et al., 2010). Activation of PAR1 increases the intracellular Ca2+ concentration in neurons as well as non-neuronal cells (Han et al., 2011), which might be associated with thrombin-mediated neurodegeneration. Numerous studies have demonstrated that activation of PAR1 initiates the signaling cascades of Rhoa/Rho kinase, protein kinase B and mitogen-activated protein kinases (MAPKs), including ERK1/2, c-Jun N-terminal kinase, p38 and p42/44 MAPK in neural and glial cells in vitro and in vivo (Luo et al., 2007; Tanuja Rohatgi et al., 2004). Considering that up-regulation of these pathways occurs in the brains of patients with intractable epilepsy (Yuan et al., 2010) and other experimental brain insults (Baraban et al., 1993; Berkeley et al., 2002; Dubreuil et al., 2006; Jeon et al., 2013; Jiang et al., 2005; Kim et al., 1994), PAR1 activation observed in the latent period after SE could trigger changes in these pathways, at least during early epileptogenesis. Finally, as PAR1 is strongly considered to be involved in a variety of pro-inflammatory effects contributing to the epileptogenic process we suggest that PAR1 activation shortly after SE can trigger retrograde pro-inflammatory signaling which can contribute to molecular, structural and synaptic changes characterizing epileptogenesis (Chapman, 2006; Cirino et al., 1996; Ravizza et al., 2011; Vergnolle et al., 2001).
CONCLUSIONS
The major thrombin receptor of the brain, PAR1, regulates many molecular signaling pathways important for development of chronic epilepsy. In the present study, we demonstrate for the first time that SE increases thrombin levels and decreases PAR1 immunoreactivity. Furthermore, the inhibition of PAR1 results in decreases of: i) post-SE animal mortality, ii) SE-induced cell loss, and iii) the likelihood of the occurrence of interictal-spikes and spontaneous seizures. While the cellular mechanism of the neuroprotective and anti-epileptogenic properties PAR1 inhibition needs to be delineated, our findings suggest that PAR1 is an attractive new target for epilepsy treatment.
HIGHLIGHTS.
Increase in thrombin level in hippocampus following lithium-pilocarpine SE.
PAR1 inhibition decreases SE-induced cell loss, delayed mortality and morbidity.
PAR1 inhibition results in suppression of epileptiform activity shortly after SE.
PAR1 inhibition reduces the likelihood of behavioral seizures at the chronic phase.
This suggests PAR1 contribution to SE-induced epileptogenesis.
Acknowledgments
Supported by National Institute of Health grants NS074450 and NS073083, and the Emmory R. Shapses Research Fund and Michael J. Pietroniro Research Fund. The authors would like to thank A. Vengrenyuk and O. Boiko for technical support.
ABBREVIATIONS
- BBB
blood-brain barrier
- BS
behavioral seizures
- COX-2
cyclooxygenase-2
- ES
electrographic seizures
- IIS
interictal spikes
- MAPK
mitogen-activated protein kinase
- PAR1
protease-activated receptor 1
- SE
status epilepticus
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Albayrak S, Zhao Q, Siesjö BK, Smith ML. Effect of transient focal ischemia on blood-brain barrier permeability in the rat: correlation to cell injury. Acta Neuropathol. 1997;94:158–163. doi: 10.1007/s004010050688. [DOI] [PubMed] [Google Scholar]
- Baraban JM, Fiore RS, Sanghera JS, Paddon HB, Pelech SL. Identification of p42 mitogen-activated protein kinase as a tyrosine kinase substrate activated by maximal electroconvulsive shock in hippocampus. J Neurochem. 1993;60:330–6. doi: 10.1111/j.1471-4159.1993.tb05855.x. [DOI] [PubMed] [Google Scholar]
- Berkeley JL, Decker MJ, Levey AI. The role of muscarinic acetylcholine receptor-mediated activation of extracellular signal-regulated kinase 1/2 in pilocarpine-induced seizures. J Neurochem. 2002;82:192–201. doi: 10.1046/j.1471-4159.2002.00977.x. [DOI] [PubMed] [Google Scholar]
- Bourgognon JM, Schiavon E, Salah-Uddin H, Skrzypiec aE, Attwood BK, Shah RS, Patel SG, Mucha M, John Challiss Ra, Forsythe ID, Pawlak R. Regulation of neuronal plasticity and fear by a dynamic change in PAR1-G protein coupling in the amygdala. Mol Psychiatry. 2013;18:1136–45. doi: 10.1038/mp.2012.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman J. Thrombin in inflammatory brain diseases. Autoimmun Rev. 2006;5:528–31. doi: 10.1016/j.autrev.2006.02.011. [DOI] [PubMed] [Google Scholar]
- Chauvière L, Doublet T, Ghestem A, Siyoucef SS, Wendling F, Huys R, Jirsa V, Bartolomei F, Bernard C. Changes in interictal spike features precede the onset of temporal lobe epilepsy. Ann Neurol. 2012;71:805–14. doi: 10.1002/ana.23549. [DOI] [PubMed] [Google Scholar]
- Chen B, Friedman B, Whitney MaMA, Van Winkle Ja, Lei IF, Olson ES, Cheng Q, Pereira B, Zhao L, Tsien RY, Lyden PD, Freidman B. Thrombin activity associated with neuronal damage during acut focal ischemia. J Neurosci. 2012;32:7622–31. doi: 10.1523/JNEUROSCI.0369-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chodobski A, Zink BJ, Szmydynger-Chodobska J. Blood-brain barrier pathophysiology in traumatic brain injury. Transl Stroke Res. 2011;2:492–516. doi: 10.1007/s12975-011-0125-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi SH, Lee DY, Kim SU, Jin BK. Thrombin-induced oxidative stress contributes to the death of hippocampal neurons in vivo: role of microglial NADPH oxidase. J Neurosci. 2005;25:4082–90. doi: 10.1523/JNEUROSCI.4306-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi SH, Lee DY, Ryu JK, Kim J, Joe EH, Jin BK. Thrombin induces nigral dopaminergic neurodegeneration in vivo by altering expression of death-related proteins. Neurobiol Dis. 2003;14:181–93. doi: 10.1016/s0969-9961(03)00085-8. [DOI] [PubMed] [Google Scholar]
- Cirino G, Cicala C, Bucci MR, Sorrentino L, Maraganore JM, Stone SR. Thrombin functions as an inflammatory mediator through activation of its receptor. J Exp Med. 1996;183:821–7. doi: 10.1084/jem.183.3.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Castro Ribeiro M, Badaut J, Price M, Meins M, Bogousslavsky J, Monard D, Hirt L. Thrombin in ischemic neuronal death. Exp Neurol. 2006;198:199–203. doi: 10.1016/j.expneurol.2005.11.017. [DOI] [PubMed] [Google Scholar]
- DeLorenzo RJ, Kirmani B, Deshpande LS, Jakkampudi V, Towne AR, Waterhouse E, Garnett L, Ramakrishnan V. Comparisons of the mortality and clinical presentations of status epilepticus in private practice community and university hospital settings in Richmond, Virginia. Seizure. 2009;18:405–11. doi: 10.1016/j.seizure.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dihanich M, Kaser M, Reinhard E, Cunningham D, Monard D. Prothrombin mRNA is expressed by cells of the nervous system. Neuron. 1991;6:575–81. doi: 10.1016/0896-6273(91)90060-d. [DOI] [PubMed] [Google Scholar]
- Donovan FM, Pike CJ, Cotman CW, Cunningham DD. Thrombin induces apoptosis in cultured neurons and astrocytes via a pathway requiring tyrosine kinase and RhoA activities. J Neurosci. 1997;17:5316–26. doi: 10.1523/JNEUROSCI.17-14-05316.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubreuil CI, Marklund N, Deschamps K, McIntosh TK, McKerracher L. Activation of Rho after traumatic brain injury and seizure in rats. Exp Neurol. 2006;198:361–9. doi: 10.1016/j.expneurol.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Friedman A. Blood-brain barrier dysfunction, status epilepticus, seizures, and epilepsy: a puzzle of a chicken and egg? Epilepsia. 2011;52(Suppl 8):19–20. doi: 10.1111/j.1528-1167.2011.03227.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto S, Katsuki H, Ohnishi M, Takagi M, Kume T, Akaike A. Thrombin induces striatal neurotoxicity depending on mitogen-activated protein kinase pathways in vivo. Neuroscience. 2007;144:694–701. doi: 10.1016/j.neuroscience.2006.09.049. [DOI] [PubMed] [Google Scholar]
- Galanopoulou AS, Jensen FE, Kaminski RM. Issues Related to Development of Anti-Epileptogenic. Therapies. 2014;54:35–43. doi: 10.1111/epi.12297.Issues. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glien M, Brandt C, Potschka H, Voigt H, Ebert U, Löscher W. Repeated low-dose treatment of rats with pilocarpine: low mortality but high proportion of rats developing epilepsy. Epilepsy Res. 2001;46:111–9. doi: 10.1016/s0920-1211(01)00272-8. [DOI] [PubMed] [Google Scholar]
- Gulati A, Srimal RC, Dhawan KN, Dhawan BN. On the mechanism of potentiation of apomorphine-induced stereotypy due to electroconvulsive shock. Neuropharmacology. 1987;26:1733–7. doi: 10.1016/0028-3908(87)90125-0. [DOI] [PubMed] [Google Scholar]
- Hamill CE, Mannaioni G, Lyuboslavsky P, Sastre AA, Traynelis F. NIH Public Access. 2010;217:136–146. doi: 10.1016/j.expneurol.2009.01.023.Protease-activated. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han KS, Mannaioni G, Hamill CE, Lee J, Junge CE, Lee CJ, Traynelis SF. Activation of protease activated receptor 1 increases the excitability of the dentate granule neurons of hippocampus. Mol Brain. 2011;4:32. doi: 10.1186/1756-6606-4-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaev D, Zhao Q, Kleen JK, Lenck-Santini PP, Adstamongkonkul D, Isaeva E, Holmes GL. Neuroaminidase reduces interictal spikes in a rat temporal lobe epilepsy model. Epilepsia. 2011;52 doi: 10.1111/j.1528-1167.2011.02988.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaeva E, Hernan A, Isaev D, Holmes GL. Thrombin facilitates seizures through activation of persistent sodium current. Ann Neurol. 2012;72:192–8. doi: 10.1002/ana.23587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon BT, Jeong EA, Park SY, Son H, Shin HJ, Lee DH, Kim HJ, Kang SS, Cho GJ, Choi WS, Roh GS. The Rho-kinase (ROCK) inhibitor Y-27632 protects against excitotoxicity-induced neuronal death in vivo and in vitro. Neurotox Res. 2013;23:238–48. doi: 10.1007/s12640-012-9339-2. [DOI] [PubMed] [Google Scholar]
- Jiang J, Quan Y, Ganesh T, Pouliot Wa, Dudek FE, Dingledine R. Inhibition of the prostaglandin receptor EP2 following status epilepticus reduces delayed mortality and brain inflammation. Proc Natl Acad Sci U S A. 2013;110:3591–6. doi: 10.1073/pnas.1218498110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang W, Van Cleemput J, Sheerin AH, Ji SP, Zhang Y, Saucier DM, Corcoran ME, Zhang X. Involvement of extracellular regulated kinase and p38 kinase in hippocampal seizure tolerance. J Neurosci Res. 2005;81:581–8. doi: 10.1002/jnr.20566. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Wu J, Hua Y, Keep RF, Xiang J, Hoff JT, Xi G. Thrombin-receptor activation and thrombin-induced brain tolerance. J Cereb Blood Flow Metab. 2002;22:404–10. doi: 10.1097/00004647-200204000-00004. [DOI] [PubMed] [Google Scholar]
- Junge CE, Sugawara T, Mannaioni G, Alagarsamy S, Conn PJ, Brat DJ, Chan PH, Traynelis SF. The contribution of protease-activated receptor 1 to neuronal damage caused by transient focal cerebral ischemia. Proc Natl Acad Sci U S A. 2003;100:13019–24. doi: 10.1073/pnas.2235594100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YS, Hong KS, Seong YS, Park JB, Kuroda S, Kishi K, Kaibuchi K, Takai Y. Phosphorylation and activation of mitogen-activated protein kinase by kainic acid-induced seizure in rat hippocampus. Biochem Biophys Res Commun. 1994;202:1163–8. doi: 10.1006/bbrc.1994.2050. [DOI] [PubMed] [Google Scholar]
- Lakshmi Thirumangalakudi, Rao Haripriya Vittal, PGA Involvement of PGE2 and PGDH but not COX-2 in thrombin- induced cortical neuron apoptosis. Neurosci Lett. 2009;452:172–175. doi: 10.1016/j.neulet.2009.01.045.Involvement. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latour LL, Kang DW, Ezzeddine MA, Chalela JA, Warach S. Early blood-brain barrier disruption in human focal brain ischemia. Ann Neurol. 2004;56:468–77. doi: 10.1002/ana.20199. [DOI] [PubMed] [Google Scholar]
- Lee KR, Drury I, Vitarbo E, Hoff JT. Seizures induced by intracerebral injection of thrombin: a model of intracerebral hemorrhage. J Neurosurg. 1997;87:73–8. doi: 10.3171/jns.1997.87.1.0073. [DOI] [PubMed] [Google Scholar]
- Levin JR, Serrano G, Dingledine R. Reduction in delayed mortality and subtle improvement in retrograde memory performance in pilocarpine-treated mice with conditional neuronal deletion of cyclooxygenase-2 gene. Epilepsia. 2012;53:1411–20. doi: 10.1111/j.1528-1167.2012.03584.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo HM, Chen CL, Tsai YJ, Wu PH, Wu WB. Thrombin induces cyclooxygenase-2 expression and prostaglandin E2 release via PAR1 activation and ERK1/2- and p38 MAPK-dependent pathway in murine macrophages. J Cell Biochem. 2009;108:1143–52. doi: 10.1002/jcb.22341. [DOI] [PubMed] [Google Scholar]
- Luo W, Wang Y, Reiser G. Protease-activated receptors in the brain: receptor expression, activation, and functions in neurodegeneration and neuroprotection. Brain Res Rev. 2007;56:331–45. doi: 10.1016/j.brainresrev.2007.08.002. [DOI] [PubMed] [Google Scholar]
- Macfarlane SR, Seatter MJ, Kanke T, Hunter GD, Plevin R. Proteinase-activated receptors. Pharmacol Rev. 2001;53:245–82. [PubMed] [Google Scholar]
- Maggio N, Cavaliere C, Papa M, Blatt I, Chapman J, Segal M. Thrombin regulation of synaptic transmission: implications for seizure onset. Neurobiol Dis. 2013;50:171–8. doi: 10.1016/j.nbd.2012.10.017. [DOI] [PubMed] [Google Scholar]
- Manaenko A, Sun X, Kim CH, Yan J, Ma Q, Zhang JH. PAR-1 antagonist SCH79797 ameliorates apoptosis following surgical brain injury through inhibition of ASK1-JNK in rats. Neurobiol Dis. 2013;50:13–20. doi: 10.1016/j.nbd.2012.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchi N, Granata T, Freri E, Ciusani E, Ragona F, Puvenna V, Teng Q, Alexopolous A, Janigro D. Efficacy of anti-inflammatory therapy in a model of acute seizures and in a population of pediatric drug resistant epileptics. PLoS One. 2011;6:e18200. doi: 10.1371/journal.pone.0018200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzuferi M, Kumar G, Rospo C, Kaminski RM. Rapid epileptogenesis in the mouse pilocarpine model: video-EEG, pharmacokinetic and histopathological characterization. Exp Neurol. 2012;238:156–67. doi: 10.1016/j.expneurol.2012.08.022. [DOI] [PubMed] [Google Scholar]
- McPhail LT, McBride CB, McGraw J, Steeves JD, Tetzlaff W. Axotomy abolishes NeuN expression in facial but not rubrospinal neurons. Exp Neurol. 2004;185:182–190. doi: 10.1016/j.expneurol.2003.10.001. [DOI] [PubMed] [Google Scholar]
- Mihály A, Bozóky B. Immunohistochemical localization of extravasated serum albumin in the hippocampus of human subjects with partial and generalized epilepsies and epileptiform convulsions. Acta Neuropathol. 1984;65:25–34. doi: 10.1007/BF00689824. [DOI] [PubMed] [Google Scholar]
- Ndode-Ekane XE, Hayward N, Gröhn O, Pitkänen A. Vascular changes in epilepsy: functional consequences and association with network plasticity in pilocarpine-induced experimental epilepsy. Neuroscience. 2010;166:312–32. doi: 10.1016/j.neuroscience.2009.12.002. [DOI] [PubMed] [Google Scholar]
- Oby E, Janigro D. The blood–brain barrier and epilepsy. Epilepsia; 2006. [DOI] [PubMed] [Google Scholar]
- Oby E, Janigro D. The blood-brain barrier and epilepsy. Epilepsia. 2006;47:1761–74. doi: 10.1111/j.1528-1167.2006.00817.x. [DOI] [PubMed] [Google Scholar]
- Olson EE, Lyuboslavsky P, Traynelis SF, McKeon RJ. PAR-1 deficiency protects against neuronal damage and neurologic deficits after unilateral cerebral hypoxia/ischemia. J Cereb Blood Flow Metab. 2004;24:964–71. doi: 10.1097/01.WCB.0000128266.87474.BF. [DOI] [PubMed] [Google Scholar]
- On NH, Mitchell R, Savant SD, Bachmeier CJ, Hatch GM, Miller DW. Examination of blood-brain barrier (BBB) integrity in a mouse brain tumor model. J Neurooncol. 2013;111:133–43. doi: 10.1007/s11060-012-1006-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racine RJ. Modification of seizure activity by electrical stimulation. II Motor seizure. Electroencephalogr Clin Neurophysiol. 1972;32:281–94. doi: 10.1016/0013-4694(72)90177-0. [DOI] [PubMed] [Google Scholar]
- Ravizza T, Balosso S, Vezzani A. Inflammation and prevention of epileptogenesis. Neurosci Lett. 2011;497:223–30. doi: 10.1016/j.neulet.2011.02.040. [DOI] [PubMed] [Google Scholar]
- Riek-Burchardt M, Striggow F, Henrich-Noack P, Reiser G, Reymann KG. Increase of prothrombin-mRNA after global cerebral ischemia in rats, with constant expression of protease nexin-1 and protease-activated receptors. Neurosci Lett. 2002;329:181–4. doi: 10.1016/s0304-3940(02)00645-6. [DOI] [PubMed] [Google Scholar]
- Rohatgi T, Sedehizade F, Goertler M, Wallesch CW, Reymann KG, Reiser G. Transient Focal Ischemia in Rat Brain Differentially Regulates mRNA Expression of Protease-Activated Receptors 1 to 4. 2004;279:273–279. doi: 10.1002/jnr.10847. [DOI] [PubMed] [Google Scholar]
- Rohatgi T, Sedehizade F, Reymann KG, Reiser G. Protease-activated receptors in neuronal development, neurodegeneration, and neuroprotection: thrombin as signaling molecule in the brain. Neuroscientist. 2004;10:501–12. doi: 10.1177/1073858404269955. [DOI] [PubMed] [Google Scholar]
- Salami P, Lévesque M, Benini R, Behr C, Gotman J, Avoli M. Dynamics of interictal spikes and high-frequency oscillations during epileptogenesis in temporal lobe epilepsy. Neurobiol Dis. 2014;67:97–106. doi: 10.1016/j.nbd.2014.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierra-Marcos A, Alvarez V, Faouzi M, Burnand B, Rossetti AO. Statins are associated with decreased mortality risk after status epilepticus. Eur J Neurol. 2014 doi: 10.1111/ene.12428. [DOI] [PubMed] [Google Scholar]
- Smirnova IV, Zhang SX, Citron BA, Arnold PM, Festoff BW. Thrombin is an extracellular signal that activates intracellular death protease pathways inducing apoptosis in model motor neurons. J Neurobiol. 1998;36:64–80. [PubMed] [Google Scholar]
- Staley KJ, White A, Dudek FE. Interictal spikes: harbingers or causes of epilepsy? Neurosci Lett. 2011;497:247–50. doi: 10.1016/j.neulet.2011.03.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolp HB, Dziegielewska KM. Review: Role of developmental inflammation and blood-brain barrier dysfunction in neurodevelopmental and neurodegenerative diseases. Neuropathol Appl Neurobiol. 2009;35:132–46. doi: 10.1111/j.1365-2990.2008.01005.x. [DOI] [PubMed] [Google Scholar]
- Strande JL, Hsu A, Su J, Fu X, Gross GJ, Baker JE. SCH 79797, a selective PAR1 antagonist, limits myocardial ischemia/reperfusion injury in rat hearts. Basic Res Cardiol. 2007;102:350–8. doi: 10.1007/s00395-007-0653-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Striggow F, Riek M, Breder J, Henrich-Noack P, Reymann KG, Reiser G. The protease thrombin is an endogenous mediator of hippocampal neuroprotection against ischemia at low concentrations but causes degeneration at high concentrations. Proc Natl Acad Sci U S A. 2000;97:2264–9. doi: 10.1073/pnas.040552897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Striggow F, Riek-Burchardt M, Kiesel a, Schmidt W, Henrich-Noack P, Breder J, Krug M, Reymann KG, Reiser G. Four different types of protease-activated receptors are widely expressed in the brain and are up-regulated in hippocampus by severe ischemia. Eur J Neurosci. 2001;14:595–608. doi: 10.1046/j.0953-816x.2001.01676.x. [DOI] [PubMed] [Google Scholar]
- Syeda F, Grosjean J, Houliston Ra, Keogh RJ, Carter TD, Paleolog E, Wheeler-Jones CPD. Cyclooxygenase-2 induction and prostacyclin release by protease-activated receptors in endothelial cells require cooperation between mitogen-activated protein kinase and NF-kappaB pathways. J Biol Chem. 2006;281:11792–804. doi: 10.1074/jbc.M509292200. [DOI] [PubMed] [Google Scholar]
- Tomkins O, Friedman O, Ivens S, Reiffurth C, Major S, Dreier JP, Heinemann U, Friedman a. Blood-brain barrier disruption results in delayed functional and structural alterations in the rat neocortex. Neurobiol Dis. 2007;25:367–77. doi: 10.1016/j.nbd.2006.10.006. [DOI] [PubMed] [Google Scholar]
- Turgeon VL, Salman N, Houenou LJ. Thrombin: a neuronal cell modulator. Thromb Res. 2000;99:417–27. doi: 10.1016/s0049-3848(00)00300-5. [DOI] [PubMed] [Google Scholar]
- Unal-Cevik I, Kilinç M, Gürsoy-Ozdemir Y, Gurer G, Dalkara T. Loss of NeuN immunoreactivity after cerebral ischemia does not indicate neuronal cell loss: a cautionary note. Brain Res. 2004;1015:169–74. doi: 10.1016/j.brainres.2004.04.032. [DOI] [PubMed] [Google Scholar]
- Van Vliet Ea, da Costa Araújo S, Redeker S, van Schaik R, Aronica E, Gorter Ja. Blood-brain barrier leakage may lead to progression of temporal lobe epilepsy. Brain. 2007;130:521–34. doi: 10.1093/brain/awl318. [DOI] [PubMed] [Google Scholar]
- Vergnolle N, Wallace JL, Bunnett NW, Hollenberg MD. Protease-activated receptors in inflammation, neuronal signaling and pain. Trends Pharmacol Sci. 2001;22:146–52. doi: 10.1016/s0165-6147(00)01634-5. [DOI] [PubMed] [Google Scholar]
- Vezzani A, Friedman A. Brain inflammation as a biomarker in epilepsy. Biomark Med. 2011;5:607–14. doi: 10.2217/bmm.11.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Jin H, Hua Y, Keep RF, Xi G. Role of protease-activated receptor-1 in brain injury after experimental global cerebral ischemia. Stroke. 2012;43:2476–82. doi: 10.1161/STROKEAHA.112.661819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein JR, Lau AL, Brass LF, Cunningham DD. Injury-related factors and conditions down-regulate the thrombin receptor (PAR-1) in a human neuronal cell line. J Neurochem. 1998;71:1034–50. doi: 10.1046/j.1471-4159.1998.71031034.x. [DOI] [PubMed] [Google Scholar]
- White A, Williams Pa, Hellier JL, Clark S, Dudek FE, Staley KJ. EEG spike activity precedes epilepsy after kainate-induced status epilepticus. Epilepsia. 2010;51:371–83. doi: 10.1111/j.1528-1167.2009.02339.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willmore LJ, Sypert GW, Munson JB. Recurrent seizures induced by cortical iron injection: a model of posttraumatic epilepsy. Ann Neurol. 1978;4:329–36. doi: 10.1002/ana.410040408. [DOI] [PubMed] [Google Scholar]
- Woitzik J, Hohenstein A, Hecht N, Juettler E, Schilling L. Short period of early reperfusion aggravates blood-brain barrier dysfunction during permanent focal ischemia in rats. Transl Stroke Res. 2011;2:67–71. doi: 10.1007/s12975-010-0042-4. [DOI] [PubMed] [Google Scholar]
- Xi G, Reiser G, Keep RF. The role of thrombin and thrombin receptors in ischemic, hemorrhagic and traumatic brain injury: deleterious or protective? J Neurochem. 2003;84:3–9. doi: 10.1046/j.1471-4159.2003.01268.x. [DOI] [PubMed] [Google Scholar]
- Yuan J, Wang L, Li J, Cao N, Wang L, Feng G, Xue T, Lu Y, Wang X. Altered expression of the small guanosine triphosphatase RhoA in human temporal lobe epilepsy. J Mol Neurosci. 2010;42:53–8. doi: 10.1007/s12031-010-9330-4. [DOI] [PubMed] [Google Scholar]