Abstract
Background: It is widely accepted that aortic valve disease is surgically managed with aortic valve replacement (AVR) using different available prostheses. The long-term survival, durability of the valve, and freedom from reoperation after AVR are well established in published literature. Over the past two decades, aortic valve repair (AVr) has evolved into an accepted surgical option for patients with aortic valve disease. We review and analyze the published literature on AVr. Methods: A systematic review of the current literature was performed through three electronic databases from inception to August 2013 to identify all relevant studies relating to aortic valve repair. Articles selected were chosen by two reviewers. Articles were excluded if they contained a pediatric population or if the patient number was less than 50. Results: Twenty-four studies conformed to the inclusion criteria for inclusion in the systematic review. In total, 4986 patients underwent aortic valve repair. 7 studies represented bicuspid aortic valve (BAV) repair, 5 studies represented cusp prolapse, and 3 studies represented valve repair with root dilation or aneurysm. Overall weighted in-hospital mortality for all studies was low (1.46% ± 1.21). Preoperative aortic insufficiency (AI) ≥ 2+ did not correlate to reoperation for valve failure (Pearson's Rs 0.2705, P = 0.2585). AI at discharge was reported in 9 studies with a mean AI ≥ 2+ in 6.1% of patients. Weighted average percentage for valve reoperation following BAV repair was 10.23% ± 3.2. Weighted average reoperation following cusp prolapse repair was 3.83 ± 1.96. Weighted average reoperation in aortic valve sparing procedures with root replacement was 4.25% ± 2.46. Although there are limitations and complications of prosthetic valves, especially for younger individuals, there is ample published literature that confers strong evidence for AVR. On the contrary, aortic valve repair may be a useful option for selected patients, but there is lack of uniformity in data and absence of compelling supporting evidence. An international multi-center study comparing and assessing the results between AVR & AVr is the next step required. Currently, higher levels of evidence do not exist for aortic valve repair.
Keywords: Aortic valve repair, Aortic valve replacement, Aortic valve surgery, Meta-analysis, Aortic valve
Introduction
It is widely accepted that aortic valve disease is surgically managed with aortic valve replacement (AVR) using different available prostheses. The long-term results and survival, durability of the valve, and freedom from reoperation after AVR are well established in published literature. Over the past two decades, aortic valve repair (AVr) has evolved into an accepted surgical option for patients with aortic valve disease. Current understanding of the mechanisms of valve dysfunction and the etiology of lesions enabled surgeons to modify their techniques in aortic valve repair. Although early results are acceptable, the long-term results, durability of the repair, and freedom from reoperation are still variable. This systemic review and meta-analysis examines the worldwide published literature to draw conclusions on the applicability, durability and outcomes of aortic valve repair as a surgical option to treat aortic valve pathology.
Methods
Search Strategy
Electronic searches were performed in PubMed, Ovid Medline and Cochrane. No limits were placed on dates and included studies from database inception to August 2013. Limits were placed for studies published in the English language. Search terms were charted to Medical Subject Headings and combined using Boolean operations. Search terms included: aortic valve repair OR aortic valve preservation OR aortic valve reconstruction. Reference lists of papers found in the literature search were manually searched to assess suitability for inclusion in this review. Articles were first screened by two reviewers (M.B. and M.F.) based on their titles and abstracts. All identified articles were systematically assessed using the inclusion and exclusion criteria for further study.
Selection Criteria
Articles deemed eligible for inclusion were those in which patient cohorts underwent surgical repair of the aortic valve for any type of pathology, including aortic regurgitation, cusp prolapse, bicuspid aortic valve, root dilation or aneurysm, infective endocarditis, rheumatic disease, or a combination of any of those listed.
Articles were excluded if they contained a pediatric population, defined as patients aged < 18 years, if the patient number was less than 50, if there was less than 100 patient years follow up, if the paper did not report mortality or morbidity, or only included patients operated on an emergency basis.
Data Extraction
All data were extracted from selected articles by two reviewers (M.B. and M.F.). Results were collected on Microsoft Excel for Windows. Statistical analysis was performed using GraphPad Prism. Patient-years (pt-yrs) were either recorded from the article or calculated if not reported by multiplying the number of patients with the mean follow-up time reported. Studies reported from the same center were addressed in data analysis, and if patient cohorts from each study overlapped, the study with the smaller cohort was excluded to prevent patient duplication in the study. Data are presented as mean ± standard deviation. Weighted means are calculated utilizing either total sample size or patient follow up years.
Results
Identification of Studies
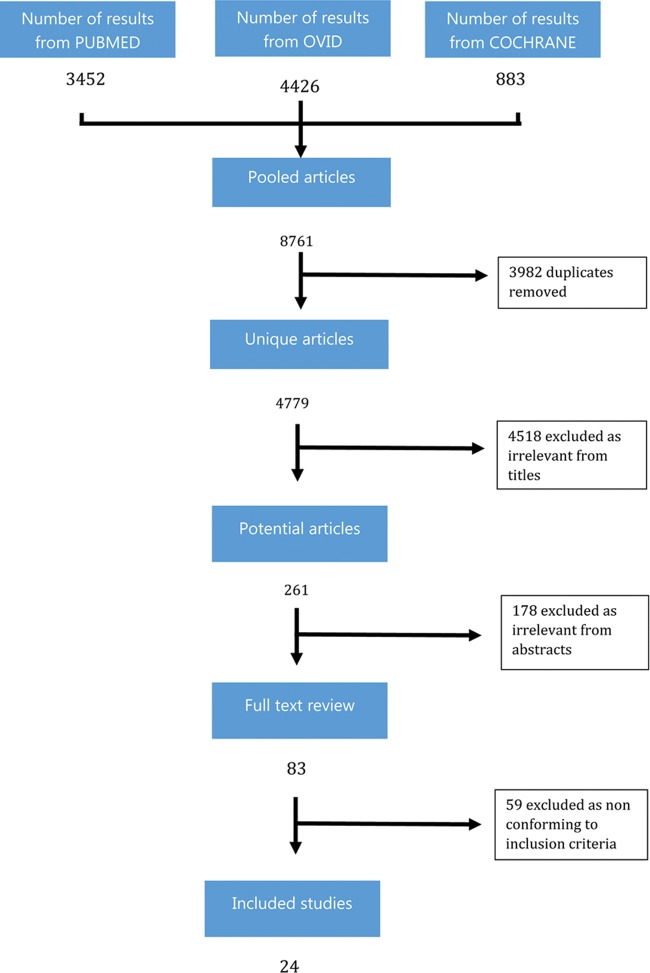
A total of 8761 studies were identified from 3 databases (PUBMED, OVID and COCHRANE) (Fig. 1). After exclusion of duplicates (3982), papers deemed irrelevant from the titles (4518), and papers deemed irrelevant from the abstracts (178), 83 papers remained for full text review. Of these, 59 were excluded as not conforming to the inclusion criteria. The remaining 24 studies were included in the systematic review and meta-analysis [1–24].
Figure 1.
Summary of search strategy, inclusion and exclusion of relevant studies.
Study Characteristics
Study characteristics are displayed in Table 1. In total, 4986 patients underwent aortic valve repair. After excluding studies that may represent overlap of patient cohorts, 15 studies remained: 7 studies representing BAV repair, 5 studies representing cusp prolapse, and 3 studies representing valve repair with root dilation or aneurysm. Studies included were published between 2004 and 2013. The majority of studies originated from 2 centers (Belgium and Germany). All studies bar one were retrospective in nature. There was a single prospective multi-center trial [6].
Table 1.
Study Characteristics
| Authors | Year | Location | Type | No. pts. (n) | Mean age (years) | Male (%) | BAV (%) | Marfan (%) | F/U (years) | F/U pt yrs |
|---|---|---|---|---|---|---|---|---|---|---|
| Kari et al (1) | 2013 | Stanford, USA | Retrospective | 50 | 45 | 80 | 100 | 9 | 3 | 190 |
| Price et al (2) | 2013 | Belgium | Retrospective | 475 | 53 | 81 | 34 | NR | 4.6 | 2152 |
| Aicher et al (3) | 2013 | Germany | Retrospective | 559 | 47.2 | 86.8 | 100 | NR | 4.6 | 2559 |
| Vohra et al (4) | 2013 | Belgium | Retrospective | 471 | 52.1 | 81.1 | 34.8 | 9.3 | 11.2 | 5275.2 |
| Luciani et al (5) | 2012 | Italy | Retrospective case control | 58 | 43 | 79.3 | 34.7 | NR | 3.8 | 220.4 |
| Fattouch et al (6) | 2012 | Italy | Retrospective | 216 | 53 | 76.8 | 27.7 | 16.6 | 3.5 | 756 |
| Baidu et al (7) | 2011 | Belgium | Retrospective case control | 100 | 47.2 | 80 | 43 | 3 | 1.7 | 167 |
| Boodhwani et al (8) | 2011 | Belgium | Retrospective | 55 | 65 | 71 | 30.9 | NR | 4.3 | 237 |
| de Kerchove et al (9) | 2011 | Germany | Retrospective | 106 | 45.5 | 93.5 | 100 | NR | 4.2 | 445 |
| Aicher et al (10) | 2011 | Germany | Retrospective | 316 | 49 | 84.8 | 100 | NR | 4 | 1253 |
| Boodhwani et al (11) | 2011 | Belgium | Retrospective | 122 | 44 | 92 | 100 | NR | 5.1 | 620 |
| Boodhwani et al (12) | 2011 | Belgium | Retrospective | 111 | 56.5 | 92 | 43.1 | 11 | 3.8 | 422 |
| Lansac et al (13) | 2010 | France | Mulitcentre prospective | 187 | 57.7 | NR | 21.4 | 39.7 | 2.4 | 455.9 |
| Aicher et al (14) | 2010 | Germany | Retrospective | 640 | 56 | 72.7 | 32 | NR | 4.7 | 3035 |
| Ashikhmina et al (15) | 2010 | Mayo | Retrospective case control | 108 | 41 | 91 | 100 | NR | 5.1 | 541 |
| David et al (16) | 2010 | Toronto, Canada | Retrospective | 64 | 46 | 78.1 | NR | NR | 4.9 | 313.6 |
| Schäfers (17) | 2010 | Germany | Retrospective | 111 | 57 | 74.6 | 0 | NR | 3.5 | 385 |
| Aicher et al (18) | 2010 | Germany | Retrospective | 427 | 53 | 70.3 | 42.4 | 2.3 | 2.9 | 1238 |
| le Polain de Waroux et al (19) | 2009 | Belgium | Blinded retrospective | 186 | 55.3 | 79 | 37 | 19 | 1.5 | 279 |
| de Kerchove et al (20) | 2008 | Germany | Retrospective | 146 | 50 | 93 | 53 | 3 | 3.5 | 185.5 |
| Jeanmart et al (21) | 2007 | Belgium | Retrospective | 71 | 51 | 83.3 | 46.4 | 4.2 | 4.2 | 298 |
| El Khoury et al (22) | 2006 | Belgium | Retrospective | 68 | 95.6 | 43 | 100 | NR | 2.8 | 190.4 |
| Minakata et al (23) | 2004 | Mayo, USA | Retrospective | 160 | 55 | 79.3 | 34 | NR | 4.2 | 672 |
| Langer et al (24) | 2004 | Germany | Retrospective | 179 | 54.5 | 73.8 | 44.1 | 5 | 2.6 | 465.4 |
NR = not reported.
In all studies, males represented the majority of treated patients (79.8% ± 10.7) and mean age was 50.8 ± 5.9 (range 41-65). Average follow up was 4.0 years ± 1.8 with average follow up-patient years 931.5 ± 1209.6. Bicuspid valves were present in approximately half of the patient cohort (52.4%). Preoperative AI greater than 2+ was present in 68.2% of patients reported in 58.3% of studies.
Early Outcomes
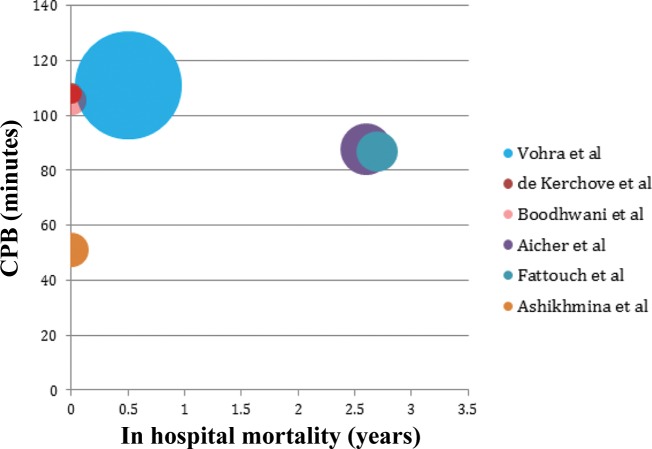
In-hospital mortality was reported in all studies. Overall weighted in-hospital mortality for all studies was low (1.46% ± 1.21) (Fig. 2). Cardiopulmonary bypass (CPB) time was reported in 6 studies (Fig. 3), which did not correlate with in-hospital mortality. Preoperative AI ≥ 2+ did not correlate to reoperation for valve failure (Pearson's Rs 0.2705, P = 0.2585) (Fig. 4). AI at discharge was reported in 9 studies, with a mean AI ≥ 2+ in 6.1% of patients (Table 2).
Figure 2.
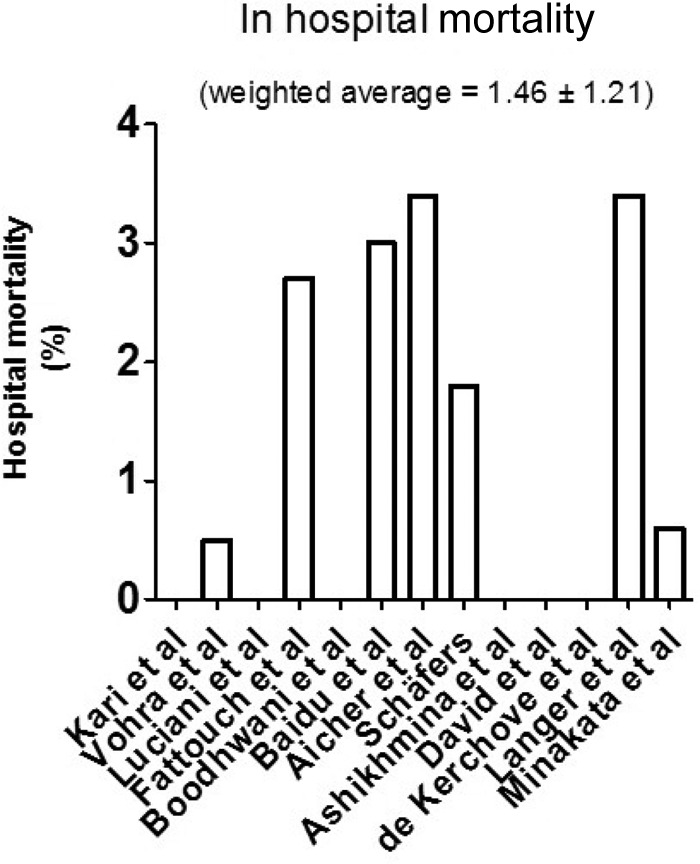
In hospital mortality per study. (Weighted average is based on study size. Average follow up for all studies was four years. Studies originated from the same center were assessed and if potential overlap in patient populations was discovered, the smaller cohort was removed to avoid duplicate patients.)
Figure 3.
Bubble chart displaying the average cardiopulmonary bypass (CPB) time (where available) per study and in hospital mortality. (Size of bubble is weighted with patient follow up years for each study. Studies from the same center that may represent similar or overlapping patients are removed to avoid duplication.)
Figure 4.
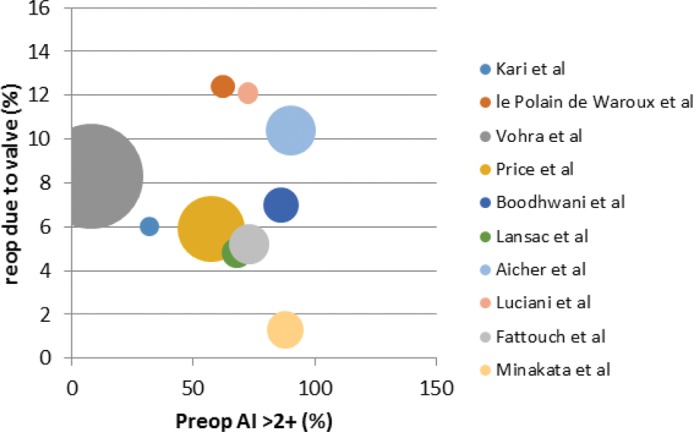
Bubble chart displaying the percentage of patients in each study with AR ≥2+ against reoperation rate for valve failure. (Size of bubble is weighted with patient follow up years for each study. Studies from the same center that may represent similar or overlapping patients are removed to avoid duplication, Pearson's r 0.2705, P = 0.2585.)
Table 2.
Study AI Characteristics Preoperative and At Discharge
| Authors | Year | No. pts. | Mean age | F/U | F/U pt yrs | LVEF >50 | Preop AI (≥2+) | Discharge AI (≥2+) | Reop due to valve (%) |
|---|---|---|---|---|---|---|---|---|---|
| Kari et al | 2013 | 50 | 45 | 3 | 190 | NR | 32 | NR | 6 |
| Vohra et al | 2013 | 471 | 52.1 | 11.2 | 5275.2 | NR | 7.9 | 5.7 | 8.3 |
| Aicher et al | 2013 | 559 | 47.2 | 4.6 | 2559 | NR | NR | NR | 10 |
| Price et al | 2013 | 475 | 53 | 4.6 | 2152 | 88.4 | 57.5 | NR | 5.9 |
| Luciani et al | 2012 | 58 | 43 | 3.8 | 220.4 | NR | 72.4 | NR | 12.1 |
| Fattouch et al | 2012 | 216 | 53 | 3.5 | 756 | NR | 73 | NR | 5.2 |
| Boodhwani et al | 2011 | 55 | 65 | 4.3 | 237 | NR | NR | 7 | 1.8 |
| Baidu et al | 2011 | 100 | 47.2 | 1.7 | 167 | 69 | NR | NR | 8 |
| de Kerchove et al | 2011 | 106 | 45.5 | 4.2 | 445 | 88.7 | 80.2 | 4 | 8.5 |
| Aicher et al | 2011 | 316 | 49 | 4 | 1253 | NR | 90.2 | NR | 10.4 |
| Boodhwani et al | 2011 | 122 | 44 | 5.1 | 620 | NR | 86 | 7 | 7 |
| Boodhwani et al | 2011 | 111 | 56.5 | 3.8 | 422 | NR | 91 | 12 | 8 |
| Lansac et al | 2010 | 187 | 57.7 | 2.4 | 455.9 | NR | 67.9 | NR | 4.8 |
| Aicher et al | 2010 | 640 | 56 | 4.7 | 3035 | NR | NR | NR | 5.6 |
| Ashikhmina et al | 2010 | 108 | 41 | 5.1 | 541 | NR | NR | NR | 17.6 |
| David et al | 2010 | 64 | 46 | 4.9 | 313.6 | NR | NR | NR | 1.5 |
| Aicher et al | 2010 | 427 | 53 | 2.9 | 1238 | NR | NR | 3 | 3 |
| Schäfers | 2010 | 111 | 57 | 3.5 | 385 | NR | NR | NR | 4 |
| le Polain de Waroux et al | 2009 | 186 | 55.3 | 1.5 | 279 | NR | 62.2 | NR | 12.4 |
| de Kerchove et al | 2008 | 146 | 50 | 3.5 | 185.5 | NR | NR | 8 | 4 |
| Jeanmart et al | 2007 | 71 | 51 | 4.2 | 298 | NR | 90.1 | NR | 3.1 |
| El Khoury et al | 2006 | 68 | 95.6 | 2.8 | 190.4 | NR | 56.5 | 0 | 10 |
| Langer et al | 2004 | 179 | 54.5 | 2.6 | 465.4 | NR | NR | NR | 2.8 |
| Minakata et al | 2004 | 160 | 55 | 4.2 | 672 | NR | 88 | 8 | 1.3 |
NR = not reported.
Late Outcomes and Valve Related Events
BAV repair represented the majority of patients undergoing aortic valve repair (Table 3). Of all studies, 7 solely assessed BAV repair. Average follow up in this cohort was for one year. In this group, reoperation required due to operated valve failure was reported in all studies. Weighted average percentage for reoperation to valve following BAV repair was 10.23% ± 3.2 (Fig. 5). Valvular endocarditis following BAV repair was reported in 5 studies with a weighted average of 1.72% ± 1.3 (Fig. 6). Other late outcomes such as stroke/TIA (transient ischemic attack) rates were reported in 4 studies with an average rate of 2.7%.
Table 3.
Outcomes in BAV Repair
| Authors | Year | No. pts. (n) | F/U (years) | F/U pt yrs | Reimplantation (%) | Remodeling (%) | In-hosp mortality (%) | Reop due to valve (%) | Valve post op endocarditis (%) | TIA (%) | Stroke (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Kari et al | 2013 | 50 | 3 | 190 | 100 | 0 | 0 | 6 | 2 | 2 | 0 |
| Aicher et al | 2013 | 559 | 4.6 | 2559 | NR | NR | 0.50 | 10 | NR | NR | NR |
| de Kerchove et al | 2011 | 106 | 4.2 | 445 | 74 | 26 | 0 | 8.5 | 2.8 | NR | 8 |
| Aicher et al | 2011 | 316 | 4 | 1253 | NR | NR | 0.63 | 10.4 | 0.6 | NR | NR |
| Boodhwani et al | 2011 | 122 | 5.1 | 620 | 34 | 10 | 0 | 7 | 1.6 | 0.8 | 2.5 |
| Ashikhmina et al | 2010 | 108 | 5.1 | 541 | NR | NR | 0 | 17.6 | 3.8 | NR | 2.8 |
| El Khoury et al | 2006 | 68 | 2.8 | 190.4 | NR | NR | 0 | 10 | NR | NR | NR |
NR = not reported.
Figure 5.
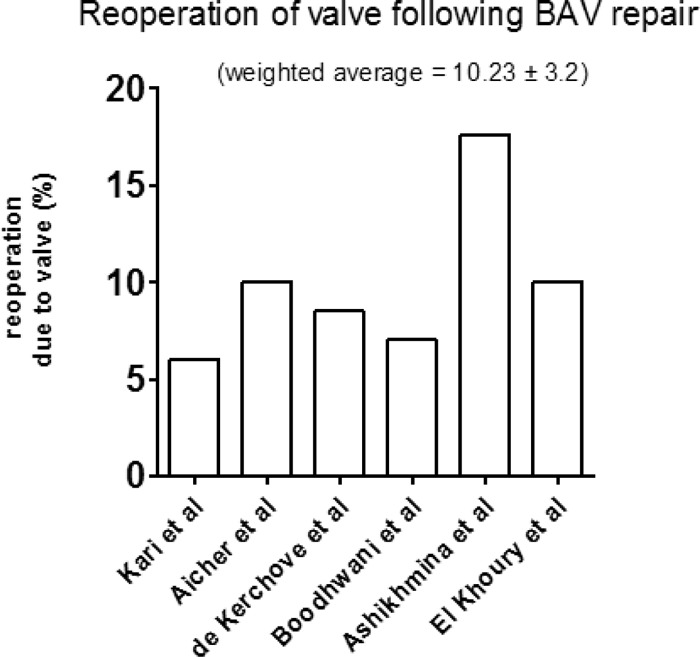
Graph displaying the percentage of patients in each study requiring reoperation due to valve failure. (Average follow up 4.1 ± 0.93 years.)
Figure 6.
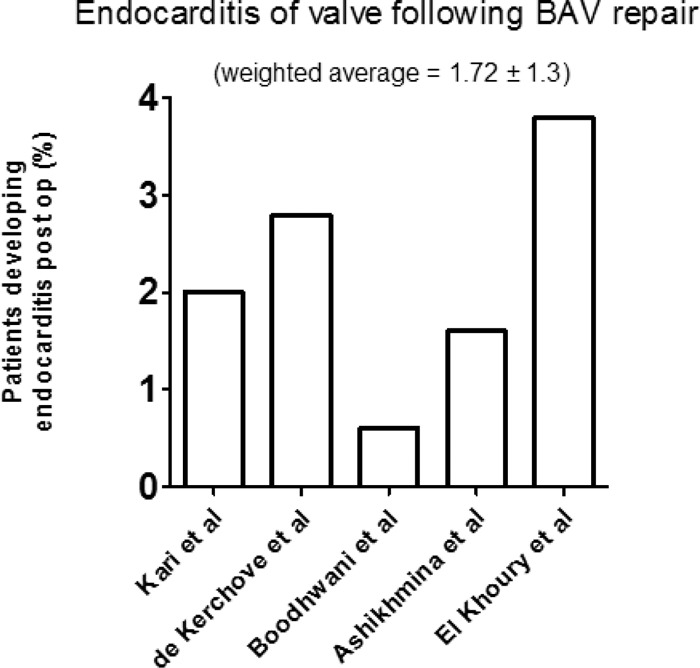
Graph displaying the percentage of patients in each study reported with endocarditis following BAV repair. (Average follow up 4.1 ± 0.93 years.)
Studies solely investigating cusp prolapse were 5 (Table 4). Average follow up in these studies was 3.72 years ± 0.74. Of these studies, 3 reported reoperation due to valve failure (Fig. 7). Weighted average reoperation following cusp prolapse repair was 3.83 ± 1.96. Negligible rates of TIA and stroke were reported in 3 studies (average 0.53%) (Table 4).
Table 4.
Outcomes in Cusp Prolapse Repair
| Authors | Year | Location | No. pts. | Mean age | Male % | % BAV | F/U | F/U pt yrs | In-hosp mort | Valve post op endocarditis | TIA | Stroke | CPB | AXC |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| David et al | 2010 | Toronto, Canada | 64 | 46 | 78.1 | NR | 4.9 | 313.6 | 0 | NR | NR | NR | NR | NR |
| de Kerchove et al | 2008 | Belgium | 146 | 50 | 93 | 53 | 3.5 | 185.5 | 0 | 0.7 | 0 | 2.1 | 108 | 88 |
| Boodhwani et al | 2011 | Belgium | 111 | 56.5 | 92 | 43.1 | 3.8 | 422 | 0 | 0 | 0.9 | 0.9 | 105.5 | NR |
| Aicher et al | 2010 | Germany | 427 | 53 | 70.3 | 42.4 | 2.9 | 1238 | 2.6 | 0.5 | NR | NR | 87.7 | 62.7 |
| Schäfers | 2010 | Germany | 111 | 57 | 74.6 | 0 | 3.5 | 385 | 1.8 | 4 | 0.9 | 0 | NR | NR |
NR = not reported.
Figure 7.
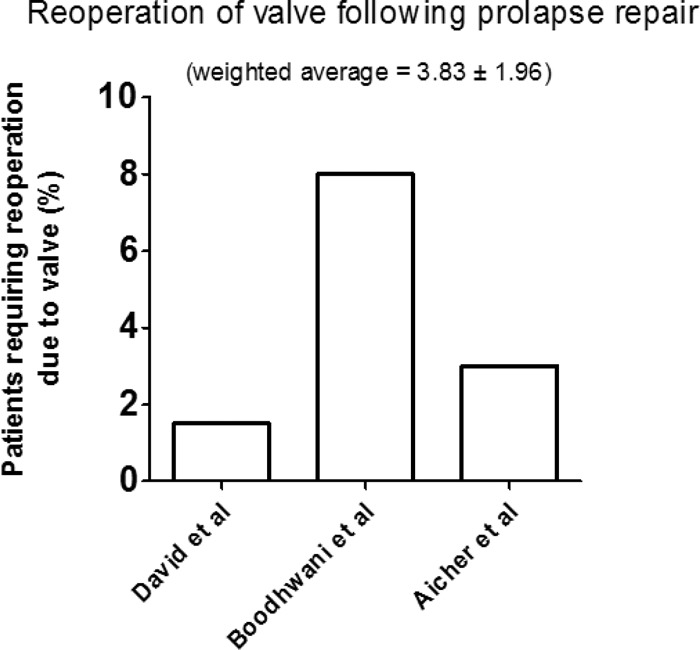
Graph displaying the percentage of patients per study requiring reoperation due to valve failure following prolapse repair. (Average follow up 3.72 ± 0.74 years.)
Aortic valve sparing procedures with root replacement were reported in 3 studies (Table 5). Of these 3 studies 2 used the remodeling technique with the other using the reimplantation technique. Average follow up in this group was 3.2 years ± 0.97. Reoperation in these studies for valve failure was reported in all 3, with a weighted average of 4.25% ± 2.46 (Fig. 8). Stroke and TIA rates were reported in all 3 studies with an average of 0.98% (Table 5).
Table 5.
Outcomes of Studies Evaluating Aortic Valve Repair in Patients with AR Secondary to Root Dilation or Aneurysm
| Authors | Year | No. pts. (n) | Mean age (n) | F/U (years) | F/U pt yrs | Reimplantation (%) | Remodeling (%) | in-hosp mort | reop due to valve | Valve post op endocarditis | TIA | Stroke |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kari et al | 2013 | 50 | 45 | 3 | 190 | 100 | 0 | 0 | 6 | 2 | 2 | 0 |
| Boodhwani et al | 2011 | 55 | 65 | 4.3 | 237 | 0 | 100 | 0 | 1.8 | 1.8 | 0 | 1.8 |
| Lansac et al | 2010 | 187 | 57.7 | 2.4 | 455.9 | 0 | 100 | NR | 4.8 | 1.1 | 1.60 | 0.5 |
Figure 8.
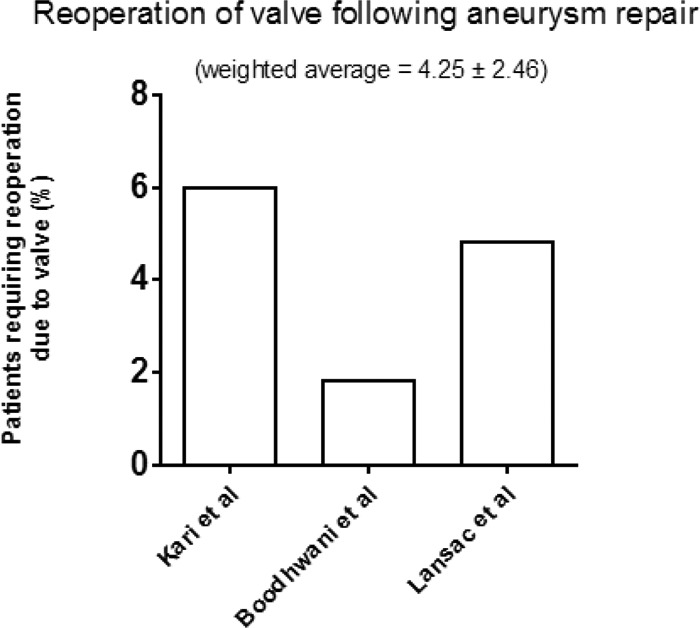
Percentage of patients requiring reoperation due to valve failure following aortic valve repair with concomitant aneurysm repair. (Average follow up 3.2 ± 0.97 years.)
Discussion
Every diseased aortic valve may ultimately require replacement. There are few, if any, medical procedures that are as effective in relieving symptoms, improving quality of life, and also increasing long-term survival as much as AVR for aortic stenosis (AS) or aortic regurgitation (AR). AVR is associated with low perioperative morbidity and mortality. The average perioperative mortality in the Society of Thoracic Surgeons database is 3.0% to 4.0% for isolated AVR and 5.5% to 6.8% for AVR plus coronary artery bypass grafting (CABG) [25,26]. A review of Medicare data, involving 684 US hospitals and more than 142,000 patients, indicates that the average in-hospital mortality for AVR in patients over the age of 65 years is 8.8% [27,28].
The use of a mechanical valve exposes the patient to lifelong need for anticoagulation and the risks of anticoagulant-related bleeding. Thromboembolic events and valve thrombosis can occur, especially if anticoagulation therapy is altered or suboptimally delivered. The risk of major bleeding with long-term anticoagulation is approximately 1% per year; however, this significantly increases with increasing age [27]. Anticoagulation in females of reproductive age poses its own complexities and risks. There are several advantages to aortic valve replacement, including ease of insertion, safety, durability, excellent hemodynamic and long-term track record of performance. However, aortic valve replacement inherently is associated with certain disadvantages, in addition to the aforementioned. These include issues of durability, infection, valve degeneration and patient-prosthesis mismatch. Banbury et al confirmed that younger age decreases durability of biological prostheses [29]. They found that freedom from explant due to structural valve damage (SVD) was 99%, 94%, and 77% at 5, 10, and 15 years. Studies analyzing factors influencing structural valve damage (SVD) post AVR conclude that SVD is promoted by the age at implantation (younger age), site of implantation (mitral position), gender (male), and valve type (porcine) [30–33]. Also, not all patients with SVD undergo reoperation within the time frame of the 15-year follow-up.
The alternative option to aortic valve replacement is aortic valve repair. This was even attempted before the advent of cardiopulmonary bypass using different techniques like circumclusion [34] and bicuspidization [35]. Lillehei in 1958 [36], using cardiopulmonary bypass, also applied the bicuspidization technique as well as single cusp enlargement using Ivalone sponge. Later, other techniques were developed, such as plication of the aortic annulus [37] and annuloplasty [38,39]. Mulder described in 1960 a variety of techniques referred to as valvuloplasty [40]. Later, Starr [41] and Spencer [42] described their techniques to repair aortic valve prolapse concomitant with VSD. Surgeons became more involved with the concept of aortic valve repair after annular disruptions and other balloon-induced injuries that caused acute insufficiency in young patients requiring immediate repair [43].
The techniques of aortic valve repair have been modified since those early times. Modern techniques have been grouped into the following categories: 1) Nonaneurysmal related annular dilation of the valve may be corrected with circular annuloplasty, commissural annuloplasty (commissural plication), and complex valve extension using pericardium. 2) Cusp prolapse is dealt with using techniques of triangular resection, leaflet resuspension, and plication of the free edge of the leaflet. 3) Valve stenosis is corrected via commissurotomy. 4) Cusp perforation is directly patch repaired.
The key questions that need to be clarified in aortic valve repair include the following.
1) What is the durability of aortic valve repair?
Given the variable strategies, the different techniques, and the short term results, the answer remains ambiguous. Initially, aortic valve repair was reserved for young patients, thus avoiding major risks related to anticoagulation and allowing better quality of life. This trend has changed and extended. AVr is now an option to be considered in a wider range of patients, with reported clinical results now extending to the early midterm stage. Our meta-analysis revealed that reoperation is 10.2% for repair of bicuspid aortic valve. BAV repair makes up the mainstay of patients undergoing aortic valve repair despite a relatively small number of studies with a relative small average follow up time. Studies looking primarily at cusp prolapse repair and root dilation with aortic valve repair represent a smaller number with reoperation rates between 3-4%. However, these studies include a much smaller average follow up.
There are no clear indications on when repair should be applied, and data showing its safety and durability are limited. AVr is confounded because most reports describe mixed groups of patients, including those with tricuspid and bicuspid valve repairs, as well as valve repair performed during procedures for aortic root reconstruction. Long-term survival data are scarce, and comparison is currently made with no control group undergoing aortic valve replacement. In the published literature, the incidence of valve-related complications is low, with recurrent aortic regurgitation being the most frequent late complication of repair. While surgical mortality is low, reoperation rates are high.
During our study, aortic insufficiency was not always reported in a standardized way. Thus comments on only a small number of studies can be made.
The majority of studies operated on patients with AI ≥ 2+. However, there was significant variation and reoperation rates were analyzed to see if there was a correlation with degree of preoperative AI. No correlation was seen between preoperative AI and reoperation rates. At discharge AI ≥ 2+ was 6.1%. AI at follow up was not reported in a standardized way, in grading technique or time, and was therefore not included in this study. However, future studies addressing both these factors would be of considerable interest in assessment of the durability of the repair.
2) What are the reasons for valve repair failure?
Our meta-analysis found a reoperation rate of 10.3% for repair of bicuspid aortic valve. Ashikhmina et al. (15) report the potential risk factors related to BAV repair failure, which are: time of operation: age at original BAV repair; sex; body mass index; year of operation; era of operation (before 2000 or after 2000); left ventricular function; concomitant cardiac pathologic factors (eg, coarctation); AV morphologic characteristics as described by the operating surgeon, including calcification; AV repair techniques; concomitant procedures; and mean AV gradient at follow-up transthoracic echocardiographic analysis.
Conclusion
Although there are limitations and complications of prosthetic valves, especially for younger individuals, there is ample published literature that provides strong evidence for AVR. On the contrary, aortic valve repair may be a useful option for selected patients, but there is lack of uniformity in data and lack of compelling long-term evidence in its favor. An international multi-center study comparing results between AVR and AVr is the next step required.
Limitations
Primarily, this study is limited due to the small number of published reports available. Furthermore, the majority of available studies are observational in nature. Currently, higher levels of evidence do not exist for aortic valve repair. Only a select number of centers and surgeons perform aortic valve repair. In this study, we identified only one prospective trial. Single-centered studies mean that patient numbers remain relatively small reducing the potential to draw definitive conclusions even when studies are combined. Analysis of type of repair is complicated by surgeon preference and valve dysfunction etiology.
There is a process and a learning curve in aortic valve repair and the relation to morbidity and mortality is a function of time and case load. . This may imply that studies published earlier do not reflect the current practice. Importantly, there are limited data on long term follow up available, particularly in regards to the need for aortic valve reoperation following repair, valve related events, and mortality. Average follow up in this study was only four years.
Conflict of Interest
The authors have no conflict of interest relevant to this publication.
EDITOR'S COMMENT
The reoperation rate for aortic valve repair is 10%. A tremendous amount of surgical experience, talent, and creativity has gone into achieving this level of success. But, we find ourselves now in a “glass half full or glass half empty” situation. Is a 10% reoperation rate a triumph or a tragedy? It is a triumph in terms of surgical science. But, for those unfortunate young people in the 10% who need an early reoperation, that is a rather tragic outcome. One's overall take on this is all a matter of point of view. Each reader needs to decide this for himself.
References
- 1.Kari FA, Liang DH, Kvitting JP, Stephens EH, Mitchell RS, Fischbein MP, et al. Tirone David valve-sparing aortic root replacement and cusp repair for bicuspid aortic valve disease. J Thorac Cardiovasc Surg. 2013;145:S35–S40.e1-2 10.1016/j.jtcvs.2012.11.043 [DOI] [PubMed] [Google Scholar]
- 2.Price J, De Kerchove L, Glineur D, Vanoverschelde JL, Noirhomme P, El Khoury G. Risk of valve-related events after aortic valve repair. Ann Thorac Surg. 2013;95:606–612. 10.1016/j.athoracsur.2012.07.016 [DOI] [PubMed] [Google Scholar]
- 3.Aicher D, Schneider U, Schmied W, Kunihara T, Tochii M, Schäfers HJ. Early results with annular support in reconstruction of the bicuspid aortic valve. J Thorac Cardiovasc Surg. 2013;145:S30–S34. 10.1016/j.jtcvs.2012.11.059 [DOI] [PubMed] [Google Scholar]
- 4.Vohra HA, Whistance RN, de Kerchove L, Glineur D, Noirhomme P, El Khoury G. Influence of higher valve gradient on long-term outcome after aortic valve repair. Ann Cardiothorac Surg. 2013;2:30–39. 10.3978/j.issn.2225-319X.2012.12.02 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Luciani GB, De Rita F, Lucchese G, Hila D, Rungatscher A, Faggian G, et al. Repair of congenitally dysplastic aortic valve by bicuspidization: midterm results. Ann Thorac Surg. 2012;94:1173–1179. 10.1016/j.athoracsur.2012.04.063 [DOI] [PubMed] [Google Scholar]
- 6.Fattouch K, Murana G, Castrovinci S, Nasso G, Mossuto C, Corrado E, et al. Outcomes of aortic valve repair according to valve morphology and surgical techniques. Interact Cardiovasc Thorac Surg. 2012;15:644–650. 10.1093/icvts/ivs195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Badiu CC, Bleiziffer S, Eichinger WB, Zaimova I, Hutter A, Mazzitelli D, et al. Are bicuspid aortic valves a limitation for aortic valve repair? Eur J Cardiothorac Surg. 2011;40:1097–1104. 10.1016/j.ejcts.2011.02.008 [DOI] [PubMed] [Google Scholar]
- 8.Boodhwani M, de Kerchove L, Glineur D, Rubay J, Vanoverschelde JL, Van Dyck M, et al. Aortic valve repair with ascending aortic aneurysms: associated lesions and adjunctive techniques. Eur J Cardiothorac Surg. 2011;40:424–428. 10.1016/j.ejcts.2010.11.053 [DOI] [PubMed] [Google Scholar]
- 9.de Kerchove L, Boodhwani M, Glineur D, Vandyck M, Vanoverschelde JL, Noirhomme P, et al. Valve sparing-root replacement with the reimplantation technique to increase the durability of bicuspid aortic valve repair. J Thorac Cardiovasc Surg. 2011;142:1430–1438. 10.1016/j.jtcvs.2011.08.021 [DOI] [PubMed] [Google Scholar]
- 10.Aicher D, Langer F, Adam O, Tscholl D, Lausberg H, Schäfers HJ. Cusp repair in aortic valve reconstruction: does the technique affect stability? J Thorac Cardiovasc Surg. 2007;134:1533–1538. 10.1016/j.jtcvs.2007.08.023 [DOI] [PubMed] [Google Scholar]
- 11.Boodhwani M, de Kerchove L, Glineur D, Rubay J, Vanoverschelde JL, Noirhomme P, et al. Repair of regurgitant bicuspid aortic valves: a systematic approach. J Thorac Cardiovasc Surg. 2010;140:276–284. 10.1016/j.jtcvs.2009.11.058 [DOI] [PubMed] [Google Scholar]
- 12.Boodhwani M, de Kerchove L, Watremez C, Glineur D, Vanoverschelde JL, Noirhomme P, et al. Assessment and repair of aortic valve cusp prolapse: implications for valve-sparing procedures. J Thorac Cardiovasc Surg. 2011;141:917–925. 10.1016/j.jtcvs.2010.12.006 [DOI] [PubMed] [Google Scholar]
- 13.Lansac E, Di Centa I, Sleilaty G, Crozat EA, Bouchot O, Hacini R, et al. An aortic ring: from physiologic reconstruction of the root to a standardized approach for aortic valve repair. J Thorac Cardiovasc Surg. 2010;140:S28–35. 10.1016/j.jtcvs.2010.08.004 [DOI] [PubMed] [Google Scholar]
- 14.Aicher D, Fries R, Rodionycheva S, Schmidt K, Langer F, Schäfers HJ. Aortic valve repair leads to a low incidence of valve-related complications. Eur J Cardiothorac Surg. 2010;37:127–132. 10.1016/j.ejcts.2009.06.021 [DOI] [PubMed] [Google Scholar]
- 15.Ashikhmina E, Sundt TM 3rd, Dearani JA, Connolly HM, Li Z, Schaff HV. Repair of the bicuspid aortic valve: a viable alternative to replacement with a bioprosthesis. J Thorac Cardiovasc Surg. 2010;139:1395–1401. 10.1016/j.jtcvs.2010.02.035 [DOI] [PubMed] [Google Scholar]
- 16.David TE, Armstrong S. Aortic cusp repair with Gore-Tex sutures during aortic valve-sparing operations. J Thorac Cardiovasc Surg. 2010;139:1340–1342. 10.1016/j.jtcvs.2009.06.010 [DOI] [PubMed] [Google Scholar]
- 17.Schäfers HJ, Langer F, Glombitza P, Kunihara T, Fries R, Aicher D. Aortic valve reconstruction in myxomatous degeneration of aortic valves: are fenestrations a risk factor for repair failure? J Thorac Cardiovasc Surg. 2010;139:660–664. 10.1016/j.jtcvs.2009.06.025 [DOI] [PubMed] [Google Scholar]
- 18.Aicher D, Kunihara T, Abou Issa O, Brittner B, Gräber S, Schäfers HJ. Valve configuration determines long-term results after repair of the bicuspid aortic valve. Circulation. 2011;123:178–185. 10.1161/CIRCULATIONAHA.109.934679 [DOI] [PubMed] [Google Scholar]
- 19.le Polain de Waroux JB, Pouleur AC, Robert A, Pasquet A, Gerber BL, Noirhomme P, et al. Mechanisms of recurrent aortic regurgitation after aortic valve repair: predictive value of intraoperative transesophageal echocardiography. J Am Coll Cardiol. Cardiovasc Imaging. 2009;2:931–939. 10.1016/j.jcmg.2009.04.013 [DOI] [PubMed] [Google Scholar]
- 20.de Kerchove L, Glineur D, Poncelet A, Boodhwani M, Rubay J, Dhoore W, et al. Repair of aortic leaflet prolapse: a ten-year experience. Eur J Cardiothorac Surg. 2008;34:785–791. 10.1016/j.ejcts.2008.06.030 [DOI] [PubMed] [Google Scholar]
- 21.Jeanmart H, de Kerchove L, Glineur D, Goffinet JM, Rougui I, Van Dyck M, Noirhomme P, El Khoury G. Aortic valve repair: the functional approach to leaflet prolapse and valve-sparing surgery. Ann Thorac Surg. 2007;83:S746–51. 10.1016/j.athoracsur.2006.10.089 [DOI] [PubMed] [Google Scholar]
- 22.El Khoury G, Vanoverschelde JL, Glineur D, Pierard F, Verhelst RR, Rubay J, et al. Repair of bicuspid aortic valves in patients with aortic regurgitation. Circulation. 2006;114:I610–6. 10.1161/CIRCULATIONAHA.105.001594 [DOI] [PubMed] [Google Scholar]
- 23.Minakata K, Schaff HV, Zehr KJ, Dearani JA, Daly RC, Orszulak TA, et al. Is repair of aortic valve regurgitation a safe alternative to valve replacement? J Thorac Cardiovasc Surg. 2004;127:645–653. 10.1016/j.jtcvs.2003.09.018 [DOI] [PubMed] [Google Scholar]
- 24.Langer F, Aicher D, Kissinger A, Wendler O, Lausberg H, Fries R, et al. Aortic valve repair using a differentiated surgical strategy. Circulation. 2004;110:II67–73. 10.1161/01.CIR.0000138383.01283.b8 [DOI] [PubMed] [Google Scholar]
- 25.Bonow RO, Carabello BA, Chatterjee K, de Leon AC Jr., Faxon DP, Freed MD, et al. ; 2006 Writing Committee Members; American College of Cardiology/American Heart Association Task Force. 2008 Focused update incorporated into the ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 1998 Guidelines for the Management of Patients With Valvular Heart Disease): endorsed by the Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. Circulation. 2008;7;118:e523–661. 10.1161/CIRCULATIONAHA.108.190748 [DOI] [PubMed] [Google Scholar]
- 26.Edwards FH, Peterson ED, Coombs LP, et al. Prediction of operative mortality after valve replacement surgery. J Am Coll Cardiol. 2001;37:885–892. 10.1016/S0735-1097(00)01202-X [DOI] [PubMed] [Google Scholar]
- 27.Svensson LG, Adams DH, Bonow RO, Kouchoukos NT, Miller DC, O'Gara PT, et al. Aortic valve and ascending aorta guidelines for management and quality measures: executive summary. Ann Thorac Surg. 2013;95:1491–1505. 10.1016/j.athoracsur.2012.12.027 [DOI] [PubMed] [Google Scholar]
- 28.Goodney PP, O'Connor GT, Wennberg DE, Birkmeyer JD. Do hospitals with low mortality rates in coronary artery bypass also perform well in valve replacement? Ann Thorac Surg. 2003;76:1131–1136. 10.1016/S0003-4975(03)00827-0 [DOI] [PubMed] [Google Scholar]
- 29.Banbury MK, Cosgrove DM, 3rd, Lytle BW, Smedira NG, Sabik JF, Saunders CR. Long-term results of the Carpentier-Edwards pericardial aortic valve: a 12-year follow-up. Ann Thorac Surg 1998;66 Suppl:S73–S76. 10.1016/S0003-4975(98)00986-2 [DOI] [PubMed] [Google Scholar]
- 30.Rahimtoola SH. Choice of prosthetic heart valve for adult patients. J Am Coll Cardiol. 2003;41:893–904. 10.1016/S0735-1097(02)02965-0 [DOI] [PubMed] [Google Scholar]
- 31.Jamieson WR, Germann E, Aupart MR, Neville PH, Marchand MA, Fradet GJ. 15-year comparison of supra-annular porcine and Perimount aortic bioprosthesis. Asian Cardiovasc Thorac Ann. 2006;14:200–205. 10.1177/021849230601400306 [DOI] [PubMed] [Google Scholar]
- 32.Gao G, Wu Y, Grunkemeier GL, Furnary AP, Starr A. Durability of pericardial versus porcine aortic valves. J Am Coll Cardiol. 2004;44:384–388. 10.1016/j.jacc.2004.01.053 [DOI] [PubMed] [Google Scholar]
- 33.Al Halees Z, Gometza B, Al Sanei A, Duran C. Repair of moderate aortic valve lesions associated with other pathology: an 11-year follow-up. Eur J Cardiothorac Surg. 2001;20:247–251. 10.1016/S1010-7940(01)00782-5 [DOI] [PubMed] [Google Scholar]
- 34.Taylor WJ, Thrower WB, Black H, Harken DE. The surgical correction of aortic insufficiency by circumclusion. J Thorac Cardiovasc Surg. 1958;35:192–205. [PubMed] [Google Scholar]
- 35.Starzl TC, Cruzat EP, Walker FB, Lewis FJ. A technique for bicuspidization of the aortic valve. J Thorac Cardiovasc Surg. 1959;38:262–270. [PMC free article] [PubMed] [Google Scholar]
- 36.Lillehei CW, Gott VL, DeWall RA, Varco RL. The surgical treatment of stenotic or regurgitant lesions of the mitral and aortic valves by direct vision using a pump oxygenator. J Thorac Cardiovasc Surg. 1958;35:154–191. [PubMed] [Google Scholar]
- 37.Hurwitt ES, Hoffert PW, Rosenblatt A. Plication of the aortic ring in the correction of aortic insufficiency. J Thorac Cardiovasc Surg. 1960;39:654–662. [PubMed] [Google Scholar]
- 38.Cabrol C, Cabrol A, Guiraudon G, Bertrand M. Le traitement de l'insuffisance aortique par l'annuloplastie aortique. Arch Mal Coeur. 1966;59:1305–1312. [PubMed] [Google Scholar]
- 39.Rocache M, Cabrol C, Cabrol A, Guiraudon G. Re′sultats e′loigne′s des annuloplasties aortiques. Arch Mal Coeur. 1971;65:1123–1128. [PubMed] [Google Scholar]
- 40.Mulder DG, Kattus AA, Longmire WP. The treatment of acquired aortic stenosis by valvuloplasty. J Thorac Cardiovasc Surg. 1960;40:713–743. [PubMed] [Google Scholar]
- 41.Starr A, Menashe V, Dotter D. Surgical correction of aortic insufficiency associated with ventricular septal defect. Surg Gynecol Obstet. 1960;111:71–76. [PubMed] [Google Scholar]
- 42.Spencer FC, Bahnson HT, Neill CA. The treatment of aortic regurgitation associated with a ventricular septal defect. J Thorac Cardiovasc Surg. 1962;43:222–227. [PubMed] [Google Scholar]
- 43.Seifert PE, Auer JE. Surgical repair of annular disruption following percutaneous balloon aortic valvuloplasty. Ann Thorac Surg. 1988;46:242–243. 10.1016/S0003-4975(10)65908-5 [DOI] [PubMed] [Google Scholar]