Abstract
An 82-year-old male presented with a 9.3 cm ascending aorta and arch aneurysm with additional aneurysms of the innominate, right subclavian, and left common carotid arteries. The patient had a history of temporal arteritis that was only briefly treated in 1989 and a 6 cm ascending aortic aneurysm that was repaired in 1993. Our operative strategy was to construct a temporary parallel cerebrovascular circuit for cerebral protection during the redo-sternotomy and aortic arch reconstruction, with the added benefit of permanently excluding the branch arch vessel aneurysms. Pathological analysis of the aortic specimen at the first operation may have identified giant cell arteritis, prompting medical therapy against further disease progression.
Keywords: Aortic aneurysm, Inflammation, Subclavian artery, Carotid artery
Case Presentation
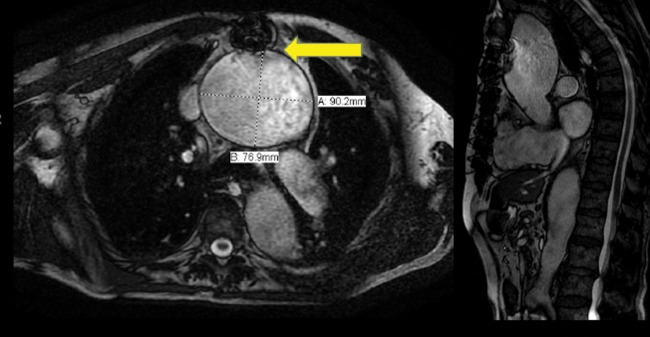
An 82-year-old male of Hungarian descent presented with dilated neck veins and pulsatility in his neck. Preoperative imaging (Fig. 1) identified a 9.3 cm ascending aortic (AscAo) and aortic arch aneurysm, as well as a 4.0 cm innominate artery (InomA), a 2.5 cm right subclavian artery (RScA), and a 2.6 cm left common carotid artery (LCCA). The RScA was at risk of rupturing. Both common carotid arteries were redundant proximally. The maximal diameters of the descending and visceral aorta were 5.8 and 5.0 cm, respectively.
Figure 1.
A 9.3 cm ascending aortic and aortic arch aneurysm, 4.0 cm innominate, 2.5 cm right subclavian (RScA) and 2.6 cm left common carotid artery (LCCA) aneurysm.
In 1989 the patient presented with malaise, extremity joint pain, and fatigue. A biopsy conducted at that time indicated temporal arteritis. The patient self-reported a 1- to 2-month course of prednisone after that admission. In 1993 a 6 cm proximal AscAo aneurysm was resected. The AscAo aneurysm diameter distal to the repair grew to 7.4 cm in 2004, 8.2 cm in 2007, and 9.3 cm in 2011. The patient did not have a history of tobacco abuse, hypertension, or emphysema. He had no family history of aneurysms, although his aunt had temporal arteritis. Three weeks prior to surgery, a percutaneous transluminal coronary angioplasty was performed and a bare metal stent was placed in the left anterior descending artery.
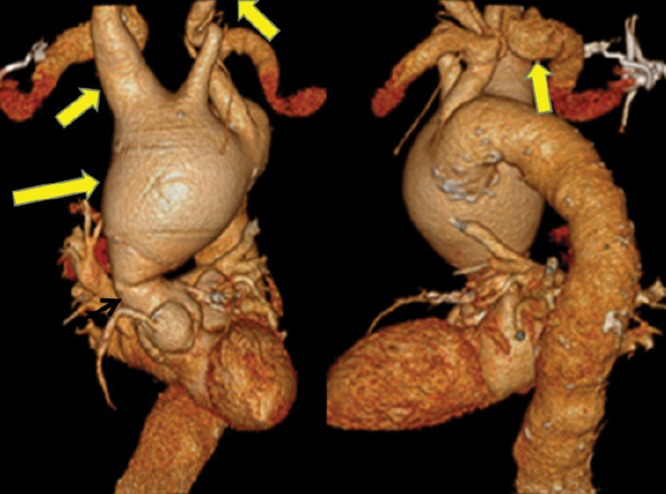
Preoperative imaging showed extremely torturous left and right carotid arteries (Fig. 2), as well as apposition of the aneurysm to the previous sternotomy with compression of the vena cava and innominate vein (Fig. 3). Our operative strategy was to (1) maintain continuous cerebrovascular perfusion during initial exclusion of the InomA, RScA, and left common carotid artery, (2) conduct a redo-sternotomy to replace the AscAo and arch aneurysms, and (3) replace the aortic valve. To these ends, a temporary extra-anatomic circuit was constructed. The first step was to perfuse the right and left common carotid arteries, and the right axillary artery (RAx) using the left subclavian artery (LScA). Bilateral supraclavicular incisions were used to expose the arch branch vessels. The left common carotid artery was divided distally to its aneurysmal portion and transposed onto the apex of the LScA.
Figure 2.
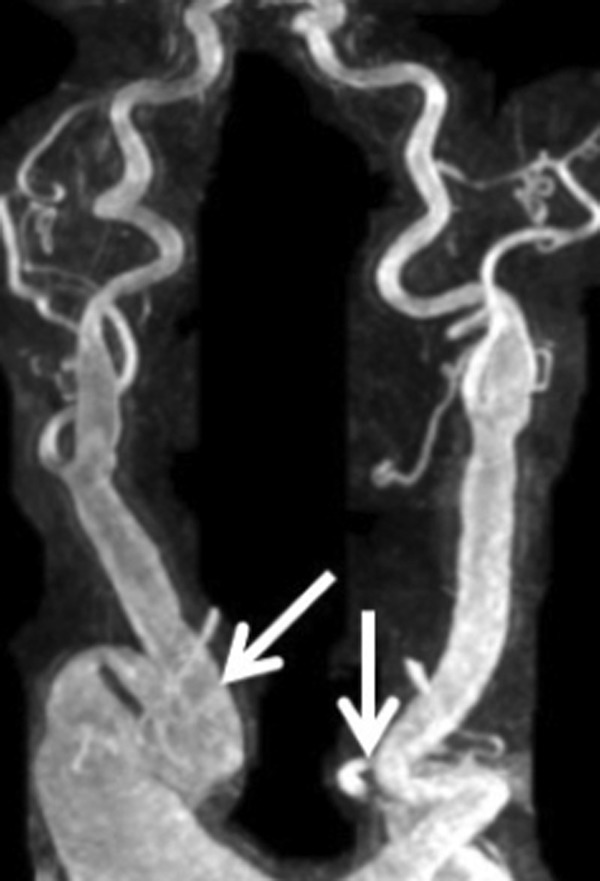
Torturous right and left carotid arteries.
Figure 3.
Apposition of the aneurysm to the previous sternotomy with compression of the vena cava and innominate veins.
A three-limbed graft was constructed from a bifurcated 16X8 mm Gore-Tex® (Flagstaff, Arizona) aortic graft by dividing one of the 8 mm limbs and sewing it to the 16 mm main body. This graft was left external to the IobanTM drape (3M, St. Paul, Minnesota) that had been placed across the operative field. The three 8 mm side-arm limbs were sewn to the circulation in the following order: end-to-side to the mid-left common carotid artery, end- to-end to the right common carotid artery after resecting the redundant proximal portion, and end-to-side to the RAx (Fig. 4). The 16 mm limb of the graft was then attached end-to-end to the cardiopulmonary bypass (CPB) circuit for arterial inflow. A 25F Bio-Medicus Multi-Stage Femoral Venous Catheter (Medtronic, Santa Rosa, California) was placed in the right common femoral vein for venous drainage into the CPB circuit.
Figure 4.
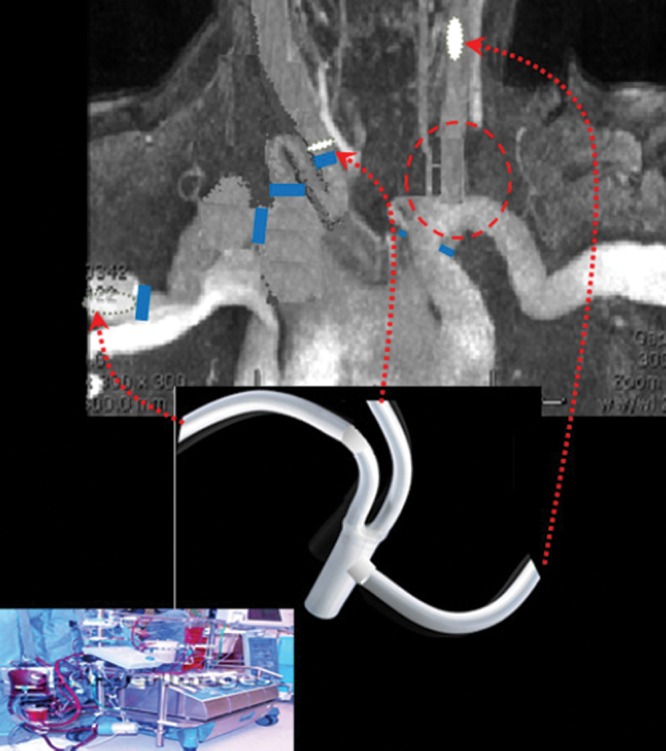
Construction of a temporary parallel cerebrovascular circuit for cerebral protection during redo-sternotomy and aortic arch reconstruction with (a) a left common carotid to left subclavian artery transposition (inside red circle); (b) a three-limbed graft to the mid-left common carotid artery, right common carotid artery, and right subclavian artery (red arrows); and (c) a pump used as inflow, connected to the three-limbed graft (bottom center and left).
Flow into the RScA was excluded with a staple line. Visualization of the RScA origin was possible only after the right common carotid artery had been transected, permitting anterior retraction of the aneurysmal InomA. The RScA branches were clipped in continuity and the proximal RAx was ligated just below the clavicle, excluding retrograde flow into the RScA. Intraoperative imaging showed no central neurological changes.
CPB was then instituted at 6 L/min, reversing flow in the Gore-Tex® external circuit into the RAx, the right and left common carotid arteries, and down the LScA into the aorta. The left ventricle (LV) was monitored for distention with transesophageal echocardiography. At 25°C the left common carotid artery was clamped between the Gore-Tex® external circuit and the LScA. This arrested arterial flow into the aorta and the rest of the cooled body, while preserving antegrade cerebral perfusion (ACP) at 2 L/min via the external Gore-Tex® circuit into the right and left common carotid arteries and the RAx.
Sternotomy followed without entry into the depressurized aneurysm and adjacent venous system. Via an intrapleural right pulmonary venotomy, a vent was placed across the mitral valve for LV decompression. The giant aneurysm was opened and the heart was arrested by giving cardioplegia directly into the left and right coronary arteries. A 30 mm Vascutek Gelweave 4 Plexus interposition graft with three arch branches and a side-arm (Teurmo, Ann Arbor, Michigan) was sewn end-to-end into the distal arch at the level of the origin of the LScA. Then the graft's side-arm conduit was attached as a second arterial inflow from the pump so that CPB could be restored to the body at 6 L/min.
While the patient was rewarmed, an aortic valve replacement was performed using a 23 mm Edwards Magna Bioprosthetic Aortic Valve (Edwards Lifesciences, Irvine, California). The proximal end of the Dacron graft was then sewn end-to-end to the remnant of the old proximal AscAo Dacron graft. The ACP and aortic cross-clamp times were 23 and 98 min, respectively. A pulse was regained, and the Gore-Tex® circuit was then excised while weaning from CPB. Its attachment to the left common carotid artery was disconnected and the artery repaired. The attachment to the right common carotid artery was excised and the artery was immediately sewn end-to-end to one of the three Dacron side-arms on the arch graft. The Gore-Tex® limb to the RAx was divided and sewn end-to-end to the second Dacron side-arm on the arch graft. The third side-arm of the Dacron arch graft was not needed.
No focal neurological deficits developed postoperatively, and the patient was extubated on postoperative day 3. The postoperative course was complicated by pneumonia, resulting in reintubation on postoperative day 7. The patient was discharged to a rehabilitation facility, neurologically intact, and with no evidence of infarcts on his follow-up brain CT scan. Cardiac function remained excellent and renal function was normal.
Discussion
This case report highlights a serious complication of temporal arteritis: aortic aneurysm. The incidence of giant cell arteritis (GCA) in the US has been reported as high as 27 cases per 100,000 persons over 50 years old. Patients with Northern European ancestry have a higher incidence [1]. The aortic root is involved in 9-18% of cases [2]. In a population study of Olmsted County, Minnesota, the incidence of GCA was 17.8 per 100,000 persons over 50 years old [3]. The incidence of thoracic aneurysm, and of isolated abdominal aortic aneurysm, was 17 times [95% CI (confidence interval), 8-33], and 2.4 times (95% CI, 0.8-5.5) higher, respectively, compared to patients without GCA. Thoracic aneurysms developed as far out as 15 years after treatment of GCA. This case report also identifies a modality to safely reenter a chest when an aneurysm is adjacent to the wired sternotomy closure, and illustrates how a combination of extra-anatomic strategies and CPB is feasible in an elderly patient with extensive aortic arch, aortic valve, and branch artery pathology.
There was no evidence of granulomatous inflammation or giant cells, hallmarks of GCA, in this patient's aorta at either operation. There was, however, patchy extensive destruction of the transmural lamellar architecture (disappearance of the elastic fibers, fibrosis of the adventitia, thickening of the intima) and prominent penetrating vasa vasora, which are features of the disease. There is one other report of mega-aorta following diagnosis of GCA [4]. However, in our case, the absence of hypertension, tobacco use, emphysema, and hyperlipidemia in this patient suggests that GCA played a significant role in the evolution of his large aneurysms. The patient was only briefly treated with steroids after a positive temporal artery biopsy for GCA in 1989. Whether this patient's aneurysms were preventable if he had had more aggressive medical management of his arteritis is unknown, since medical management of temporal arteritis is still evolving, and there are isolated reports of aneurysm development even after prolonged immunosuppression. Notably, his first 6 cm proximal AscAo aneurysm was treated four years after diagnosis of temporal arteritis.
Perhaps the progression of the aneurysm disease could have been avoided if pathological analysis had been conducted at the first operation in order to yield a diagnosis and commence aggressive medical management. Genetic analysis could also have been conducted as individuals homozygous for HLA-DR4 and the FCGR2A-131RR allele have a 6-fold greater risk of developing temporal arteritis. Further, both the HLA-DR1, HLA-DR3, and HLA-DR5 genes and polymorphisms in immune-modulating proteins such as interleukin-1 receptor antagonist, tumor necrosis factor-α, and intracellular adhesion molecule-1 are associated with a greater incidence of temporal arteritis.
Although the axial growth of this patient's aneurysms was remarkable, the longitudinal growth was equally impressive. The InomA extended significantly into the patient's neck. Both common carotid arteries were so redundant they each developed two nearly 180 degree turns. Arteriogenesis, the maturation of collaterals around an occluded large artery, also generates (on a much smaller scale) angiographically visible “corkscrew” collaterals due to both longitudinal and radial growth. While they both generate “corkscrew” vessels on a macroscopic level, GCA and arteriogenesis also have similar biochemical mechanisms. In both processes, endothelial cells express adhesion molecules which bind circulating inflammatory cells that are then brought subendothelially. There, they secrete membrane metalloproteinases and nonspecific serine proteases that digest the underlying supportive protein matrix, allowing for growth of the artery. Further elucidation of the mechanism of arterial growth in GCA may be dually beneficial. Insight into the mechanism of arterial growth may help design therapeutic strategies for arterial collateral growth in chronic severe limb-threatening ischemia, but also therapeutic inhibition of aneurysm expansion.
Regarding the operative approach, a temporary parallel cerebrovascular circuit was constructed rather than performing a simple axillary cannulation for two reasons. First, given the patient's multiple aneurysms in the great vessels, extensive replacement of the great vessels was not indicated. Second, the temporary parallel circuit provided the added benefit of increased cerebral protection. We believed that there was a significant likelihood that we would enter the aorta on sternotomy. Given the patient's age and the complexity of the procedure, we wanted to ensure cerebral protection. The patient was cooled before opening; however, due to the patient's significant aortic valve insufficiency, there was concern that the ventricle would dilate and the patient would fibrillate, without being sufficiently cool for adequate cerebral protection.
In summary, this case report presents an 82-year-old patient who developed progressive aneurysmal aortic degeneration due to giant cell arteritis. The patient underwent initial ascending aortic aneurysm repair, and presented to our center over 15 years later with multiple, large aneurysms. The patient required a high-risk redo-reconstruction that necessitated the use of a temporary parallel cerebrovascular circuit. The operation was successful, and the patient was discharged to a rehabilitation facility on postoperative day 7, neurologically intact, with no evidence of infarcts on his follow-up brain CT scan.
Conflict of Interest
The authors have no conflict of interest relevant to this publication.
References
- 1. Goodwin JS. Progress in gerontology: polymyalgia rheumatica and temporal arteritis. J Am Geriatr Soc. 1992;40:515–525. [DOI] [PubMed] [Google Scholar]
- 2. Cid MC, García-Martínez A, Lozano E, Espígol-Frigolé G, Hernández-Rodríguez J. Five clinical conundrums in the management of giant cell arteritis. Rheum Dis Clin North Am. 2007;33:819–834, vii 10.1016/j.rdc.2007.08.001 [DOI] [PubMed] [Google Scholar]
- 3. Salvarani C, Gabriel SE, O'Fallon WM, Hunder GG. The incidence of giant cell arteritis in Olmsted County, Minnesota: apparent fluctuations in a cyclic pattern. Ann Intern Med. 1995;123:192–194. 10.7326/0003-4819-123-3-199508010-00006 [DOI] [PubMed] [Google Scholar]
- 4. Morgan AW, Robinson JI, Barrett JH, Martin J, Walker A, Babbage SJ, Ollier WE, Gonzalez-Gay MA, Isaacs JD. Association of FCGR2A and FCGR2A-FCGR3A haplotypes with susceptibility to giant cell arteritis. Arthritis Res Ther. 2006;8:R109 10.1186/ar1996 [DOI] [PMC free article] [PubMed] [Google Scholar]