Abstract
Background: Marfan syndrome (MFS), an inherited disorder of connective tissue characterized by abnormalities in the skeletal, ocular, and cardiovascular systems, is caused by mutations in the gene for fibrillin-1 (FBN1). The high mortality in untreated patients is primarily due to aneurysm and dissection of the ascending aorta. The complex pathogenesis of MFS involves changes in transforming growth factor β (TGF-β) signaling, increased matrix metalloproteinase (MMP) expression, and fragmentation of the extracellular matrix. A number of studies have demonstrated increased counts of macrophages and T cells in the ascending aorta of persons or mouse models of MFS, but the efficacy of anti-inflammatory therapy in mouse models of MFS has not yet been assessed. Methods: FBN1 underexpressing mgR/mgR Marfan mice were treated with oral indomethacin. Treatment was begun at the age of three weeks and continued for 8 weeks, following which the aorta of wild type as well as treated and untreated mgR/mgR mice was compared. Results: Indomethacin treatment led to a statistically significant reduction of aortic elastin degeneration and macrophage infiltration, as well as a lessening of MMP-2, MMP-9, and MMP-12 upregulation. Additionally, indomethacin decreased both cyclooxygenases 2 (COX-2) expression and activity in the aorta of mgR/mgR mice. COX-2-mediated inflammatory infiltrate contributes to the progression of aortic aneurysm in mgR/mgR mice, providing evidence that COX-2 is a relevant therapeutic target in MFS-associated aortic aneurysmal disease. Conclusions: COX-2 mediated inflammatory infiltration plays an important role in the pathogenesis of aortic aneurysm disease in MFS.
Keywords: Aorta, Aneurysm, Inflammatory infiltrate, Marfan syndrome
Introduction
Marfan syndrome (MFS) is a hereditary connective tissue disorder caused by mutations in fibrillin-1 gene (FBN1). The most life threatening symptom of MFS is thoracic aortic aneurysm (TAA) [1]. Multiple factors such as haploinsufficiency, FBN1 proteolysis, abnormal TGF-β signaling, increased matrix metalloproteinase (MMP) expression, and changes in cell-matrix interaction contribute to the complex pathogenesis of this disorder.
Collagens, laminins, and elastin have multiple motifs that are able to interact with cell-surface receptors on macrophages and other inflammatory cells, thereby eliciting reactions including chemotactic activity, phagocytic functions, upregulation of matrix metalloproteinases (MMP), gene expression, and immune responses [2,3]. Recently, evidence is accumulating to support the notion that inflammation may also play an important role in the development of TAA in MFS. For instance, studies have found inflammatory and immune cells around focal medial degeneration in patients with MFS and isolated TAA [4–6]. In our previous study, CD68 immunostaining of the surgical aortic specimen in 28 patients with MFS showed significant increase in the number of macrophages in the tunica media [7]. Moreover, immunohistochemical investigations of the aorta from patients with MFS, familial or sporadic TAA consistently shows the presence of T cells (CD3+) and macrophages (CD68+), particularly in the adventitia, along with an increase in the density of the vasa vasorum and local endothelial activation [6]. Recently, an increased serum concentration of macrophage-colony stimulating factor (M-CSF) was found to correlate with progressive aortic disease in MFS, and increased expression of a number of inflammatory genes was identified in skin cultures of MFS patients and found to correlate with a number of clinical features [8].
In FBN1 underexpressing mgR/mgR Marfan mice, as early as 8 weeks, monocytes begin to infiltrate the medial layer, followed by adventitial inflammation with fragmentation of the medial elastic network and fibroblast hyperplasia [9,10]; these findings suggest that inflammatory infiltration is relevant to the progression of aortic aneurysm in this Marfan mouse model. Our previous work has demonstrated that both recombinant glycine-x-x-proline-glycine (GxxPG) containing FBN1 fragments and Marfan aortic extracts induce macrophage chemotaxis through the elastin-binding protein signal pathway, providing a possible explanation of the inflammatory infiltration observed in the aortic wall of mgR/mgR mice and MFS patients [7,11]. Moreover, we showed that treatment of mgR/mgR mice with the monoclonal antibody BA4, which binds to glycine-x-x-proline-glycine (GxxPG) fragments and thereby reduced elastin-binding protein-mediated signaling [7] efficiently attenuates progression of aortic aneurysm in Marfan mouse model [12].
Prostanoids are a class of inflammatory mediators induced by cyclooxygenases (COX), which are present as two isoforms, termed COX-1 and COX-2 [13]. Increased COX-2 expression was observed in the thoracic aorta of Fbn1C1039G/+ MFS mouse model, but by contrast, COX-1 was downregulated [14]. Indomethacin is a nonsteroidal anti-inflammatory drug (NSAID) with nonspecific COX inhibitory activity and anti-inflammatory effects that are mediated by inhibition of the activity of the COX-2 isoform [15]. Indomethacin has been shown to be an effective treatment in animal models of abdominal aortic aneurysm (AAA), a disease that can be characterized by a substantial degree of inflammation [15]. Macrophages isolated from human AAA biopsies stain strongly for cyclooxygenase 2, and secrete levels of Prostaglandin E2 (PGE2) that can cause apoptosis of smooth muscle cells derived from the AAA specimen [16]. In light of these results suggesting a role of inflammation in MFS [4–6,8] and the results of our previous studies showing that GxxPG fragments of the MFS aorta can induce macrophage chemotaxis [7,11], we were motivated to investigate the effects of oral indomethacin treatment on the progression of aortic abnormalities in the aorta of the mgR/mgR MFS mouse model. Our results demonstrate that indomethacin efficiently blocks elastin degeneration and decreases macrophage infiltration and unregulated MMP expression in the aortic wall of mgR/mgR mice. Moreover, it also decreases both TGF-β activity and COX-2 activity. These findings indicate that indomethacin is an effective treatment in this mouse model of MFS and shows an interrelation between inflammation and TGF-β activity in MFS.
Materials and Methods
Animals and Study Design
Heterozygous mgR FBN1 underexpressing mice [9] were mated to generate wild-type, heterozygous and homozygous mgR mice. All animals were genotyped with polymerase chain reaction as described previously [7]. Indomethacin at 6 mg/L (Sigma-Aldrich) was added to the drinking water. The treatment started at 3 weeks of age and continued for a total of 8 weeks. At the end of treatment, mice were euthanized and aortic root, ascending aorta, and arch were collected and either flash frozen in liquid nitrogen and stored at −80°C or treated in 1× Phosphate Buffered Saline (PBS) with 4% paraformaldehyde (PFA) overnight at 4°C for preparing sections. Mice were maintained under pathogen-free conditions, and the animal study was reviewed and approved by the Landesamt für Gesundheit und Soziales in Berlin (LaGeSo, Reg 0137/07).
Blot of Whole Aorta Protein
Whole aorta tissue (n = 5 or 6 per group) was ground in 200 μl lysis buffer (20 mM Tris, pH 7.4, 150 mM NaCl, 1% TritonX-100, 1 mM Ethylenediaminetetraacetic acid [EDTA]) with protease and phosphatase inhibitors (Roche Applied Science, Mannheim, Germany) and homogenized in a homogenizer. Samples were then mixed thoroughly by vortexing four times for 20 seconds and cooling on ice for 10–15 minutes and, subsequently, centrifuged at 10,000 g at 4°C for 15 minutes. The supernatant was then transferred to a fresh tube and stored at −20°C. Protein concentration was determined with the bicinchoninic acid (BCA) assay kit (Pierce) and equal amounts (15 μg) were resolved by 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS PAGE) before transfer onto polyvinylidene fluoride (PVDF) membranes (Millipore). Immunodetection was performed by incubating at 4°C with antibodies against MMP-2 (Abcam), MMP-9 (Abcam), MMP-12 (Abcam), pSMAD2 (Cell Signaling Technology), ERK1/2 and pERK1/2 (Cell Signaling Technology), and β-actin (Abcam) as a loading control, followed by subsequent incubation with antimouse Immunoglobulin G (IgG) or antirabbit IgG horseradish peroxidase-conjugated secondary antibody. Protein expression was quantified using the ImageJ program.
Histology and Immunohistochemistry
Freshly prepared specimens of mouse ascending aorta and aortic arch were fixed in 1× PBS with 4% PFA overnight at 4°C. Tissues were embedded in paraffin. The solidified paraffin-embedded tissues were then stored at 4°C. These blocks were then sectioned into 5 μm microsections and used for histological analysis after drying for at least one night. To visualize the elastic fibers of the aorta, sections were stained with modified Verhoeff-van Giesen's stain. Free ends of elastic lamellae per millimeter aorta length and aortic medial thickness were counted by two independent observers at 10–15 representative locations and averaged. Immunohistochemical examinations were carried out using the Avidin Biotin Complex (ABC) streptavidin-biotin method with the Vectastain ABC kit (Vector Laboratories, Burlingame, CA) according to the manufacturer's protocol. Briefly, after deparaffinization and rehydration, endogenous peroxidase activity was quenched by a 5-minute incubation in 3% H2O2. After trypsin-mediated antigen retrieval, tissue sections were preincubated in prediluted blocking horse serum for 10 minutes to block nonspecific binding. Binding of primary rabbit anti-pSMAD2 (1:150, Cell Signaling Technology), rat anti-F4/80 antibody (1:150, Abcam), or rabbit anti COX-2 was carried out overnight at 4°C. The sections were then washed and subsequently incubated with biotinylated panspecific universal secondary antibody for 10 minutes. After washing, streptavidin peroxidase complex was added for 5 minutes. Antigen detection was performed by a 3-minute incubation with 0.1% diaminobenzidine tetrahydrochloride (DAB) and 0.01% hydrogen peroxide. The sections were then counterstained with hematoxylin, mounted in Entellan (Merck), and analyzed on a Leica MZ 12.5 stereomicroscope (Leica) coupled to the AxioCam HRc camera and the AxioVision 4.2 image analysis software. Counts of infiltrated adventitial macrophages were calculated in 10–15 random 200× fields by two independent observers. Morphometric analysis of histologic sections was used to determine the COX-2 positive area in the aortic wall.
ELISA for Measuring Prostaglandin E2 (PGE2)
The PGE2 content in whole aorta protein was measured by using an enzyme immunoassay (ELISA) kit (R&D) according to the manufacturer's instructions. The results were expressed as nanograms of PGE2 per milligram protein.
Statistics
The results are displayed as mean ± standard error (s.e.m.). Because the sample size is small in some experiments, tests of significance were conducted using the Mann–Whitney U test. P < 0.05 was considered statistically significant.
Results
Indomethacin Decreases COX-2 Expression and PGE2 Levels in mgR/mgR Mice
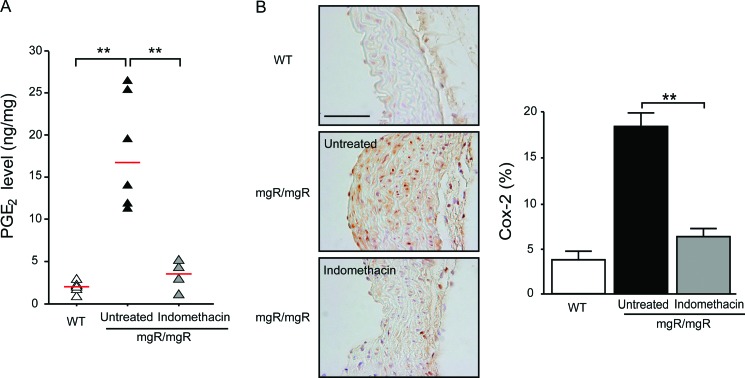
Local production of PGE2 is often taken as an indicator of COX-2 activity [17]. We, therefore, measured PGE2 in aorta lysate by ELISA. Significantly higher PGE2 production was measured in the aorta lysate of mgR/mgR mice than in wild-type mice (Fig. 1A). The PGE2 levels decreased significantly in the indomethacin-treated group (3.4 ± 0.77 ng/mg) versus the untreated group (18.1 ± 2.5 ng/mg), indicating that indomethacin treatment decreased COX-2 activity in mgR/mgR mice. Because COX-2 expression has been reported to be increased in the aorta of Fbn1C1039G/+ mice [14], we next investigated COX-2 expression in mgR/mgR mice. Immunostaining showed a nearly fourfold increase of COX-2 positive area in the aortic media and adventitia in mgR/mgR mice compared with age-matched wild-type mice. Compared with the untreated group, the indomethacin-treated group showed a statistically significant reduction in COX-2 expression (Fig. 1B). These results document the pharmacological effectiveness of the chosen indomethacin dosage.
Figure 1.
Indomethacin decreases both PGE2 levels and COX-2 expression in mgR/mgR mice. (A) PGE2 levels were measured by ELISA in aortic homogenates from wild-type (WT) (n = 6), untreated (n = 6), and indomethacin (n = 4) treated mgR/mgR mice. PGE2 levels were significantly higher in mgR/mgR mice than wild-type mice. Indomethacin significantly reduced PGE2 concentration in mgR/mgR mice compared with untreated mice. Red bars indicate the median levels of PGE2. (B) Representative immunostaining with the COX-2 antibody in the aorta from three groups of mice. Scale bar indicates 50 μm. COX-2 expression is increased in untreated mgR/mgR mice, whereas indomethacin treatment led to a marked decrease in COX-2 positive area. Number of animals per group was five to six. **P < 0.01.
Indomethacin Attenuates Elastin Degeneration and Inhibits Macrophage Infiltration
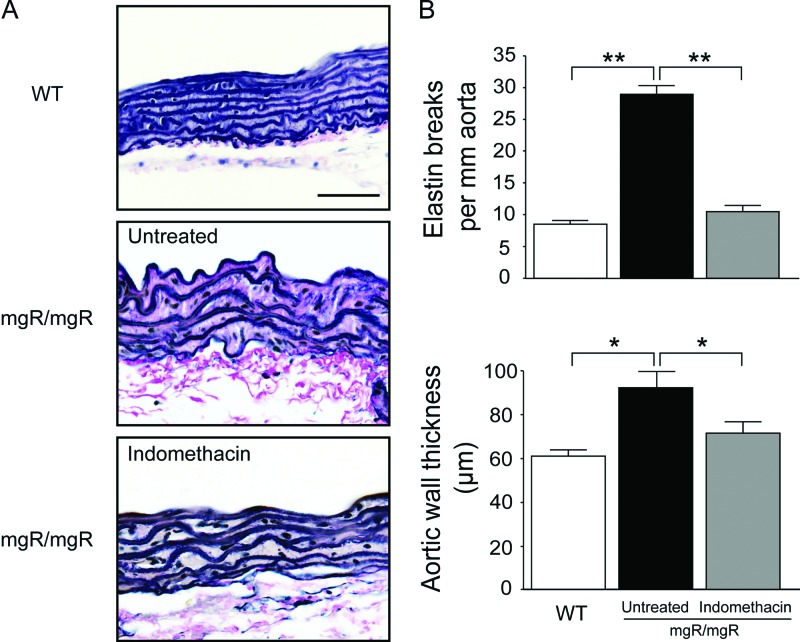
Substantial fragmentation and disorganization of elastic lamellae were observed in the aortic media of untreated mgR/mgR mice, but treatment with indomethacin attenuated elastin degeneration and restored the architecture of the aortic media (Fig. 2A). Quantification of the numbers of elastin breaks revealed that indomethacin resulted in a statistically significant reduction of elastin breaks compared to that of the untreated group. In addition, the aortic media thickness decreased nearly to that value of wild-type mice after indomethacin treatment (Fig. 2B).
Figure 2.
Indomethacin rescues elastin fragmentation in the aorta of mgR/mgR mice. (A) Representative van Giesen stained aorta sections from wild-type (WT), untreated or indomethacin treated mgR/mgR mice. Scale bar indicates 50 μm. (B) Quantitative analysis of elastin breaks and aortic medial thickness. mgR/mgR mice treated with indomethacin had significantly fewer elastin breaks and reduced aortic medial thickness compared with untreated littermates. Number of animals per group was five to six. Statistically significant differences between untreated and indomethacin-treated mgR/mgR mice. *P < 0.05; **P < 0.01.
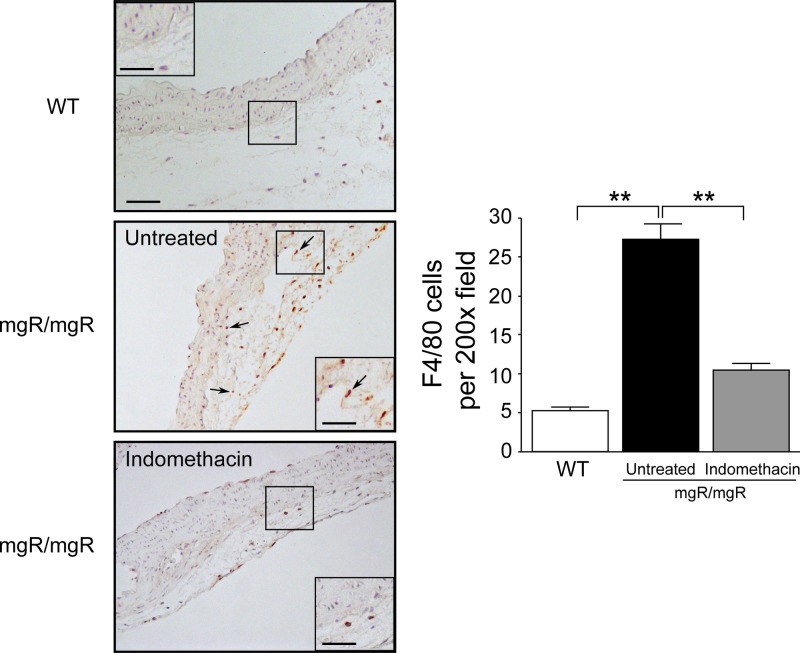
Macrophage migration could be observed as early as 8 weeks in the aortic wall of mgR/mgR mice. To investigate whether indomethacin could inhibit macrophage infiltration, we performed immunohistochemistry by using the macrophage marker F4/80. There were very few macrophages in the aortic wall of wild-type mice, whereas substantial numbers of F4/80 positive macrophages were observed in aortic adventitia of untreated mgR/mgR mice; indomethacin treatment was effective in reducing macrophage numbers (Fig. 3). Quantitative analysis of the macrophage numbers revealed a more than 60% reduction of cells numbers after indomethacin treatment; the reduction was statistically significant (Fig. 3).
Figure 3.
Indomethacin reduces macrophage infiltration in aortic adventitia of mgR/mgR mice. F4/80 immunohistochemistry in representative aorta sections from WT, untreated and indomethacin treated mgR/mgR mice. Arrowheads indicate F4/80 positive cells. WT mice exhibited nearly no macrophage infiltrate in the aortic wall. Macrophage infiltrates aortic adventitia of mgR/mgR mice, whereas there are decreased macrophages in indomethacin-treated mgR/mgR mice. Scale bar indicates 50 μm. Inset bar indicates 25 μm. Quantitative analysis of F4/80 macrophage showed statistically significant differences between untreated and indomethacin treated mgR/mgR mice. Number of animals per group was five to six. **P < 0.01.
Indomethacin Decreases Upregulated MMP Expression
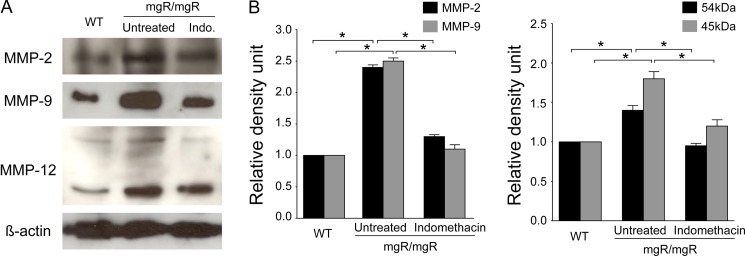
Upregulation of the gelatinases MMP-2 and MMP-9 is thought to be involved in the pathogenesis of aortic elastic fiber degeneration in MFS [18,19]. Additionally, MMP-12 (a macrophage-specific metalloproteinase) was shown to be modestly upregulated in MFS aortic samples taken during aortic replacement surgery [20]. To determine whether indomethacin treatment has an effect on MMP expression, we performed Western blot of aortic homogenates for MMP expression. As shown in Figure 4A, MMP-2, −9, and −12 expression was higher in mgR/mgR mice than in wild-type mice. Treatment with indomethacin resulted in decreased intensities of MMP-2, −9, and −12 expression (Fig. 4A). The results were further confirmed by quantification of Western blot signals, which showed that indomethacin significantly decreased MMP expression in mgR/mgR mice compared with untreated mice (Fig. 4B).
Figure 4.
Indomethacin decreases MMP expression in mgR/mgR mice. (A) Representative Western blots of MMP-2, −9, and −12 from aortic homogenates from each group (wild-type, untreated and indomethacin-treated mgR/mgR mice). β-actin was used as a loading control. (B) Quantitative analysis of MMP expression by densitometry of the corresponding bands and represented as the ratio of MMP expression to β-actin. MMP expression decreased significantly after indomethacin treatment. The bands are representative of findings in three subjects. *P < 0.05.
Decreased Phosphorylated Smad2 (pSMAD2) and Phosphorylated ERK1/2 (pERK1/2) Activity After Indomethacin
TGF-β is a potent inducer of SMAD2 phosphorylation and nuclear translocation and alterations in both canonical and noncanonical TGF-β signaling, including activation of extracellular signal-regulated kinase (ERK), are an important aspect of the pathogenesis of MFS [21–24].
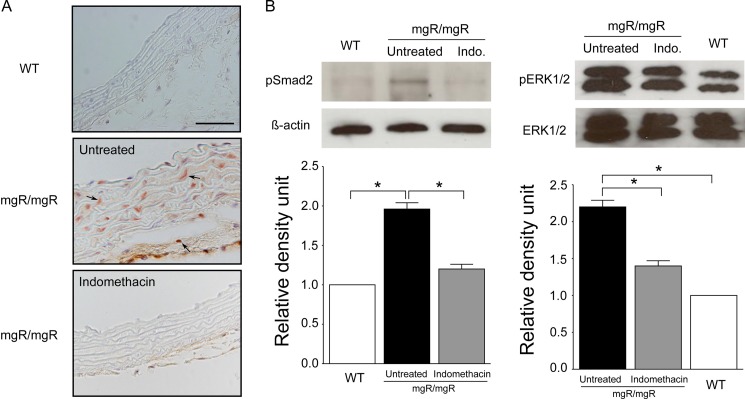
To assess TGF-β activity, we measured pSMAD2 expression by immunohistochemistry. In wild-type mice, nearly no signal for pSMAD2 was observed. In contrast, substantial numbers of SMAD2 positive cells were present in both aortic media and adventitia of mgR/mgR mice, and aorta from indomethacin treated group showed almost no pSMAD2 signal (Fig. 5A). Consistently, Western blot showed a significant reduction of pSMAD2 expression in the indomethacin-treated group compared with the untreated group (Fig. 5B). Additionally, we also measured both ERK1/2 and pERK1/2 activity by Western blot. pERK1/2 is the active (phosphorylated) form of ERK. There was no significant difference in ERK1/2 expression between wild-type and mgR/mgR mice. In contrast, pERK1/2 expression was increased in mgR/mgR mice compared with wild-type mice, and indomethacin significantly reduced pERK1/2 activity (Fig. 5B).
Figure 5.
Indomethacin reduces both pSMAD2 and pERK1/2 activity in the aorta of mgR/mgR mice. (A) Immunostaining for pSMAD2 in representative aortic section from wild-type, untreated, and indomethacin-treated mgR/mgR mice. Arrowheads indicate pSMAD2 staining cells. Indomethacin reduced the pSMAD2 signaling in mgR/mgR mice to an almost undetectable level of wild-type mice. Number of animals per group was five to six. (B) Representative Western blot of pSMAD2 and pERK1/2 protein in aortic homogenates from each group of mice. Quantitative analysis of pSMAD2 expression normalized with β-actin. Relative levels of pERK1/2 protein expression were normalized to ERK1/2. Indomethacin significantly decreased both SMAD2 and ERK1/2 phosphorylation in mgR/mgR mice. The bands are representative of findings in three subjects. *P < 0.05.
Discussion
In the present study, we demonstrated that the nonsteroidal anti-inflammatory drug indomethacin significantly improved elastin integrity and reduced the numbers of macrophages in the aortic adventitia of mgR/mgR mice, which coincided with decreased MMP-2, −9, and −12 expression. Based on our previous and recent studies, we speculate that macrophage infiltration observed in the aortic wall of mgR/mgR Marfan mice participates in a kind of vicious cycle, in which matrix fragments induce deleterious effects, including upregulation of MMP activity and macrophage infiltration, which in turn reinforces the pathological processes associated with matrix degradation and defects in TGF-β sequestration [7,11,12].
Treatment of MFS mice with doxycycline, a nonspecific inhibitor of MMP activity, slows progression of aortic disease and improves survival [19]. The importance of inflammation in TAA of MFS was further confirmed in a recent study, which demonstrated that pravastatin, a cholesterol-lowering agent, prevents aortic root dilation in a mouse model of MFS, an effect that may be associated with the anti-inflammatory effect of pravastatin [25]. In the present study, we aimed to investigate whether a treatment regimen based on indomethacin aimed primarily at reducing inflammation would have a similarly beneficial therapeutic effect in preventing TAA progression in mgR/mgR mice.
The efficacy of indomethacin was further demonstrated in our study by its ability to block increased TGF-β activity in Marfan aorta. Similar to previous findings that treating mice with losartan or with inhibitors of noncanonical TGF-β reduced pSMAD2 activity [22–24], treatment with indomethacin led to a clear reduction in pSMAD2 as well as pERK1/2 activity. We suggest that decreased TGF-β activity after indomethacin treatment may be due to the maintenance of the elastic lamellae architecture, thus restoring the ability of the extracellular matrix (ECM) to control TGF-β activity.
PGE2 is a primary product of arachidonic metabolism and is reported to be abundantly synthesized in AAA via the COX-2 dependent pathway [17]. Macrophage-derived PGE2 in AAA tissue may contribute to the expansion of AAA [16]. Our study demonstrated that PGE2 levels were significantly increased in the aorta of mgR/mgR mice and that indomethacin reduces PGE2 concentration to a level that is not significantly different from that of wild-type mice. Consistent with increased PGE2 levels, we found strong COX-2 immunostaining in the aortic wall of mgR/mgR mice; indomethacin decreased COX-2 expression nearly to the level seen in wild-type mice. The reason for increased COX-2 expression is still unclear. It has been suggested that loss of vessel elasticity and increase in pulse wave velocity in the Marfan aorta may induce COX-2 expression [14]. Based on our findings, we believe that macrophages in Marfan aorta may be also responsible for increased COX-2 activity and expression. The decreased COX-2 expression and PGE2 levels may, therefore, be due to reduced macrophage infiltration after indomethacin treatment, thus providing us a possible therapeutic mechanism for indomethacin in MFS. In aortic explant, indomethacin has been proven to improve the contractility of aorta in Fbn1C1039G/+ mice [14]. Though no experiments were performed to test aortic contractility in the current study, complementary beneficial effects of indomethacin on aortic vasomotor function in mgR/mgR mice cannot be excluded.
Indomethacin can inhibit both COX-1 and COX-2 [15], and thus, any beneficial effects that indomethacin may have on aneurysm expansion are not necessarily solely related to inhibition of COX-2 activity. COX-1 has been shown to be downregulated in the aorta of Fbn1C1039G/+ mice [14]. Reduced COX-1 levels were thought to effect aortic aneurysm in MFS by decreasing COX-1 derived constricting prostanoid thromboxane A2, in turn causing an imbalance between endothelial relaxant and constricting prostanoids [14]. Future studies may focus on using isoform-selective inhibitors of COX to elucidate the separate role of both COX-1 and COX-2 in the TAA progression in MFS.
In conclusion, our observations support the suggestion that COX-2 mediated inflammatory infiltration contributes to further destruction of aortic wall in mgR/mgR mice. Moreover, these findings are consistent with the premise that macrophages are participating in the pathogenesis of TAA via a synergistic combination of MMPs that degrade the matrix of the aorta. A limitation of the current study is the lack of a survival analysis of the treated and untreated mgR/mgR animals. Although more work will be needed to determine whether COX-2 antagonism might be a useful component of future treatment regimens of individuals with MFS, our results do clearly show that COX-2 inhibition can dramatically ameliorate a series of abnormalities encountered in the aorta of the mgR/mgR mouse, which together with the many indications of the role of inflammation in MFS, isolated TAA, and AAA [4,5,7,15–17], supports the notion that COX-2 is a relevant therapeutic target for aortic aneurysmal disease in MFS.
Acknowledgments
Acknowledgments
This study was supported by a grant from the European Commission (FAD; HEALTH-F2-2008-200647).
Editor's Comments and Questions
Dr. Robert Thompson, Professor, Washington University School of Medicine, MO, USA
The authors report a study of the effects of indomethacin on ascending aortic aneurysm formation in the mgR/mgR mouse model of Marfan syndrome. The experiments are comprehensive and well-designed, and the results demonstrate important suppressive effects of indomethacin on a number of different aspects of aneurysmal degeneration. The data are presented exceptionally well and the figures are superb. The Discussion highlights the mechanistic implications of this study and there are several points of speculation that should spark new directions in research. These findings have important translational implications as well, with regard to potential use of nonsteroidal anti-inflammatory agents in prevention or treatment of aortic disease in Marfan syndrome.
Questions
How strongly can we rely on the mouse model to mimic the human MFS disease and predict response to therapy?
This is of course a major question in any animal study, and no mouse model is a perfect mimic of any human disease. We have performed our studies on the mgR mouse model of Marfan syndrome, because this model does mimic the human disease in that it develops lethal aortic dissection. On the other hand, the degree of inflammatory infiltration is more than that which we see in human disease.
In light of the strong supportive findings for your hypothesis, do you feel a human therapeutic trial with indomethacin is warranted in MFS? If not, what other investigative steps would you like to see taken before human application of this relatively innocuous drug?
Given that our study does have some limitations, including mainly the fact that we were not able to perform a formal survival study, we do not think it is appropriate to propose a human therapeutic study at this time. It seems reasonable to us to think that indomethacin might be a useful adjunct to losartan therapy.
References
- 1. von Kodolitsch Y, Robinson PN. Marfan syndrome: An update of genetics, medical and surgical management. Heart. 2007;93:755–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Adair-Kirk TL, Senior RM. Fragments of extracellular matrix as mediators of inflammation. Int J Biochem Cell Biol. 2008;40:1101–1110. 10.1016/j.biocel.2007.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. O'Reilly PJ, Gaggar A, Blalock JE. Interfering with extracellular matrix degradation to blunt inflammation. Curr Opin Pharmacol. 2008;8:242–248. 10.1016/j.coph.2008.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nataatmadja M, West M, West J, Summers K, Walker P, Nagata M, et al. Abnormal extracellular matrix protein transport associated with increased apoptosis of vascular smooth muscle cells in Marfan syndrome and bicuspid aortic valve thoracic aortic aneurysm. Circulation. 2003;108(suppl 1):I329–I334. [DOI] [PubMed] [Google Scholar]
- 5. Segura AM, Luna RE, Horiba K, Stetler-Stevenson WG, McAllister HA Jr., Willerson JT, et al. Immunohistochemistry of matrix metalloproteinases and their inhibitors in thoracic aortic aneurysms and aortic valves of patients with Marfan's syndrome. Circulation. 1998;98:II331–II337. [PubMed] [Google Scholar]
- 6. He R, Guo DC, Sun W, Papke CL, Duraisamy S, Estrera AL, et al. Characterization of the inflammatory cells in ascending thoracic aortic aneurysms in patients with Marfan syndrome, familial thoracic aortic aneurysms, and sporadic aneurysms. J Thorac Cardiovasc Surg. 2008;136:922–929. 10.1016/j.jtcvs.2007.12.063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Guo G, Booms P, Halushka M, Dietz HC, Ney A, Stricker S, et al. Induction of macrophage chemotaxis by aortic extracts of the mgR Marfan mouse model and a GxxPG-containing fibrillin-1 fragment. Circulation. 2006;114:1855–1862. [DOI] [PubMed] [Google Scholar]
- 8. Radonic T, de Witte P, Groenink M, de Waard V, Lutter R, van Eijk M, et al. Inflammation aggravates disease severity in Marfan syndrome patients. PLoS ONE. 2012;7:e32963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pereira L, Lee SY, Gayraud B, Andrikopoulos K, Shapiro SD, Bunton T, et al. Pathogenetic sequence for aneurysm revealed in mice underexpressing fibrillin-1. Proc Natl Acad Sci USA. 1999;96:3819–3823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bunton TE, Biery NJ, Myers L, Gayraud B, Ramirez F, Dietz HC. Phenotypic alteration of vascular smooth muscle cells precedes elastolysis in a mouse model of Marfan Syndrome. Circ Res. 2001;88:37–43. 10.1161/01.RES.88.1.37 [DOI] [PubMed] [Google Scholar]
- 11. Guo G, Gehle P, Doelken S, Martin-Ventura JL, von Kodolitsch Y, Hetzer R, et al. Induction of macrophage chemotaxis by aortic extracts from patients with Marfan syndrome is related to elastin binding protein. PLoS ONE. 2011;6:e20138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Guo G, Muñoz-García B, Ott C, Grünhagen J, Shaaban A, Pletschacher A, et al. Antagonism of GxxPG fragments ameliorates manifestations of aortic disease in Marfan syndrome mice. Hum Mol Genet. 2013;22(3):433–443. [DOI] [PubMed] [Google Scholar]
- 13. Warner TD, Mitchell JA. Cyclooxygenases: New forms, new inhibitors, and lessons from the clinic. FASEB J. 2004;18:790–804. 10.1096/fj.03-0645rev [DOI] [PubMed] [Google Scholar]
- 14. Chung AW, Yeung KA, Cortes SF, Sandor GG, Judge DP, Dietz HC, et al. Endothelial dysfunction and compromised eNOS/Akt signaling in the thoracic aorta during the progression of Marfan syndrome. Br J Pharmacol. 2007;150(8):1075–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Miralles M, Wester W, Sicard GA, Thompson R, Reilly JM. Indomethacin inhibits expansion of experimental aortic aneurysms via inhibition of the COX2 isoform of cyclooxygenase. J Vasc Surg. 1999;29:884–892. 10.1016/S0741-5214(99)70216-8 [DOI] [PubMed] [Google Scholar]
- 16. Walton LJ, Franklin IJ, Bayston T, Brown LC, Greenhalgh RM, Taylor GW, et al. Inhibition of prostaglandin E2 synthesis in abdominal aortic aneurysms: Implications for smooth muscle cell viability, inflammatory processes, and the expansion of abdominal aortic aneurysms. Circulation. 1999;100:48–54. [DOI] [PubMed] [Google Scholar]
- 17. Holmes DR, Wester W, Thompson RW, Reilly JM. Prostaglandin E2 synthesis and cyclooxygenase expression in abdominal aortic aneurysms. J Vasc Surg. 1997;25:810–815. 10.1016/S0741-5214(97)70210-6 [DOI] [PubMed] [Google Scholar]
- 18. Chung AW, Yeung KA, Sandor GG, Judge DP, Dietz HC, van Breemen C. Loss of elastic fiber integrity and reduction of vascular smooth muscle contraction resulting from the upregulated activities of matrix metalloproteinase-2 and -9 in the thoracic aortic aneurysm in Marfan syndrome. Circ Res. 2007;101:512–522. 10.1161/CIRCRESAHA.107.157776 [DOI] [PubMed] [Google Scholar]
- 19. Chung AW, Yang HH, Radomski MW, van Breemen C. Long-term doxycycline is more effective than atenolol to prevent thoracic aortic aneurysm in Marfan syndrome through the inhibition of matrix metalloproteinase-2 and -9. Circ Res. 2008;102(8):e73–e85. 10.1161/CIRCRESAHA.108.174367 [DOI] [PubMed] [Google Scholar]
- 20. Ikonomidis JS, Jones JA, Barbour JR, Stroud RE, Clark LL, Kaplan BS, et al. Expression of matrix metalloproteinases and endogenous inhibitors within ascending aortic aneurysms of patients with Marfan syndrome. Circulation. 2006;114(suppl 1):I365–I370. [DOI] [PubMed] [Google Scholar]
- 21. Neptune ER, Frischmeyer PA, Arking DE, Myers L, Bunton TE, Gayraud B, et al. Dysregulation of TGF-beta activation contributes to pathogenesis in Marfan syndrome. Nat Genet. 2003;33:407–411. 10.1038/ng1116 [DOI] [PubMed] [Google Scholar]
- 22. Habashi JP, Judge DP, Holm TM, Cohn RD, Loeys B, Cooper TK, et al. Losartan, an AT1 antagonist, prevents aortic aneurysm in a mouse model of Marfan syndrome. Science. 2006;312:117–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Holm TM, Habashi JP, Doyle JJ, Bedja D, Chen Y, van Erp C, et al. Noncanonical TGFβ signaling contributes to aortic aneurysm progression in Marfan syndrome mice. Science. 2011;332:358–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Habashi JP, Doyle JJ, Holm TM, Aziz H, Schoenhoff F, Bedja D, et al. Angiotensin II type 2 receptor signaling attenuates aortic aneurysm in mice through ERK antagonism. Science. 2011;332:361–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. McLoughlin D, McGuinness J, Byrne J, Terzo E, Huuskonen V, McAllister H, et al. Pravastatin reduces Marfan aortic dilation. Circulation. 2011;124:S168–S173. [DOI] [PubMed] [Google Scholar]