Abstract
Aims
Aldosterone activation is central to the sodium-fluid retention that marks the progression of heart failure (HF). The actions of dietary sodium restriction, a mainstay in HF management, on cardiorenal and neuroendocrine adaptations during the progression of HF are poorly understood. The study aim was to assess the role of dietary sodium during the progression of experimental HF.
Methods and Results
Experimental HF was produced in a canine model by rapid right ventricular pacing which evolves from early mild HF to overt, severe HF. Dogs were fed one of three diets: 1) high sodium [250 mEq (5.8 grams) per day, n=6]; 2) standard sodium [58 mEq (1.3 grams) per day, n=6]; and 3) sodium restriction [11 mEq (0.25 grams) per day, n=6]. During the 38 day study hemodynamics, renal function, renin activity (PRA), and aldosterone were measured. Changes in hemodynamics at 38 days were similar in all three groups, as were changes in renal function. Aldosterone activation was demonstrated in all three groups, however, dietary sodium restriction, in contrast to high sodium, resulted in early (10 days) activation of PRA and aldosterone. High sodium demonstrated significant suppression of aldosterone activation over the course of HF progression.
Conclusions
Excessive dietary sodium restriction particularly in early stage HF results in early aldosterone activation, while normal and excess sodium intake are associated with delayed or suppressed activation. These findings warrant evaluation in humans to determine if dietary sodium manipulation, particularly during early stage HF, may have a significant impact on neuroendocrine disease progression.
Keywords: heart failure, canine model, aldosterone, renin, dietary sodium, renal function
Introduction
Aldosterone has emerged as a key hormone in the development of heart failure (HF) based upon its pleiotropic actions mediated by mineralocorticoid receptors. These actions include pro-myocardial fibrosis effects and the induction of oxidative stress in the vasculature.1 Also, of importance is the avid renal sodium-water retaining effect of aldosterone which contributes to the development of congestion in HF. The clinical importance of aldosterone activation in HF has also been established by pivotal clinical trials utilizing mineralocorticoid receptor antagonists. In the landmark trial RALES, 2 aldosterone receptor blockade in advanced symptomatic HF improved mortality. More recently, in the EMPHASIS-HF trial 3 of mild symptomatic systolic HF, aldosterone antagonism also improved mortality. These results support the concept that aldosterone activation across the spectrum of HF from mild to severe symptomatic ventricular systolic dysfunction is deleterious.
Further, in the context of renal sodium homeostasis and neurohormonal activation in HF, a well articulated commentary by Gupta et al. 4 addressed the need to better understand a mainstay in HF therapy – the impact of dietary sodium on HF progression. While discussing the restriction of dietary sodium as a fundamental guideline recommended early step in HF management, Gupta et al. commented that little is known about the full impact of dietary sodium restriction on neurohormonal function and clinical outcomes. They called for more studies to advance the understanding of the physiology of dietary sodium changes in HF and also the need for studies defining actions on symptoms and disease progression. Indeed, the findings of some studies suggest that excessive sodium restriction may be detrimental and result in excess cardiovascular morbidity and mortality, 5-8 whereas moderate sodium intake may actually reduce hospitalizations and adverse outcomes. 5,8,9 The modulating actions of dietary sodium in HF upon aldosterone to date remain undefined across the spectrum of ventricular systolic dysfunction prompting the current study in experimental HF.
The objective of this investigation was to address the role of dietary sodium manipulation in the activation of aldosterone and associated renal function and clinical symptoms during the development and progression of experimental HF. We hypothesized that normal and high dietary sodium intake would be associated with delayed activation of aldosterone compared to dietary sodium restriction. In contrast, marked dietary sodium restriction would be characterized by early activation of aldosterone. To address this hypothesis, a chronic conscious dog model without confounding medication therapy was established by rapid right ventricular pacing 10 with stringent dietary sodium restriction, normal (moderate) sodium intake, and a high dietary sodium intake during experimental HF. This model evolves from a state of compensated early left ventricular dysfunction which simulates Stage B HF to, with increasing pacing rates over a 38 day period, a model of overt CHF. We also employed a clinical score which we have previously reported 11 to assess the overall clinical well being of the animals.
Methods
Animal Model
Studies were conducted in 20 kg adult male mongrel dogs in accordance with the Animal Welfare Act. All dogs were allowed free access to water and fed one of the three specified diets: 1) high sodium diet (n=6) containing 250 mEq (5.8 grams) of sodium per day (58 mEq/day from Hills I-D plus an additional 192 mEq of sodium); 2) a standard sodium diet (n=6) containing 58 mEq (1.3 grams) of sodium per day (Hills I-D); and 3) a sodium restricted diet (n=6) containing 11 mEq (0.25 grams) of sodium per day (Hills I-D). The specified diets were initiated one week before obtaining baseline data. Each animal was individually housed in a metabolic cage for the period of the study to allow for continuous urine collections and intake monitoring.
Surgical Preparation
In a sterile surgical suite, adult male mongrel dogs were anesthetized utilizing pentobarbital sodium (30 mg/kg IV) and ventilated with supplemental oxygen (5 L/min) via an endotracheal tube utilizing a Harvard respirator (Harvard Apparatus, Millis, MA). An epicardial lead (Medtronic, Minneapolis, MN) was implanted in the right ventricle via a left thoracotomy with a 1-2 cm pericardiotomy. The pacemaker lead was connected to a pulse generator (Medtronic, Minneapolis, MN, model 8329) which was then implanted subcutaneously in the chest wall. Pacing capture was verified intraoperatively prior to closing the chest cavity. The pericardium was sutured closed with great care not to distort the anatomy of the pericardium. The chest cavity with deep and superficial incisions was closed in layers.
A femoral artery catheter (Model GPV Vascular-Access Port, Access Technologies, Skokie, IL) was placed chronically for mean arterial pressure monitoring and blood sampling. The catheter was implanted into the left femoral artery with a self-sealing silicone rubber septum port tunneled subcutaneously to the left upper hind limb. Dogs received pre and postoperative prophylactic antibiotic treatment with 225 mg clindamycin subcutaneously and 400,000 U procaine penicillin G plus 500 mg dihydrostreptomycin intramuscularly (Combiotic, Pfizer, Inc., New York NY). Postoperative prophylactic antibiotic was continued through the first two postoperative days.
Ventricular Pacing
Experimental HF was produced by incremental rapid right ventricular pacing. 10 Following a 14 day postoperative recovery period, rapid ventricular pacing was initiated at 180 beats per minute (bpm) for ten days. The pacing rate was then increased at seven day intervals to 200, 210, 220 and 240 bpm. At the completion of each pacing interval mean arterial pressure (MAP) and blood for neuroendocrine analysis was obtained and immediately placed on ice and processed. During the final two days of each pacing interval, two consecutive 24 hour urine collections were obtained for urine volume and sodium excretion.
Echocardiography
A two-dimensional and two-dimensional guided M-mode echocardiogram (Toshiba, Japan) was performed from the right parasternal window of the dog at Baseline, day 10, day 24, and day 38. Left ventricular end-diastolic (LVEDd) and end-systolic (LVESd) dimensions were measured from the two-dimensional guided M-mode tracings. Echocardiograms were performed in the conscious state with the dog unrestrained and standing quietly. All images were obtained with the pacemaker deprogrammed for a total imaging period of less than ten minutes. Three cardiac cycles were measured and the average of the three measurements recorded. No cycles after a premature or paced premature beat were used for analysis. The ejection fraction was calculated using a modified version from Quinones 12 as follows: [(LVEDd2 – LVESd2)/LVEDd2].
Clinical Profile Score
A canine clinical assessment scale has been developed and serves as an additional means of assessing the effects of progressive experimental heart failure. 11 This score was assigned to each dog by two investigators independently at baseline and at each interval that echocardiography was performed. The six criteria were as follows: 1) Slows/stops with brisk 100 yard walk, 2) Eating <100% of daily meal, 3) Respiratory rate doubled from baseline. 4) Muscle atrophy/loss of body fat. 5) Ascites, and 6) Left ventricular ejection fraction <50% of baseline. For each criterion that the dog manifested, a score of 1 was given. Scores ranged from zero if all the criteria were absent to a maximum of six if all the criteria were present.
Study Protocol
The following protocol was undertaken for each of the animals at baseline prior to initiation of experimental HF and at completion of the chronic protocol during overt CHF. Each dog was anesthetized with thiopental sodium (15 mg/kg IV) to allow sterile percutaneous placement of a flow-directed balloon tip pulmonary artery catheter (model 93131-7F; American Edwards Laboratories, AHS del Caribe, Anasco, PR) via an internal jugular vein. The chronic indwelling arterial catheter was connected to a pressure monitor for on-line measurement of aortic pressure and for blood sampling. A second balloon tip catheter was inserted in the urinary bladder for urine collection. After recovery from anesthesia, each dog was allowed to stand freely in a minimally restricting body sling. Each dog was allowed to stabilize for a 60 minute period prior to parameter measurements. For characterization of renal function, a loading dose of PAH and/or inulin followed by a PAH and/or inulin solution was infused at a rate of 1 ml/min. For assessment of renal tubular function, lithium carbonate 300 mg p.o. was given the night prior to the acute protocol. The acute protocol consisted of four 30 minute clearances with hemodynamics and blood sampling via the arterial port occurring at the midpoint of the clearance.
During the experimental periods, the following hemodynamic data were collected: mean arterial pressure (MAP), right atrial pressure (RAP), pulmonary artery pressure (PAP), pulmonary capillary pressure (PCWP), and cardiac output (CO). Cardiac output was measured by thermodilution (American Edwards Cardiac Output Computer model 9510-A, Irvine, CA). Cardiac output was averaged from four measurements. Systemic vascular resistance (SVR) was calculated as [(mean arterial pressure-right atrial pressure)/cardiac output]. Pulmonary vascular resistance (PVR) was calculated as [(mean pulmonary artery pressure-pulmonary capillary wedge pressure)/cardiac output]. Renal vascular resistance (RVR) was calculated as [(mean arterial pressure-right atrial pressure)/renal blood flow]. MAP was assessed via direct measurement from the chronic arterial catheter. Glomerular filtration rate (GFR) was measured by inulin clearance. Renal blood flow (RBF) was calculated from estimated renal plasma flow (PAH clearance) and hematocrit. Urine was collected on ice during the entire clearance period for assessment of urine volume, electrolytes, inulin, and PAH.
Plasma Analyses
Arterial blood for hormone analysis was obtained during the chronic studies at baseline and during each pacing interval change. Plasma samples were collected in heparin and EDTA tubes and immediately placed on ice. After centrifugation at 2,500 rpm at 4°C, the plasma was decanted and stored at -20°C until analysis. Specific plasma radioimmunoassay included plasma renin activity (PRA) and aldosterone as previously described. 13 Blood for hormone analysis was collected in an ethylenediaminetetraacetic acid (EDTA) tube and immediately placed on ice. After centrifugation of 2,500 rpm at 4°C, plasma was separated and stored at -20°C until assay. Urinary and plasma lithium and electrolytes were determined by flame emission spectrophotometry (model 357, Instrumentation Laboratory, Wilmington, MA). Lithium clearances were used as an indirect method to calculate proximal and distal fractional tubular reabsorption [(clearance of lithium-clearance of sodium)/clearance of lithium] × 100.
Statistical Analysis
Results of the quantitative studies were expressed as mean ± SEM. Data were assessed by Student’s unpaired t test and factorial ANOVA for comparison between groups. Student’s paired t tests for single comparisons of absolute changes within each group and by ANOVA for repeated measures followed by Bonferroni correction post-test when appropriate. Statistical significance was accepted as p<0.05.
Results
Clinical Profile Score During the Progression to Congestive Heart Failure
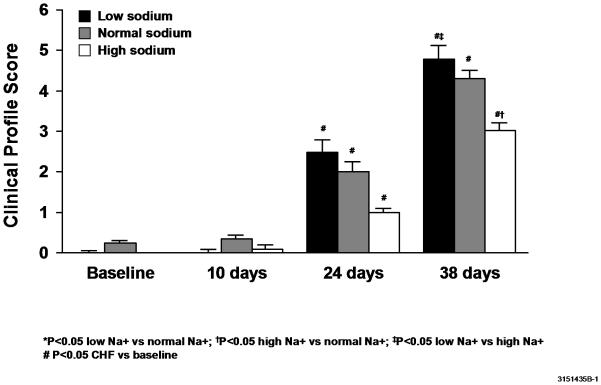
In an attempt to quantify the clinical status of each animal, a clinical score was devised and assigned to each animal. Dogs that received either a low sodium diet (4.8 ± 0.3) or a normal sodium diet (4.3 ±0.2) had a significantly higher (worse) score than dogs receiving a high sodium diet (3.0 ± 0.2) during experimental CHF (Figure 1).
Figure 1.
Canine clinical profile scores.
Sodium Balance during the Progression of HF
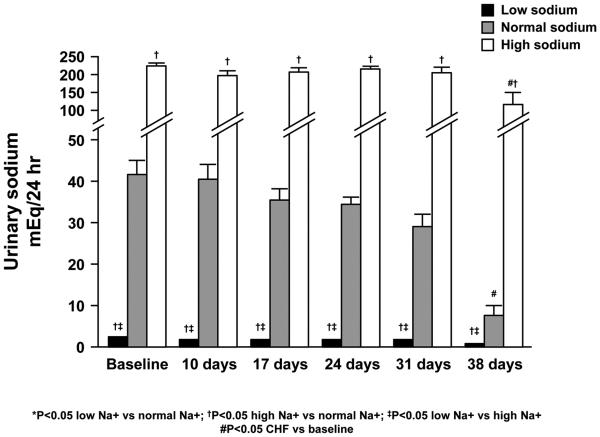
Twenty four hour urine collections to quantitate sodium excretion and assess sodium homeostasis were obtained at baseline and at the conclusion of each pacing interval (Figure 2). All three groups of dogs were able to maintain sodium balance through 24 days of CHF as measured by daily urine sodium excretion. At 31 days of CHF, however, the group with dietary sodium restriction demonstrated a significant decrease in urinary sodium excretion as compared to baseline (3.2 ± 0.4 vs. 1.3 ± 0.5 mEq/24 hours). Then at 38 days of CHF (compared to baseline) both the normal sodium diet (42.3 ± 4.5 vs. 9.2 ± 4.9 mEq/24 hours) and the high sodium diet (224.5 ± 6.6 vs. 119.4 ± 35.1 mEq/24 hours) were associated with a significant decrease in sodium excretion. Although the percent decrease in sodium excretion was greatest in the low sodium group, the magnitude of the absolute decrease in sodium excretion was greatest in dogs receiving the high sodium diet (105 ± 30 mEq/24 hours).
Figure 2.
24 hour sodium excretion.
Plasma Renin Activity and Aldosterone during the Progression to HF
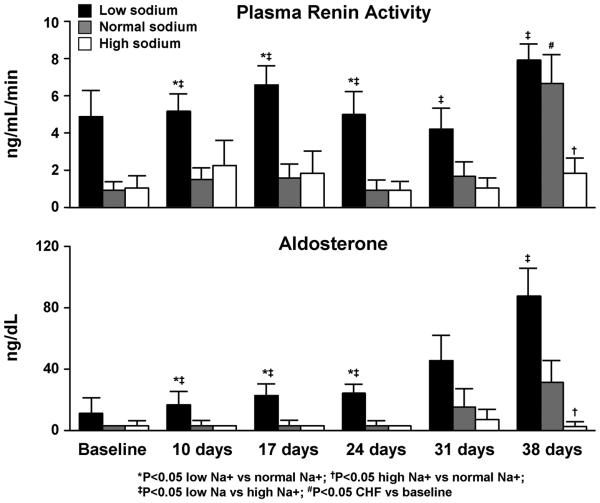
Dietary sodium restriction relative to normal and high sodium intake resulted in early stimulation (baseline and day 10) of PRA and aldosterone (Figure 3). PRA and aldosterone concentrations did not further increase with HF progression within the respective sodium intake groups.
Figure 3.
Modulating actions of dietary sodium on plasma rennin activity and aldosterone activation in experimental heart failure.
Cardiovascular and Renal Function at Baseline and Overt CHF
The modulating actions of dietary sodium intake on hemodynamic and renal function parameters at baseline and with CHF are shown in Table 1. Baseline cardiovascular hemodynamics were similar in all three dietary sodium groups. Similar decreases in CO and MAP and increases in PCWP and SVR were observed in association with 38 days of CHF in all three groups. Neither dietary sodium restriction, nor high dietary sodium intake as compared to a normal sodium diet resulted in statistically significant differences in MAP, CO, PCWP, or SVR among the groups at baseline or when compared in overt CHF.
Table 1.
Cardiovascular and Renal Function
| Low Sodium | Normal Sodium | High Sodium | |
|---|---|---|---|
| MAP, mmHg | |||
| Baseline | 121 ± 4 | 116 ± 5 | 118 ± 2 |
| CHF | 99 ± 5 * | 97 ± 6 * | 94 ± 2 * |
|
| |||
| CO, L/min | |||
| Baseline | 5.0 ± 0.4 | 5.2 ± 0.4 | 5.3 ± 0.4 |
| CHF | 2.0 ± 0.1 * | 2.4 ± 0.3 * | 2.5 ± 0.3 * |
|
| |||
| PCWP, mmHg | |||
| Baseline | 8.3 ± 0.5 | 10.2 ± 1.1 | 9.3 ± 0.7 |
| CHF | 21.3 ± 1.3 * | 22 ± 1.9 * | 24 ± 2 * |
|
| |||
| RAP, mmHg | |||
| Baseline | 4.2±0.5 | 5.2±0.8 | 5.3±0.9 |
| CHF | 14.8±0.8 * | 12.5±0.8 * | 14.0±1.0 * |
| SVR, WU | |||
| Baseline | 22.4 ± 0.9 | 20.1 ± 2.1 | 19.1 ± 0.8 |
| CHF | 42 ± 3.4 * | 34 ± 4.1 * | 33 ± 4.2 * |
|
| |||
| RBF, ml/min | |||
| Baseline | 321 ± 36 | 331 ± 78 | 378 ± 151 |
| CHF | 157 ± 49 * | 156 ± 16 * | 142 ± 19 * |
|
| |||
| GFR, ml/min | |||
| Baseline | 57 ± 4 | 72 ± 10 | 79 ± 15 |
| CHF | 49 ± 12 | 56 ± 5 | 69 ± 21 |
|
| |||
| Urine Volume, mL/24 hours |
|||
| Baseline | 535±103† | 494±38 | 1138±161‡ |
| CHF | 509±79 | 316±20 * | 606±60 ‡ * |
|
| |||
| FENa+, % | |||
| Baseline | 0.053 ± 0.09†§ | 0.28 ± 0.08 | 1.07 ± 0.2‡ |
| CHF | 0.028 ± 0.004 †§ | 0.38 ± 0.33 | 0.86 ± 0.67 |
|
| |||
| Body weight, kg | |||
| Baseline | 21.1±0.7 | 19.6±0.8 | 20.3±0.7 |
| CHF | 19.3±0.7 | 21.5±1.1 | 20.1±1.1 |
CO = cardiac output; MAP = mean arterial pressure; PCWP = pulmonary capillary wedge pressure; SVR = systemic vascular resistance; WU = Wood units (mPAP-PCWP/CO) RBF = renal blood flow; GFR = glomerular filtration rate; FENa+ = fractional excretion of sodium
(Low Sodium n=6; Normal Sodium n=6; High Sodium n=6)
p<0.05 CHF vs Baseline
p<0.05 High Sodium vs. Low Sodium
p<0.05 High Sodium vs. Normal Sodium
p<0.05 Low Sodium vs. Normal Sodium
Baseline and CHF renal hemodynamics and function are also shown in Table 1. RBF was significantly reduced in all three sodium groups with CHF, but neither dietary sodium restriction nor high sodium intake resulted in changes in GFR; RVR was significantly increased with CHF only in the low sodium group (0.81±0.18 vs 0.35±0.03 Wood units, p<0.05). Sodium excretion (24 hour) was significantly decreased in all three sodium intake groups in CHF. At baseline, dietary sodium restriction did result in a decrease in FENa+ as compared to normal and high sodium diets. Distal tubular sodium reabsorption was increased as compared to dogs receiving the high sodium diet (99.4 ± 0.29% vs. 94.5 ± 0.6%, p<0.05). High dietary sodium intake resulted in an increase in FENa+ (1.07 ± 0.2% vs. 0.275 ± 0.08%, p<0.05) and a decrease in distal tubular reabsorption of sodium as compared to normal sodium diet (94.5 ± 0.6% vs. 97.8 ± 0.66%, p<0.05).
As shown in Table 2, all three groups demonstrated a gradual decrease in left ventricular ejection fraction (LVEF) during the progression of experimental HF with significant differences noted as early as 10 days. The absolute decrease in LVEF in the low sodium group was most significant at 38 days (17.6 ± 1.9% vs. 23.5 ± 2.4%) of CHF as compared to the high sodium group. The end diastolic diameter, however, was significantly increased in all three groups by 24 days. Animals receiving a high sodium diet demonstrated a significantly greater degree of dilation as compared to the low sodium diet (49.6±1.3mm vs 44.0±1.2mm) which was an approximately 21% increase with the high sodium diet compared to 14% increase with the low sodium diet.
Table 2.
Echocardiographic Parameters in Experimental Heart Failure
| Baseline | 10 Days CHF |
24 Days CHF |
38 Days CHF |
|
|---|---|---|---|---|
| Ejection Fraction, % | ||||
| Low Sodium | 55.1 ± 2.3 | 38.6 ± 4.3 * | 23.9 ± 1.9 * | 17.6 ± 1.9 * |
| Normal Sodium | 52.0 ± 2.7 | 29.2 ± 3.8 * | 23.4 ± 3.1 * | 21.2 ± 2.3 * |
| High Sodium | 48.3 ± 2.7 | 33.0 ± 3.8 * | 26.7 ± 3.1 * | 23.5 ± 2.4† * |
|
| ||||
| End Diastolic | ||||
| Diameter, mm | ||||
| Low Sodium | 38.7 ± 0.7 | 41.3 ± 1.8 | 44.5 ± 1.1 * | 44.0 ± 1.2 * |
| Normal Sodium | 38.8 ± 0.9 | 42.1 ± 2.0 | 46.0 ± 1.3 * | 48.0 ± 1.2 * |
| High Sodium | 40.8 ± 0.9 | 45.7 ± 2.0 * | 48.9 ± 1.4 * | 49.6 ± 1.3 † * |
Ejection fraction expressed in percent; End diastolic diameter in millimeters
(Low Sodium n=6; Normal Sodium n=6; High Sodium n=6)
p<0.05 CHF vs Baseline
p<0.05 High Sodium vs. Low Sodium
Discussion
Dietary sodium restriction has emerged as a guideline recommended step in the management of patients with HF, but the heterogeneity in the recommended level of sodium restriction and a lack of accounting for differences in the physiology of sodium homeostasis in the different stages of HF may contribute to a lack in consistency and compliance in patient and provider adherence to the recommendations. 4 Dietary sodium excess has conventionally been viewed as being detrimental, but the modulating actions of dietary sodium and the effects of chronic dietary sodium manipulation during the development and progression of HF remain poorly understood. The most striking finding of our study demonstrating the pathophysiologic progression of HF in a canine model without the confounding issues of diuretic or neuroendocrine inhibiting drug therapy was that high dietary sodium intake (5.8 g/day) suppressed, and very low dietary sodium (0.25g/day) accelerated the aldosterone activation that characterizes the development to symptomatic CHF. Our model of HF allowed full hemodynamic monitoring during the progression of HF and while changes were noted from baseline to overt CHF, there were no significant hemodynamic differences among the three dietary sodium groups with established CHF. LVEF declined over the course of the pacing period in all groups although the decline was most significant in the restricted dietary sodium group with a relatively higher EF maintained in the high dietary sodium group (at 38 days of pacing 24±2% vs 18±2%, p<0.05). LVEF did not predict CO, RBF, or RAAS activation, nor the presence or clinical severity of CHF which appear to reflect the magnitude of salt and water retention, rather than LVEF per se.
The mechanism of the early activation of aldosterone with low sodium diet and/or the delayed activation of aldosterone on higher sodium intakes may be multifactorial but may be importantly related to the macula densa regulation of intrarenal renin production. With low dietary sodium intake, and comparable levels of GFR, there may be less filtered sodium with a greater uptake in the proximal tubule, and, therefore, less sodium delivered to the macula densa. Such decreased delivery of solute to the macula densa may result in more stimulation of renin release leading to increased levels of angiotensin II resulting in increased secretion of aldosterone. In contrast, more filtered sodium with moderate and high dietary sodium diet would result in greater sodium delivery to the macula densa with suppression of renin release and ultimately of aldosterone. In contrast to these early findings, any contribution of reduced water intake and associated intravascular volume contraction to further neurohormonal activation and renal dysfunction would be a late even in the progression of HF occurring at the time when anorexia was observed to develop in the restricted dietary sodium group.
Sodium balance as monitored by 24 hour urine sodium collection was maintained in all three groups, albeit at different levels, until the development of more advanced HF as reflected in the excess PRA and aldosterone activation and worsening clinical profile scores. At 38 days all three groups demonstrated significant declines in sodium excretion relative to baseline values and this corresponded to the most marked sodium-retaining aldosterone activation observed. This finding is contrasted by the results in the high dietary sodium group where aldosterone activation was suppressed even relative to normal dietary sodium intake. Also, of note is that high dietary sodium intake during HF did not result in elevated LV filling pressures or increased systemic vascular resistance relative to the normal or restricted dietary sodium groups; this may, in part, reflect the relatively short duration of established CHF in this model. The reductions in 24 hour urine sodium observed in the setting of advancing HF, particularly at day 38, may have reflected a reduction in sodium intake in association with anorexia of advanced HF. Importantly, anorexia and, therefore sodium intake reduction, was noted only in dogs on the very restricted sodium diet whereas the dogs on a high sodium diet retained a good appetite and consumed all food daily. Also of value to recognize is that in all groups there was an increase in sodium reabsorption at the tubular level as demonstrated in the acute studies at baseline and 38 days in which the fractional excretion of sodium was reduced in all three groups.
The findings of our study support the concept that the manipulation of dietary sodium intake is highly important in the management of HF and that strict sodium restriction may be detrimental particularly in the early stages of HF and promote early excess activation and acceleration of neuroendocrine pathways. The result is an inappropriate elevation in plasma aldosterone relative to dietary sodium intake (hyperaldosteronism) which has significant pathophysiologic consequences that contribute to the progression of HF. Our findings in experimental HF build upon the elegant studies of Braunwald et al.14, Cody et al.15, and Volpe et al.16 Specifically, previous studies have demonstrated the failure of the kidney to be able to excrete a sodium load in the presence of HF14. Cody et al. 15 elegantly demonstrated that dietary sodium restriction comparable to that used in our study markedly activated the RAAS system in human HF, and that the renin-angiotensin system illustrated by Volpe et al.16 mediated mild and early sodium retention even in the early stages of HF which could be reversed with captopril. Our findings now strongly demonstrate that aldosterone is also not only activated in the early stages of experimental HF by marked dietary sodium restriction, but that high sodium intake, as well as, moderate sodium intake can appropriately retard the activation of aldosterone, which has fundamental direct actions not only on the kidney, but also structural changes in the heart. Moderation in sodium intake has been reported to be associated with longer event-free survival in patients with compensated HF 5,7 and sodium restriction with an increased risk of myocardial infarction in hypertensive patients. 17 Such studies taken together underscore the importance of further research which is required to fully appreciate the modulating actions of dietary sodium excess and dietary sodium restriction upon the progression of HF.
This study also has important implications for clinical trials in HF in that it underscores the importance of further defining in our practice the role of dietary sodium intake and where the most favorable dietary sodium cut-point lies. It certainly needs to be appreciated that a high sodium diet will contribute to edema and possible decompensation, particularly in advanced stage HF. Therefore, any clinical design must account for the development of congestion which would mean the use of diuretics and not withholding critical medications to control for volume overload.
Limitations of the Study
This study was conducted in a canine model of HF which is well established, but nonetheless, does not substitute for data in human subjects with HF. The advantages of this model are that it allows detailed assessment of the development and progression of HF in its early stages and permits a pure evaluation of hemodynamic and neuroendocrine responses without the confounding influences of drug therapy. The influences of dietary sodium manipulation can, therefore, be more clearly assessed. The clinical correlate might be Stage B to compensated Stage C HF patients who often, when identified, have yet to be initiated or maximized on medical therapy and, therefore, might benefit with a pause in treatment to assess sodium excretion and volume status and address dietary sodium intake and management concomitant with the approach to medication therapy.
Thus, contrary to current practice guideline recommendations, dietary sodium restriction may be detrimental particularly in early stage HF and contribute to the premature acceleration of PRA and excess aldosterone activation, and a more moderate sodium diet may aid in delaying the neurohormonal progression of early HF. It should be noted that in the study by Costello-Boerrigter et al.18, in a canine model of early asymptomatic left ventricular dysfunction, exogenous administration of the mineralocorticoid DOCA to mimic aldosterone failed to induce sodium retention but did induce early cardiac fibrosis. While findings are variable in the reported literature and our data do not support recommendation for a specific desirable dietary sodium cut-point in humans, the current studies support the conclusion that careful assessment of dietary sodium in the setting of HF merits further prospective investigation, particularly in patients with Stage B and compensated Stage C HF receiving optimal medical therapy.
Footnotes
Disclosures
This research was supported by National Institutes of Health grant HL36634.
References
- 1.Weber KT. Aldosterone in congestive heart failure. N Engl J Med. 2001;345:1689–1697. doi: 10.1056/NEJMra000050. [DOI] [PubMed] [Google Scholar]
- 2.Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, Palensky J, Wittes J. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. N Engl J Med. 1999;341:709–717. doi: 10.1056/NEJM199909023411001. [DOI] [PubMed] [Google Scholar]
- 3.Zannad F, McMurry JJV, Krum H, van Veldhuisen DJ, Swedberg K, Shi H, Vincent J, Pocock SJ, Pitt B, EMPHASIS Heart Failure Study Group Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med. 2011;364:11–21. doi: 10.1056/NEJMoa1009492. [DOI] [PubMed] [Google Scholar]
- 4.Gupta D, Georgiopoulou VV, Kalpgeropoulous AP, Dunbar SB, Reilly CM, Sands JM, Fonarow GC, Jessup M, Gheorghiade M, Yancy C, Butler J. Dietary sodium intake in heart failure. Circulation. 2012;126:479–485. doi: 10.1161/CIRCULATIONAHA.111.062430. [DOI] [PubMed] [Google Scholar]
- 5.Lennie TA, Song EK, Wu J-R, Chung ML, Dunbar SB, Pressler SJ, Moser DK. Three gram sodium intake is associated with longer event-free survival only in patients with advanced heart failure. J Cardiac Fail. 2011;17(4):325–330. doi: 10.1016/j.cardfail.2010.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mori T, Kurumazuka D, Matsumoto C, Shirakawa H, Kimura S, Kitada K, Kobayashi K, Matsuda H, Hayashi T, Kitaura Y, Matsumura Y. Dietary salt restriction activates mineralocorticoid receptor signaling in volume-overloaded heart failure. Eur J Pharm. 2009;623:84–88. doi: 10.1016/j.ejphar.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 7.Parrinello G, Di Pasquale P, Licata G, Torres D, Giammanco M, Fasullo S, Mezzero M, Paterna S. Long-term effects of dietary sodium intake on cytokines and neurohormonal activation in patients with recently compensated heart failure. J Cardiac Fail. 2009;15:864–873. doi: 10.1016/j.cardfail.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 8.Damgaard M, Norsk P, Gustafsson F, Kanters JK, Christensen NJ, Bie P, Friberg L, Gadsboll N. Hemodynamic and neuroendocrine responses to changes in sodium intake in compensated heart failure. Am J Physiol - Regul Integr Comp Physiol. 2006;290:R1294–R1301. doi: 10.1152/ajpregu.00738.2005. [DOI] [PubMed] [Google Scholar]
- 9.Paterna S, Fasullo S, Parrinello G, Cannizzaro S, Basile I, Vitrano G, Terrazzino G, Maringhini G, Ganci F, Sealzo S, Sarullo F, Cice G, Di Pasquale P. Short-term effects of hypertonic saline solution in acute heart failure and long-term effects of a moderate sodium restriction in patients with compensated heart failure with New York Heart Association Class III (SMAC-HF Study) Am J Med Sci. 2011;342:27–37. doi: 10.1097/MAJ.0b013e31820f10ad. [DOI] [PubMed] [Google Scholar]
- 10.Stevens TL, Burnett JC, Kinoshita M, Matsuda Y, Redfield MM. A functional role for endogenous atrial natriuretic peptide in a canine model of early left ventricular dysfunction. J Clin Invest. 1995;95:1101–1108. doi: 10.1172/JCI117757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martin FL, Steven TL, Cataliotti A, Schirger JA, Borgeson DD, Redfield MM, Luchner A, Burnett JC., Jr Natriuretic and antialdosterone actions of chronic oral NEP inhibition during progressive congestive heart failure. Kidney International. 2005;67:1723–1730. doi: 10.1111/j.1523-1755.2005.00269.x. [DOI] [PubMed] [Google Scholar]
- 12.Quinones MA, Waggoner AD, Reduto LA, Nelson JG, Young JB, Winters WL, Ribeiro LG, Miller RR. A new simplified and accurate method for determining ejection fraction with two-dimensional echocardiography. Circulation. 1981;64:744–753. doi: 10.1161/01.cir.64.4.744. [DOI] [PubMed] [Google Scholar]
- 13.Haber E, Koerner D, Page T, Kliman B, Purnode A. Application of radioimmunoassay for angiotensin I to the physiological measurement of plasma renin activity in normal human subjects. J Clin Endocrin Metab. 1969;29:1349–1355. doi: 10.1210/jcem-29-10-1349. [DOI] [PubMed] [Google Scholar]
- 14.Braunwald E, Plauth WH, Morrow AF. A method for the detection and quantification of impaired sodium excretion. Results of an oral sodium tolerance test in normal subjects and in patients with heart failure. Circulation. 1965;32:223–231. doi: 10.1161/01.cir.32.2.223. [DOI] [PubMed] [Google Scholar]
- 15.Cody RJ, Covit AB, Schaer GL, Laragh JH, Sealey JE, Feldschuh J. Sodium and water balance in chronic congestive heart failure. J Clin Invest. 1986;77:1441–1452. doi: 10.1172/JCI112456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Volpe M, Tritto C, DeLuca N, Rubattu S, Rao MA, Lamenza F, Mirante A, Enea I, Redina V, Mele AF. Abnormalities of sodium handling and of cardiovascular adaptions during high salt diet in patients with mild heart falure. Circulation. 1993;88:1620–27. doi: 10.1161/01.cir.88.4.1620. [DOI] [PubMed] [Google Scholar]
- 17.Alderman MH, Madhaven S, Cohen H, Sealey JE, Laragh JH. Low urinary sodium is associated with a greater risk of myocardial infarction among treated hypertensive men. Hypertension. 1995;25:1142–1152. doi: 10.1161/01.hyp.25.6.1144. [DOI] [PubMed] [Google Scholar]
- 18.Costello-Boerriger LC, Boerrigter G, Harty GJ, Cataliotti A, Reffield MM, Burnett JC., Jr Mineralocorticoid escape by the kidney but not the heart in experimental asymptomatic left ventricular dysfunction. Hypertension. 2007;50:481–488. doi: 10.1161/HYPERTENSIONAHA.107.088534. [DOI] [PubMed] [Google Scholar]