SUMMARY
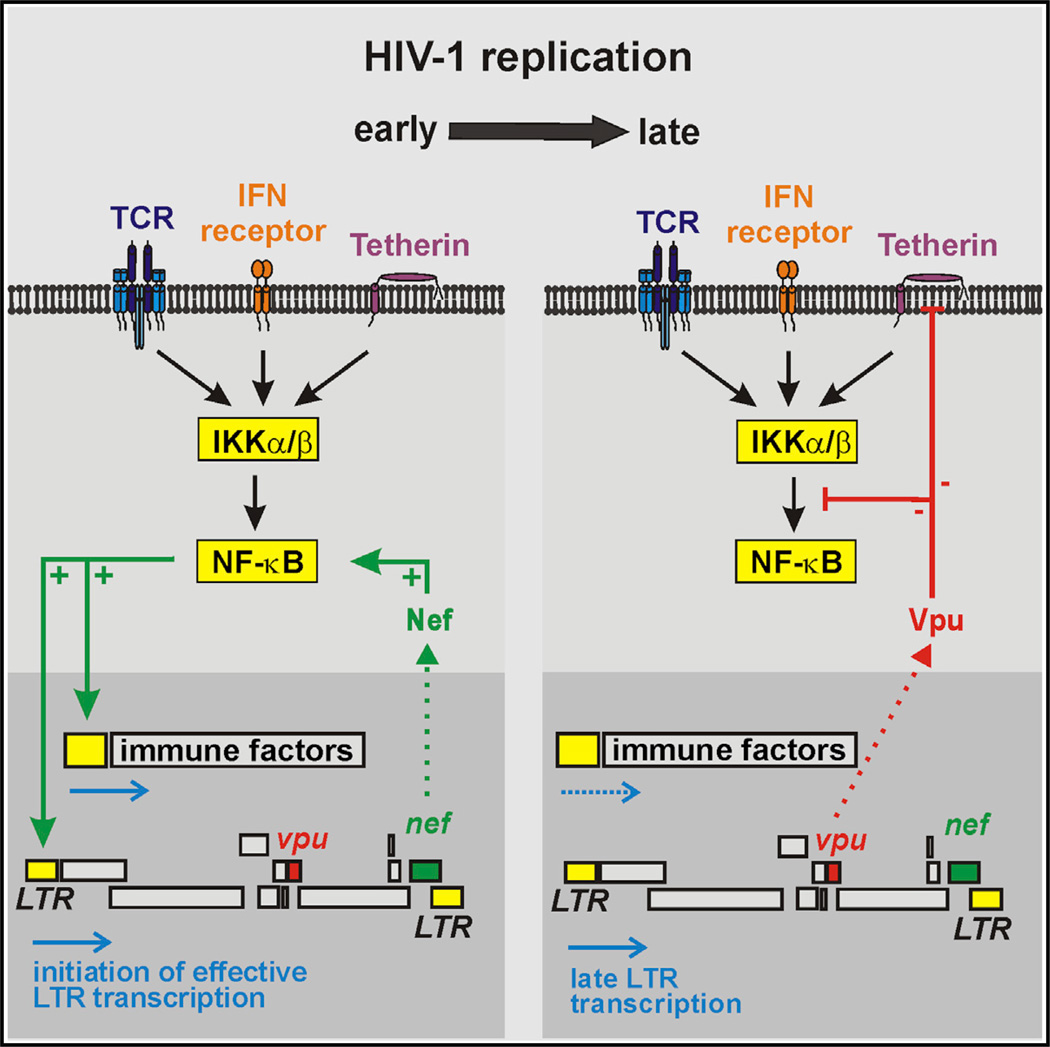
NF-κB is essential for effective transcription of primate lentiviral genomes and also activates antiviral host genes. Here, we show that the early protein Nef of most primate lentiviruses enhances NF-κB activation. In contrast, the late protein Vpu of HIV-1 and its simian precursors inhibits activation of NF-κB, even in the presence of Nef. Although this effect of Vpu did not correlate with its ability to interact with β-TrCP, it involved the stabilization of IκB and reduced nuclear translocation of p65. Interestingly, however, Vpu did not affect casein kinase II-mediated phosphorylation of p65. Lack of Vpu was associated with increased NF-κB activation and induction of interferon and interferon-stimulated genes (ISGs) in HIV-1-infected T cells. Thus, HIV-1 and its simian precursors employ Nef to boost NF-κB activation early during the viral life cycle to initiate proviral transcription, while Vpu is used to downmodulate NF-κB-dependent expression of ISGs at later stages.
Graphical Abstract
INTRODUCTION
NF-κB (nuclear factor kappa-light-chain-enhancer of activated B cells) is an inducible transcription factor that is ubiquitously expressed and regulates the expression of numerous genes involved in cell survival, inflammation, and immunity (Ghosh and Hayden, 2012; Napetschnig and Wu, 2013). NF-κB also regulates the antimicrobial immune response, including the expression of interferon-stimulated genes (ISGs) that protect against viral pathogens (Pfeffer, 2011).
Not only is NF-κB a key mediator of antiviral immune responses, but it is also exploited by viruses for efficient transcription of viral genes (Chan and Greene, 2012). For example, binding of NF-κB p50/p65 heterodimers to the tandem κB sites in the HIV-1 long terminal repeats (LTRs) is critical for viral replication. The p50/p65 dimers initiate HIV-1 transcription by associating with p300, thereby increasing the accessibility of the LTR for the cellular RNA polymerase II (RNAPII) (Williams et al., 2006). Furthermore, p50/p65 dimers recruit the P-TEFb complex to increase the processivity of RNAPII and to support RNA elongation (Williams et al., 2007).
The opposing roles of NF-κB on virus transcription and innate responses make it necessary for HIV-1 and other primate lentiviruses to tightly regulate its activation. For the accessory protein Nef, enhancing (Herbein et al., 2008; Mangino et al., 2011), inhibitory (Bandres and Ratner, 1994; Niederman et al., 1992), and no (Yoon and Kim, 1999) effects on NF-κB activity have been reported. Nef is abundantly expressed throughout the viral life cycle and induces changes in protein trafficking, signal transduction, and gene expression to promote viral replication and immune evasion. Many simian immunodeficiency viruses (SIVs) use Nef to counteract the restriction factor tetherin that retains nascent virions at the cell surface (Jia et al., 2009; Sauter et al., 2009; Zhang et al., 2009). In contrast, pandemic HIV-1 and SIVs infecting Cercopithecus monkeys utilize their Vpu protein to counteract tetherin (Van Damme et al., 2008; Neil et al., 2008; Sauter et al., 2009). It has also been reported that tetherin acts as an innate sensor that activates an NF-κB-mediated antiviral immune response and that this effect is counteracted by the anti-tetherin activity of Vpu (Cocka and Bates, 2012; Galão et al., 2012, 2014; Tokarev et al., 2013). However, earlier studies suggested that Vpu suppresses NF-κB activation by preventing the polyubiquitination and degradation of IκB through sequestration of the adaptor protein β-TrCP (Akari et al., 2001; Bour et al., 2001).
While many studies investigated the effect of HIV-1 on NF-κB activation, results were often discrepant and mostly obtained using the T cell line-adapted NL4-3 molecular clone. Thus, it remains unknown how primary HIV-1 strains and other primate lentiviruses modulate NF-κB activity and how they ensure effective proviral transcription while minimizing the activation of antiviral responses. To address these questions, we analyzed nef and vpu alleles representing nearly the entire spectrum of primate lentiviruses. We focused on these accessory genes because their products have been implicated in the modulation of NF-κB activity and cooperate in other functions, such as downmodulation of CD4 (Lindwasser et al., 2007). We show that the vast majority of Nef proteins increase NF-κB activity, while Vpu proteins inhibit the activation of NF-κB independently of their anti-tetherin function by stabilizing IκBand preventing nuclear translocation of p65. Notably, Vpu-mediated inhibition of NF-κB activation is dominant over the stimulatory effect of Nef and associated with decreased expression of ISGs. Thus, Nef appears to increase NF-κB activity early during the viral life cycle (i.e., from viral entry to expression of Tat, Rev, and Nef from completely spliced viral RNAs) to initiate proviral transcription, whereas Vpu downmodulates NF-κB activity during later stages (i.e., during expression of Vpu, Vpr, Vif, and structural proteins from singly and unspliced viral RNAs) to suppress the antiviral immune response.
RESULTS
Primate Lentiviral Nef Proteins Boost IKKβ-Mediated NF-κB Activation
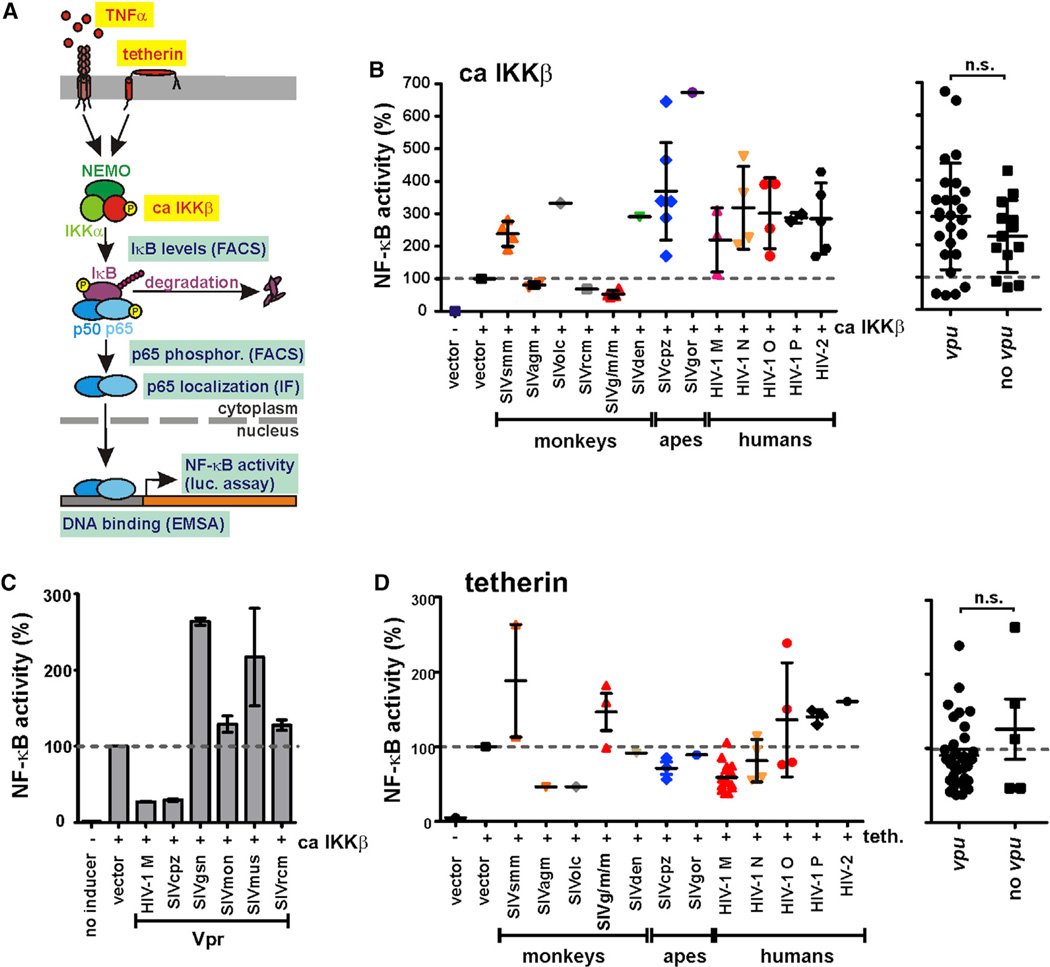
Activation of NF-κB involves ubiquitination and proteasomal degradation of its inhibitor IκB (Figure 1A). In the absence of IκB, NF-κB is translocated into the nucleus, where it binds to the promoter regions of its target genes. To analyze NF-κB activation, we took advantage of a reporter vector expressing firefly luciferase under the control of three NF-κB binding sites and determined DNA binding and nuclear translocation of p65 by electrophoretic mobility shift assay (EMSA) and fluorescence microscopy. Furthermore, we monitored the expression levels of IκB and activating phosphorylation of p65 at serine 529 by flow cytometric analyses (Figure 1A).
Figure 1. Stimulation of NF-κB Activity by Primate Lentiviral Nef Proteins.
(A) Schematic of the canonical NF-κB signaling pathway. Inducers used in this study are highlighted by yellow boxes and methods used to monitor the activation levels of NF-κB in light blue boxes.
(B) Nef boosts IKKβ-induced NF-κB activation. 293T cells were cotransfected with the indicated nef alleles, a firefly luciferase reporter construct under the control of three NF-κB binding sites, a Gaussia luciferase construct for normalization, and expression vectors for a constitutively active mutant of IKKβ (ca IKKβ) as inducer of NF-κB. Luciferase activities were determined 40 hr posttransfection. Each data point represents one nef allele from the respective groups of primate lentiviruses. Mean values of three to six transfections are shown in (B) and (D). In the right panel, nef alleles were grouped based on the presence or absence of a vpu gene in the respective viruses. HIV-1 group N was excluded from this analysis because their Vpus are poorly active.
(C) Vpr proteins of SIVgsn/mus/mon promote NF-κB activation. 293T cells were transfected and analyzed as described above. Results show mean values (±SEM) from six transfections.
(D) Effect of Nef on tetherin-induced NF-κB activation. 293T cells were transfected as described for (B), using human tetherin as inducer of NF-κB. Each data point represents one nef allele.
See also Figure S1.
To determine the effect of primate lentiviral Nef proteins on NF-κB activity, we analyzed 39 HIV and SIV nef alleles representing all four groups of HIV-1 (M, N, O, and P), their direct precursors SIVcpz from chimpanzees and SIVgor from gorillas, as well as a variety of additional SIVs, including the vpu-containing SIVgsn, SIVmus, and SIVmon strains. To determine the impact of Nef on NF-κB activation, we cotransfected 293T cells with vectors coexpressing Nef and enhanced GFP (EGFP) together with the NF-κB-dependent firefly luciferase reporter construct and a constitutively active mutant of IKKβ. Coexpression of most Nef proteins from SIVcpz, SIVgor, and HIV-1 enhanced IKKβ-mediated NF-κB activation ~2- to 4-fold. Similarly, HIV-2, SIVsmm, SIVolc, and SIVden Nefs boosted NF-κB activity (Figure 1B). In contrast, SIVgsn/mus/mon, SIVagm, and SIVrcm nef alleles did not promote NF-κB activation (Figure 1B). To identify possible reasons for these differences in Nef function, we also examined the effect of the Vpr protein on NF-κB activity. In agreement with published data (Ayyavoo et al., 1997), HIV-1 and SIVcpz Vprs inhibited NF-κB, but the opposite was observed for SIVgsn/mus/mon Vprs (Figure 1C). Thus, some primate lentiviruses may utilize Vpr instead of Nef to boost NF-κB activation, although it will be important to confirm these effects in cells from the respective host species.
Since Vpu has been shown to affect NF-κB activation (Akari et al., 2001; Bour et al., 2001; Galão et al., 2012), we next examined whether the presence of a vpu gene, which is specific to HIV-1 and its simian precursors (Schindler et al., 2006), is associated with differences in the ability of Nef to modulate NF-κB activation. We found that nef alleles derived from primate lentiviruses encoding vpu were not significantly more active (287.3% ± 32.2%, n = 26) than those lacking this accessory gene (226.1% ± 30.9%, n = 13) (Figure 1B).
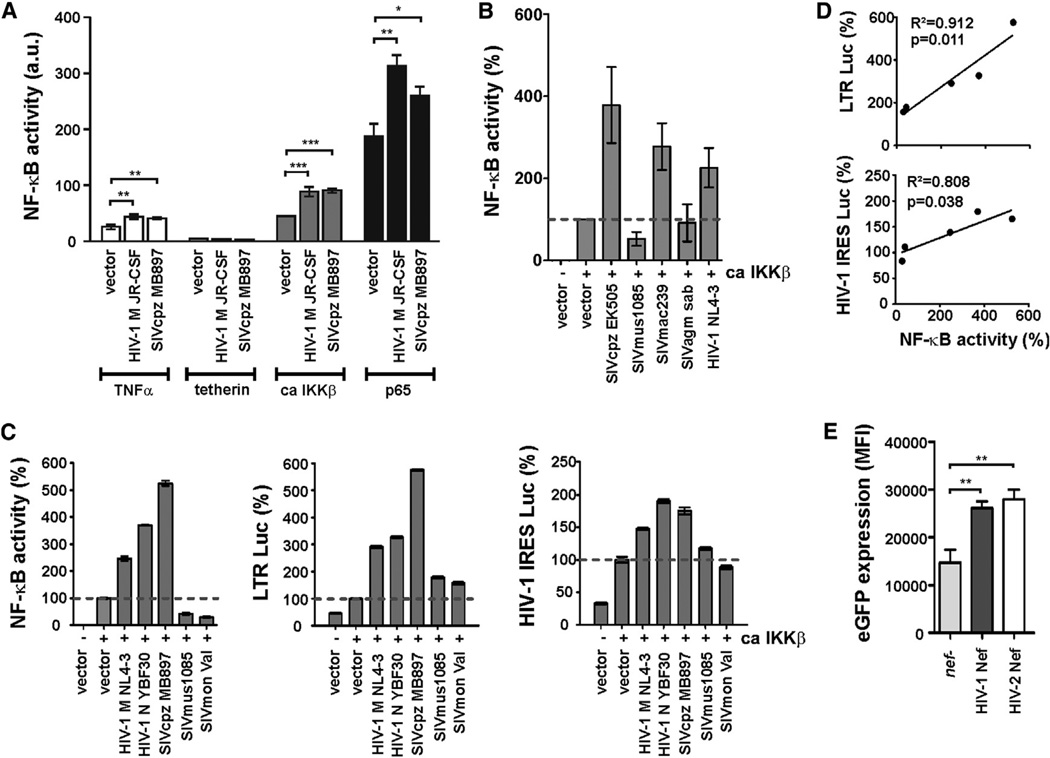
Overexpression of human tetherin activates the canonical NF-κB pathway (Cocka and Bates, 2012; Galão et al., 2012; Tokarev et al., 2013). To determine whether Nef modulates tetherin-induced NF-κB activation, we cotransfected 293T cells with the NF-κB dependent firefly luciferase reporter construct, expression vectors for human tetherin, and various nef alleles. In general, the effects of Nef on tetherin-dependent NF-κB activation were modest and did not differ significantly between viruses encoding vpu or not (Figures 1D). In contrast, HIV-1 and SIVcpz Nefs clearly enhanced TNFα and p65-induced NF-κB activation (Figures S1A and 2A). Notably, Nef did not affect NF-κB activity in the absence of other stimuli (Figure S1B), indicating that it modulates responsiveness to stimulation rather than activating NF-κB directly.
Figure 2. Primate Lentiviral Nef Proteins Stimulate Viral LTR Activity.
(A) Nef-mediated modulation of NF-κB activation by different inducers. 293T cells were transfected with the indicated nef alleles as described for Figure 1B and activated via TNFα stimulation (25 ng/ml) or cotransfection of tetherin, ca IKKβ, or p65. Stars indicate statistically significant differences (*p < 0.05, **p < 0.01, ***p < 0.001). Mean values of three to six transfections (±SEM) are shown in (A)–(C).
(B) 293T cells were transfected with nef alleles differing strongly in their effect on NF-κB activity as described for Figure 1B using ca IKKβ as inducer.
(C) 293T cells were cotransfected with Nef expression vectors, ca IKKβ, a Gaussia luciferase construct for normalization, and an NF-κB-dependent firefly luciferase reporter vector (left), an HIV-1 LTR firefly luciferase reporter construct (middle), or a nef and env defective proviral HIV-1 NL4-3 IRES luciferase construct (right).
(D) Correlations between the levels of NF-κB activity and (upper) LTR-driven or (lower) proviral expression of a luciferase reporter gene.
(E) Levels of EGFP expression in PBMC cultures infected with HIV-1 IRES EGFP constructs expressing the NL4-3 or HIV-2 BEN nef alleles or containing a disrupted nef gene (nef−). Results were obtained from infections of three PBMC donors.
See also Figure S2.
To obtain insights into the mechanisms underlying Nef-mediated modulation of NF-κB activity, we analyzed a subset of five nef alleles that differed the most in their effect on NF-κB activation (Figure 2B). EMSA and immunofluorescence microscopy revealed that Nef does not affect the quantity of nuclear p65 (Figures S1C and S1D). To elucidate the impact on viral transcription, we compared LTR activation in the presence of Nefs that enhanced (HIV-1 and SIVcpz) or suppressed (SIVmus and SIVmon) NF-κB activation (Figure 2C, left). We found that the former enhanced LTR promoter activity ~3- to 6-fold, whereas the latter had only modest effects (Figure 2C, middle). Similarly, transcription of the HIV-1 provirus was enhanced by HIV-1 and SIVcpz, but not by SIVmus or SIVmon Nefs (Figure 2C, right). Increased NF-κB activation correlated with enhanced LTR promoter activity and proviral transcription (Figure 2D). To further examine the effect of Nef on proviral transcription, we infected human peripheral blood mononuclear cells (PBMCs) with HIV-1 IRES EGFP constructs. In these constructs, EGFP expression is an indicator of proviral transcription, since EGFP is expressed together with Nef from a biscistronic RNA via the regular LTR promoter and splice sites. We found that intact HIV-1 and HIV-2 nef genes increased LTR-dependent EGFP expression ~2-fold in infected primary cells (Figure 2E). Thus, many primate lentiviruses including HIV-1, HIV-2, and their SIV precursors use Nef to boost viral transcription through NF-κB activation.
To determine which domains in Nef are involved in increasing NF-κB activity, we examined 21 mutants of an HIV-1 nef allele (NA7) derived directly from an infected patient (Figure S2A) (Greenberg et al., 1997). All mutant Nefs were efficiently expressed (Figure S2B). Functional analyses showed that multiple amino acid residues across the Nef sequence are important for efficient enhancement of NF-κB activity (Figure S2C). For example, mutations in the (PxxP)3 region that interacts with SH3 domain-containing cellular kinases as well as two acidic C-terminal residues (E154/E155) were critical for Nef-mediated enhancement of NF-κB activity. Moreover, enhancement of NF-κB activity was separable from other Nef functions, such as downmodulation of CD4 and MHC-I and stimulation of HIV-1 replication in PBMC cultures, which also contribute to efficient replication in human PBMCs (Kirchhoff et al., 2008). However, we found a significant correlation (R2 = 0.3208; p = 0.0054) between Nef-mediated enhancement of NF-κB activity and virion infectivity, suggesting that both are mediated by overlapping domains. For example, changes of R71A, D86A, and E154A/E155A disrupted these two activities but had little if any effect on other Nef functions (Figure S2C).
Primate Lentiviral Vpus Inhibit NF-κB Activation
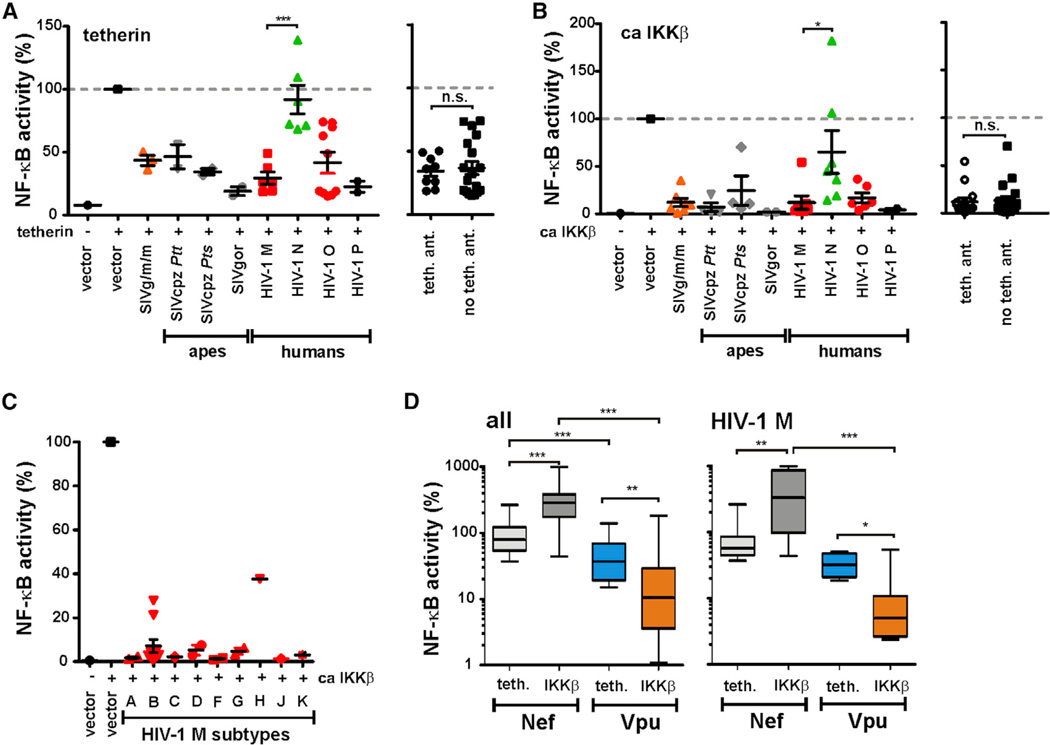
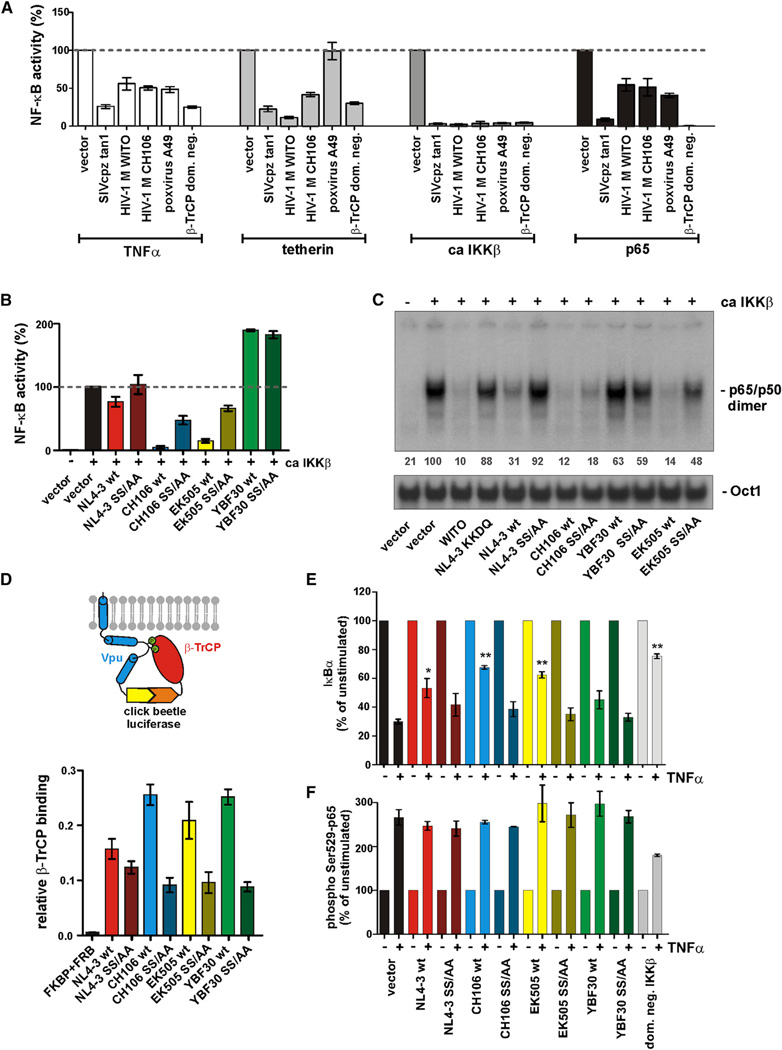
Next, we examined the effects of Vpu on NF-κB activity. Initially, we focused on tetherin-induced NF-κB activation because it has been shown that HIV-1 group M Vpus suppress this effect (Galão et al., 2012; Tokarev et al., 2013). To determine whether suppression of NF-κB activation by Vpu is conserved among primate lentiviruses, we analyzed 33 vpu alleles from essentially all groups of HIV-1 and SIV encoding this accessory gene. As shown in the left panel of Figure 3A, most primate lentiviral Vpus inhibited tetherin-induced NF-κB activation. The exception were Vpu proteins from HIV-1 group N, which are also poor tetherin antagonists (Sauter et al., 2012) (Figure 3A). Nonetheless, the finding that SIVcpz, SIVgor, and HIV-1 group O Vpus were active in this assay came as a surprise, since these viruses use Nef instead of Vpu to antagonize tetherin in their respective hosts (Sauter et al., 2009; Kluge et al., 2014). Thus, vpu alleles known to differ in their ability to antagonize tetherin did not differ in their ability to suppress tetherin-induced NF-κB activation (Figure 3A, right panel), suggesting that Vpu targets a later step in the NF-κB signaling pathway. Indeed, we found that most Vpu proteins blocked IKKβ-induced NF-κB activation by >90% (Figure 3B), which was conserved in all lineages of SIV and HIV-1 (except group N), including all group M subtypes (Figures 3A–3C). We also examined the effects of tetherin and IKKβ at different doses and found that Vpu inhibits both of them with similar efficacy (Figure S3A). While primate lentiviral Nef proteins enhanced IKKβ-induced (but not tetherin-induced) NF-κB activation (324.7% ± 34.5%, n = 44; versus 95.1% ± 9.0%, n = 37; mean values ± SEM), HIV-1 and SIV Vpus suppressed this process (Figure 3D, left). These differences were particularly striking for Nef and Vpu proteins from pandemic HIV-1 M strains: the former increased IKKβ-induced NF-κB up to 10-fold (mean 462.7% ± 119.6%, n = 10), and the latter reduced it by up to 50-fold (mean 11.9% ± 7.1%, n = 7) (Figure 3D, right).
Figure 3. Inhibition of NF-κB Activation by Vpu.
(A and B) 293T cells were transfected with the indicated vpu alleles as described for Figure 1B using (A) human tetherin or (B) ca IKKβ as inducer. Each data point represents one vpu allele. Mean values of three to nine transfections are shown in (A)–(C). In the right panels, the data sets were grouped based on the ability of Vpu to antagonize human tetherin. HIV-1 N Vpus cannot be clearly assigned to the active or defective group and are thus not shown.
(C) Inhibition of NF-κB activation by Vpus of different subtypes of HIV-1 group M. 293T cells were transfected with vpu alleles from the indicated HIV-1Msubtypes as described for Figure 1B using ca IKKβ as inducer.
(D) Comparison of Nef- and Vpu-mediated modulation of ca IKKβ- and tetherin-induced NF-κB activation.
See also Figure S3.
Recruitment of β-TrCP by Vpu Is Not Sufficient for Inhibition of NF-κB
Our finding that Vpu suppressed IKKβ-induced NF-κB activation suggested that it may target the IKK complex directly or interfere with a factor downstream in the NF-κB signaling pathway. The effects of Vpu on NF-κB activation were similar to those of a transdominant-negative mutant of β-TrCP and the poxvirus A49 protein, which sequesters β-TrCP (Figure 4A) (Mansur et al., 2013). It has been suggested that NL4-3 Vpu inhibits the degradation of IκB by acting as a transdominant inhibitor of β-TrCP (Bour et al., 2001). This effect was proposed to be dependent on a DSGxxS motif in Vpu that is phosphorylated at the serine residues by casein kinase II (CKII) and interacts with β-TrCP to recruit the E3 ubiquitinligase SCF (Skp1, Cullin, F-box) complex (Douglas et al., 2009; Mangeat et al., 2009; Margottin et al., 1998). Thus, it was unexpected that mutation of these serine residues to alanine in two HIV-1 (CH106) and SIVcpz (EK505) Vpus reduced, but not fully disrupted, their ability to suppress NF-κB activation (Figures 4B and S3B). Consistent with this, the mutant Vpus maintained some activity in reducing the binding of p65/p50 dimers to DNA (Figure 4C). Of note, the Vpu of the T cell line-adapted NL4-3 clone, which has been utilized in most previous studies, was substantially less active in reducing IKKβ-induced NF-κB activation (62.0% ± 9.5%) than the Vpu proteins of 28 other HIV-1 M strains analyzed (5.3% ± 1.3%; p < 0.0001).
Figure 4. Mechanism of Vpu-Mediated Inhibition of NF-κB Activation.
(A) 293T cells were cotransfected with luciferase constructs as described for Figure 1B and expression vectors for the indicated vpu alleles, poxvirus protein A49, or a dominant-negative mutant of β-TrCP1. TNFα stimulation (25 ng/ml), overexpression of tetherin, ca IKKβ, or p65 were used to activate NF-κB. (A) and (B) show mean values (±SEM) derived from three to nine transfections.
(B) Effect of mutations in the DSGxxS β-TrCP interaction site of various Vpus on IKKβ-induced NF-κB stimulation. 293T cells were transfected with the indicated vpu alleles as described for Figure 1B, using ca IKKβ as inducer.
(C) Vpu reduces p65-DNA association. 293T cells were transfected with the indicated expression vectors. Nuclear extracts were prepared and EMSA was performed 24 hr posttransfection. The 50-labeled oligonucleotide probes corresponding to the HIV-1 NF-κB sites or to an Oct-1 consensus were incubated with nuclear extracts. Numbers provide the mean intensities of the p65/p50 signals normalized to Oct1 of two independent experiments.
(D) Interaction of Vpu with β-TrCP. 293T cells were transfected with equal amounts of plasmids expressing β-TrCP N-terminally fused to the C-terminal fragment of click beetle green and Vpu C-terminally fused to the N-terminal fragment of click beetle green. After 40 hr, click beetle luciferase activity was determined in living cells by addition of D-luciferin and quantification of bioluminescence. The mean values of three transfections ±SEM are shown.
(E) Vpu stabilizes IκBα. 293T cells were transfected with plasmids expressing the indicated vpu alleles or a dominant-negative mutant of IKKβ (dom. neg. IKKβ). Cells were stimulated 24 hr posttransfection with TNFα (10 ng/ml) or left untreated. Fifteen minutes after stimulation, cells were harvested, fixed, and permeabilized and levels of IκBα were analyzed by flow cytometry. Stars indicate a statistically significant stabilization of IκB compared to the vector control (*p < 0.05; **p < 0.01).
(F) Vpu does not affect phosphorylation of p65 at Ser529. Levels of phosphorylated p65 (Ser529) were determined by flow cytometry as described for Figure 4E.
See also Figure S4.
To examine whether the capability of various Vpus to suppress NF-κB activation correlated with their ability to interact with β-TrCP, we fused the N-terminal fragment of a click beetle luciferase to the C terminus of various Vpus and the C-terminal fragment of this luciferase to the N terminus of β-TrCP (Figure 4D, upper). We found that both wild-type HIV-1 (CH106) and SIVcpz (EK505) Vpus that block NF-κB activation as well as a group N (YBF30) Vpu that increases its activation interacted efficiently with β-TrCP (Figure 4D, lower). As expected (Sauter et al., 2012), mutation of the serine phosphorylation sites impaired the interaction of Vpu with β-TrCP. However, the effects of various Vpu proteins on NF-κB activity did not correlate with their β-TrCP binding capacity. Furthermore, Vpu stabilized IκBα upon stimulation with TNFα (Figures 4E and S4A). This effect was partially abrogated by serine to alanine mutations in the DSGxxS β-TrCP binding motif. Since p65 and Vpu are both phosphorylated by CKII (Schubert et al., 1992), we also examined whether phosphorylation of p65 at Ser529, a known target of CKII (Wang et al., 2000), is inhibited by Vpu, but we found that this was not the case (Figures 4F and S4B). Thus, Vpu stabilizes IκB in a β-TrCP-dependent manner but also inhibits NF-κB activation by mechanisms that are independent of β-TrCP and CKII sequestration.
Vpu Inhibits Nuclear Translocation of p65
To define the determinants within Vpu that are involved in NF-κB inhibition, we performed triple-alanine scan mutagenesis in the cytoplasmic domains of two HIV-1 (WITO, CH106) Vpus. Western blot analyses showed that all mutant Vpus were efficiently expressed (Figure S4C). Whereas mutations in the DSGxxS β-TrCP binding motif abolished the ability of WITO Vpu to block NF-κB activation, the effects of these mutations were less pronounced in CH106 Vpu (Figure S4D). In addition to mutations of the serine residues that are phosphorylated and critical for β-TrCP binding, some alanine mutations in the first α-helix of Vpu (i.e., RAE49-51AAA) impaired inhibition of NF-κB activation. This is in agreement with recent findings showing that mutations of not only the β-TrCP binding site but also adjacent residues R45, R49, E51, G59, and E62 are required for efficient Vpu-mediated inhibition of tetherin signaling (Pickering et al., 2014). Thus, this region in Vpu appears to affect NF-κB activity by both β-TrCP-dependent and independent mechanisms.
We next analyzed whether subcellular localization is important for the ability of Vpu to suppress NF-κB activation. To address this, we fused the NL4-3, CH106, and EK505 Vpus to a signal (KKDQ) previously shown to retain Vpu in the endoplasmic reticulum (ER) (Skasko et al., 2011). This completely disrupted the modest anti-NF-κB activity of NL4-3 Vpu (Figure S4E). In comparison, the CH106 and EK505 KKDQ-Vpus still inhibited NF-κB activation, albeit with an ~4-fold lower potency than the parental forms. Thus, transport of Vpu to a post-ER compartment is required for full anti-NF-κB activity.
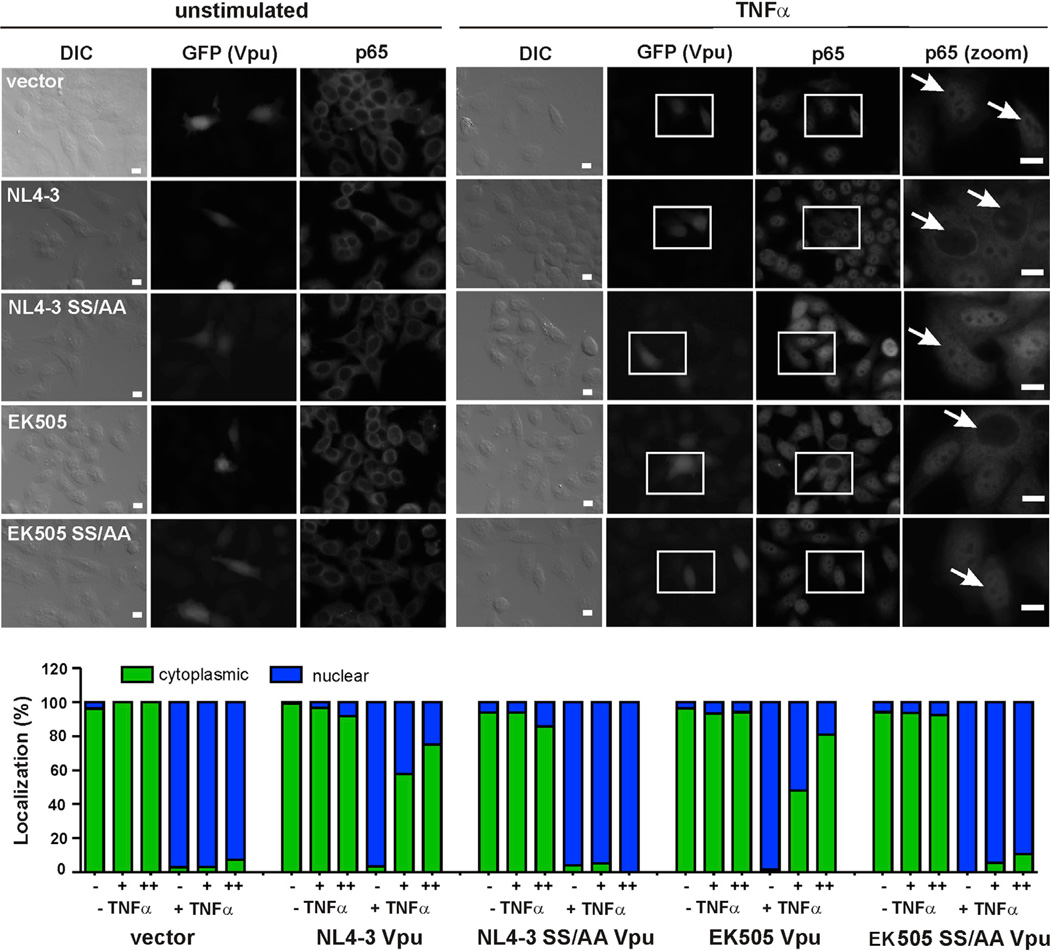
Finally, we microscopically analyzed the subcellular localization of p65 in the presence of wild-type (WT) and mutant Vpus before and after activation with TNFα. These analyses showed that WT HIV-1 (NL4-3, EK505, and CH106) Vpu proteins efficiently inhibited nuclear translocation of p65 (Figures 5 and S5). The SS/AA mutations in the DSGxxS motif of these Vpus largely abolished this activity. Thus, Vpu is a potent inhibitor of nuclear translocation of p65.
Figure 5. Vpu Inhibits Nuclear Translocation of p65.
HeLa cells were transfected with plasmids expressing EGFP alone (vector) or also the indicated Vpu proteins. Cells were treated or not with TNFα for 15 min and analyzed by microscopy 24 hr after transfection. Subcellular localization of endogenous p65 was monitored by indirect immunofluorescence. Cells transfected with the Vpu expression or control constructs were identified by detection of EGFP expression. Nuclei of transfected cells are indicated by white arrows in the close-up images on the right. The lower panel shows the quantity of nuclear and cytoplasmic p65 in cells expressing no (−), medium (+), or high (++) levels of EGFP (and hence Vpu). All samples were blinded, to avoid an experimenter-caused bias in the results. Scale bars indicate 10 µm. The results were derived from the analysis of 213–416 individual cells.
See also Figure S5.
Vpu Suppresses NF-κB-Dependent Immune Activation in a Dominant Manner
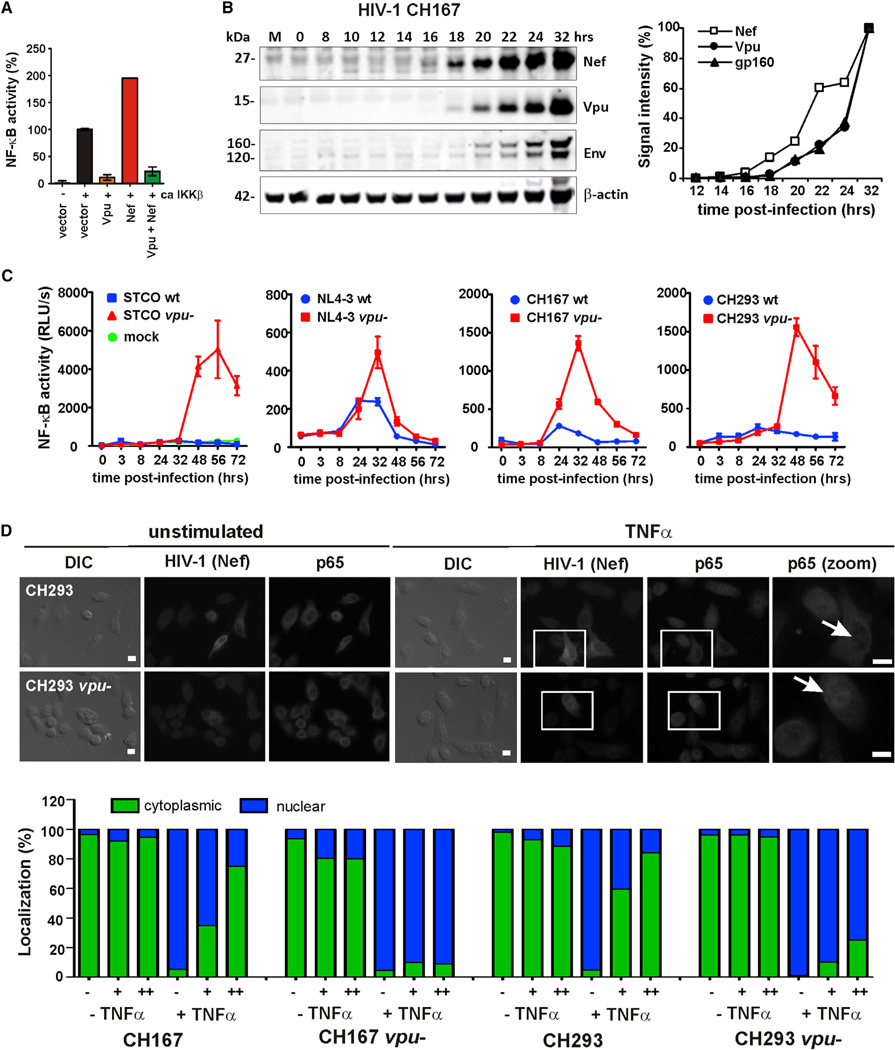
Our results suggested that Nef increases NF-κB activity early during the viral life cycle, whereas Vpu suppresses it during the late stage. Since Nef is expressed throughout the viral life cycle, we reasoned that the effect of Vpu must be dominant. Consistent with this, Vpu prevented IKKβ-induced NF-κB activation even in the presence of Nef (Figure 6A). To examine the effects of Nef and Vpu on NF-κB activity in HIV-1-infected cells, we generated a derivative of the SupT1 T cell line stably expressing a short-lived version of the firefly luciferase under the control of an NF-κB-dependent promoter. This cell line, named SupD1, was infected with VSV-G pseudotyped HIV-1 M molecular clones as well as vpu-defective mutants thereof, and the levels of NF-κB- dependent luciferase activity were measured at different times posttransduction. We found that Nef was expressed prior to Vpu (Figure 6B) and that defective vpu genes were associated with substantially higher levels of NF-κB activation (Figure 6C). While the decline of NF-κB activity at later time points was likely due to virus-induced cytopathic effects, the observed differences in NF-κB activation were not due to different replication rates (Figure S6A). Finally, analysis of the subcellular localization of p65 in cells transduced with WT and vpu-defective HIV-1 (CH167 and CH293) infectious molecular clones before and after activation with TNFα showed that Vpu blocks translocation of p65 from the cytoplasm to the nucleus in HIV-1-infected cells (Figure 6D).
Figure 6. Vpu Suppresses NF-κB Activation in HIV-1-Infected Cells in a Dominant Manner.
(A) 293T cells were transfected with the indicated HIV-1MJR-CSF nef and/or vpu alleles as described for Figure 1B. Mean values of three transfections ±SEM are shown.
(B and C) SupD1 cells stably expressing a short-lived version of the firefly luciferase under the control of an NF-κB-dependent promoter were infected with (B) HIV-1 CH167 or (C) the indicated VSV-G pseudotyped HIV-1 strains. Cells were harvested at the indicated time points posttransduction to determine (B) viral protein expression or (C) the activation levels of NF-κB. The graph in (B) shows the signal intensities of Vpu, Nef, and Env expression relative to those obtained at 32 hr posttransduction (100%).
(D) HeLa cells were transduced with wild-type or vpu-defective HIV-1 CH167 and CH293 constructs and analyzed as described in the legend to Figure 5. HIV-1-infected cells were identified by staining with a Nef-specific antibody. Nuclei of transduced cells are indicated by white arrows in the close-up images on the right. The lower panel shows the quantity of nuclear and cytoplasmic p65 in cells expressing no (−), medium (+), or high (++) levels of Nef (and hence HIV-1). All samples were blinded to avoid an experimenter-caused bias in the results. Scale bars indicate 10 µm. The results were derived from the analysis of 354–519 individual cells.
See also Figure S6.
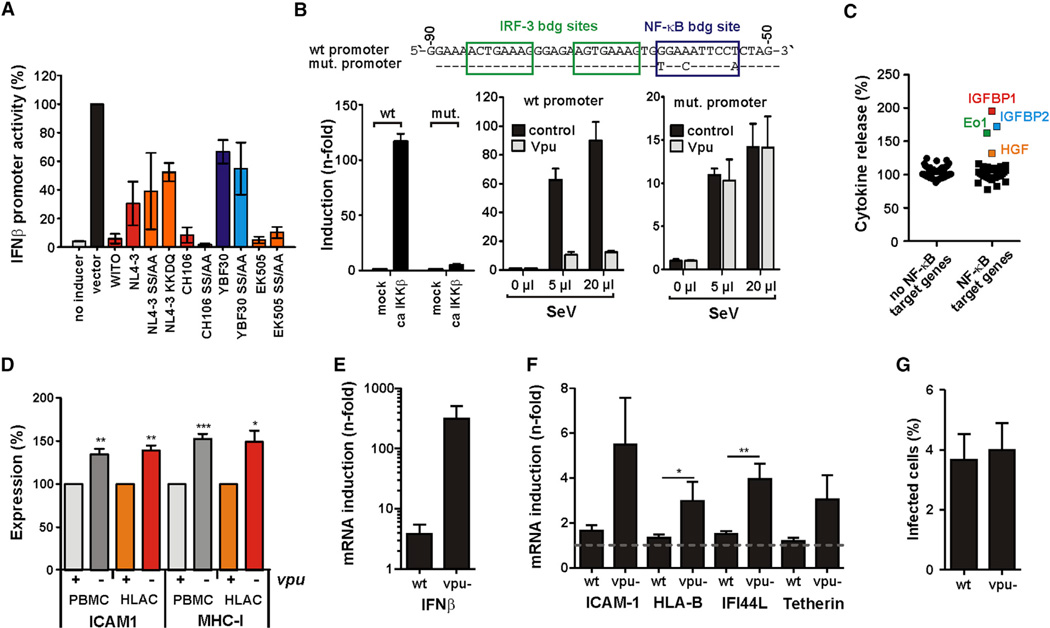
To examine whether Vpu affects the expression of antiviral genes via modulation of NF-κB, we analyzed the effect of various Vpus on the transcriptional activity of the IFNβ promoter upon stimulation with Sendai virus. Vpu proteins from both primary HIV-1 group M (WITO, CH106) and SIVcpz (EK505) strains reduced IFNβ promoter activity by ~95% (Figure 7A). In contrast, NL4-3 and group N (YBF30) Vpus achieved only 70% and 33% inhibition, respectively. Most importantly, mutations in the two-serine phosphorylation sites in the DSGxxS β-TrCP interaction motif did not reduce the ability of HIV-1 (NL4-3, CH106) and SIVcpz (EK505) Vpu proteins to suppress IFNβ promoter activity. The effects of these Vpu proteins on NF-κB activation and IFNβ promoter-dependent gene expression correlated significantly (R2 = 0.89; p = 0.0001).
Figure 7. Vpu Suppresses the Induction of IFNβ and ISGs.
(A) 293T cells were cotransfected with the indicated vpu alleles, a firefly luciferase reporter construct under the control of the IFNβ promoter, and a Gaussia luciferase construct for normalization. Cells were infected with Sendai virus 16 hr posttransfection to activate the IFNβ promoter. Luciferase activities were determined 40 hr posttransfection. The mean values of two independent experiments in triplicates ±SEM are shown.
(B) Top: mutation introduced into the IFNβ promoter firefly reporter construct. 293T cells were cotransfected with plasmids expressing the WITO Vpu (or an empty vector control) together with wild-type or NF-κB unresponsive IFNβ promoter firefly and control Gaussia luciferase constructs. Cells were stimulated by infection with Sendai virus (SeV) 24 hr posttransfection and luciferase assays were performed 16 hr later. Firefly luciferase signals were normalized to the corresponding Gaussia luciferase signals.
(C) Cytokine release from SupD1 cells infected with the HIV-1 M CH167 construct or a vpu-deficient mutant thereof. Shown are cytokines levels obtained for the vpu-deficient mutant relative to the wild-type virus (100%), grouped into target and nontarget genes of NF-κB. Cytokines that were markedly induced in the absence of Vpu are highlighted Eo1, Eotaxin1.
(D) Effect of Vpu on ICAM-1 and MHC-I expression in HIV-1-infected primary cells. PBMCs or HLACs were transduced with HIV-1 M CH167 wild-type or a vpu-deficient mutant thereof. Three days postinfection, ICAM-1 and MHC-I surface levels were analyzed by flow cytometry. The mean values of three to five independent experiments ±SEM are shown.
(E and F) PBMCs from six different donors were transduced with wild-type or vpu-defective HIV-1 CH167 and CH198 constructs. Cells were harvested 72 hr posttransduction. Total cellular RNA was isolated and reversely transcribed, and mRNA expression levels of (E) IFNβ or (F) the indicated NF-κB target genes and ISGs were measured by quantitative real-time PCR. (E)–(G) show mean values of 12 transductions ±SEM.
(G) The percentage of HIV-1-infected PBMCs used in (E) and (F) was determined by flow cytometry after intracellular p24 staining.
It is controversial whether Vpu inhibits innate immune activation by degradation of IRF-3 (Doehle et al., 2012) and/or inhibition of NF-κB (Hotter et al., 2013). To further examine this, we mutated the single NF-κB site in the IFNβ promoter (Figure 7B, upper panel). As expected, this mutation abolished responsiveness of the IFNβ promoter to NF-κB activation via IKKβ (Figure 7B, left panel) and reduced induction by Sendai virus (Figure 7B, middle and right panel). However, the mutated promoter was not inhibited by Vpu (Figure 7B, right panel). These results show that Sendai virus induces IFNβ promoter activity in 293T cells through both NF-κB and IRF-3 and confirmed that Vpu suppresses activation through inhibition of NF-κB and not IRF-3.
We also quantified the release of 80 cytokines from SupD1 cells infected with WT and vpu-defective HIV-1 (CH167). A quantitative protein array of the supernatants (Figure S6B) revealed that production of four genes (eotaxin 1, insulin-like growth factor-binding proteins IGFBP1 and IGFBP2, as well as hepatocyte growth factor [HGF]) was markedly increased in cells infected with the vpu-deficient virus (Figures 7C and S6C). Notably, these four genes are known to be induced by NF-κB (Pahl, 1999). Furthermore, HGF and eotaxin 1 are both ISGs, and high plasma levels of the latter are associated with reduced susceptibility to infection in the SIV/macaque model (Promadej-Lanier et al., 2010). Interestingly, it has been reported that a single-nucleotide polymorphism in the eotaxin 1 promoter affects susceptibility to HIV-1 infection (Modi et al., 2003). In contrast, none of 48 genes known not to be regulated by NF-κB were affected by the absence of Vpu.
Finally, we analyzed the expression of ICAM-1 and MHC-I on infected primary cells. Both proteins are key players of inflammatory immune responses and established targets of NF-κB (Baumann et al., 2007; Girdlestone et al., 1993). The surface levels of ICAM-1 and MHC-I increased by 35% to 73% on PBMCs and human lymphoid aggregate cells (HLAC) infected with vpu-deficient HIV-1 compared to cells infected with the wild-type virus (Figures 7D and S6D). Next, we infected PBMCs with wild-type or vpu-defective HIV-1 CH167 and CH198 constructs and determined the effect of Vpu on the transcriptional levels of IFNβ and several NF-κB- and IFN-dependent genes. On average, lack of Vpu was associated with ~100-fold increased induction of IFNβ and ~3- to 5-fold increased expression of ICAM-1, HLA-B, IFI44L, and tetherin (Figures 7E and 7F). These alterations were observed in bulk mRNA preparations and only ~4% of the cells were infected by HIV-1 (Figure 7G). Thus, the results suggest that lack of Vpu is associated with ~75- to 125-fold increased expression levels of these ISGs in HIV-1-infected primary T cells.
DISCUSSION
In this study, we show that the great majority of HIV-1 and SIV Nefs increase NF-κB activity, including in primary PBMCs. It is well established that Nef is abundantly expressed during the earliest stages of the viral replication cycle and that NF-κB activation is essential for effective proviral transcription. Thus, our results suggest that most primate lentiviral Nef proteins activate NF-κB to initiate efficient viral transcription. However, NF-κB also induces the expression of many antiviral host factors. To suppress this induction, HIV-1 and its SIV precursors employ Vpu during the later stages of the viral life cycle. The inhibitory effect of Vpu is dominant over the stimulatory effect of Nef (Figure 6A), and vpu-defective HIV-1 constructs induce substantially higher levels of NF-κB activity (Figure 6C) as well as IFNβ and ISG expression (Figure 7) in infected T cells than the corresponding wild-type viruses. Thus, it seems clear that HIV-1 and its SIV precursors use Nef and Vpu to fine-tune NF-κB activity during the viral life cycle to achieve both efficient viral replication and immune evasion.
Vpu proteins from the rare HIV-1 group N strains showed significantly lower activity in suppressing NF-κB activation than those from pandemic HIV-1 M strains and SIVcpz. This further supports the hypothesis that HIV-1 N Vpus lost important functions following cross-species transmission, such as the ability to reduce the surface expression of CD4 (Sauter et al., 2009, Sauter et al., 2012). Lack of Vpu-mediated inhibition of NF-κB was associated with increased transcriptional activity of the IFNβ promoter. It will be interesting to determine whether HIV-1 N strains induce stronger innate immune responses than pandemic HIV-1 group M strains.
Vpu proteins suppressed tetherin-mediated NF-κB activation irrespective of their ability to antagonize this protein’s effect on virus release, suggesting multiple interactions with the signaling pathway. We found that Vpu-mediated degradation of tetherin and inhibition of nuclear translocation of p65 both contribute to the suppression of NF-κB activity. However, the exact mechanism underlying Vpus ability to inhibit NF-κB activation remains to be established. It has been suggested that Vpu prevents degradation of IκB by acting as a transdominant-negative inhibitor of β-TrCP (Bour et al., 2001). In agreement with this, IκB stabilization and inhibition of p65 nuclear translocation depend on the presence of an intact β-TrCP binding motif and interaction of Vpu with β-TrCP was essential for full inhibition. However, Vpu proteins that failed to recruit β-TrCP were still able to inhibit binding of p65 to its target sequences and to reduce NF-κB-dependent gene expression (Figures 4B and 4C). Thus, the interaction of Vpu with β-TrCP is neither required nor sufficient for the inhibition of NF-κB activation. A possible explanation is that the NL4-3 Vpu used in most studies is a much weaker inhibitor of NF-κB activation than vpu alleles derived from primary HIV-1 strains. Furthermore, the disruptive effect of SS/AA mutations in the DSGxxS β-TrCP interaction motif is more severe in the NL4-3 Vpu than in most primary HIV-1 Vpus, further emphasizing the importance of using primary HIV-1 genes. Notably, mutations in the DSGxxS site may not only prevent β-TrCP binding but also alter the structure and function of the C-terminal Vpu domain (Coadou et al., 2003).
The results of the present study add to the evidence that early HIV-1 gene products, i.e., Nef and Tat, promote NF-κB activation, whereas late products, such as Vpu, suppress it (Akari et al., 2001; Bour et al., 2001; Felzien et al., 1998; Fiume et al., 2012; Herbein et al., 2008; Leulier et al., 2003; Liu et al., 2013; Mangino et al., 2011; Roux et al., 2000; Varin et al., 2005). However, we also show that there is a tight interplay between Nef and Vpu function, strongly suggesting that the opposing effect of these two viral factors optimize viral transcription in the face of innate antiviral responses. The importance of timing, along with the fact that accessory proteins from primary HIV-1 show functional differences from the lab-adapted NL4-3 strain, thus provide an explanation for previous seemingly discrepant results.
Finally, we provide new insight into why Nef proteins of vpu-containing primate lentiviruses lost their ability to downmodulate TCR-CD3 from the cell surface. Stimulation via the TCR-CD3 receptor also induces NF-κB activation and thus expression of immune genes. It seems that most primate lentiviruses prevent this by Nef-mediated downmodulation of the TCR-CD3 receptor from the cell surface, whereas HIV-1 and its vpu-containing simian precursors utilize Vpu to prevent NF-κB activation at a later step of the signaling pathway. However, Nef proteins that downmodulate TCR-CD3 boost IKKβ-induced NF-κB activation as efficiently as Nefs lacking this function. Thus, these Nef proteins uncouple stimulation of NF-κB activation in infected T cells from the interaction with antigen-presenting cells. It is thus possible that Nef may stimulate NF-κB more rapidly than it takes to efficiently remove TCR-CD3 from the cell surface.
CONCLUSIONS
In summary, our data show that Nef and Vpu exert opposing effects on NF-κB activity and tightly regulate the activation of this transcription factor to ensure efficient viral transcription while minimizing the expression of antiviral genes. Nef does not enhance NF-κB activation on its own, but it augments the responsiveness to other stimuli, most likely because a certain state of cellular activation is needed to allow productive virus infection. We also show that primate lentiviral Vpu proteins inhibit NF-κB activation and antiviral gene expression independently of their anti-tetherin activity. Thus, a tight regulation of NF-κB by Nef and Vpu seems critical for viral replication, immune evasion, and pathogenesis.
EXPERIMENTAL PROCEDURES
Expression Vectors
Cloning of vpu, nef, and tetherin alleles into the bicistronic cytomegalovirus promoter-based pCG expression vector coexpressing the GFP was performed as described previously (Sauter et al., 2009). PCR with primers introducing XbaI and MluI restriction sites flanking the reading frames was used to generate mutant or chimeric vpu and nef alleles for cloning into the pCG vector.
Proviral HIV-1 Constructs
Generation of HIV-1 NL4-3-based proviral constructs and infectious molecular clones of T/F and CC HIV-1 strains has been described previously (Parrish et al., 2013; Schindler et al., 2006).
Cell Culture and Transfections
Cells were cultured and transfected as described in Supplemental Experimental Procedures.
NF-κB Activation
Dual luciferase assays with an NF-κB-dependent firefly luciferase and a Gaussia luciferase construct under the control of a minimal pTAL promoter for normalization were performed to determine the effect of Nef or Vpu on NF-κB activity as described in Supplemental Experimental Procedures.
EMSA
Nuclear extracts were prepared, and EMSAs with an HIV-1 NF-κB probe were performed as previously described (Van Lint et al., 1996). See Supplemental Experimental Procedures for details.
Viral LTR Activity
To determine whether Nef enhances viral promoter activity via modulation of NF-κB, 293T cells were cotransfected with expression vectors for nef and a constitutively active mutant of IKKβ, a Gaussia luciferase construct for normalization, and an HIV-1 LTR firefly luciferase reporter construct (LTR_Luc) or an NL4-3 nef- and env-deficient proviral construct coexpressing firefly luciferase via an IRES (HIV-1_IRES_Luc). Luciferase activities were determined 40 hr posttransfection.
Microscopic Analyses of p65
Subcellular localization of p65 in the presence of Nef or Vpu was monitored by indirect immunofluorescence as outlined in Supplemental Experimental Procedures.
Click Beetle Luciferase Assay
Generation of Vpu constructs fused to fragments of click beetle luciferase and a protocol of this assay have been described previously (Sauter et al., 2012).
NF-κB Activity in HIV-1-Infected Cells
The effect of HIV infection on NF-κB activity was determined as outlined in Supplemental Experimental Procedures.
IFNβ Promoter Activity
To examine whether Vpu suppresses the expression of innate immunity genes, 293T cells were cotransfected with a firefly luciferase reporter construct under the control of the IFNβ promoter or a variant thereof with mutated NF-κB binding site, a Gaussia luciferase construct for normalization, and expression vectors for vpu or an empty vector control. Cells were infected 16 hr posttransfection with Sendai virus to activate the IFNβ promoter. Luciferase activities were determined 40 hr posttransfection.
Cytokine Array
Differences in cytokine release from cells infected with WT or vpu-deficient CH167 were determined using a cytokine array as described in Supplemental Experimental Procedures.
qRT-PCR
Gene expression levels were determined by qRT-PCR as outlined in Supplemental Experimental Procedures.
Flow Cytometry
Flow cytometric analyses were performed as outlined in Supplemental Experimental Procedures.
Statistical Analysis
All statistical calculations were performed with a two-tailed unpaired Student’s t test using Graph Pad Prism Version 5.03. p values < 0.05 were considered significant. Correlations were calculated with the linear regression module.
Supplementary Material
Highlights.
The early protein Nef boosts and the late protein Vpu suppresses NF-κB activation
Vpu inhibits nuclear translocation of p65 and stabilizes cytoplasmic IκB
Vpu-mediated inhibition of NF-κB activation suppresses innate immune activation
Primate lentiviruses use Nef and Vpu to fine-tune viral and antiviral gene expression
ACKNOWLEDGMENTS
We thank Susanne Engelhart, Nadége Delacourt, Daniela Krnavek, Kerstin Regensburger, and Martha Mayer for excellent technical assistance. Sendai virus was kindly provided by Georg Kochs. Poxvirus protein A49 was kindly provided by Andrew Bowie. C.V.L. is directeur de recherches of the Belgian Fund for Scientific Research (FRS-FNRS, Belgium). B.V.D is fellow of the CIBLES Excellence Program of the Walloon region. Work in C.V.L.’s lab was supported by grants from the FRS-FNRS (Belgium), the Télévie-Programme of the FRS-FNRS, the CIBLES Excellence Programme of the Walloon Region, the NEAT (European AIDS treatment network) integration grant, the International Brachet Stiftung, the Fondation Roi Baudouin (Belgium), and the ANRS (Agence Nationale de Recherche sur le SIDA, France). J.C.P. is funded by the Rouen University Hospital. This work was further supported by the Deutsche Forschungsgemeinschaft, European FP7 “HIT HIDDEN HIV” (305762), and an ERC advanced grant to F.K. and by grants from the NIH to B.H.H. (R37 AI50529, R01 AI58715, R37 AI066998, P30 AI045008) and the International Graduate School in Molecular Medicine Ulm to D.H.
Footnotes
Supplemental Information includes Supplemental Experimental Procedures and six figures and can be found with this article online at http://dx.doi.org/10.1016/j.celrep.2014.12.047.
AUTHOR CONTRIBUTIONS
D.S. and D.H. contributed equally to this manuscript and performed most experiments. B.V.D. and C.V.L. analyzed nuclear translocation and DNA binding of p65; S.W. functionally characterized mutant Nefs; H.Y. contributed fluorescence- activated cell sorting data; S.F.K. characterized ER-resident Vpus; and C.M.S., B.B., T.W., and B.H.H. provided viral constructs, reagents, and protocols. J.C.P. and M.L. contributed unpublished HIV-1 sequence data. D.S., D.H. and F.K. analyzed and interpreted data, assembled the figures, and wrote the manuscript.
REFERENCES
- Akari H, Bour S, Kao S, Adachi A, Strebel K. The human immunodeficiency virus type 1 accessory protein Vpu induces apoptosis by suppressing the nuclear factor kappaB-dependent expression of antiapoptotic factors. J. Exp. Med. 2001;194:1299–1311. doi: 10.1084/jem.194.9.1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayyavoo V, Mahboubi A, Mahalingam S, Ramalingam R, Kudchodkar S, Williams WV, Green DR, Weiner DB. HIV-1 Vpr suppresses immune activation and apoptosis through regulation of nuclear factor kappa B. Nat. Med. 1997;3:1117–1123. doi: 10.1038/nm1097-1117. [DOI] [PubMed] [Google Scholar]
- Bandres JC, Ratner L. Human immunodeficiency virus type 1 Nef protein down-regulates transcription factors NF-kappa B and AP-1 in human T cells in vitro after T-cell receptor stimulation. J. Virol. 1994;68:3243–3249. doi: 10.1128/jvi.68.5.3243-3249.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann B, Wagner M, Aleksic T, von Wichert G, Weber CK, Adler G, Wirth T. Constitutive IKK2 activation in acinar cells is sufficient to induce pancreatitis in vivo. J. Clin. Invest. 2007;117:1502–1513. doi: 10.1172/JCI30876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bour S, Perrin C, Akari H, Strebel K. The human immunodeficiency virus type 1 Vpu protein inhibits NF-kappa B activation by interfering with beta TrCP-mediated degradation of Ikappa B. J. Biol. Chem. 2001;276:15920–15928. doi: 10.1074/jbc.M010533200. [DOI] [PubMed] [Google Scholar]
- Chan JK, Greene WC. Dynamic roles for NF-κB in HTLV-I and HIV-1 retroviral pathogenesis. Immunol. Rev. 2012;246:286–310. doi: 10.1111/j.1600-065X.2012.01094.x. [DOI] [PubMed] [Google Scholar]
- Coadou G, Gharbi-Benarous J, Megy S, Bertho G, Evrard-Todeschi N, Segeral E, Benarous R, Girault J-P. NMR studies of the phosphorylation motif of the HIV-1 protein Vpu bound to the F-box protein beta-TrCP. Biochemistry. 2003;42:14741–14751. doi: 10.1021/bi035207u. [DOI] [PubMed] [Google Scholar]
- Cocka LJ, Bates P. Identification of alternatively translated Tetherin isoforms with differing antiviral and signaling activities. PLoS Pathog. 2012;8:e1002931. doi: 10.1371/journal.ppat.1002931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doehle BP, Chang K, Rustagi A, McNevin J, McElrath MJ, Gale M., Jr Vpu mediates depletion of interferon regulatory factor 3 during HIV infection by a lysosome-dependent mechanism. J. Virol. 2012;86:8367–8374. doi: 10.1128/JVI.00423-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas JL, Viswanathan K, McCarroll MN, Gustin JK, Früh K, Moses AV. Vpu directs the degradation of the human immunodeficiency virus restriction factor BST-2/Tetherin via a betaTrCP-dependent mechanism. J. Virol. 2009;83:7931–7947. doi: 10.1128/JVI.00242-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felzien LK, Woffendin C, Hottiger MO, Subbramanian RA, Cohen EA, Nabel GJ. HIV transcriptional activation by the accessory protein VPR, is mediated by the p300 co-activator. Proc. Natl. Acad. Sci. USA. 1998;95:5281–5286. doi: 10.1073/pnas.95.9.5281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiume G, Vecchio E, De Laurentiis A, Trimboli F, Palmieri C, Pisano A, Falcone C, Pontoriero M, Rossi A, Scialdone A, et al. Human immunodeficiency virus-1 Tat activates NF-κB via physical interaction with IκB-α and p65. Nucleic Acids Res. 2012;40:3548–3562. doi: 10.1093/nar/gkr1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galão RP, Le Tortorec A, Pickering S, Kueck T, Neil SJD. Innate sensing of HIV-1 assembly by Tetherin induces NFκB-dependent proinflammatory responses. Cell Host Microbe. 2012;12:633–644. doi: 10.1016/j.chom.2012.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galão RP, Pickering S, Curnock R, Neil SJD. Retroviral retention activates a Syk-dependent HemITAM in human tetherin. Cell Host Microbe. 2014;16:291–303. doi: 10.1016/j.chom.2014.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S, Hayden MS. Celebrating 25 years of NF-κB research. Immunol. Rev. 2012;246:5–13. doi: 10.1111/j.1600-065X.2012.01111.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girdlestone J, Isamat M, Gewert D, Milstein C. Transcriptional regulation of HLA-A and -B: differential binding of members of the Rel and IRF families of transcription factors. Proc. Natl. Acad. Sci. USA. 1993;90:11568–11572. doi: 10.1073/pnas.90.24.11568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg ME, Bronson S, Lock M, Neumann M, Pavlakis GN, Skowronski J. Co-localization of HIV-1 Nef with the AP-2 adaptor protein complex correlates with Nef-induced CD4 down-regulation. EMBO J. 1997;16:6964–6976. doi: 10.1093/emboj/16.23.6964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbein G, Varin A, Larbi A, Fortin C, Mahlknecht U, Fulop T, Aggarwal BB. Nef and TNFalpha are coplayers that favor HIV-1 replication in monocytic cells and primary macrophages. Curr. HIV Res. 2008;6:117–129. doi: 10.2174/157016208783884985. [DOI] [PubMed] [Google Scholar]
- Hotter D, Kirchhoff F, Sauter D. HIV-1 Vpu does not degrade interferon regulatory factor 3. J. Virol. 2013;87:7160–7165. doi: 10.1128/JVI.00526-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia B, Serra-Moreno R, Neidermyer W, Rahmberg A, Mackey J, Fofana IB, Johnson WE, Westmoreland S, Evans DT. Species-specific activity of SIV Nef and HIV-1 Vpu in overcoming restriction by tetherin/ BST2. PLoS Pathog. 2009;5:e1000429. doi: 10.1371/journal.ppat.1000429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchhoff F, Schindler M, Specht A, Arhel N, Münch J. Role of Nef in primate lentiviral immunopathogenesis. Cell. Mol. Life Sci. 2008;65:2621–2636. doi: 10.1007/s00018-008-8094-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kluge SF, Mack K, Iyer SS, Pujol FM, Heigele A, Learn GH, Usmani SM, Sauter D, Joas S, Hotter D, et al. Nef proteins of epidemic HIV-1 group O strains antagonize human tetherin. Cell Host Microbe. 2014;16:639–650. doi: 10.1016/j.chom.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leulier F, Marchal C, Miletich I, Limbourg-Bouchon B, Benarous R, Lemaitre B. Directed expression of the HIV-1 accessory protein Vpu in Drosophila fat-body cells inhibits Toll-dependent immune responses. EMBO Rep. 2003;4:976–981. doi: 10.1038/sj.embor.embor936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindwasser OW, Chaudhuri R, Bonifacino JS. Mechanisms of CD4 downregulation by the Nef and Vpu proteins of primate immunodeficiency viruses. Curr. Mol. Med. 2007;7:171–184. doi: 10.2174/156652407780059177. [DOI] [PubMed] [Google Scholar]
- Liu R, Tan J, Lin Y, Jia R, Yang W, Liang C, Geng Y, Qiao W. HIV-1 Vpr activates both canonical and noncanonical NFκB pathway by enhancing the phosphorylation of IKKα/β. Virology. 2013;439:47–56. doi: 10.1016/j.virol.2013.01.020. [DOI] [PubMed] [Google Scholar]
- Mangeat B, Gers-Huber G, Lehmann M, Zufferey M, Luban J, Piguet V. HIV-1 Vpu neutralizes the antiviral factor Tetherin/BST-2 by binding it and directing its beta-TrCP2-dependent degradation. PLoS Pathog. 2009;5:e1000574. doi: 10.1371/journal.ppat.1000574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangino G, Percario ZA, Fiorucci G, Vaccari G, Acconcia F, Chiarabelli C, Leone S, Noto A, Horenkamp FA, Manrique S, et al. HIV-1 Nef induces proinflammatory state in macrophages through its acidic cluster domain: involvement of TNF alpha receptor associated factor 2. PLoS ONE. 2011;6:e22982. doi: 10.1371/journal.pone.0022982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansur DS, Maluquer de Motes C, Unterholzner L, Sumner RP, Ferguson BJ, Ren H, Strnadova P, Bowie AG, Smith GL. Poxvirus targeting of E3 ligase β-TrCP by molecular mimicry: a mechanism to inhibit NFκB activation and promote immune evasion and virulence. PLoS Pathog. 2013;9:e1003183. doi: 10.1371/journal.ppat.1003183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margottin F, Bour SP, Durand H, Selig L, Benichou S, Richard V, Thomas D, Strebel K, Benarous R. A novel human WD protein, h-beta TrCp, that interacts with HIV-1 Vpu connects CD4 to the ER degradation pathway through an F-box motif. Mol. Cell. 1998;1:565–574. doi: 10.1016/s1097-2765(00)80056-8. [DOI] [PubMed] [Google Scholar]
- Modi WS, Goedert JJ, Strathdee S, Buchbinder S, Detels R, Donfield S, O’Brien SJ, Winkler C. MCP-1-MCP-3-Eotaxin gene cluster influences HIV-1 transmission. AIDS. 2003;17:2357–2365. doi: 10.1097/00002030-200311070-00011. [DOI] [PubMed] [Google Scholar]
- Napetschnig J, Wu H. Molecular basis of NF-κB signaling. Annu. Rev. Biophys. 2013;42:443–468. doi: 10.1146/annurev-biophys-083012-130338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neil SJD, Zang T, Bieniasz PD. Tetherin inhibits retrovirus release and is antagonized by HIV-1 Vpu. Nature. 2008;451:425–430. doi: 10.1038/nature06553. [DOI] [PubMed] [Google Scholar]
- Niederman TM, Garcia JV, Hastings WR, Luria S, Ratner L. Human immunodeficiency virus type 1 Nef protein inhibits NF-kappa B induction in human T cells. J. Virol. 1992;66:6213–6219. doi: 10.1128/jvi.66.10.6213-6219.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pahl HL. Activators and target genes of Rel/NF-kappaB transcription factors. Oncogene. 1999;18:6853–6866. doi: 10.1038/sj.onc.1203239. [DOI] [PubMed] [Google Scholar]
- Parrish NF, Gao F, Li H, Giorgi EE, Barbian HJ, Parrish EH, Zajic L, Iyer SS, Decker JM, Kumar A, et al. Phenotypic properties of transmitted founder HIV-1. Proc. Natl. Acad. Sci. USA. 2013;110:6626–6633. doi: 10.1073/pnas.1304288110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeffer LM. The role of nuclear factor κB in the interferon response. J. Interferon Cytokine Res. 2011;31:553–559. doi: 10.1089/jir.2011.0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickering S, Hué S, Kim E-Y, Reddy S, Wolinsky SM, Neil SJD. Preservation of tetherin and CD4 counter-activities in circulating Vpu alleles despite extensive sequence variation within HIV-1 infected individuals. PLoS Pathog. 2014;10:e1003895. doi: 10.1371/journal.ppat.1003895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Promadej-Lanier N, Hanson DL, Srinivasan P, Luo W, Adams DR, Guenthner PC, Butera S, Otten RA, Kersh EN. Resistance to Simian HIV infection is associated with high plasma interleukin-8, RANTES and Eotaxin in a macaque model of repeated virus challenges. J. Acquir. Immune Defic. Syndr. 2010;53:574–581. doi: 10.1097/QAI.0b013e3181d3521f. [DOI] [PubMed] [Google Scholar]
- Roux P, Alfieri C, Hrimech M, Cohen EA, Tanner JE. Activation of transcription factors NF-kappaB and NF-IL-6 by human immunodeficiency virus type 1 protein R (Vpr) induces interleukin-8 expression. J. Virol. 2000;74:4658–4665. doi: 10.1128/jvi.74.10.4658-4665.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauter D, Schindler M, Specht A, Landford WN, Münch J, Kim K-A, Votteler J, Schubert U, Bibollet-Ruche F, Keele BF, et al. Tetherin- driven adaptation of Vpu and Nef function and the evolution of pandemic and nonpandemic HIV-1 strains. Cell Host Microbe. 2009;6:409–421. doi: 10.1016/j.chom.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauter D, Unterweger D, Vogl M, Usmani SM, Heigele A, Kluge SF, Hermkes E, Moll M, Barker E, Peeters M, et al. Human tetherin exerts strong selection pressure on the HIV-1 group N Vpu protein. PLoS Pathog. 2012;8:e1003093. doi: 10.1371/journal.ppat.1003093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler M, Münch J, Kutsch O, Li H, Santiago ML, Bibollet-Ruche F, Müller-Trutwin MC, Novembre FJ, Peeters M, Courgnaud V, et al. Nef-mediated suppression of T cell activation was lost in a lentiviral lineage that gave rise to HIV-1. Cell. 2006;125:1055–1067. doi: 10.1016/j.cell.2006.04.033. [DOI] [PubMed] [Google Scholar]
- Schubert U, Schneider T, Henklein P, Hoffmann K, Berthold E, Hauser H, Pauli G, Porstmann T. Human-immunodeficiency-virus-type-1-encoded Vpu protein is phosphorylated by casein kinase II. Eur. J. Biochem. 1992;204:875–883. doi: 10.1111/j.1432-1033.1992.tb16707.x. [DOI] [PubMed] [Google Scholar]
- Skasko M, Tokarev A, Chen C-C, Fischer WB, Pillai SK, Guatelli J. BST-2 is rapidly down-regulated from the cell surface by the HIV-1 protein Vpu: evidence for a post-ER mechanism of Vpu-action. Virology. 2011;411:65–77. doi: 10.1016/j.virol.2010.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokarev A, Suarez M, Kwan W, Fitzpatrick K, Singh R, Guatelli J. Stimulation of NF-κB activity by the HIV restriction factor BST2. J. Virol. 2013;87:2046–2057. doi: 10.1128/JVI.02272-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Damme N, Goff D, Katsura C, Jorgenson RL, Mitchell R, Johnson MC, Stephens EB, Guatelli J. The interferon-induced protein BST-2 restricts HIV-1 release and is downregulated from the cell surface by the viral Vpu protein. Cell Host Microbe. 2008;3:245–252. doi: 10.1016/j.chom.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Lint C, Emiliani S, Ott M, Verdin E. Transcriptional activation and chromatin remodeling of the HIV-1 promoter in response to histone acetylation. EMBO J. 1996;15:1112–1120. [PMC free article] [PubMed] [Google Scholar]
- Varin A, Decrion A-Z, Sabbah E, Quivy V, Sire J, Van Lint C, Roques BP, Aggarwal BB, Herbein G. Synthetic Vpr protein activates activator protein-1, c-Jun N-terminal kinase, and NF-kappaB and stimulates HIV-1 transcription in promonocytic cells and primary macrophages. J. Biol. Chem. 2005;280:42557–42567. doi: 10.1074/jbc.M502211200. [DOI] [PubMed] [Google Scholar]
- Wang D, Westerheide SD, Hanson JL, Baldwin AS., Jr Tumor necrosis factor alpha-induced phosphorylation of RelA/p65 on Ser529 is controlled by casein kinase II. J. Biol. Chem. 2000;275:32592–32597. doi: 10.1074/jbc.M001358200. [DOI] [PubMed] [Google Scholar]
- Williams SA, Chen L-F, Kwon H, Ruiz-Jarabo CM, Verdin E, Greene WC. NF-kappaB p50 promotes HIV latency through HDAC recruitment and repression of transcriptional initiation. EMBO J. 2006;25:139–149. doi: 10.1038/sj.emboj.7600900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams SA, Kwon H, Chen L-F, Greene WC. Sustained induction of NF-kappa B is required for efficient expression of latent human immunodeficiency virus type 1. J. Virol. 2007;81:6043–6056. doi: 10.1128/JVI.02074-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon K, Kim S. Lack of negative influence on the cellular transcription factors NF-kappaB and AP-1 by the nef protein of human immunodeficiency virus type 1. J. Gen. Virol. 1999;80:2951–2956. doi: 10.1099/0022-1317-80-11-2951. [DOI] [PubMed] [Google Scholar]
- Zhang F, Wilson SJ, Landford WC, Virgen B, Gregory D, Johnson MC, Munch J, Kirchhoff F, Bieniasz PD, Hatziioannou T. Nef proteins from simian immunodeficiency viruses are tetherin antagonists. Cell Host Microbe. 2009;6:54–67. doi: 10.1016/j.chom.2009.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.