Abstract
Pathology of the aorta has been recognized for nearly three and a half millennia, dating back to the first recorded description in the scrolls of Ebers, circa 1550 BC. Since that time, treatment has evolved from magical medicinal remedies and incantations to nearly outpatient percutaneous interventions. From the first attempts at open surgical reconstruction in the 1700s and 1800s, to the latest generations of endovascular devices, innovative pioneers have pushed the envelope of surgical technique in developing unique and novel strategies to treat the ever complex pathology of the aorta. We are just now beginning to understand these pathologies at the molecular and genetic levels, and with that expansive extent of investigation enters a journal, dedicated solely to the aorta. With this article, we hope to illuminate the rich and deep history of aortic pathology, and the innovations leading to the technology of today. A firm understanding of our past provides a strong foundation for further growth into the future.
Introduction
Aneurysma, aneurysmos … the etymologic roots of Latin and Greek origin, meaning widening or dilation, form the foundation of the modern day word aneurysm. Today, the term aneurysm applied in its most strict sense describes a blood vessel one and a half times the diameter of age matched individuals, with loss of vessel wall parallelism. Vessels of less dilation are described as ectatic, derived from the Greek origin ektasis, or to stretch out. Abdominal aortic aneurysms (AAAs) account for nearly two-thirds of all diagnosed aneurysms, with thoracic aneurysms (TAAs) accounting for another 20%. Each year, nearly 200,000 new AAAs are diagnosed in the United States, and another 15,000 reach size criterion for repair. The SAAAVE Act of 2007 (Screening Abdominal Aortic Aneurysm Very Efficiently) has brought aneurysms to a more visible public and political perspective in recent years, but the knowledge of aortic aneurysms dates back much farther in history.
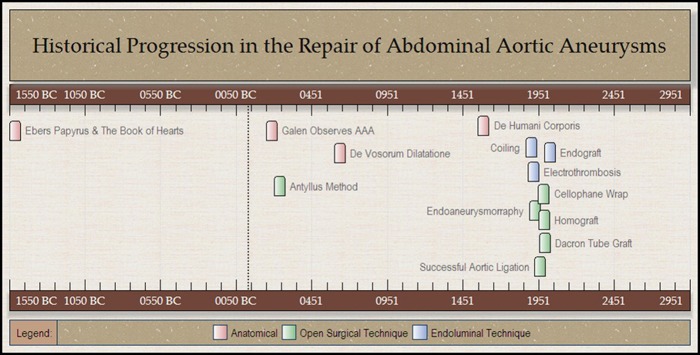
The first documented description of aortic pathology appears circa 1550 BC. Over the following three and a half millennia, our understanding of aortic aneurysms has progressed from a mystical and uniformly lethal disease process to one that focuses on preventative intervention and minimally invasive, even percutaneous, repair (Fig. 1). In this time, we have progressed from palliative options to nearly outpatient surgical interventions. Our understanding of the disease processes has zoomed down to the cellular, genetic, and molecular level with such areas of research including matrix metalloprotenases, cell signaling pathways, and collagen gene transcript products. On the other end of the spectrum, academia and industry have pushed the envelope in the development of new and novel implantable devices to treat a wider base of patients with increasingly complex anatomy via endovascular techniques. An only natural progression is the birth of a new specialty journal dedicated solely to this, AORTA. A journal dedicated to the multidisciplinary approach to treatment and research in diseases of the aorta and its first-order branches. A journal dedicated to the future of this increasing complex field. However, as Alexis de Tocqueville, a French political thinker and historian once stated: “when the past no longer illuminates the future, the spirit walks in darkness.” In the following pages, we hope to illuminate the history of aortic surgery.
Figure 1.
Advancements in aortic surgery over three and a half millennia. Anatomic, open surgical, and endovascular advancements are all illustrated. The first rudimentary description of the cardiovascular system and aneurysms dates back to 1550 BC.
Ancient Anatomic History
The term aorta was first applied by Aristotle in the 4th century BC, used to describe the great vessel of the heart. Prior to this description, the term had been used by Hippocrates to describe the bronchial tree, consistent with the belief that the vital “pneuma” derived from respiration was delivered to the body by these vessels. The respiratory and circulatory systems were seen as one continuous circuit until the early 1600s when separate blood circulation was described by Harvey. Since Aristotle's time however, the term aorta has continued to define the primary arterial outflow of the left ventricle. Pathology of the great vessel, however, had been recognized for nearly a millennium before the times of Hippocrates and Aristotle. The first preserved written account of the aorta dates back to 1550BC. The Ebers Papyrus (Fig. 2) was a hieratic script of Pharaonic Egypt, thought to be a transcription of an even earlier text. It consisted of a 110 page long papyrus scroll containing more than 700 magical and medicinal remedies for ailments of all organ systems [1,2]. The book of hearts was the largest and most highly regarded of these scrolls. It described the heart and great vessels as the center of all being and existence. Within this script lies the first recorded mention of aortic aneurysms, quoted as “… only magic can cure tumors of the major arteries.” [2] The scrolls go on to describe other conditions of the cardiovascular system, including peripheral arterial aneurysms:
Figure 2.
Excerpt from the Ebers Papyrus. Thought to have been based on even more ancient texts, the Ebers Papyrus contains the first documented description of the human heart, aorta, and aneurysms in general. Courtesy of the online catalogue of the research archives of the Oriental Institute, University of Chicago. Located at: http://oilib.uchicago.edu/books/bryan_the_papyrus_ebers_1930.pdf. Originally appears in: [2].
“When thou meetest a tumor of the vessels in any part of the body of a person and thou findest it round in form, growing under thy finger … … Treat it with the Knife and burn it with Fire so that it bleeds not too much. Heal it like the Cautery heals.” [2]
This script likely references the development of traumatic pseudoaneurysms but remains the earliest recorded description of major vascular pathology. Nearly 1,000 years pass before the next mention of these vessels again. Galen, a Greek physician practicing in Rome, served as the physician to the gladiators. Galen holds the reputation as the first trauma surgeon, caring for those injured in combat. He developed rudimentary anatomic charts which were based on canine vivisection [3]. While the only anatomic reference of his time, and for hundreds of years later, they were somewhat incomplete and inaccurate in their representation of human anatomy. In his writings, he describes aneurysms on physical examination as “localized pulsatile swellings.” Furthermore, he goes on to describe the first documented ruptured aneurysm as when “an aneurysm is wounded, the blood is spouted out with so much violence that it can scarcely be arrested.” [4,5] A contemporary to Galen was Antyllus, another Greek surgeon practicing in Rome. He is considered the true father of vascular surgery. He described both true and false aneurysms in his writings and documented the first attempted aneurysm repair in the year 200AD [6]. The “Antyllus method” consisted of proximal and distal ligation, central incision of the aneurysm, and evacuation of the thrombotic materials [1]. This remained the standard treatment of aneurysms for over 1,000 years to follow.
A few hundred years later, Aetius of Amida, a 7th century Byzantine physician and medical writer authored the manuscript De Vasorum Dilatatione, loosely translated “on the dilation of the vessels.” This was a detailed manuscript on the development and repair of abdominal aortic aneurysms utilizing a technique similar to the Antyllus method [6]. Unfortunately, as many of the time did, Aetius of Amida believed no wound heals properly without the formation of pus, and to encourage this, the aneurysm sac was packed with incense.
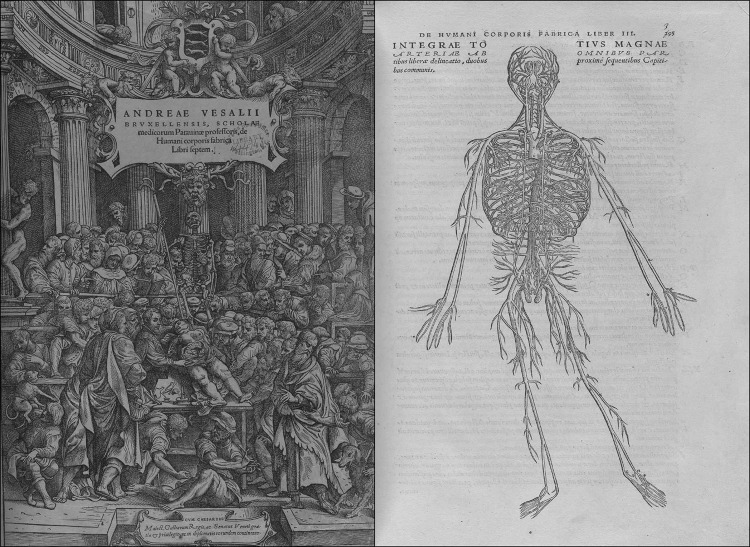
Andreas Vesalius lived from 1514–1564 AD, a Flemish physician, who traveled to Paris to study anatomy, medicine, and surgery. In 1554, he authored a seven-volume text of anatomic plates, De humani corporis fabrica (On the Structure of the Human Body, Fig. 3). These were the first human anatomic charts of their time, based on actual human anatomic dissections [4]. He taught his students by direct observation of dissection, and thus, he became known as the founding father of modern human anatomy. His seven-volume anatomic text provided new, detailed descriptions of the heart, great vessels, and vascular system. These volumes provided a level of detail never before seen and helped lay the foundation for future surgical pioneers that would follow in the centuries to come.
Figure 3.
Title plate and anatomic plate from Andreas Vesalius's “De humani corporis fabrica libri septem.” This text revolutionized the understanding of human anatomy and disease. They were the first documented anatomic charts based on direct human dissection and observation. Courtesy of the National Library of Medicine: Historical* anatomies on the web. Located at: http://www.nlm.nih.gov/exhibition/historicalanatomies/home.html.
Early Operative History
John Hunter is perhaps best known for his famed ligation of the popliteal artery; however, his older brother, William, also studied aneurysms throughout the vascular system [1]. In 1757, William published the manuscript “The History of an Aneurysm of the Aorta with Some Remarks on Aneurysms in General.” He described these aneurysms as dilated and pulsatile vessels. He was also one of the first to describe arteriovenous fistulae, along with the hissing noise heard on auscultation [6]. One of Hunter's pupils, Sir Astley Cooper, went on to further the field of aortic surgery. He experimented with and developed a retroperitoneal exposure of the aorta in a cadaveric model. In 1817, he was summoned urgently to care for a 38-year-old porter with a large external iliac artery aneurysm that had eroded the overlying skin and freely ruptured [1]. He explored the patient transperitoneally and ligated the distal aorta with a single heavy silk tie. The patient survived 48 hours postoperatively. The post mortem specimen remains on display in the Gordon Museum of Pathology at King's College London (Fig. 4) [6]. Around the same period, Jean-Nicolas Corvisart, personal physician to Emperor Napoleon I, published his essay on disease of the heart and great vessels (1806, translated to English by Jacob Gates 1812) [7]. He is now known as the father of cardiology, with the first detailed description of dilative cardiomyopathy, congestive failure, and other valvular heart disorders. In addition to these diseases, he also provided a detailed evolution of aneurysms of the aorta [7].
Figure 4.
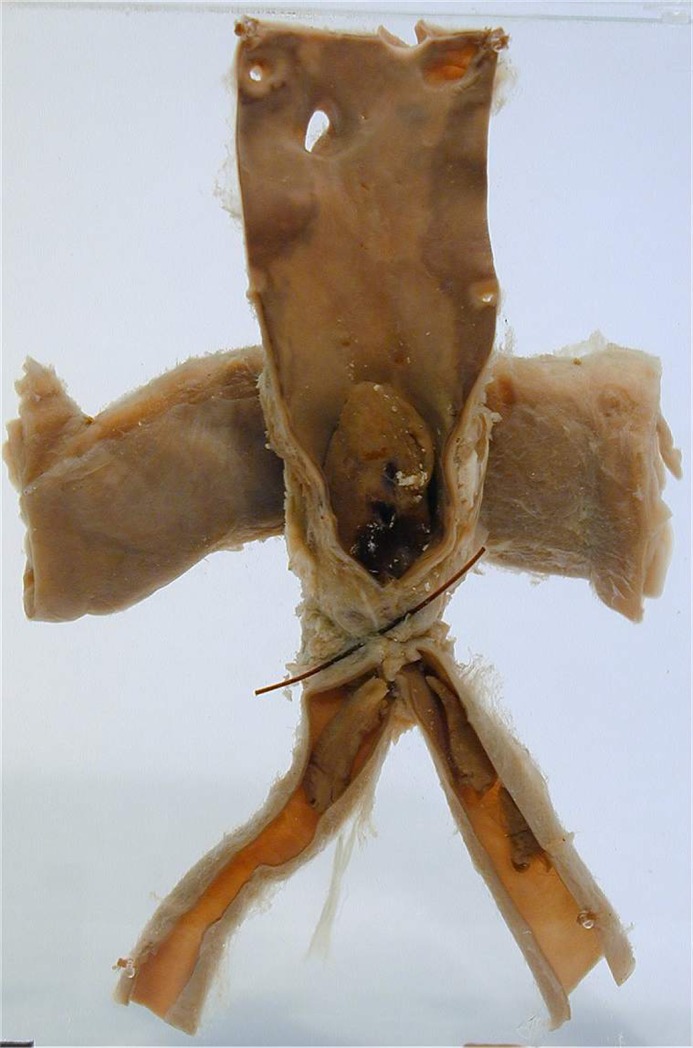
Original postmortem specimen from Sir Astley Cooper's aortic ligation. Courtesy of the Gordon Museum of Pathology, King's College London. Exhibit located online at: http://www.kcl.ac.uk/gordon/collection/specimens.aspx#APCoopersLigationAbdominalAorta.
In 1865, the first attempts at percutaneous endovascular aneurysm repairs were made (Fig. 5). Moore and Murchison attempted aneurysm sac thrombosis by direct needle cannulation and wire packing. Via direct aneurysm puncture, 26 yards of wire coils were introduced into a large thoracic aneurysm [8]. The patient ultimately expired, but the aneurysm had partially thrombosed. Sepsis and distal embolism were obvious complication. In 1879, the addition of electricity was included, and the Moore–Corradi Method was born. This electrothrombosis entailed the coiling with silver and copper wire, and before complete packing, the passage of current through this wire to encourage thrombosis [8,9].
Figure 5.
Wire introduced into aortic aneurysm to promote thrombosis. Originally appears in: [8].
Rudolf Matas (1860–1957) attempted to use electrothrombosis in 1900 for the treatment of a large abdominal aneurysm. Prior to this, he had described the use of endoaneurysmorraphy in the treatment of peripheral aneurysms (Fig. 6) [10]. He described three forms of aneurysmorraphy: obliterative, restorative, and reconstructive [6]. In 1923, he used these techniques to successfully treat an infrarenal AAA [11]. The patient survived for 18 months postoperatively, eventually succumbing to pulmonary tuberculosis complications [1].
Figure 6.
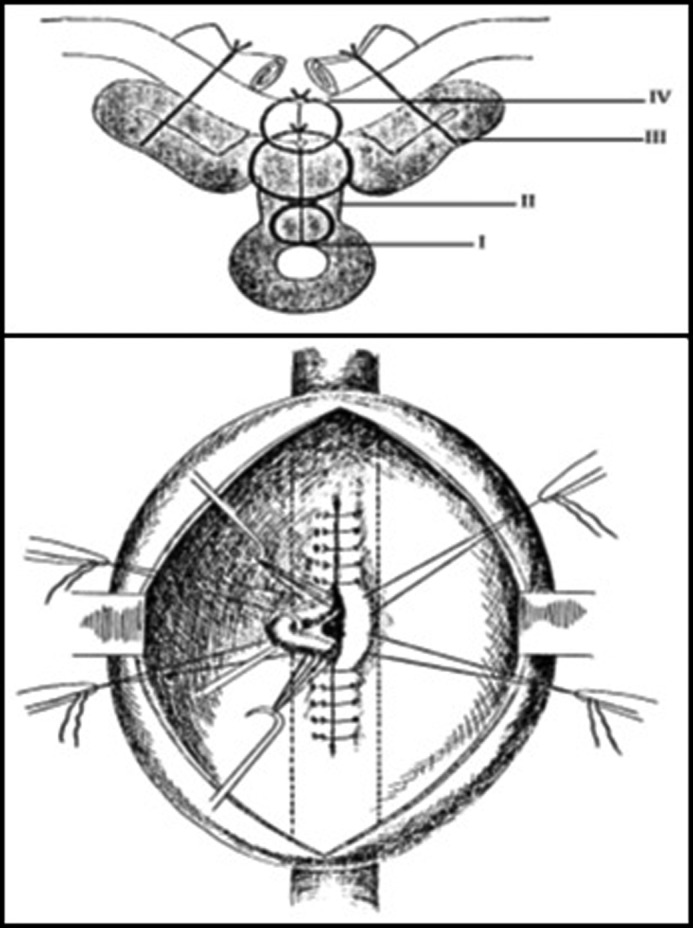
Technique for Matas endoaneursymorraphy; the lumen was preserved by plicating the aneurysm over an inner silastic tube, which was removed prior to complete endoaneursymorraphy. Originally appears in: [10].
Rea suggested an alternative form of plication, utilizing cellophane wrapping around abdominal aneurysms [12]. This technique gained wide exposure after Rudolf Nissen used it to wrap an abdominal aneurysm of Albert Einstein in 1949. He survived for more than 5 years after this intervention but ultimately succumb to a ruptured aneurysm April 18, 1955. Frustrated by the poor results with wraps, coiling, and ligation, Charles Dubost performed the first successful aneurysm resection and interposition graft using cadaveric aortic allograft on March 29, 1951 [1,13]. This technique included the complete resection of the aneurysm sac, an often bloody, morbid, and dangerous procedure. The next major advancement in aortic surgery came with the combination of Dubost's interposition grafting and Matas's endoaneursymorraphy. In 1966, Oscar Creech described this combined procedure that remains the operation of choice nearly 50 years later [14]. This simple synthesis greatly simplified open aortic operations and removed much or the associated morbidity from complete aneurysm sac resection.
Contemporary Surgical History
The use of cadaveric allografts was, however, of limited wide-scale utility. These conduits were of obvious limited supply and were also wrought with the complications of alloimmunity and late aneurysmal degeneration. Because of this, the search for a more stable, long-term, synthetic conduit material was undertaken. Through an intense combined effort of investigation, including surgeons, textile engineers, and mechanical engineers, the Dacron vascular graft was born, including a completely new manufacturing process and knitting machine that allowed for the seamless construction of these novel branching vascular grafts. This new arterial substitute was officially announced to the medical community in 1958 with the landmark paper authored by our contemporary cardiovascular surgical giants: Michael E. DeBakey and colleagues [15]. DeBakey and Cooley, however, had been developing techniques for complex aneurysm repair and spinal cord protection during thoracic aortic surgery for some years prior, with their first successful resection of a fusiform thoracic aneurysm on January 5, 1953. They went on to expand their experience, with a series of 245 successful aneurysm repairs by July 1955. Now, with the addition of a stable and suitable synthetic conduit, the Dacron interposition graft became the mainstay of modern vascular surgery, allowing complex operations for thoracic, abdominal, and thoracoabdominal aortic aneurysms. In 1974, Stanley Crawford reported his experience of thoracoamdominal aneurysm repairs [16]. Initially involving side arm branches to the renovisceral vessels, and ultimately evolving to incorporate small visceral patches sewn directly to the graft, these open techniques have only recently been challenged by the newest generation of endovascular technologies.
While others had dabbled in “endovascular techniques” in aortic surgery for nearly 125 years prior, including attempts at wire coiling and electrothrombosis, the true breakthrough came with the work of Parodi and Palmaz. They initially experimented with stainless steel stents hand sewn to thin-walled Dacron tube grafts (Fig. 7). Their handmade endografts were used in canine models before initial human trials. On September 6, 1990, the first successful human endovascular aneurysm repair was performed. They went on to describe their first five patients in the landmark paper “Transfemoral intraluminal graft implantation for abdominal aortic aneurysms.” [17] Since then, endovascular devices have progressed to be more readily available, lower profile, and more complex in configuration.
Figure 7.
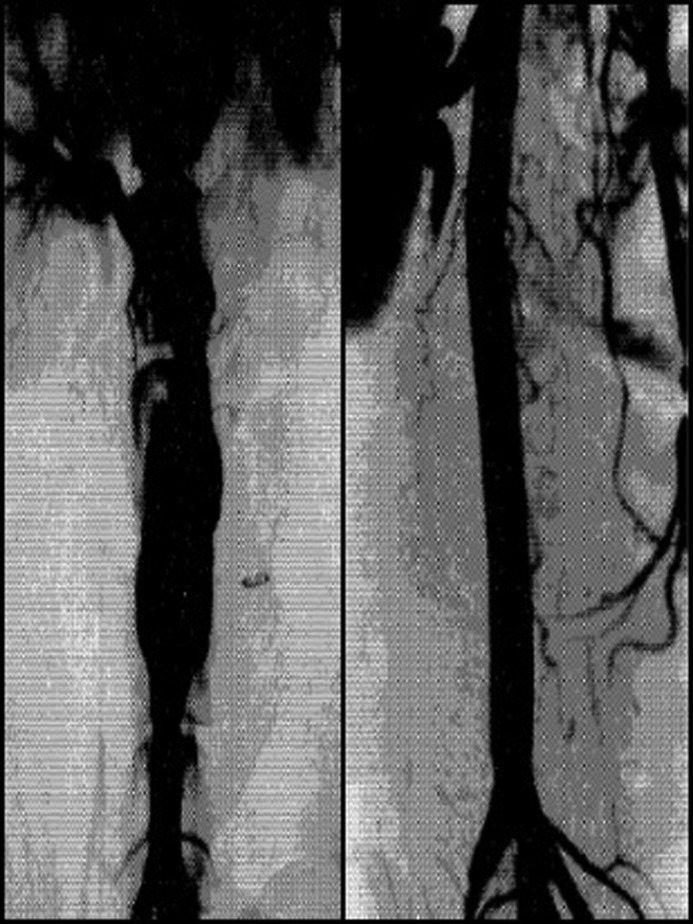
Before and after images of the initial Parodi–Palmaz endovascular aneurysm repair stent and graft system. Originally appears in: [17].
Conclusions
From the Ebers Papyrus to endografts, over three and a half millennia, pathology of the aorta has plagued mankind and been an area for bold and progressive innovations. From a disease once treated with magical medicinal remedies to a pathology that can now be treated percutaneously, pathology of the aorta continues to provide challenging opportunities for innovative and landmark research. It is with this understanding that the journal AORTA has come to fruition. A forum dedicated solely to advancing the knowledge in this specific niche. We hope that this article has helped to illuminate the deep, rich history of this field and that this journal will help to elucidate the future of it.
References
- 1. Thompson JE. Early history of aortic surgery. J Vasc Surg. 1998;28:746–752. 10.1016/S0741-5214(98)70107-7 [DOI] [PubMed] [Google Scholar]
- 2. Bryan CP. The Papyrus Ebers. London: The Garden City Press LTD; 1930. [Google Scholar]
- 3. Singer C. A Short History of Anatomy and Physiology from the Greeks to Harvey: The Evolution of Anatomy. Mineola, NY: Dover Publications, Inc; 1957. [Google Scholar]
- 4. Cooley DA. Surgical Treatment of Aortic Aneurysms. Philadelphia, PA: W.B. Saunders Company; 1986. [Google Scholar]
- 5. Galen J. Observations on Aneurysms. Translated by Erichsen JE. London: Syndenham Society; 1944. [Google Scholar]
- 6. Westaby S, Bosher C. Landmarks in Cardiac Surgery. Oxford: Isis Medical Media; 1997. [Google Scholar]
- 7. Karamanou M, Vlachopoulos C, Stefanadis C, et al. Professor Jean-Nicolas Corvisart des Marets (1755–1821): founder of modern cardiology. Hellenic journal of cardiology: HJC = Hellenike kardiologike epitheorese. 2010;51:290–293. [PubMed] [Google Scholar]
- 8. Cooley DA. Aortic aneurysm operations: past, present, and future. Ann Thorac Surg. 1999;67:1959–1962; discussion 1979–1980 10.1016/S0003-4975(99)00393-8 [DOI] [PubMed] [Google Scholar]
- 9. Finney JM. The wiring of otherwise inoperable aneurisms: with report of cases. Ann Surg. 1912;55:661–681. 10.1097/00000658-191205000-00002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Matas RI. An operation for the radical cure of aneurism based upon arteriorrhaphy. Ann Surg. 1903;37:161–196. [PMC free article] [PubMed] [Google Scholar]
- 11. Matas R. Ligation of the abdominal aorta: report of the ultimate result, one year, five months and nine days after ligation of the abdominal aorta for aneurism at the bifurcation. Ann Surg. 1925;81:457–464. 10.1097/00000658-192502010-00004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rea CE. The surgical treatment of aneurysm of the abdominal aorta. Minnesota medicine. 1948;31:153–156. [PubMed] [Google Scholar]
- 13. Dubost C, Allary M, Oeconomos N. Resection of an aneurysm of the abdominal aorta: reestablishment of the continuity by a preserved human arterial graft, with result after five months. AMA Arch Surg. 1952;64:405–408. 10.1001/archsurg.1952.01260010419018 [DOI] [PubMed] [Google Scholar]
- 14. Creech O Jr. Endo-aneurysmorrhaphy and treatment of aortic aneurysm. Ann Surg. 1966;164:935–946. 10.1097/00000658-196612000-00001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. De Bakey ME, Cooley DA, Crawford ES. Clinical application of a new flexible knitted Dacron arterial substitute. Am Surg. 1958;24:862–869. [PubMed] [Google Scholar]
- 16. Crawford ES. Thoraco-abdominal and abdominal aortic aneurysms involving renal, superior mesenteric, celiac arteries. Ann Surg. 1974;179:763–772. 10.1097/00000658-197405000-00032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Parodi JC, Palmaz JC, Barone HD. Transfemoral intraluminal graft implantation for abdominal aortic aneurysms. Ann Vasc Surg. 1991;5:491–499. 10.1007/BF02015271 [DOI] [PubMed] [Google Scholar]