Abstract
Objective
To determine if active listening modulates the strength of the medial olivocochlear (MOC) reflex in children.
Design
Click-evoked otoacoustic emissions (CEOAEs) were recorded from the right ear in quiet and in four test conditions: one with contralateral broadband noise (BBN) only, and three with active listening tasks wherein attention was directed to speech embedded in contralateral BBN.
Study sample
Fifteen typically-developing children (ranging in age from 8 to 14 years) with normal hearing.
Results
CEOAE levels were reduced in every condition with contralateral acoustic stimulus (CAS) when compared to preceding quiet conditions. There was an additional systematic decrease in CEOAE level with increased listening task difficulty, although this effect was very small. These CEOAE level differences were most apparent in the 8–18 ms region after click onset.
Conclusions
Active listening may change the strength of the MOC reflex in children, although the effects reported here are very subtle. Further studies are needed to verify that task difficulty modulates the activity of the MOC reflex in children.
Keywords: Click-evoked otoacoustic emissions (CEOAE), contralateral inhibition, medial olivocochlear (MOC) reflex, auditory Stroop task, attention
Accruing evidence suggests that active listening influences cochlear mechanics, making it possible for the brain to fine-tune peripheral auditory processing in real time (de Boer & Thornton, 2007; Garinis et al, 2011; Harkrider & Bowers, 2009; Maison et al, 2001; Meric & Collet, 1994). This effect likely arises from an efferent coupling between the cortex and the medial olivocochlear (MOC) bundle, a neural circuit in the auditory brainstem with inhibitory synapses terminating directly on outer hair cells. The MOC bundle provides the neural substrate for the MOC reflex, which decreases the gain of the cochlear amplifier when activated (Cooper & Guinan, 2006; Guinan, 2006). In animal models, it has been demonstrated that the MOC reflex improves the encoding of masked signals at the level of the auditory nerve (Kawase & Liberman, 1993; May et al, 2004; Winslow & Sachs, 1988). It is hypothesized that the human MOC reflex may be involved in speech-in-noise perception and that corticofugal modulation of this mechanism is advantageous in difficult listening situations (Garinis et al, 2011; Giraud et al, 1997; Kumar & Vanaja, 2004; Maison et al, 2001; Micheyl & Collet, 1996).
The inhibitory effect of the human MOC reflex (Guinan, 2010) has been studied with otoacoustic emission (OAE) measurements. Many experiments have employed a ‘contralateral inhibition1 of OAEs’ technique, where in OAEs are first measured in quiet and then during presentation of a contralateral acoustic stimulus (CAS). The OAE level, phase, and/or spectral differences between quiet and CAS conditions are used to quantify the inhibitory effect of the MOC reflex (Velenovsky & Glattke, 2002). Various types of CAS have been employed to elicit contralateral inhibition of OAEs including continuous or pulsed broadband and narrowband noise, speech babble, and steady-state and amplitude-modulated tones (Berlin et al, 1993; Maison et al, 1997, 1999; Smith et al, 2001). In general, broadband CAS, such as white noise, is more effective as an inhibitor than narrowband CAS (Berlin et al, 1993; Norman & Thornton, 1993). CAS can be presented in a steady-state fashion throughout OAE recording (Hood et al, 1996) or in trials with short duration CAS temporally preceding the evoking stimulus (Berlin et al, 1995).
Contralateral inhibition of both distortion product (see Guinan, 2010 and Wagner & Heyd, 2011 for reviews) and click-evoked (e.g. Berlin et al, 1993, 1994) OAEs (DPOAE and CEOAE, respectively) yield an average decrease in emission level of approximately 1–4 dB, although inhibition of up to 10–20 dB has been reported (e.g. Müller et al, 2005; Wagner et al, 2007) at particular points in DPOAE fine structure.
CEOAE inhibition can be quantified in both time and frequency domains (waveform and spectrum, respectively) and it is typically reported as the difference in overall CEOAE level with and without CAS. Because this approach may underestimate CAS inhibition that occurs in restricted frequency regions (Berlin et al, 1994; Garinis et al, 2011), MOC effects on the CEOAE waveform have been examined within short analysis epochs (1–3 ms) of the response. This ‘microstructure’ analysis reveals that the maximum amount of CEOAE inhibition with MOC reflex activation is seen within the 8–18-ms post-stimulus range of the waveform (Hood et al, 1996). Similarly, spectral analyses of CEOAE waveforms indicate maximum inhibition within the 1–4 kHz range (Collet et al, 1990). The preponderance of inhibition in the mid-frequencies is thought to reflect the relatively high density of radial efferent fibers terminating on outer hair cells in this tonotopic range (Guinan, 2006).
Corticofugal effects of attention on the MOC reflex have been studied in adults by combining contralateral inhibition of CEOAEs with active listening paradigms (de Boer & Thornton, 2007; Garinis et al, 2011; Harkrider & Bowers, 2009). While the attention tasks have differed across studies, it is apparent that attention can influence MOC reflex strength. No study, to our knowledge, has examined the effect of auditory attention on the MOC reflex in children. In this communication, we report the results of a pilot study testing the hypothesis that active listening increases the magnitude of MOC reflex inhibition in typically developing children. Because cortical auditory areas are not fully mature until the late teenage years (Wunderlich & Cone-Wesson, 2006), we predicted that the magnitude of MOC reflex inhibition would be smaller than that reported in adults.
Methods
Participants and screening procedures
This experiment was approved by the Human Subjects Protection Program at the University of Arizona. Fifteen typically developing children (eight boys and seven girls), ages 8–14 years (M = 12.25 years), participated in this study. Informed consent was obtained from their parents and all children gave verbal assent to the procedures. The children had normal bilateral hearing sensitivity from 0.25–8 kHz when tested using supra-aural earphones in a double-walled sound booth. At the time of testing, all children demonstrated normal middle-ear function as determined by otoscopic inspection and tympanograms using a 0.226 kHz probe tone. A parental questionnaire verified that the children were not taking medications that could make them drowsy or hyperactive and that they had not been diagnosed with behavioral, cognitive, neurological, or auditory processing deficits.
Contralateral acoustic reflex thresholds to BBN were measured bilaterally for each participant using a Grason Stadler GSI-Tympstar immittance system (Madison, USA); this value was defined as the lowest level of BBN (bandwidth = 0.1–6 kHz) needed to elicit a 0.03 mm hos decrease in an ongoing acoustic admittance measurement. Only children with contralateral acoustic reflex thresholds greater than 65 dB SPL (the level of the contralateral CEOAE suppressor stimulus) were included, to limit the influence of stapedius muscle contraction on reductions in CEOAE levels in the presence of contralateral noise.
The HearID Auditory Diagnostics System (Mimosa Acoustics Inc., Champaign, USA) was used to acquire CEOAEs in a double-walled sound booth. First, each ear underwent a CEOAE screening test using 100-μs non-linear click trains presented at 50/s at a level of 80 dB peSPL. CEOAEs had to be ≥ 3 dB above the noise floor for four out of five half-octave-wide frequency bands in the 1–4 kHz range and waveform reproducibility had to be > 70% (Hood et al, 1996) for a response to be considered present.
Stimuli
CEOAEs were recorded from the right ear, and CAS was delivered to the left ear during four test conditions (Noise-only, Control, Easy Stroop, and Hard Stroop) summarized in Figure 1. Linear click trains (i.e. groupings of four clicks with equal amplitude and polarity) at a level of 60 dB peSPL were used to evoke CEOAEs. CEOAE waveforms were averaged over 2048 sweeps or approximately 2 minutes of recording time (Hood et al, 1996; Velenovsky & Glattke, 2002). In-ear calibration was performed before each measurement to ensure that the peak signal level was 60 dB SPL and that probe position remained stable across trials.
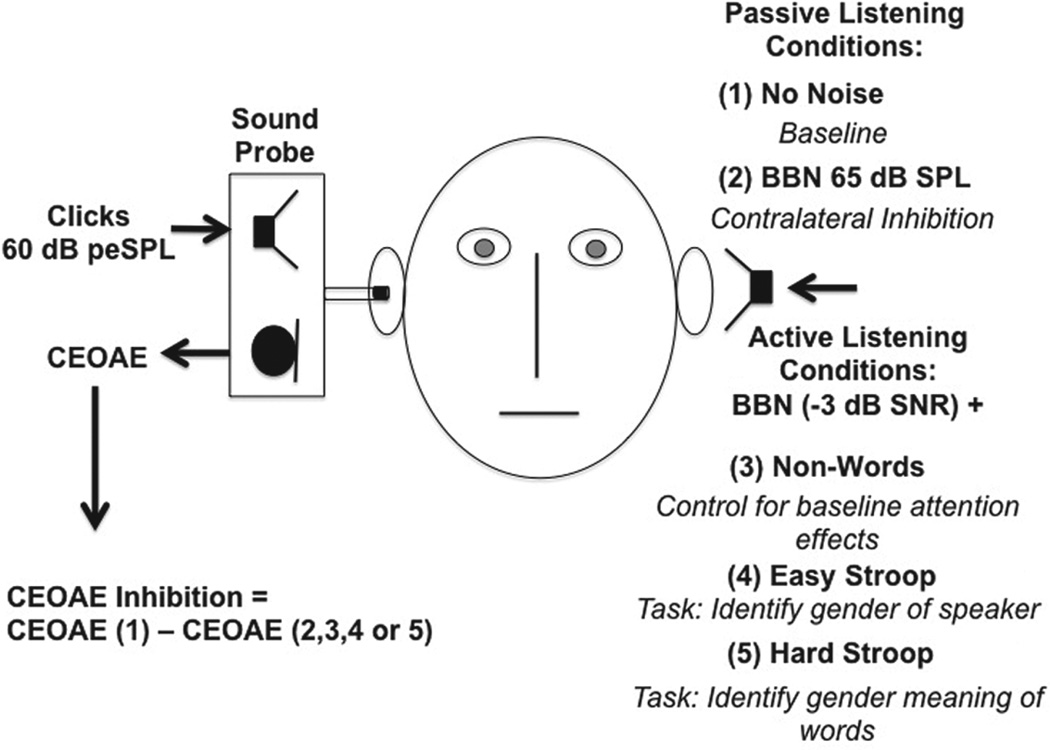
Figure 1.
CEOAE test conditions and purpose. Summary of stimulus parameters, test conditions, and purpose/prompt for each condition (italics). In all cases, the right ear was the probe ear in which 60 dB peSPL linear click stimuli were used to evoke CEOAEs, and CAS was presented to the left ear. For active listening tasks, attention was directed to speech in the left ear. The four test conditions and preceding quiet trials were presented in random order.
In each of the four test conditions, BBN (bandwidth = 0.1–6 kHz) with a level of 65 dB SPL was used as the CAS. This noise was generated by a Grason Stadler GSI-16 audiometer (Madison, USA) and was introduced 3–5 seconds before the initiation of the CEOAE recording. In three of the four test conditions (Control, Easy Stroop, and Hard Stroop), words were acoustically mixed in the ear canal with the BBN at a signal-to-noise ratio (SNR) of − 3 dB. This SNR value was set by first equalizing the root-mean-squared (RMS) amplitudes of each word using Sound Forge software (Sony Creative Software Inc., New York, USA). The equalized words were then used to create a sound file with no inter-stimulus interval between the words. This file was looped continuously through ER-3A headphones in a 2-cc coupler connected to a Larsen Davis sound level meter (Model 824, Provo, USA) and the level was adjusted until an average RMS level of 62 dB SPL (i.e. 3 dB below the level of BBN) was achieved. Word lists for each condition consisted of 50 monosyllabic words, which were randomized and presented at an inter-stimulus interval of 3.5 s via E-Prime software (Version 2.0, Psychology Software Tools Inc., Northridge, USA). This interval was also the limit within which a participant was required to make a behavioral response (described below) before the next word was presented.
Procedures
CEOAE recordings were made without CAS (quiet condition) and with CAS (test conditions). A quiet condition preceded each of the four test conditions (Figure 1). These quiet recordings served as ‘baseline’ measurements for quantifying the magnitude of inhibition in the test conditions with CAS that followed. The use of alternating quiet and test conditions has been documented as a valid control for any long-term shifts in emission amplitude across trials (Berlin et al, 1993). The repeatability of MOC reflex assays in these conditions is high (Mishra & Lutman, 2013).
The four test conditions with their preceding quiet trials were presented to each participant in random order. In the noise-only condition, listeners were asked to remain still during CEOAE acquisition with CAS. In the active listening conditions (Control, Easy, and Hard Stroop), listeners were asked to respond to words embedded in CAS using a keypad. The words for the Easy and Hard Stroop conditions were identical; only the instructions for each task differed and word order was randomized. Both Stroop tasks required participants to listen for single-syllable words spoken by male or female voices. These words had gender-specific meanings, such as ‘king, queen, mom, dad, cow, or bull’. The Easy Stroop condition required listeners to identify the gender of the speaker. In the Hard Stroop condition, listeners were asked to ignore the speaker’s gender and to respond to the gender meaning of the word instead. Response time and accuracy were calculated in five subjects.
A Control condition was used to account for minimal attention effects on MOC reflex inhibition that may be ascribed to listening for words and responding on a keypad. The Control condition consisted of a list of nonsense words that comprised minimal pairs to those used in the Stroop tasks (e.g. ‘briest’ for ‘priest’), spoken by male and female talkers. These nonsense words were designed to minimize the acoustic variability between the Stroop and Control conditions. The listener’s task was to press any response button each time a nonsense word was heard.
Analyses
CEOAE levels calculated by the HearID software were used for initial comparison between quiet and test conditions with CAS. Analyses of variance were used to test the hypothesis that CEOAE level varied as a function of condition. The pressure-by-time CEOAE waveforms were then exported to IgorPro waveform analysis software (Wave-Metrics Inc., Lake Oswego, USA) to derive time-domain difference waveforms. The difference waveforms were created by subtracting the Noise-only, Easy Stroop, Hard Stroop, and Control waveforms from the preceding quiet condition waveforms. The derived waveforms underwent additional ‘microstructure’ analyses by calculating dB RMS levels for five 2-ms epochs within the 8–18 ms range of the difference waveforms (Figure 2). Average CEOAE inhibition over the entire 8–18 ms epoch was also calculated for each difference waveform. These analyses provide a fine-grained assessment of MOC reflex effects in the approximate tonotopic range of 1–4 kHz frequency (Garinis et al, 2011; Norton & Neely, 1987), where the greatest amount of inhibition is known to occur (Berlin et al, 1993, 1994). Paired-comparison t-tests were used to test the hypothesis that the CEOAE levels obtained in the microstructure analyses varied as a function of test condition.
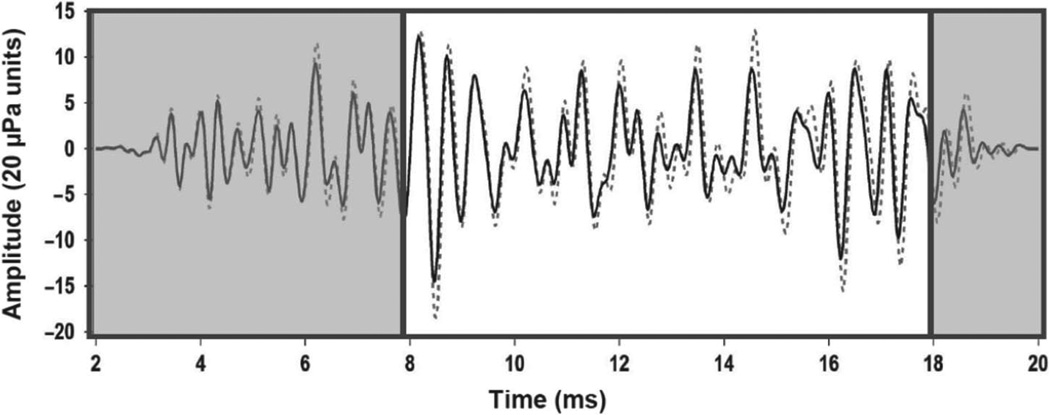
Figure 2.
Example of Quiet and Noise-only waveform comparison. The dashed line indicates the CEOAE waveform obtained in quiet (without CAS) and the solid line is the waveform obtained with contralateral BBN at 65 dB SPL. Amplitude and latency/phase shifts relative to the quiet condition can be observed in the waveform obtained with CAS. The unshaded waveform portion, from 8 to18 ms, indicates the time epoch of greatest inhibition over which microstructure analyses were completed.
As demonstrated in Figure 2, CAS can sometimes alter waveform peak latencies in addition to amplitude. Because this latency shift is not constant across all waveform peaks, applying a constant adjustment to one waveform would result in temporal alignment at some, but not all waveform peaks. In keeping with previous research utilizing similar techniques (e.g. Berlin et al, 1993; Garinis et al, 2011), we did not adjust for this latency shift at the risk of over- or underestimating inhibition in some cases.
Spectral analyses were carried out by using the CEOAE levels and SNRs calculated by HearID software within five half-octave frequency bands centered at 1.0, 1.4, 2.0, 2.8, and 4.0 kHz. Analyses of variance were used to determine if CEOAE level or SNR by frequency band varied as a function of listening condition.
Results
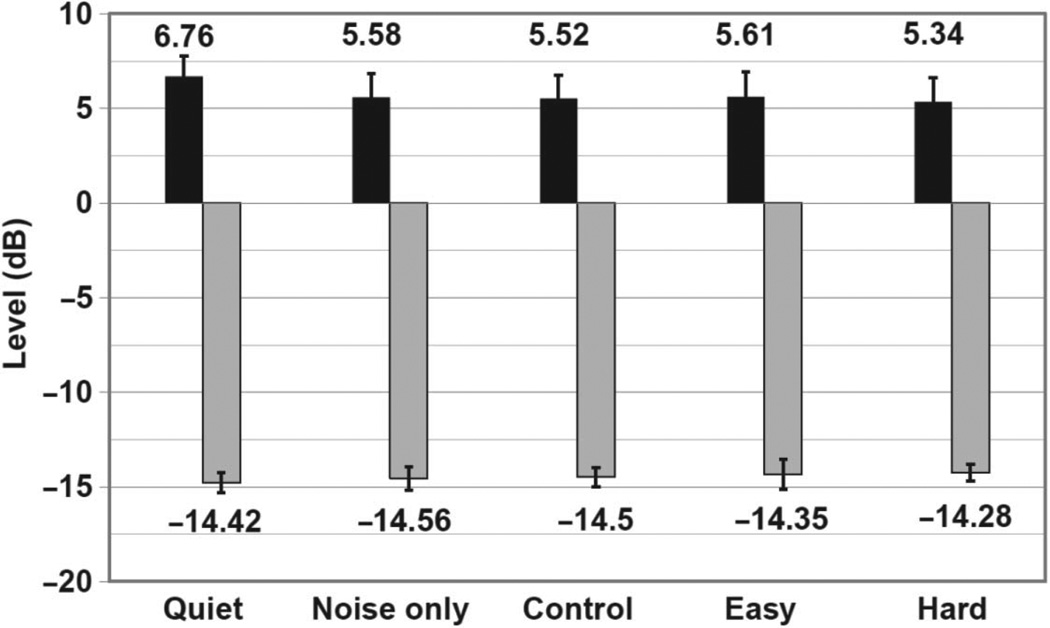
CEOAE levels and associated noise floors, as calculated by HearID, are graphed by condition in Figure 3. CEOAE levels were largest in the quiet condition, with a mean of 6.76 dB, and were reduced on average by 1.25 dB in all four test conditions with the introduction of contralateral noise. A repeated-measures analysis of variance for CEOAE level as a function of listening condition indicated that any condition with contralateral noise resulted in significantly reduced CEOAE levels relative to quiet (F4,14 = 9.46, p < 0.0001). These results suggest an inhibitory effect of the MOC reflex in any condition with CAS. Although CEOAE levels were lower in the active listening conditions (Easy and Hard Stroop) compared to the Control and Noise-only conditions, differences in CEOAE level and noise floors between each of these test conditions (with CAS) were not statistically significant.
Figure 3.
Comparison of whole-wave CEOAE and noise floor levels for each condition. Mean whole-wave CEOAE and corresponding noise floor levels are plotted for each condition (Error bars = 1 SEM). Values for the quiet condition were averaged over the four trials that preceded each trial with CAS.
CEOAE levels and noise floors for the four quiet conditions that preceded each of the test conditions were also compared. Analyses of variance were used to test the hypothesis that CEOAE levels and noise floors varied over the course of the experiment. The results for both CEOAE level (F3,56 = 0.002, p = 0.99), and for noise floor (F3,56 = 0.021, p = 0.99) in the four quiet conditions were not significant, suggesting that variability in these values over the time course of the experiment could not account for variability due to experimental manipulation.
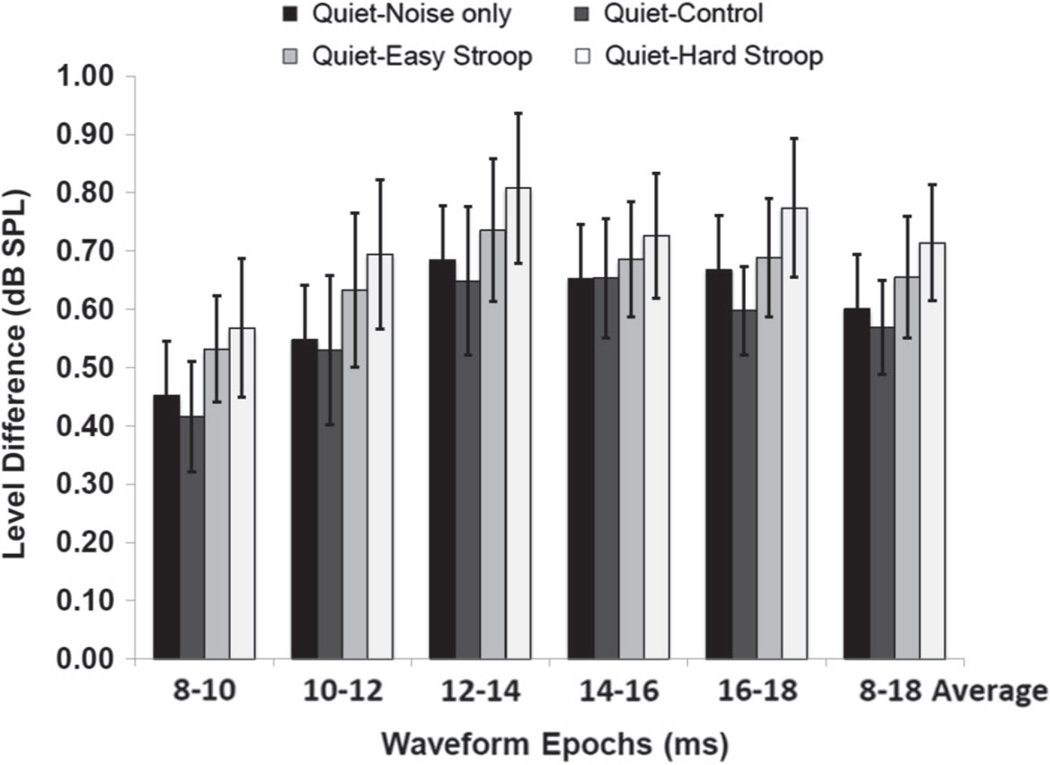
The results of the microstructure analyses for the derived waveforms are shown in Figure 4, with higher levels indicating greater inhibition between each test condition and its preceding quiet trial. The Easy and Hard Stroop conditions both resulted in greater inhibition within each epoch when compared to the Noise-only and Control conditions; however, mean difference in inhibition between the Easy and Hard Stroop conditions was quite small (0.06 dB). Given such small differences, the Easy and Hard Stroop data were combined as one active listening condition. Paired-comparison t-tests were used to test the hypothesis that there were differences in average CEOAE level in the 8–18 ms range between Noise-only, Control, and combined Stroop conditions. A summary of these paired-comparisons with their associated significance values is reported in Table 1. Average inhibition over the 8–18 ms epoch (in dB) for the combined Stroop tasks was significantly greater than Control (t = 4.87, p < 0.0001) and Noise-only (t = 3.87, p = 0.0002) conditions, although these level differences were also quite small: 0.11 dB and 0.09 dB, respectively. The average difference between Control and Noise-only conditions, −0.02 dB, was not significant.
Figure 4.
Derived inhibition levels obtained for each CAS condition in 2-ms epochs. Magnitudes of inhibition from the MOC reflex relative to quiet are graphed by condition for each 2-ms epoch of an 8–18 ms window. Average inhibition over the entire 8–18 ms epoch is also shown. Higher levels indicate greater inhibition. Error bars = 1 SEM.
Table 1.
Mean inhibition effect (in dB) for different test conditions over the 8–18 ms epoch. These levels represent the amount of MOC reflex inhibition of CEOAE relative to quiet. For each condition, the mean and standard deviation are given. The paired t-test difference scores and associated p-values (in parenthesis) for each condition comparison are shown.
| Condition | Mean inhibition 8–18 ms (dB) |
Paired-comparison mean difference (dB) |
|
|---|---|---|---|
| Noise-only | Control | ||
| Noise-only | 0.62 (± 0.05) | ||
| Control | 0.59 (± 0.05) | −0.02 (p = 0.4064) | |
| Combined Stroop | 0.71 (± 0.05) | 0.09 (p = 0.0002) | 0.11 (p < 0.0001) |
In the frequency domain, CEOAE levels and SNRs measured for five half-octave bands were significantly reduced for all conditions with CAS compared to quiet tests (F3,14 = 6.63, p = 0.0002); however, there were no significant differences in level or SNR between Stroop, Control, or Noise-only conditions. The largest differences between quiet and test conditions were in the 1.0, 1.4, and 2.0 kHz bands.
Behavioral data were available for five subjects2. In the Easy Stroop task, performance accuracy was 97.6% (± 2.5 s.d.) with an average reaction time of 1068 ms (± 105.7 s.d). In the Hard Stroop task, performance accuracy was 78.2% (± 15.4 s.d.) with an average reaction time of 1436. 9 ms (± 100.45 s.d.). The longer reaction times and decreased accuracy suggest that the Hard Stroop condition was more difficult than the Easy Stroop condition, which is consistent with adult performance using the same stimuli (Christensen et al, 2011).
Discussion
The objective of this study was to test the hypothesis that active listening influences the strength of MOC reflex inhibition in children. The greatest amount of CEOAE inhibition relative to quiet occurred during the Stroop tasks in the 8–18 ms epochs of the CEOAE waveforms. Although quite small (< 0.15 dB), it is possible that this effect reflects corticofugal recruitment of the MOC reflex during tasks requiring higher signal fidelity. The two Stroop listening conditions used the same word list in randomized order, and the Control condition used words that were minimal pairs to those of the Stroop conditions. The differences between the Stroop and Control conditions were specifically in the instructions on how to respond (i.e. the cognitive task), while the long-term spectro-temporal stimulus characteristics were the same for each condition. These listening conditions were presented in random order; therefore, the consistently observed effect of greater inhibition of CEOAEs during Stroop tasks, although small, would be unexpected due purely to stimulus-driven factors.
The current findings are generally consistent with Maison et al (2001) and Garinis et al (2011), who found that active listening to tones or speech in the contralateral ear led to greater CEOAE inhibition in adult listeners. These findings differ from the results of Harkrider & Bowers (2008), who observed enhancement of CEOAE level when listeners were asked to attend to clicks in the probe ear or to perform a detection task using ‘sham’ speech stimuli in the CAS ear. This discrepancy may indicate that corticofugal modulation increases inhibition only when it is advantageous for the task (i.e. detecting actual tones or understanding words-in-noise), but this speculation needs further investigation.
It is not possible to compare the magnitude of the effects obtained in the present study to those previously published due to differences in instrumentation, data analysis techniques, and stimulus paradigms. The CEOAE response and noise levels obtained using the HearID system were significantly lower than those reported by others using the Otodynamics ILO-88 OAE analyser (e.g. Prieve et al, 1997; Garinis et al, 2011). A preliminary investigation undertaken in our lab indicates that there is not a simple correction factor that could be applied to the data to reconcile these instrumentation differences. The hypothesis regarding auditory cortex immaturity resulting in weaker MOC reflex inhibition in children compared to adults cannot be addressed until such reconciliation is accomplished or the experiment is repeated in adults using identical instrumentation and methods.
Given the small size of the observed changes in MOC reflex inhibition during active listening, alternative explanations for the data must also be considered. An acoustic factor that may have altered the CEOAE level across conditions was the effect of amplitude modulation caused by the contralateral speech-in-noise stimuli. Although the BBN was held at a constant level of 65 dB SPL, it was effectively amplitude modulated by speech envelopes at approximately 0.3 Hz, or every time a word was presented. Furthermore, the fine structure of the words may have added faster amplitude modulation to the CAS on the order of approximately 0.1–0.2 kHz (Dimitrijevic et al, 2004). Maison and colleagues (1997, 1999) have demonstrated that both tones and BBN amplitude-modulated at around 0.1 kHz are potent suppressors, especially with larger modulation depths of 75 to 100%. However, a paired-comparison t-test of the Noise-only and Control CEOAE-derived waveforms revealed that there was not a significant difference in average CEOAE level between these two conditions (Table 1), suggesting that the slow modulation rate induced by speech in the Control condition did not have an additional inhibitory effect than BBN alone. It is also recognized that small changes in CEOAE level can be attributed to changes in probe placement in the outer ear canal or changes in middle-ear admittance due to movement and swallowing of the subject during the test procedure. In the current study, efforts were made to control for these effects by re-calibrating the stimulus level in the ear before each trial, and by measuring CEOAE levels in each test condition relative to the preceding quiet condition.
This pilot study of the MOC reflex in children during active listening raises questions that will be addressed in future experiments. It is unknown if these very small changes in CEOAE amplitude during active listening reflect behaviorally significant cochlear activity that shapes the neural encoding of speech-in-noise or if they are simply artifacts. Planned experiments will seek to determine if performance accuracy on the listening tasks is related to the amplitude changes in MOC reflex inhibition. Determining how attention influences early auditory processing in typically developing children is critical for understanding issues such as speech-in-noise deficits and the neurobiology of auditory processing disorders. Some research indicates that speech-in-noise or learning deficits are related to physiologic hypofunction of the efferent system (Garinis et al, 2008; Muchnik et al, 2004; Sanches & Carvallo, 2006; Veuillet et al, 2007) and/or a global inability to attend to and filter auditory signals at various levels of the auditory nervous system (Moore et al, 2010, 2012). It is possible that dysfunction in the efferent pathway, either at cortical (top-down) or brainstem (bottom-up) levels may disrupt speech-in-noise perception while leaving hearing sensitivity intact. The long-term goal of the current research is to elucidate the neurophysiologic bases of listening impairments in normal-hearing individuals with auditory processing or learning disorders.
Acknowledgements
The authors would like to thank Dr. David Velenovsky for his theoretical contributions to this work and Holden Sanders for his assistance with data collection. We also thank Dr. Jacek Smurzynski and two anonymous reviewers for their critique of earlier versions of this paper, and for Figure 1, provided by one of the anonymous reviewers.
This preliminary work was supported by NIH T32 Training Grant #DC009398-04 and NIH-NIDCD K-24 Grant #DC-8826, Barbara Cone, PI. Portions of these data were presented at the 2013 American Auditory Society meeting in Scottsdale, Arizona and at the Audiology Now! 2013, in Anaheim, USA. Ongoing support is provided by a Student Research Grant to Spencer Smith from the American Academy of Audiology Foundation.
Abbreviations
- BBN
Broadband noise
- CAS
Contralateral acoustic stimulus
- CEOAE
Click-evoked otoacoustic emissions
- MOC
Medial olivocochlear
Footnotes
Also known as suppression (Velenovsky & Glattke, 2002).
Behavioral data on the remaining subjects were corrupted by a software-related storage error.
Declaration of interest: The authors have no conflicts of interests to declare.
References
- Berlin CI, Hood LJ, Wen H, Szabo P, Cecola RP, et al. Contralateral inhibition of non-linear click-evoked otoacoustic emissions. Hear Res. 1993;71(1):1–11. doi: 10.1016/0378-5955(93)90015-s. [DOI] [PubMed] [Google Scholar]
- Berlin CI, Hood LJ, Hurley A, Wen H. Contralateral inhibition of otoacoustic emissions: An index of the function of the medial olivocochlear system. Otolaryngol Head Neck Surg. 1994;110(1):3–21. doi: 10.1177/019459989411000102. [DOI] [PubMed] [Google Scholar]
- Berlin CI, Hood LJ, Hurley AE, Wen H, Kemp DT. Binaural noise suppresses linear click-evoked otoacoustic emissions more than ipsilateral or contralateral noise. Hear Res. 1995;87(1):96–103. doi: 10.1016/0378-5955(95)00082-f. [DOI] [PubMed] [Google Scholar]
- Christensen TA, Lockwood JL, Almryde KR, Plante E. Neural substrates of attentive listening assessed with a novel auditory Stroop task. Front Hum Neurosci. 2011;4:1–12. doi: 10.3389/fnhum.2010.00236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collet L, Kemp DT, Veuillet E, Duclaux R, Moulin A, Morgon A. Effect of contralateral auditory stimuli on active cochlear micromechanical properties in human subjects. Hear Res. 1990;43(2):251–261. doi: 10.1016/0378-5955(90)90232-e. [DOI] [PubMed] [Google Scholar]
- Cooper NP, Guinan JJ. Efferent-mediated control of basilar membrane motion. J Physiol. 2006;576(1):49–54. doi: 10.1113/jphysiol.2006.114991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Boer J, Thornton ARD. Effect of subject task on contralateral inhibition of click evoked otoacoustic emissions. Hear Res. 2007;233(1):117–123. doi: 10.1016/j.heares.2007.08.002. [DOI] [PubMed] [Google Scholar]
- Dimitrijevic A, John MS, Picton TW. Auditory steady-state responses and word recognition scores in normal-hearing and hearing-impaired adults. Ear Hear. 2004;25(1):68–84. doi: 10.1097/01.AUD.0000111545.71693.48. [DOI] [PubMed] [Google Scholar]
- Garinis AC, Glattke T, Cone-Wesson BK. CEOAE inhibition in adults with learning disabilities. Int J Audiol. 2008;47(10):607–614. doi: 10.1080/14992020802129402. [DOI] [PubMed] [Google Scholar]
- Garinis AC, Glattke T, Cone BK. The MOC reflex during active listening to speech. J Speech Lang Hear Res. 2011;54(5):1464–1476. doi: 10.1044/1092-4388(2011/10-0223). [DOI] [PubMed] [Google Scholar]
- Giraud AL, Garnier S, Micheyl C, Lina G, Chays A, et al. Auditory efferents involved in speech-in-noise intelligibility. Neuroreport. 1997;8(7):1779–1783. doi: 10.1097/00001756-199705060-00042. [DOI] [PubMed] [Google Scholar]
- Guinan JJ., Jr Olivocochlear efferents: Anatomy, physiology, function, and the measurement of efferent effects in humans. Ear Hear. 2006;27(6):589–607. doi: 10.1097/01.aud.0000240507.83072.e7. [DOI] [PubMed] [Google Scholar]
- Guinan JJ., Jr Cochlear efferent innervation and function. Curr Opin Otolaryngol Head Neck Surg. 2010;18(5):447–453. doi: 10.1097/MOO.0b013e32833e05d6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harkrider AW, Bowers CD. Evidence for a cortically mediated release from inhibition in the human cochlea. J Am Acad Audiol. 2009;20(3):208–215. doi: 10.3766/jaaa.20.3.7. [DOI] [PubMed] [Google Scholar]
- Hood LJ, Berlin CI, Hurley A, Cecola RP, Bel B. Contralateral inhibition of transient-evoked otoacoustic emissions in humans: Intensity effects. Hear Res. 1996;101(1):113–118. doi: 10.1016/s0378-5955(96)00138-4. [DOI] [PubMed] [Google Scholar]
- Kawase T, Liberman MC. Antimasking effects of the olivocochlear reflex. I. Enhancement of compound action potentials to masked tones. J Neurophysiol. 1993;70(6):2519–2532. doi: 10.1152/jn.1993.70.6.2519. [DOI] [PubMed] [Google Scholar]
- Kumar UA, Vanaja CS. Functioning of olivocochlear bundle and speech perception in noise. Ear Hear. 2004;25(2):142–146. doi: 10.1097/01.aud.0000120363.56591.e6. [DOI] [PubMed] [Google Scholar]
- Maison S, Micheyl C, Collet L. Medial olivocochlear efferent system in humans studied with amplitude-modulated tones. J Neurophysiol. 1997;77(4):1759–1768. doi: 10.1152/jn.1997.77.4.1759. [DOI] [PubMed] [Google Scholar]
- Maison S, Micheyl C, Collet L. Sinusoidal amplitude modulation alters contralateral noise suppression of evoked otoacoustic emissions in humans. Neuroscience. 1999;91(1):133–138. doi: 10.1016/s0306-4522(98)00608-3. [DOI] [PubMed] [Google Scholar]
- Maison S, Micheyl C, Collet L. Influence of focused auditory attention on cochlear activity in humans. Psychophysiology. 2001;38(1):35–40. [PubMed] [Google Scholar]
- May BJ, Budelis J, Niparko JK. Behavioral studies of the olivocochlear efferent system: Learning to listen in noise. Arch Otolaryngol Head Neck Surg. 2004;130(5):660. doi: 10.1001/archotol.130.5.660. [DOI] [PubMed] [Google Scholar]
- Meric C, Collet L. Attention and otoacoustic emissions: A review. Neurosci Biobehav Rev. 1994;18(2):215–222. doi: 10.1016/0149-7634(94)90026-4. [DOI] [PubMed] [Google Scholar]
- Micheyl C, Collet L. Involvement of the olivocochlear bundle in the detection of tones in noise. J Acoust Soc Am. 1996;99(3):1604–1610. doi: 10.1121/1.414734. [DOI] [PubMed] [Google Scholar]
- Mishra SK, Lutman ME. Repeatability of click-evoked otoacoustic emission-based medial olivocochlear efferent assay. Ear Hear. 2013;34(6):789–798. doi: 10.1097/AUD.0b013e3182944c04. [DOI] [PubMed] [Google Scholar]
- Moore DR, Ferguson MA, Edmondson-Jones AM, Ratib S, Riley A. Nature of auditory processing disorder in children. Pediatrics. 2010;126(2):382–390. doi: 10.1542/peds.2009-2826. [DOI] [PubMed] [Google Scholar]
- Moore DR. Listening difficulties in children: Bottom-up and top-down contributions. J Commun Disord. 2012;45(6):411–418. doi: 10.1016/j.jcomdis.2012.06.006. [DOI] [PubMed] [Google Scholar]
- Muchnik C, Ari-Even Roth DE, Othman-Jebara R, Putter-Katz H, Shabtai EL, et al. Reduced medial olivocochlear bundle system function in children with auditory processing disorders. Audiol Neurootol. 2004;9(2):107–114. doi: 10.1159/000076001. [DOI] [PubMed] [Google Scholar]
- Müller J, Janssen T, Heppelmann G, Wagner W. Evidence for a bipolar change in distortion product otoacoustic emissions during contralateral acoustic stimulation in humans. J Acoust Soc Am. 2005;118(6):3747–3756. doi: 10.1121/1.2109127. [DOI] [PubMed] [Google Scholar]
- Norman M, Thornton ARD. Frequency analysis of the contralateral suppression of evoked otoacoustic emissions by narrow-band noise. Br J Audiol. 1993;27(4):281–289. doi: 10.3109/03005369309076705. [DOI] [PubMed] [Google Scholar]
- Norton SJ, Neely ST. Tone-burst-evoked otoacoustic emissions from normal - hearing subjects. J Acoust Soc Am. 1987;81(6):1860–1872. doi: 10.1121/1.394750. [DOI] [PubMed] [Google Scholar]
- Prieve BA, Fitzgerald TS, Schulte LE. Basic characteristics of click-evoked otoacoustic emissions in infants and children. J Acoust Soc Am. 1997;102(5):2860–2870. doi: 10.1121/1.420341. [DOI] [PubMed] [Google Scholar]
- Sanches SGG, Carvallo RM. Contralateral inhibition of transient evoked otoacoustic emissions in children with auditory processing disorder. Audiol Neurootol. 2006;11(6):366–372. doi: 10.1159/000095898. [DOI] [PubMed] [Google Scholar]
- Smith S, Kei J, McPherson B, Smyth V. Effects of speech babble on transient evoked otoacoustic emissions in normal-hearing adults. J Am Acad Audiol. 2001;12(7):371–378. [PubMed] [Google Scholar]
- Velenovsky DS, Glattke TJ. The effect of noise bandwidth on the contralateral inhibition of transient evoked otoacoustic emissions. Hear Res. 2002;164(1):39–48. doi: 10.1016/s0378-5955(01)00393-8. [DOI] [PubMed] [Google Scholar]
- Veuillet E, Magnan A, Ecalle J, Thai-Van H, Collet L. Auditory processing disorder in children with reading disabilities: Effect of audiovisual training. Brain. 2007;130(11):2915–2928. doi: 10.1093/brain/awm235. [DOI] [PubMed] [Google Scholar]
- Wagner W, Heppelmann G, Müller J, Janssen T, Zenner HP. Olivocochlear reflex effect on human distortion product otoacoustic emissions is largest at frequencies with distinct fine structure dips. Hear Res. 2007;223(1):83–92. doi: 10.1016/j.heares.2006.10.001. [DOI] [PubMed] [Google Scholar]
- Wagner W, Heyd A. Measurement of medial olivocochlear efferent activity in humans: Comparison of different distortion product otoacoustic emission-based paradigms. Otolol Neurotol. 2011;32(8):1379–1388. doi: 10.1097/MAO.0b013e31822f1548. [DOI] [PubMed] [Google Scholar]
- Winslow RL, Sachs MB. Single-tone intensity discrimination based on auditory-nerve rate responses in backgrounds of quiet, noise, and with stimulation of the crossed olivocochlear bundle. Hear Res. 1988;35(2):165–189. doi: 10.1016/0378-5955(88)90116-5. [DOI] [PubMed] [Google Scholar]
- Wunderlich JL, Cone-Wesson BK. Maturation of CAEP in infants and children: A review. Hear Res. 2006;212(1):212–223. doi: 10.1016/j.heares.2005.11.008. [DOI] [PubMed] [Google Scholar]