Abstract
BACKGROUND
It has been demonstrated that regular exercise improves the quality of life in patients undergoing treatment for lung cancer and has been associated with reductions in cancer-specific mortality in patients with colon and breast cancer. The direct effects of cardiovascular exercise on lung cancer tumor biology, however, remain unknown. The authors evaluated the effects of cardiovascular exercise in a mouse model of lung adenocarcinoma.
METHODS
Luciferase-tagged A549 lung adenocarcinoma cells were injected through the tail vein of nude male mice. Then, the mice underwent weekly bioluminescent imaging until lung tumors were clearly identified. After lung tumors were identified, the mice were randomized to daily wheel running versus no wheel running, and they were imaged weekly. After 4 weeks, all mice were killed, and the lung tumors were harvested. Western blot and immunohistochemical analyses were conducted on tumor tissues to identify potential differences in protein expression levels in exercising mice versus sedentary mice.
RESULTS
Lung tumors in exercising mice grew significantly more slowly relative to sedentary mice. There was no change in the development of metastatic lesions between the 2 groups. Protein analysis by Western blot or immunohistochemical analysis demonstrated increased p53 protein levels in exercising mice relative to sedentary mice as well as increased mediators of apoptosis, including Bax and active caspase 3, in tumor tissues. In both groups of mice, no normal tissue toxicity was observed in other organs.
CONCLUSIONS
Daily cardiovascular exercise appears to mitigate the growth of lung adenocarcinoma tumors, possibly by activation of the p53 tumor suppressor function and increased apoptosis.
Keywords: lung cancer, exercise, apoptosis, p53, adenocarcinoma
INTRODUCTION
Regular exercise provides immense health benefits, including reduced rates of many chronic health conditions such as obesity, cardiovascular disease, and diabetes.1,2 Current recommendations set forth by the World Health Organization include at least 150 minutes of moderate-intensity aerobic physical activity throughout the week for adults ages 18 to 64 years to promote cardiorespiratory and muscular fitness and bone health and to reduce the risk of depression.
The relation between cancer and exercise continues to evolve. It is well known that regular exercise is associated with a reduced risk of developing a variety of cancers, including colon, breast, esophageal, pancreas, endometrial, and ovarian cancers.3,4 In addition, large epidemiologic studies have demonstrated that regular exercise after a diagnosis of breast or colon cancer significantly decreases the rate of cancer-related mortality.5,6 Thus, it appears that exercise may have tumor-mitigating properties across a spectrum of malignancies; however, the molecular mechanisms behind such clinical findings are poorly understood. Specifically in relation to lung cancer, it has been demonstrated that exercise improves quality of life in patients undergoing active treatment.7 However, it is unknown whether exercise after a diagnosis of lung cancer plays any role in the biology of the disease itself.
Non-small cell lung cancer remains 1 of the most deadly malignancies, with a 5-year survival rate of only 15%. Approximately 80% of newly diagnosed lung cancers are non-small cell, and approximately 50% of such cancers harbor a mutation in the tumor suppressor p53,8 which plays an important role in a multitude of biologic pathways, including DNA repair, cell-cycle arrest, apoptosis, senescence, autophagy, and metabolism.9–11 In addition, p53 maintains genomic integrity by responding to cellular stress and DNA damage through cell-cycle arrest and DNA repair or induction of apoptosis. In lung cancers with wild-type p53, proper function of p53 is often inhibited by negative regulators such as mouse double minute 2 homolog/E3 ubiquitin-protein ligase Mdm2 (MDM2) and the p53 regulator MDMX. Thus, anticancer therapies aimed at activating wild-type p53 could prove useful in the treatment of lung cancer.
The current study examines the effects of daily exercise on a mouse model of lung adenocarcinoma, examining the clinically relevant endpoint of primary lung tumor growth. We demonstrate that daily cardiovascular exercise abrogates the growth of lung tumors. Furthermore, we explore potential biologic mechanisms underlying the tumor-mitigating effects of exercise and demonstrate that increased p53 levels and apoptosis in tumors from exercising mice may be a potential mechanism by which exercise reduces lung adenocarcinoma tumor growth.
MATERIALS AND METHODS
Materials
D-Luciferin was purchased from BD Biosciences (San Jose, Calif). Ketamine hydrochloride/xylazine hydrochloride solution was purchased from Sigma-Aldrich (St. Louis, Mo). Active caspase 3-specific antibodies were purchased from Cell Signaling Technology (Danvers, Mass). Bax, Actin, and p53 antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, Calif), and B-cell leukemia/lymphoma 2 (Bcl2) antibody was obtained from Calbiochem (Darmstadt, Germany). B-cell lymphoma-extra large (Bcl-XL) and Bak antibodies were purchased from Epitomics (Burlingame, Calif). All other reagents were obtained from commercial sources unless otherwise stated.
Cell Line and Cell Culture
The luciferase-expressing cell line A549-luc-C8, which was derived from A549 human lung adenocarcinoma cells by stable transfection of the North American firefly luciferase gene, was purchased from Caliper Life Sciences, Inc. (Hopkinton, Mass). Cells were cultured in RPMI 1640 medium supplemented with 10% fetal bovine serum in 5% CO2 at 37°C.
Lung Cancer Mouse Model
Six-week-old, male, severe combined immunodeficiency (SCID)-beige mice were purchased from Charles River Laboratories (Wilmington, Mass) and housed under pathogen-free conditions. All animal treatments were undertaken in accordance with protocols approved by the Institutional Animal Care and Use Committee at Emory University. A549-luc-C8 cells (5 × 105) in Dulbecco phosphate-buffered saline (Mediatech Inc., Manassas, Va) were injected intravenously through the tail vain of mice.
Establishment of A549 Lung Adenocarcinoma Mouse Xenografts
Six-week old, male, SCID-beige mice underwent tail vein injection with A549 lung adenocarcinoma cells (5×105 cells/200 μL Dulbecco phosphate-buffered saline) that were previously transfected with luciferase. Mice underwent bioluminescent imaging twice weekly until lung tumors were confirmed. Exercising mice and sedentary mice were fed the same diet, which consisted of 23% crude protein, 4.5% crude fat 4.5%, 6% crude fiber, and 2.5% added minerals.
Exercise and Lung Tumor Growth
After lung tumors were detected with bioluminescent imaging, 10 mice were randomized to no activity (cages with no running wheels), and 10 mice were randomized to exercise (cages with running wheels present). The physical activity of the sedentary mice was not specifically controlled for, because cages with no running wheels provide minimal opportunity for activity. Running wheels were obtained from Mini Mitter (Bend, Ore). Exercise was monitored by a digital wheel revolution counter that converted revolutions to kilometers. Mice underwent whole-body bioluminescent imaging weekly, and they were euthanized after 28 days. Lung tumor volume was assessed using weekly bioluminescent imaging to detect the mean photon count, which has been strongly correlated with tumor volume.12 Bioluminescent imaging also was used to gain a temporal understanding of tumor growth at various time points. This methodology has been proven useful in animal models of cancer secondary to an increase in the amount of biologic information obtained relative to tumor weight measurements, the noninvasive approach of bioluminescent imaging, and foregoing the need to euthanize multiple groups of mice to obtain information for various time points throughout a study.12
Lung Tumor Tissue Analysis
After the mice were euthanized, tumors were removed from the lungs, snap-frozen in liquid nitrogen, and stored at −80°C. Immunohistochemistry and Western blot techniques were used to analyze specific proteins within tumors.
Statistics
Comparisons of bioluminescent mean photon counts between exercise and sedentary mice were made using 2-tailed paired t tests. Comparisons between the numbers of active caspase 3-positive cells in exercising versus sedentary mice also were made using 2-tailed paired t tests. Quantification of Western blot bands was performed using ImageJ software (National Institutes of Health, 1997–2012; available at: imagej.nih.gov/ij/; Accessed March 14, 2014).
RESULTS
Establishment of Lung Tumors and Daily Exercise Regimen
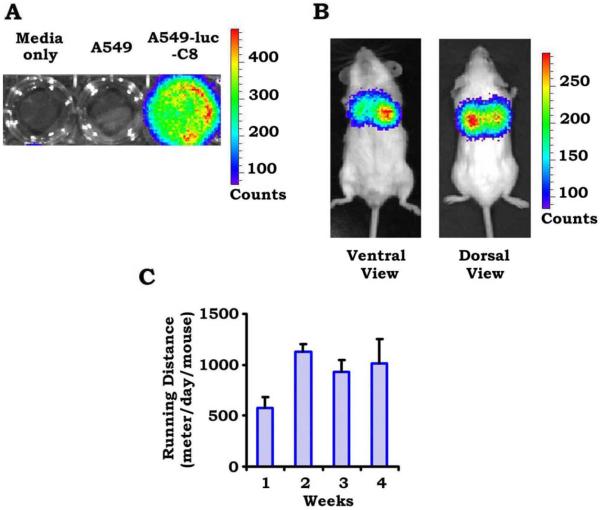
To study the effects of daily cardiovascular exercise on lung cancer progression, lung tumors were established in 20 male, 6-week-old, SCID-beige mice after tail vein injections of luciferase-tagged A549 lung adenocarcinoma cells. Luciferase-tagged A549 cells that had an appropriate bioluminescent imaging signal are displayed in Figure 1A, and lung tumors that were established in mice after tail vein injection are displayed in Figure 1B. Tumors are observed localized to the lung on both dorsal and ventral views. After lung tumors were confirmed by bioluminescence imaging, mice were randomized to sedentary living in a cage with no running wheel versus daily, voluntary cardiovascular exercise by placement in cages with running wheels. Wheel revolutions were tracked, and the average number of meters per day traversed by exercising mice was calculated (Fig. 1C). The average number of meters ran per day per mouse ranged from 600 to 1200 meters and was fairly consistent from week to week. Mice underwent weekly bioluminescent imaging throughout the 4-week course to assess for tumor growth and the development of distant metastasis.
Figure 1.
A mouse lung adenocarcinoma model was established with luciferase-tagged A549 cells (A549-luc-C8) injected through the tail veins of immunocompromised mice. (A) This is an example of successful bioluminescent imaging of luciferase-tagged A549 cells. (B) Lung tumors were established within mice using bioluminescent imaging. (C) This chart illustrates the average number of meters per day traversed by exercising mice throughout the course of the experiment.
The Effects of Cardiovascular Exercise on Lung Tumor Growth
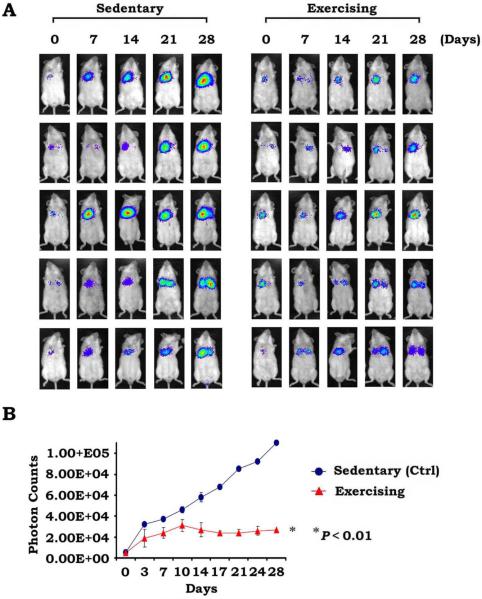
Figure 2A displays weekly bioluminescent images from 5 representative mice in the exercise and sedentary cohorts throughout the 28-day period. Lung tumors appear to be growing less rapidly in the exercising mice, and an easily observed, less robust tumor burden is observed within the lungs. We observed slower growth of lung tumors in exercising mice compared with sedentary mice, as indicated in Figure 2B, which compares the change in mean photon counts over time between 10 exercising mice and 10 sedentary mice (P < .01). The photon counts from sedentary mice continued to rise steadily throughout the experimental time course; however, photon counts from exercising mice stabilize after 10 days and do not increase after that time point. There was no difference in the development of distant metastasis between the 2 groups, because no mouse developed distant disease.
Figure 2.
Exercise repressed lung tumor growth in vivo. (A) Whole-body images were taken weekly of 5 representative mice in each cohort (individual pictures of all mice are not shown because of space limitations). Each row depicts lung tumor growth of an individual mouse tumor over 4 weeks. (B) Changes in mean photon counts in lung tumors over time are compared between sedentary (control [Ctrl]) mice versus exercising mice (all mice; there were 10 mice in each group), with sedentary mice exhibiting a significantly more rapid increase in mean photon counts.
Analysis of Intratumoral Proteins
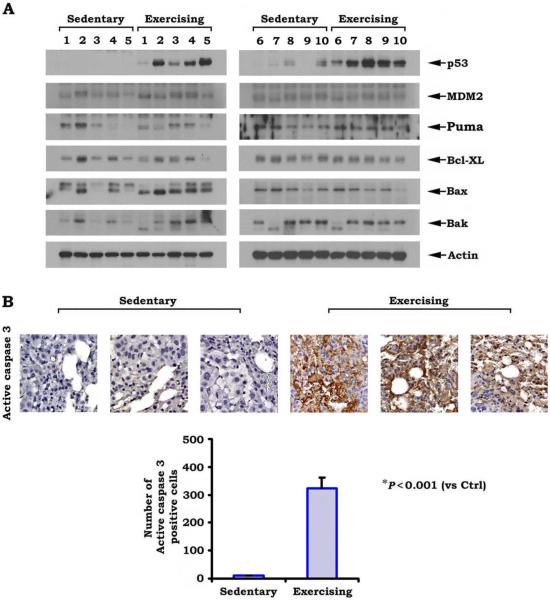
Four weeks after the establishment of lung tumors, the mice were euthanized, and the tumors were extracted from the lungs. Studies were undertaken to determine possible biologic mechanisms leading to the slower tumor growth observed in exercising mice. Western blot analyses for specific proteins were performed on homogenized lung tumor tissue. We speculated that exercise may be inducing apoptosis and examined the proteins involved in p53 driven apoptosis. Figure 3A displays protein expression of p53, MDM2, Puma (a Bcl2 binding component), Bcl-XL, Bak, and Bax. Levels of the p53 protein were strongly increased in exercising mouse lung tumors relative to sedentary mouse lung tumors, and ImageJ quantification revealed up-regulation by 1103% (P < .01) relative to sedentary mouse lung tumors. In addition, levels of the proapoptotic proteins Bax and Bak were higher in exercising mouse tumors relative to sedentary mouse tumors, indicating that p53-driven apoptosis is occurring in exercising mouse tumors. ImageJ quantification demonstrated Bax up-regulation by 179% relative to sedentary mice (P < .02) and Bak up-regulation by 140% (P=.1; non-significant). Figure 3B displays the results from an immunohistochemical analysis for active caspase 3 and demonstrates increased levels of this apoptotic intermediary in lung tumors from exercising mice relative to lung tumors from sedentary mice. T tests were performed to compare the number of cells that stained positive for active caspase 3, and significantly more staining was observed in tumors from exercising mice (P < .001). These findings suggest that cardiovascular exercise leads to impaired tumor growth because of increased levels of p53 and subsequent p53-driven apoptosis.
Figure 3.
Exercise induces the up-regulation of p53 and apoptosis in tumor tissues. (A) Western blots for various proteins involved in apoptosis are shown. Tumors from exercising mice had increased levels of p53 and Bax relative to sedentary mice. MDM2 indicates mouse double minute 2 homolog/E3 ubiquitin-protein ligase Mdm2; Puma, B-cell leukemia/lymphoma 2 (Bcl2) binding component; BCL-XL, B-cell lymphoma-extra large; Bax and Bak, proapoptotic Bcl2 family members. (B) In immunohistochemical analysis for active caspase 3, significantly higher levels of this apoptotic intermediary were observed in lung tumors from exercising mice compared with lung tumors from sedentary (control [Ctrl]) mice.
Physiologic Changes in Weight, Organ Function, and Normal Tissue Morphology
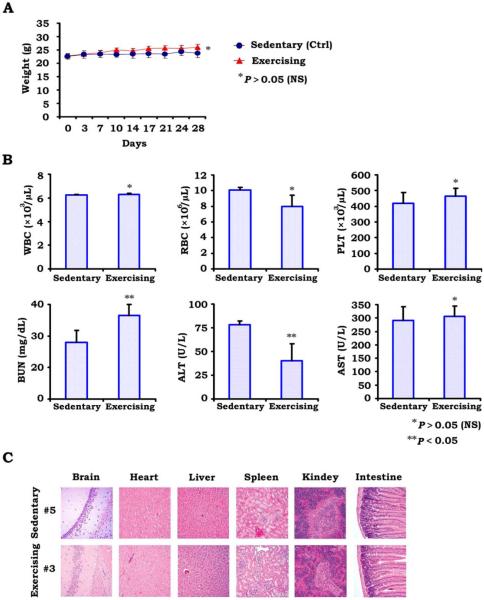
An ideal anticancer therapy differentially attacks tumor cells relative to normal tissues. To determine whether exercise-induced apoptosis was selective to tumor cells, we examined both the morphology and the function of normal tissues from exercising and sedentary mice as well as changes in body weight. Figure 4A demonstrates the change in weight of exercising versus sedentary mice, with no statistically significant differences in weight. Surprisingly, exercising mice maintained slightly higher weights than sedentary mice. This is an important point, because weight loss is a well known factor associated with poor prognosis in humans with lung cancer, and exercise and the increased caloric expenditure could lead to unintended weight loss. However, we did not observe weight loss in exercising mice.
Figure 4.
Toxicity analyses are illustrated. (A) The average change in weight of sedentary (control [Ctrl]) mice versus exercising mice is illustrated. No statistically significant differences were observed. (B) White blood cell (WBC) counts, erythrocyte (RBC) counts, platelets (PLT), blood urea nitrogen (BUN) levels, alanine transaminase (ALT) levels, and aspartate transaminase (AST) levels for exercising versus sedentary mice. No significant differences were observed, with the exception of slightly elevated BUN and slightly decreased ALT in exercising mice. These changes, however, remain clinically insignificant and fall closely within the normal range for mice. (C) Normal tissue morphology is illustrated in various organs. No differences were appreciated between exercising and sedentary mice.
No differences were observed in the bone marrow function of mice, and the levels of white blood cells and erythrocytes were equivalent between exercising mice and sedentary mice. Alanine transaminase levels were slightly lower and blood urea nitrogen levels were slightly higher in exercising mice, however these levels still fall closely within the normal ranges for these laboratory values, and such changes are not clinically significant, indicating that exercise is not adversly affecting liver or kidney function (Fig. 4). Figure 4 displays microscopy of organs, including the brain, heart, liver, spleen, kidney, and small intestine, with no major morphologic changes observed between organs from exercising and sedentary mice. Thus, exercise does not appear to convey any adverse effects on normal organ function.
DISCUSSION
Exercise and improved cardiorespiratory fitness decrease the risk of developing cancer, as made evident by large, population-based studies.13 The mechanisms behind the protective effect of exercise on carcinogenesis is likely multifactorial and includes boosting host immunity14 and reducing chronic inflammation, both of which have been reported to reduce carcinogenesis.15 Additional plausible mechanisms include the activation of DNA repair enzymes and a reduction in oxidative stress by exercise-induced antioxidants.16
Beyond reducing the risk of developing cancer, large population-based studies have demonstrated the protective effects of exercise on mortality from breast cancer17 and gastrointestinal cancer.18 The mechanisms behind such observed protective effects are largely unknown, and hypotheses include an improved innate immune response, alterations in insulin levels, and changes in endogenous reproductive hormones. In lung cancer specifically, the influence of exercise on cancer-related mortality is less defined. Although it has been demonstrated that exercise decreases the risk of developing lung cancer,19 it is unknown whether exercise after a diagnosis of lung cancer affects cancer-related mortality.
To our knowledge, an association between exercise and tumor apoptosis has not been previously reported. However, apoptosis is a well described mechanism of action for a multitude of anticancer therapies, including radiation therapy and a variety of chemotherapeutic agents. Apoptosis, or programmed cell death, involves orchestrated cellular changes, including nuclear and cytoplasmic condensation, DNA degradation, membrane blebbing, and cellular fragmentation into apoptotic bodies.20 Suppression of apoptosis is a well described hallmark of cancer,21 and targeting both the extrinsic22 and intrinsic pathways of apoptosis23 is an active area of ongoing cancer research.
In the current study, we have demonstrated that daily exercise significantly reduces local tumor growth in a mouse model of lung cancer. We cannot draw any conclusions regarding the effects of exercise on the development of distant metastases, because no animal developed distant disease. Mechanistically, tumors from exercising mice exhibit higher levels of p53 and Bax, suggesting that exercise may be increasing p53-driven apoptosis. Apoptosis is the hallmark of p53 tumor suppression; and, in our study, tumors from exercising mice had increased apoptosis with higher levels of active caspase 3. However, to more definitively determine whether p53 function is necessary for exercise to confer a reduction in tumor burden, genetic manipulation of p53 would be required. Our group is currently undertaking further experiments, including a study of the effects of exercise in a mouse model of lung cancer derived from p53 mutant cell lines. This will likely clarify whether wild-type p53 is necessary for exercise to reduce tumor burden.
In healthy patients, exercise is a known promoter of p53 translocation into the mitochondria in skeletal muscle as well as increased p53 phosphorylation, which is associated with increased protein stability and activity.24,25 To our knowledge, exercise-induced increases in p53 protein levels within a malignant tumor have not been reported. It is well known that p53 acts as a quintessential tumor suppressor gene by inducing cell-cycle arrest, apoptosis and senescence. Within cancer research, drugs aimed at improving tumor suppression through p53 have largely been unsuccessful. Thus, demonstrating that exercise increases p53 levels in lung tumors may have broadly reaching implications for a wide range of malignancies.
These results represent novel findings, because increased levels of functional p53 protein were observed in lung tumors from exercising mice. Although A549 lung adenocarcinoma cells do not contain a recognized p53 mutation, our sedentary mouse lung tumors did demonstrate impaired p53 levels, with no measurable p53 protein and a phenotype consistent with a rapidly growing lung tumor. Many p53 wild-type tumors exhibit similar behavior because of increases in negative regulators of p53, such as MDM2.26 In the current study, exercise increased p53 protein levels in mouse lung tumors and subsequent apoptosis within lung tumors, without any changes in normal tissue morphology or normal organ function. Thus, exercise is likely stabilizing wild-type p53 proteins and rendering them more effective at tumor suppression. Essentially, cardiovascular exercise is restoring function of the p53 tumor suppressor in nonmutated p53 lung adenocarcinoma tumors (A549 adenocarcinoma cells do not harbor a p53 mutation). It is unknown, however, whether exercise is advantageous in tumors that harbor a p53 mutation, and further work to clarify this important point is ongoing.
The activation of p53 within lung tumors in mice undergoing exercise is a novel finding and lends a biologic mechanism to population data suggesting an association between exercise and improved cancer outcomes. The current study demonstrates reduction of lung tumor growth in exercising mice, possibly through restoration of p53. Furthermore, we demonstrate increased apoptosis in lung tumors from exercising hosts, a key function of p53 tumor suppression. Future research directions include studying cell-cycle arrest and the less well defined metabolic functions modulated by p53.
Central to the idea of exercise as adjunct therapy is the ability to use an exercise regimen in the clinical setting. Cancer patients often suffer from both disease-related and treatment-related fatigue, weight loss, and generalized malaise. This collection of symptoms may make it difficult to engage in daily exercise. However, a phase 2 randomized trial has been conducted in women with breast cancer who were receiving neoadjuvant chemotherapy in which they were randomized to receive either doxorubicin plus cyclophosphamide or the same chemotherapy regimen with 3 weekly, supervised, moderate-intensity to high-intensity aerobic training sessions. The results demonstrated an 82% rate of attendance to aerobic training sessions and a 66% rate of adherence to the planned exercise routine along with improvements in cardiopulmonary function in exercising women.27 Thus, it appears that exercise can be safely implemented in patients with cancer under controlled and carefully monitored conditions.
Caveats to this study include other potential mechanisms at play that may influence lung tumor growth beyond apoptosis. It is likely that, although our findings support apoptosis, other mechanisms also may be involved, including the endocrine system and the modulation of complex hormone pathways by exercise. An association between elevated insulin-like growth factor 1 (IGF1) and breast cancer risk has been demonstrated in both premenopausal and postmenopausal women.28,29 It is plausible that reductions in IGF1caused by exercise could be involved in promoting apoptosis, because it is known that IGF1 inhibits apoptosis.30 IGF1 and other endocrine pathways were not analyzed in this study, but future directions for research include an investigation of these pathways in relation to exercise, apoptosis, and reduced tumor growth.
Our current study demonstrates the beneficial effects of exercise on lung adenocarcinoma tumor growth in mice. Targeting tumor suppressors by pharmacologic means has proven to be difficult, but exercise appears to be a promising anticancer therapy that improves p53 tumor suppressor function. However, further studies are needed to determine whether the mechanism behind exercise-induced tumor reduction is reliant on p53 alone. Future research directions include exploratory studies in humans examining the effects of cardiovascular exercise on lung cancer progression.
Acknowledgments
FUNDING SUPPORT
This work was supported by a Winship Cancer Institute Kennedy Seed Grant (to K.A.H.) and by a grant from the National Cancer Institute, National Institutes of Health (R01CA136534; to X.D.).
Footnotes
CONFLICT OF INTEREST DISCLOSURES
The authors made no disclosures.
This study demonstrates that daily cardiovascular exercise reduces lung tumor growth in mice, and tumor apoptosis is increased. Therefore, exercise should be explored further as a potential anticancer therapy.
REFERENCES
- 1.Strath SJ, Kaminsky LA, Ainsworth BE, et al. Guide to the assessment of physical activity: Clinical and research applications: a scientific statement from the American Heart Association. Circulation. 2013;128:2259–2279. doi: 10.1161/01.cir.0000435708.67487.da. [DOI] [PubMed] [Google Scholar]
- 2.Dhaliwal SS, Welborn TA, Howat PA. Recreational physical activity as an independent predictor of multivariable cardiovascular disease risk [serial online] PLoS One. 2013;8:e83435. doi: 10.1371/journal.pone.0083435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eheman C, Henley SJ, Ballard-Barbash R, et al. Annual Report to the Nation on the status of cancer, 1975–2008, featuring cancers associated with excess weight and lack of sufficient physical activity. Cancer. 2012;118:2338–2366. doi: 10.1002/cncr.27514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wen CP, Wai JP, Tsai MK, et al. Minimum amount of physical activity for reduced mortality and extended life expectancy: a prospective cohort study. Lancet. 2011;378:1244–1253. doi: 10.1016/S0140-6736(11)60749-6. [DOI] [PubMed] [Google Scholar]
- 5.Irwin ML, Smith AW, McTiernan A, et al. Influence of pre- and postdiagnosis physical activity on mortality in breast cancer survivors: the Health, Eating, Activity, and Lifestyle Study. J Clin Oncol. 2008;26:3958–3964. doi: 10.1200/JCO.2007.15.9822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meyerhardt JA, Heseltine D, Niedzwiecki D, et al. Impact of physical activity on cancer recurrence and survival in patients with stage III colon cancer: findings from CALGB 89803. J Clin Oncol. 2006;24:3535–3541. doi: 10.1200/JCO.2006.06.0863. [DOI] [PubMed] [Google Scholar]
- 7.Lin YY, Wu YC, Rau KM, Lin CC. Effects of physical activity on the quality of life in Taiwanese lung cancer patients receiving active treatment or off treatment. Cancer Nurs. 2013;36:E35–E41. doi: 10.1097/NCC.0b013e31826fb8bf. [DOI] [PubMed] [Google Scholar]
- 8.Takahashi T, Nau MM, Chiba I, et al. p53: a frequent target for genetic abnormalities in lung cancer. Science. 1989;246:491–494. doi: 10.1126/science.2554494. [DOI] [PubMed] [Google Scholar]
- 9.Vogelstein B, Lane D, Levine AJ. Surfing the p53 network. Nature. 2000;408:307–310. doi: 10.1038/35042675. [DOI] [PubMed] [Google Scholar]
- 10.Levine AJ. p53, the cellular gatekeeper for growth and division. Cell. 1997;88:323–331. doi: 10.1016/s0092-8674(00)81871-1. [DOI] [PubMed] [Google Scholar]
- 11.Jin S, Levine AJ. The p53 functional circuit. J Cell Sci. 2001;114(pt 23):4139–4140. doi: 10.1242/jcs.114.23.4139. [DOI] [PubMed] [Google Scholar]
- 12.Jenkins DE, Oei Y, Hornig YS, et al. Bioluminescent imaging (BLI) to improve and refine traditional murine models of tumor growth and metastasis. Clin Exp Metastasis. 2003;20:733–744. doi: 10.1023/b:clin.0000006815.49932.98. [DOI] [PubMed] [Google Scholar]
- 13.Laukkanen JA, Pukkala E, Rauramaa R, Makikallio TH, Toriola AT, Kurl S. Cardiorespiratory fitness, lifestyle factors and cancer risk and mortality in Finnish men. Eur J Cancer. 2010;46:355–363. doi: 10.1016/j.ejca.2009.07.013. [DOI] [PubMed] [Google Scholar]
- 14.Woods JA, Davis JM, Kohut ML, Ghaffar A, Mayer EP, Pate RR. Effects of exercise on the immune response to cancer. Med Sci Sports Exerc. 1994;26:1109–1115. [PubMed] [Google Scholar]
- 15.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rogers CJ, Colbert LH, Greiner JW, Perkins SN, Hursting SD. Physical activity and cancer prevention: pathways and targets for intervention. Sports Med. 2008;38:271–296. doi: 10.2165/00007256-200838040-00002. [DOI] [PubMed] [Google Scholar]
- 17.Peel JB, Sui X, Adams SA, Hebert JR, Hardin JW, Blair SN. A prospective study of cardiorespiratory fitness and breast cancer mortality. Med Sci Sports Exerc. 2009;41:742–748. doi: 10.1249/MSS.0b013e31818edac7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peel JB, Sui X, Matthews CE, et al. Cardiorespiratory fitness and digestive cancer mortality: findings from the aerobics center longitudinal study. Cancer Epidemiol Biomarkers Prev. 2009;18:1111–1117. doi: 10.1158/1055-9965.EPI-08-0846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee IM, Sesso HD, Paffenbarger RS., Jr Physical activity and risk of lung cancer. Int J Epidemiol. 1999;28:620–625. doi: 10.1093/ije/28.4.620. [DOI] [PubMed] [Google Scholar]
- 20.Kerr JF, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972;26:239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 22.Ashkenazi A, Pai RC, Fong S, et al. Safety and antitumor activity of recombinant soluble Apo2 ligand. J Clin Invest. 1999;104:155–162. doi: 10.1172/JCI6926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Benimetskaya L, Miller P, Benimetsky S, et al. Inhibition of potentially anti-apoptotic proteins by antisense protein kinase C-alpha (Isis 3521) and antisense bcl-2 (G3139) phosphorothioate oligodeoxynucleotides: relationship to the decreased viability of T24 bladder and PC3 prostate cancer cells. Mol Pharmacol. 2001;60:1296–1307. doi: 10.1124/mol.60.6.1296. [DOI] [PubMed] [Google Scholar]
- 24.Bartlett JD, Louhelainen J, Iqbal Z, et al. Reduced carbohydrate availability enhances exercise-induced p53 signaling in human skeletal muscle: implications for mitochondrial biogenesis. Am J Physiol Regul Integr Comp Physiol. 2013;304:R450–R458. doi: 10.1152/ajpregu.00498.2012. [DOI] [PubMed] [Google Scholar]
- 25.Saleem A, Hood DA. Acute exercise induces p53 translocation to the mitochondria and promotes a p53-Tfam-mtDNA complex in skeletal muscle. J Physiol. 2013;59(pt 14):3625–3636. doi: 10.1113/jphysiol.2013.252791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Toledo F, Wahl GM. Regulating the p53 pathway: in vitro hypotheses, in vivo veritas. Nat Rev Cancer. 2006;6:909–923. doi: 10.1038/nrc2012. [DOI] [PubMed] [Google Scholar]
- 27.Hornsby WE, Douglas PS, West MJ, et al. Safety and efficacy of aerobic training in operable breast cancer patients receiving neoadjuvant chemotherapy: a phase II randomized trial. Acta Oncol. 2014;53:65–74. doi: 10.3109/0284186X.2013.781673. [DOI] [PubMed] [Google Scholar]
- 28.Fletcher O, Gibson L, Johnson N, et al. Polymorphisms and circulating levels in the insulin-like growth factor system and risk of breast cancer: a systematic review. Cancer Epidemiol Biomarkers Prev. 2005;14:2–19. [PubMed] [Google Scholar]
- 29.Key TJ, Appleby PN, Reeves GK, Roddam AW. Insulin-like growth factor 1 (IGF1), IGF binding protein 3 (IGFBP3), and breast cancer risk: pooled individual data analysis of 17 prospective studies. Lancet Oncol. 2010;11:530–542. doi: 10.1016/S1470-2045(10)70095-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pollak M. Insulin and insulin-like growth factor signalling in neoplasia. Nat Rev Cancer. 2008;8:915–928. doi: 10.1038/nrc2536. [DOI] [PubMed] [Google Scholar]