Abstract
Objectives
C-terminal Agrin Fragment (CAF) has been proposed as a potential circulating biomarker for predicting changes in physical function among older adults. To determine the effect of a one-year PA intervention on changes in CAF concentrations and to evaluate baseline and longitudinal associations between CAF concentrations and indices of physical function.
Design
Ancillary study to the Lifestyle Interventions and Independence for Elders Pilot (LIFE-P), a multi-site randomized clinical trial designed to evaluate the effects of chronic exercise on the physical function of older adults at risk for mobility disability.
Setting
Four academic research centers within the U.S.
Participants
Three hundred thirty three older adults aged 70 to 89 with mild to moderate impairments in physical function.
Intervention
A 12-month intervention of either structured physical activity (PA) or health education promoting successful aging (SA).
Measurements
Serum CAF concentrations and objectives measures of physical function – i.e. gait speed and performance on the Short Physical Performance Battery (SPPB).
Results
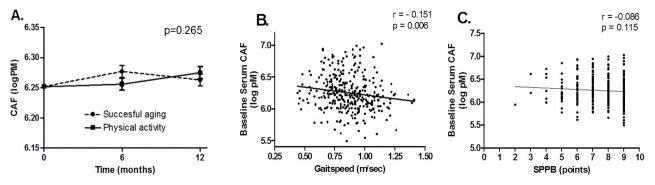
The group*time interaction was not significant for serum CAF concentrations (p=0.265), indicating that the PA intervention did not significantly reduce serum CAF levels compared to SA. Baseline gait speed was significantly correlated with baseline CAF level (r = −0.151, p= 0.006), however the association between CAF and SPPB was not significant. Additionally, neither baseline nor the change in CAF concentrations strongly predicted the change in either performance measure following the PA intervention.
Conclusion
In summary, the present study shows that a one-year structured PA program did not reduce serum CAF levels among mobility-limited older adults. However, further study is needed to definitively determine the utility of CAF as a biomarker of physical function.
Keywords: Aging, Physical Function, Neuromuscular function, mobility, LIFE study
INTRODUCTION
As the number of older men and women continues to rise worldwide, (1) the maintenance of physical independence is an important public health challenge (2,3). The capacity to perform basic physical tasks is a central tenet of health-related quality of life (4) and a key predictor of adverse health outcomes including hospitalization, post-surgical morbidity, and mortality (5–8). Interest has recently grown in discovering non-invasive biomarkers capable of predicting declines in physical function that ultimately lead to disability (9). The identification of such biomarkers may allow for improved identification of individuals at risk for becoming disabled and/or facilitate the development of novel therapeutic approaches to combat functional decline (10).
Recently, scientists have demonstrated increased interest in the contribution of the nervous system to age-related declines in function. The nervous system may play an important role in age-related functional decline due to its significant contributions to force production and movement. Some authors have proposed that declines in neuromuscular junction integrity may contribute to the development of sarcopenia and age-related functional decline (11). As such, indicators of neuromuscular junction (NMJ) integrity may serve as potential biomarkers for the progression of age-related functional decline.
One such potential biomarker is C-terminal Agrin Fragment (CAF). This 22 kDa protein is derived from the proteolytic cleavage of agrin by neurotrypsin (12). Agrin is a large heparin sulfate proteoglycan involved in the development and maintenance of the NMJ. Agrin also binds proteins on the extracellular surface of muscle such as dystroglycan complex and laminin, (13) making it an important component in maintaining proper musculoskeletal force transduction. Though relatively little is known regarding the precise mechanisms through which CAF may influence neuromuscular function, several studies have demonstrated that elevations in CAF may negatively impact NMJ and skeletal muscle integrity. For example, neurotrypsin overexpression in young mice was previously shown to reduce muscle fiber number and increased the proportion of type I fibers compared to control (14). Moreover, Hettwer et al. (15) also reported that older adults with low muscle mass and grip strength had significantly greater serum CAF concentrations than age-matched controls. These findings suggest that excessive cleavage of agrin results in NMJ fragmentation and fiber denervation and may contribute to skeletal muscle atrophy and loss of muscle strength. Given the importance of muscle mass and strength to maintenance of physical function in advanced age, it seems plausible that CAF concentrations may also be used as a biomarker of functional status among older adults. To our knowledge, however, no study to date has reported the association of CAF levels in older adults with objective performance measures of physical function.
Furthermore, given the importance of neuromuscular function to maintaining physical function, we speculate that CAF may also have utility in predicting function improvements in response to efficacious interventions. For instance, physical exercise is known to improve both neuromuscular and overall physical function – thus we hypothesize that CAF may predict functional responses to longer-term exercise interventions. However, data are lacking regarding longitudinal changes in circulating CAF levels following physical exercise. The present work is an ancillary study to the Lifestyle Interventions and Independence for Elders Pilot (LIFE-P). LIFE-P was a multi-site clinical trial that tested the effects of a one year structured physical activity (PA) intervention relative to a successful aging (SA) health education program in older adults at risk for becoming physically disabled. The objectives of the present study were to determine the effect of a one-year PA intervention on changes in circulating CAF concentrations and to evaluate associations between CAF concentrations and indices of physical function.
METHODS
Participant recruitment
Details about specific study inclusion and exclusion criteria of LIFE-P have been reported previously (16, 17). Briefly, subjects were eligible for the study if they were aged between 70–89 years, were sedentary, had a SPPB score ≤ 9, and were able to walk 400 meters within 15 minutes. A total of 424 participants were randomized into the PA and SA arms at four sites (Cooper Institute, Stanford University, University of Pittsburgh, and Wake Forest University) and followed for at least 12 months. For the present study, stored blood samples were available at baseline and each follow-up timepoint from a total of 333 participants. All participants provided written informed consent and the study protocol was approved by the Institutional Review Boards of all participating institutions.
Successful aging intervention
The SA intervention was designed to provide attention and health education (16). Study participants randomly assigned to SA group attended weekly classes for the first 26 weeks and then monthly until the end of the trial. Workshops were held on various relevant health topics to older adults such as nutrition, medication use, foot care, and preventive medicine. Each class was concluded with a short instructor-led program of upper extremity stretching exercises and regular telephone contact was made to encourage further participation.
Physical Activity Intervention
Details of the PA intervention have been reported previously (17). Briefly, participants randomized to PA performed walking, strength, flexibility, and balance training in center- and home-based settings. Moderate-intensity exercise was promoted and assessed using the Borg scale (18). Participants were asked to walk at a target intensity of 13 (somewhat hard) and perform strength training at an intensity of 15 to 16 (hard). Staff monitored the volume and intensity of exercise throughout the study by recording the completed walking time and overall rating of perceived exertion (RPE) for each session.
Measures of Physical Function
The primary measures of physical function for this analysis were walking speed during a 400-m test and score on the SPPB. During the 400-m test, participants were asked to walk 10 laps of a 40-m course at their usual pace. The SPPB is based on timed measures of standing balance, short-duration walking (4 m), and ability to rise from a chair. Each task was scored on a scale from 0 to 4 depending on the ability and time needed to complete each task. A summary score (range 0–12) was subsequently calculated by summing the three scores (19).
Blood Collection and CAF
Blood was collected and processed at each assessment visit (baseline, 6 months, and 12 months) according to standard clinical procedures. Serum samples were evaluated for CAF concentrations using a commercially available enzyme-linked immunoabsorbent assay (ELISA) kit (NTCAF Elisa Kit; Neurotune, Schlieren, Switzerland) as published previously (20). CAF values are expressed as log picomolar (pM) concentrations.
Statistical analysis
An an priori alpha level of 0.05 was set to determine statistical significance. Data were initially analyzed for normality and homogeneity of variance and descriptive statistics calculated. Baseline characteristics were compared using Student’s t-test for continuous variables and the chi-square test for discrete variables. When continuous variables were non-normally distributed, the Mann-Whitney test was used. Longitudinal changes in serum CAF concentrations were evaluated using linear mixed effects modeling. Covariates included in the model were age, sex, baseline CAF concentration, and an index of co-morbidity as published previously (21, 22). The log transformation was applied to serum CAF concentrations because they were non-normally distributed.
Pearson’s correlation coefficient (r) was determined to assess the association between baseline serum CAF concentration and baseline walking speed. Because SPPB was non-normally distributed, the association of baseline CAF concentrations of with baseline SPPB score was determined using Spearman’s rank correlation (ρ). Because of the close relationship between age and baseline CAF levels, partial correlations controlling for the effect of age were performed as an a posteriori analysis. Linear regression models were created to evaluate the relationships between baseline CAF concentrations and longitudinal changes in CAF concentrations with changes in SPPB score and 400 m walking speed at 12 months. Regression models were created using the stepwise procedure as published previously (23, 24). Variables includes in the models include age, co-morbidity, baseline CAF concentrations, and change in CAF concentration at 12 months.
RESULTS
Data from a total of 333 persons were included in the present study. The mean age was 76.7 (±4.21) years, 68.2 % were women, and 23.7% were racial/ethnic minorities. Demographics characteristics are shown by intervention group in Table 1. Across groups, the main effect for time did not reach statistical significance (p =0.062). However, a significant age*time effect was observed (p=0.016) indicating that older age was associated with greater increases in serum CAF concentrations from baseline to 12 months. However, we did not observe a significant group*time interaction (p=0.265), indicating that a one-year structured physical activity program did not significantly reduce serum CAF levels compared to health education (Figure 1A). Likewise, the sex * time interaction did not suggest sex-related differences in the response to the intervention (p = 0.167).
Table 1.
Baseline characteristics of study participants by randomized group.
| Characteristics | PA (n = 166) | SA (n = 167) | P-value |
|---|---|---|---|
| Age, years | 77.0 ± 4.3 | 76.3.0 ± 4. | 0.156 |
| Body mass index, kg/m2 | 29.6 ± 5.4 | 31.0 ± 5.9 | 0.045 |
| Systolic blood pressure, mm Hg | 133.0 ± 17.8 | 132.0 ± 17.7 | 0.604 |
| Diastolic blood pressure, mm Hg | 69.9 ± 10.6 | 68.6 ± 10.7 | 0.252 |
| Women | 112 (67.5) | 115 (68.9) | 0.785 |
| Fair/Poor health, self-rated | 37 (22.3) | 24 (14.4) | 0.062 |
| Co-morbidity index, score* | 1.6± 1.1 | 1.7 ± 1.1 | 0.337 |
| Prevalent Health Conditions | |||
| Myocardial infarction | 11 (6.6) | 18 (10.8) | 0.179 |
| Congestive heart failure | 9 (5.4) | 9 (5.4) | 0.990 |
| Lung disease | 23 (13.8) | 25 (15.1) | 0.738 |
| Cancer | 26 (15.6) | 28 (16.9) | 0.748 |
| Diabetes | 43 (25.7) | 25 (15.1) | 0.016 |
| Stroke | 7 (4.2)) | 9 (5.4) | 0.600 |
| Osteoarthritis | 36 (21.6) | 36 (21.6) | 0.977 |
Values are expressed as means ± S.D. or n (%). PA = Physical Activity; SA = Successful Aging Health Education.
Calculated as a composite score based on the presence/absence of 10 prevalent comorbidities: hypertension, heart attack, heart failure, stroke, cancer, diabetes, broken hip, arthritis, liver disease, and lung disease. A higher score indicates greater co-morbidity burden.
Figure 1.
Figure 1A. Serum CAF levels according to randomized groups at baseline and during follow-up. Means estimated from repeated measures analysis of covariance adjusted for gender, intervention assignment and visit. p-value indicates that for the group*time interaction.
Figure 1B. Scatterplot demonstrating the linear relationships between baseline serum CAF levels and self-selected gait speed. Correlation coefficient indicates bivariate associations.
Figure 1C. Scatterplot demonstrating the linear relationships between baseline serum CAF levels and score on the Short Physical Performance Battery. Correlation coefficient indicates bivariate associations.
Gait speed was the only functional indicator significantly correlated with baseline CAF level (r = −0.151, p= 0.006) (Figure 1B), indicating that higher baseline gait speeds were associated with lower serum CAF levels. Results were consistent when controlling for the effects of age as gait speed (r = −0.114, p = 0.038), but not SPPB (ρ = −0.047, p = 0.196), was significantly correlated with baseline CAF concentrations. Likewise, comorbidity index was correlated positively with total CAF level (r= 0.172, p= 0.002). Our findings suggest that SPPB score does not appear to be correlated with baseline serum CAF level (r=−0.086, p=0.115) (Figure 1C). In agreement with the findings of the correlation analyses, stepwise regression models indicated that comorbidity index and age were the only significant contributors to the final models as age and co-morbidity predicted change in gait speed while only contributed to the final model for SPPB.(Table 2a and 2b). Indeed, the standardized beta coefficients for both baseline concentrations of and changes in circulating CAF were small (range: −0.06 to 0.01) and thus did not significantly contribute to the final model.
Table 2a.
Results of the stepwise regression model for the change in gait speed following the exercise intervention
| Betaa | T | Prob(t) | 95%CI | Partial R2 | Model R2 | Probability>F | |
|---|---|---|---|---|---|---|---|
| Included variables | 0.045 | 0.002 | |||||
| Co-morbidity index | −0.152 | −2.561 | 0.011 | −0.03, −0.04 | 0.033 | ||
| Age | −0.151 | −2.536 | 0.012 | 0.00, 0.00 | 0.023 | ||
| Excluded variables | |||||||
| Gender | −0.15 | −0.258 | 0.797 | ||||
| Baseline CAF | −0.061 | −1.013 | 0.312 | ||||
| Δ CAF* | 0.012 | 0.196 | 0.845 | ||||
Standardized β;
CAF = C-terminal Agrin Fragment.
indicates change from baseline CAF concentration at 12 month
Table 2b.
Results of the stepwise regression model for the change in SPPB following the exercise intervention
| Betaa | T | Prob(t) | 95%CI | Partial R2 | Model R2 | Probability >F | |
|---|---|---|---|---|---|---|---|
| Included variables | 0.030 | 0.004 | |||||
| Age | −0.174 | −2.922 | 0.004 | −0.13, −0.02 | 0.030 | ||
| Excluded variables | |||||||
| Co-morbidity index | −0.067 | −1.119 | 0.264 | ||||
| Gender | 0.002 | 0.042 | 0.967 | ||||
| BaselineCAF | −0.058 | −0.967 | 0.334 | ||||
| ΔCAF* | −0.026 | −0.425 | 0.671 | ||||
Standardized β;
CAF = C-terminal Agrin Fragment.
indicates change from baseline CAF concentration at 12 months
DISCUSSION
Forty-two percent of the 37.3 million adults over the age of 65 years report having one or more physical limitations performing daily tasks that are essential for maintaining independence in the community (25). Preserving physical function has become a major public health priority as it would drastically reduce health care costs and improve quality of life for many older Americans and their families (26). In recent years, scientists have shown considerable interest in the discovery of non-invasive biomarkers capable of predicting declines in physical function that ultimately lead to disability (1, 7). The identification of such biomarkers may facilitate the development of novel therapeutic approaches to combat functional decline (10).
Recent findings indicate that declines in muscle strength occur more rapidly than declines in muscle mass and that the change in muscle size explains less than 10% of the between-subject variability in the change in muscle strength (27). As a result of these observations, interest has increased in understanding contributions of the nervous system to declines in muscle strength and overall physical function. Because they indicate neuromuscular integrity, circulating CAF concentrations have been proposed as such a biomarker for functional decline (28). The present study is the first to our knowledge to report on the association of CAF levels in older adults with objective performance measures of physical function as well as changes in serum concentrations of CAF following long-term exercise training.
Compared with health education, a one-year structured PA intervention did not reduce serum CAF levels among older adults at risk for becoming physically disabled. These data indicate that the change in serum CAF level was similar in the PA intervention group compared with that in the health education group. It should be noted, however, that prior studies have reported both increases and decreases in CAF concentrations in response to shorter term (12–20 weeks) exercise interventions. Drey et al. (28) previously that resistance exercise training (along with Vitamin D supplementation) was associated with significant decreases in CAF among 69 older adults – particularly among those with high initial CAF concentrations. Conversely, Fragala et al. (29) noted that a 6-week resistance training intervention significantly increased CAF concentrations among 23 older adults. Several possibilities may explain the differences in results between these studies and those of the present study. First, Drey et al. utilized western blotting to quantify CAF while we and Fragala et al. utilized an immunoassay. Secondly, the prior studies both utilized resistance exercise modalities whereas the present study utilized a multimodal intervention which emphasized aerobic training as the primary modality. Thirdly, though our sample size was significantly larger, the longer duration of the present study could have influenced results as adherence was lower in the latter part of the trial.
Though we observed a significant bivariate correlation between gait speed and baseline CAF concentrations, the present study generally indicated that circulating CAF concentrations were not strongly associated with functional outcomes. These data suggest that CAF may not be the strongest biomarker for predicting functional decline in this population. These data are similar to findings from Drey et al. (28) indicating that gait speed and grip strength were not significantly associated with serum CAF concentrations. Interestingly, however, this group did report that CAF concentrations were negatively correlated with appendicular lean mass (aLM) among men, but not women. This is important to note given that the majority of participants in the present study were women. Future studies may be warranted which are specifically designed determine if CAF is a more useful biomarker for men relative women.
Major strengths of this study include the use of a simple and broadly applicable PA intervention, a large sample size, and relatively long period of intervention and follow-up. However, like any study, the present investigation is not without limitations. For instance, because prior studies have reported that older adults with sarcopenic characteristics had significantly greater serum CAF concentrations than age-matched controls, the addition of a direct measure of muscle mass or muscle strength would have strengthened this study’s findings. Additionally, the inclusion of more than one outcome measure could have benefited the study, however few viable serum biomarkers have been proposed to date.
In summary, the present study shows that a one-year structured physical activity program did not reduce serum CAF levels among mobility-limited older adults. These findings add to prior literature which has indicated the need for circulating biomarkers related to changes in physical function among older adults. This study adds to a relative lack of clarity in the literature regarding the utility of CAF as a biomarker of physical function is mixed. Thus, further study is needed to definitively determine the utility of CAF as a biomarker of physical function. Future studies are particularly needed which specifically evaluate the effects of sex and exercise modality on longitudinal changes in CAF concentrations and potential biologic mediators of these responses.
Acknowledgments
This work was supported by the following grants from the National Institutes of Health/National Institute on Aging: Lifestyle Interventions and Independence for Elders Pilot (U01AG-22376), Claude D. Pepper Older Americans Independence Centers (University of Florida, P30AG-028740; Wake Forest, P30AG-21332; Pittsburgh, P30AG-024827). Neurotune, LLC donated biochemical assays and provided labor to conduct these assays. All data analyses were conducted by investigators unaffiliated with Neurotune.
Footnotes
Conflict of Interest
Dr. Hettwer and Dr. Dahinden report grants from Eurostars project “DISARCO”, outside the submitted work; In addition, Dr. Hettwer has a patent PCT/EP2012/000137 pending, a patent PCT/EP2008/003406 pending, and a patent PCT/EP2013/000520 pending.
Ethical Standards
All ethical standards were adhered to of the countries in which the experiments were performed.
References
- 1.Anderson GF, Hussey PS. Population aging: A comparison among industrialized countries. Health Aff (Millwood) 2000;19(3):191–203. doi: 10.1377/hlthaff.19.3.191. [DOI] [PubMed] [Google Scholar]
- 2.Brady AO, Straight CR, Schmidt MD, Evans EM. Impact of body mass index on the relationship between muscle quality and physical function in older women. J Nutr Health Aging. 2014;18(4):378–382. doi: 10.1007/s12603-013-0421-0. [DOI] [PubMed] [Google Scholar]
- 3.Ng TP, Aung KC, Feng L, Feng L, Nyunt MS, Yap KB. Tea consumption and physical function in older adults: A cross-sectional study. J Nutr Health Aging. 2014;18(2):161–166. doi: 10.1007/s12603-013-0354-7. [DOI] [PubMed] [Google Scholar]
- 4.Muszalik M, Dijkstra A, Kedziora-Kornatowska K, Zielinska-Wieczkowska H, Kornatowski T, Kotkiewicz A. Independence of elderly patients with arterial hypertension in fulfilling their needs, in the aspect of functional assessment and quality of life (QoL) Arch Gerontol Geriatr. 2011;52(3):e204–9. doi: 10.1016/j.archger.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 5.Afilalo J, Eisenberg MJ, Morin JF, et al. Gait speed as an incremental predictor of mortality and major morbidity in elderly patients undergoing cardiac surgery. J Am Coll Cardiol. 2010;56(20):1668–1676. doi: 10.1016/j.jacc.2010.06.039. [DOI] [PubMed] [Google Scholar]
- 6.Giaccaglia V, Nicklas B, Kritchevsky S, et al. Interaction between angiotensin converting enzyme insertion/deletion genotype and exercise training on knee extensor strength in older individuals. Int J Sports Med. 2008;29(1):40–44. doi: 10.1055/s-2007-964842. [DOI] [PubMed] [Google Scholar]
- 7.Perera S, Mody SH, Woodman RC, Studenski SA. Meaningful change and responsiveness in common physical performance measures in older adults. J Am Geriatr Soc. 2006;54(5):743–749. doi: 10.1111/j.1532-5415.2006.00701.x. [DOI] [PubMed] [Google Scholar]
- 8.Studenski S, Perera S, Patel K, et al. Gait speed and survival in older adults. JAMA. 2011;305(1):50–58. doi: 10.1001/jama.2010.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nedergaard A, Karsdal MA, Sun S, Henriksen K. Serological muscle loss biomarkers: An overview of current concepts and future possibilities. J Cachexia Sarcopenia Muscle. 2013;4(1):1–17. doi: 10.1007/s13539-012-0086-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Kan GA, Cderbaum JM, Cesari M, et al. Sarcopenia: Biomarkers and imaging (international conference on sarcopenia research) J Nutr Health Aging. 2011;15(10):834–846. doi: 10.1007/s12603-011-0365-1. [DOI] [PubMed] [Google Scholar]
- 11.Shigemoto K, Kubo S, Mori S, Yamada S, Akiyoshi T, Miyazaki T. Muscle weakness and neuromuscular junctions in aging and disease. Geriatr Gerontol Int. 2010;10(Suppl 1):S137–47. doi: 10.1111/j.1447-0594.2010.00608.x. [DOI] [PubMed] [Google Scholar]
- 12.Stephan A, Mateos JM, Kozlov SV, et al. Neurotrypsin cleaves agrin locally at the synapse. FASEB J. 2008;22(6):1861–1873. doi: 10.1096/fj.07-100008. [DOI] [PubMed] [Google Scholar]
- 13.Bezakova G, Lomo T. Muscle activity and muscle agrin regulate the organization of cytoskeletal proteins and attached acetylcholine receptor (AchR) aggregates in skeletal muscle fibers. J Cell Biol. 2001;153(7):1453–1463. doi: 10.1083/jcb.153.7.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Butikofer L, Zurlinden A, Bolliger MF, Kunz B, Sonderegger P. Destabilization of the neuromuscular junction by proteolytic cleavage of agrin results in precocious sarcopenia. FASEB J. 2011;25(12):4378–4393. doi: 10.1096/fj.11-191262. [DOI] [PubMed] [Google Scholar]
- 15.Hettwer S, Dahinden P, Kucsera S, et al. Elevated levels of a C-terminal agrin fragment identifies a new subset of sarcopenia patients. Exp Gerontol. 2013;48(1):69–75. doi: 10.1016/j.exger.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 16.LIFE Study Investigators. Pahor M, Blair SN, et al. Effects of a physical activity intervention on measures of physical performance: Results of the lifestyle interventions and independence for elders pilot (LIFE-P) study. J Gerontol A Biol Sci Med Sci. 2006;61(11):1157–1165. doi: 10.1093/gerona/61.11.1157. [DOI] [PubMed] [Google Scholar]
- 17.Katula JA, Kritchevsky SB, Guralnik JM, et al. Lifestyle interventions and independence for elders pilot study: Recruitment and baseline characteristics. J Am Geriatr Soc. 2007;55(5):674–683. doi: 10.1111/j.1532-5415.2007.01136.x. [DOI] [PubMed] [Google Scholar]
- 18.Borg G, editor. Perceived Exertion and Pain Scales. Champaign, IL: Human Kinetics; 1988. [Google Scholar]
- 19.Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: Association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49(2):M85–94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 20.Steubl D, Hettwer S, Vrijbloed W, et al. C-terminal agrin fragment--a new fast biomarker for kidney function in renal transplant recipients. Am J Nephrol. 2013;38(6):501–508. doi: 10.1159/000356969. [DOI] [PubMed] [Google Scholar]
- 21.Buford TW, Hsu FC, Brinkley TE, et al. Genetic influence on exercise-induced changes in physical function among mobility-limited older adults. Physiol Genomics. 2014;46(5):149–158. doi: 10.1152/physiolgenomics.00169.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Buford TW, Manini TM, Hsu FC, et al. Angiotensin-converting enzyme inhibitor use by older adults is associated with greater functional responses to exercise. J Am Geriatr Soc. 2012;60(7):1244–1252. doi: 10.1111/j.1532-5415.2012.04045.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Buford TW, Lott DJ, Marzetti E, et al. Age-related differences in lower extremity tissue compartments and associations with physical function in older adults. Exp Gerontol. 2012;47(1):38–44. doi: 10.1016/j.exger.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Buford TW, MacNeil RG, Clough LG, et al. Active muscle regeneration following eccentric contraction-induced injury is similar between healthy young and older adults. J Appl Physiol (1985) 2014;116(11):1481–1490. doi: 10.1152/japplphysiol.01350.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Seeman TE, Merkin SS, Crimmins EM, Karlamangla AS. Disability trends among older americans: National health and nutrition examination surveys, 1988–1994 and 1999–2004. Am J Public Health. 2010;100(1):100–107. doi: 10.2105/AJPH.2008.157388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hoffman C, Rice D, Sung HY. Persons with chronic conditions. their prevalence and costs. JAMA. 1996;276(18):1473–1479. [PubMed] [Google Scholar]
- 27.Delmonico MJ, Harris TB, Visser M, et al. Longitudinal study of muscle strength, quality, and adipose tissue infiltration. Am J Clin Nutr. 2009;90(6):1579–1585. doi: 10.3945/ajcn.2009.28047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Drey M, Sieber CC, Bauer JM, Uter W, Dahinden P, Fariello RG, Vrijbloed JW FiAT intervention group. C-terminal Agrin Fragment as a potential marker for sarcopenia caused by degeneration of the neuromuscular junction. Exp Gerontol. 2013;48:76–80. doi: 10.1016/j.exger.2012.05.021. [DOI] [PubMed] [Google Scholar]
- 29.Fragala MS, Jatner AR, Beyer KS, Townsend JR, Emerson NS, Scanlon TC, Oliveira LP, Hoffman JR, Stout JR. Biomarkers of muscle quality: N-terminal propeptide of type III procollagen and C-terminal agrin fragment responses to resistance exercise training in older adults. J Cachexia Sarcopenia Muscle. 2014;5:139–148. doi: 10.1007/s13539-013-0120-z. [DOI] [PMC free article] [PubMed] [Google Scholar]